Abstract
Sentinel lymph node biopsy has become the routine procedure in axilla-negative breast cancer patients at most medical centers for axillary staging and local control in the recent years. Sentinel lymph node is the only focus in axillary lymph metastasis in a large portion of patients. In our trial, we investigated the clinical and pathological factors that affect the positive status of sentinel lymph node. We included 89 patients, who underwent sentinel lymph node biopsy (SLNB) with methylene blue and/or technetium-99 m Sulphur Colloid due to early-stage breast cancer. Five patients, in whom SLN was not detected and who underwent axillary dissection, were excluded from the trial. The patient age, location of the tumor, the type of the tumor, the T stage by the TNM staging system, the histological grade and type of the tumor, the status of multifocality, the lymphovascular invasion status of the tumor, and the ER, PR, and HER-neu2 status were recorded. The median age of the 89 patients was 52, 9 (±10) years. Fifty-seven (64 %) and 32 (36 %) of the 89 patients were detected to have positive and negative SLN, respectively. Assessing the SLNB positivity and the patient age, tumor size, tumor grade, multifocality, tumor localization, the T stage by the TNM staging, the ER/PR positivity/negativity, and the HER/neu2 and p53 status, the data revealed no statistically significant results with respect to SLN metastasis. The lymphovascular invasion status (LVI) was observed to statistically affect the SLN positivity (p < 0.016). We showed that LVI could be an important marker in predicting the SLN positivity in patients with axilla-negative early-stage breast cancer. In the future, upon introduction of new biomarkers and with relevant studies, it may be possible to predict the SLNB status of patients at an adequately high accuracy and a low risk.
Keywords: Sentinel lymph node biopsy, Breast cancer, Axillary lymph metastasis, Lymphovascular invasion
Introduction
The axillary lymph node status is considered as a significant prognostic factor in predicting the clinical outcome in invasive breast cancer. Starting from the 1990s, sentinel lymph node biopsy (SLNB) has been routinely performed to evaluate the axillary lymph node (ALN) status and reduce the complications associated with axillary lymph node dissection (ALND). Sentinel lymph nodule biopsy is a method that provides data on axillary involvement and prognosis. Sentinel lymph node is described as the first lymph nodule that receives the lymphatic flow in the axilla. Theoretically, in case of absence of metastatic involvement in the sentinel lymph nodule, no metastasis is considered to occur also in the other non-sentinel lymph nodules (NSLN), which are located in the axilla [1]. Sentinel lymph node biopsy has been detected to enable a shorter duration of hospitalization, lower costs, and a more accurate and precise determination of the adjuvant therapy as well as reduce complications secondary to routine axillary dissection such as pain in the arm and numbness [2, 3]. In order to obtain an accurate axillary nodal status of clinically axillary node-negative breast cancer patients, it is important to successfully identify the SLN. Thus far, the factors known to affect SLN identification rates include the age, lymphovascular invasion (LVI), body mass index (BMI), tumor grade, SLN mapping methods, and tumor location.
Material and Method
We included 89 patients diagnosed with early-stage breast cancer at Izmir KatipCelebi University Ataturk Training and Research Hospital, 1st General Surgery Clinic between January 2011 and October 2013, who were not detected to have clinically axillary lymph node metastasis and who were administered a successful SLNB procedure. The patient files were retrospectively investigated. Among 94 patients, in whom SLNB was planned for breast cancer within the time interval indicated, methylene blue and technetium-99 m Sulphur Colloid (combination technique) was used in 80 patients; methylene blue alone was used in 8 patients and technetium-99 m Sulphur alone was used in 6 patients. Of these patients, four patients who used the combination technique and one patient who used methylene blue only were not observed to have SLN and underwent axillary dissection; these five patients were excluded from the trial. All patients underwent physical examination (PE), mammography (MMG), and ultrasonography (USG) for the purpose of diagnosis and therapeutic planning; seven patients additionally underwent magnetic resonance imaging (MRI). Forty-six, 29, and 14 patients were diagnosed using excisional biopsy, fine needle aspiration biopsy (FNAB), and tru-cut biopsy, respectively, with 15 patients undergoing the procedure in company with stereotactic marking and three patients in company with the radionuclide occult lesion localization (ROLL) method. Performing nuclear lymphoscintigraphy in patients, in whom radiocolloids were used, the day before the operation (peri-areolar, 0.1 cc × 4 RK), SLNB was conducted via small axillary incision or mastectomy incision using gamma probe while seven patients underwent SLNB with methylene blue. Methylene blue injection was performed 10 min before incision following appropriate surgical preparation using a 20-gauge needle via subareolar and subdermal routes. Five to 10 min of massage was applied followed by penetration into the axillary region via small axilla incision or breast incision (using a probe beforehand where activity was detected over the skin). Lymph nodes, which showed activity as detected by gamma probe and/or stained in blue, were found. Tracking the blue-stained lymph canal towards the axilla and breast, the lymph node with the highest activity was detected. All the lymph nodes, which were detected to have an activity exceeding the activity value measured in this lymph node via gamma probe by 10 %, were considered to be SLN and excised and put under pathological investigation. During the pathological inspection of the sentinel lymph nodes, lymph nodes >5 mm were transferred to cassettes in two pieces and the nodes >5 mm were transferred in serial sections at 3-mm intervals. Each section was processed and imprinted and evaluated using hematoxylene-eosin (HE). All tissues were subject to frozen incisions followed by fixation and paraffin blocking of all sentinel lymph nodes. From these sections, five from each level were transferred onto empty slides at 25-μm intervals. One section was transferred to lysine-added slide for immunohistochemical staining. All the sentinel lymph nodes were put under immunohistochemical investigation after staining with cytokeratin. If the patient was detected to have a change in the disease stage as shown by these investigations, the patient was administered axillary dissection. The patient age, location of the tumor, the type of the tumor, the T stage by the TNM (T: tumor, N: node, M: metastasis) staging system, the histological grade and type of the tumor, the status of multifocality, the lymphovascular invasion status of the tumor, and the ER, PR, and HER-neu2 status were recorded. The numbers of the SLN positive and negative patients were determined and the SLN ratio was calculated. The recordings were translated into statistical data using SPSS 15 software. To detect the factors that are effective on the SLNB metastasis, single variable analyses were conducted with Mann-Whitney U, chi-square tests using the SPSS 15.0 software.
Results
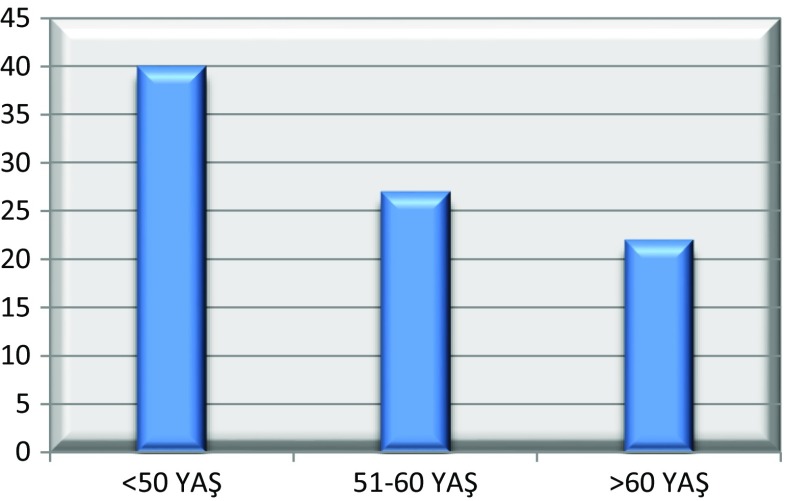
Eighty-nine patients were included in the trial. The mean age was 52, 9 (±10) (33–79). The distribution of the patients by age range is given in Graphic 1 and the histopathological characteristics of the tumor are presented in Table 1. The mean menarche age of the patients was 12, 9 (±1), and 81 patients had a history of breastfeeding (91 %). Thirty-three patients (37 %) were pre-menopausal and 56 were post-menopausal (63 %). The mean age of menopause was 46, 8 (±2, 7). Eight patients (9 %) had familial history of breast cancer. The mean tumor diameter was 2.24 cm (diameter range: 0.13–5 cm), and while the tumor diameter was 2.51 cm in SLN positive patients, it was 2.05 cm in SLN negative patients. Thirty-two patients (36 %) had a positive SLN biopsy, and the mean number of SLNs extracted per patient was 3.17 (number range: 1–8). Reviewing the effect of the patient age on the SLNB positivity, no statistically significant correlation was detected (Pearson chi-square) (p: 0.133); however, patients above 50 years of age were observed to have a markedly increased SLNB positivity compared to those below 50 years of age (Table 2). The effect of the histopathological tumor factors on lymph node positivity is presented in Table 3. Among these parameters, only the presence of lymphovascular invasion was detected to have a statistically significant effect on the SLNB positivity (p: 0.016).
Fig. 1.
Distribution of age in patients by the age range
Table 1.
Histopathological features of the tumors
| Tumor localization | Number of patients | Percentage (%) |
|---|---|---|
| Upper outer quadrant | 53 | 59, 6 % |
| Lower outer quadrant | 20 | 22, 5 % |
| Upper inner quadrant | 7 | 7, 9 % |
| Lower inner quadrant | 5 | 5, 6 % |
| Central | 4 | 4, 5 % |
| Total | 89 | 100 % |
| Tumor size | ||
| T1 | 56 | 63, 9 % |
| T1a | 1 | 1, 1 % |
| T1b | 11 | 12, 4 % |
| T1c | 44 | 49, 4 % |
| T2 | 33 | 37, 1 % |
| Total | 89 | 100 % |
| ER | ||
| Negative | 17 | 19 % |
| Positive | 71 | 81 % |
| Total | 89 | 100 % |
| PR | ||
| Negative | 23 | 24, 8 % |
| Positive | 66 | 74, 2 % |
| Total | 88 | 100 % |
| p53 | ||
| Negative | 27 | 46, 6 % |
| Positive | 31 | 53, 4 % |
| Total | 58 | 100 % |
| Her2/neu | ||
| Negative | 50 | 61, 7 % |
| Positive | 31 | 38, 3 % |
| Total | 81 | 100 % |
| LVI | ||
| Negative | 81 | 91 % |
| Positive | 8 | 9 % |
| Total | 89 | 100 % |
Table 2.
Effect of the patient age on SLNB positivity (Pearson chi-square)
| Age | SLNB | Total | |
|---|---|---|---|
| Negative | Positive | ||
| ≤50 | 29 (72, 5 %) | 11 (27, 5 %) | 40 (100 %) |
| >50 | 28 (57, 1 %) | 21 (42, 9 %) | 49 (100 %) |
| TOTAL | 57 (64 %) | 32 (36 %) | 89 (100 %) |
Table 3.
The effect of the tumor’s histopathological factors on the sentinel lymph node positivity (Pearson chi-square)
| Variables | SLN | p value | |
|---|---|---|---|
| Negative % (n:57) | Positive % (n:32) | ||
| Age | |||
| ≤50 | 29 (72, 5 %) | 11 (27, 5 %) | 0, 133 |
| >50 | 28 (57, 1 %) | 21 (42, 9 %) | |
| Tumor size | |||
| T1 | 39 (69, 6 %) | 17 (30, 4 %) | 0, 330 |
| T1a | 1 (100 %) | 0 (0 %) | |
| T1b | 9 (81, 8 %) | 2 (18, 2 %) | |
| T1c | 29 (65, 9 %) | 15 (34, 1 %) | |
| T2 | 18 (54, 5 %) | 15 (45, 5 %) | |
| Multifocality | |||
| Yes | 3 (75 %) | 1 (25 %) | 0, 640 |
| No | 54 (63, 5 %) | 31 (36, 5 %) | |
| Tumor localization | |||
| Upper outer quadrant | 29 (54, 7 %) | 24 (45, 3 %) | 0, 069 |
| Lower outer quadrant | 17 (85 %) | 3 (15 %) | |
| Upper inner quadrant | 6 (85, 7 %) | 1 (14, 3 %) | |
| Lower inner quadrant | 2 (40 %) | 3 (60 %) | |
| Central | 3 (75 %) | 1 (25 %) | |
| Histopathology | |||
| Ductal | 49 (69 %) | 22 (31 %) | 0, 068 |
| Lobular | 1 (16, 7 %) | 5 (83, 3 %) | |
| Mix | 3 (50 %) | 3 (50 %) | |
| Other | 4 (66, 7 %) | 2 (33, 3 %) | |
| Lymphovascular invasion | |||
| No | 55 (67, 9 %) | 26 (32, 1 %) | 0, 016 |
| Yes | 2 (25 %) | 6 (75 %) | |
| Histological grade | |||
| 1 | 9 (69, 2 %) | 4 (30, 8 %) | 0, 786 |
| 2 | 35 (61, 4 %) | 22 (38, 6 %) | |
| 3 | 13 (68, 4 %) | 6 (31, 6 %) | |
| ER | |||
| Positive | 44 (62 %) | 5 (29, 4 %) | 0, 507 |
| Negative | 12 (70,6 %) | 27 (38 %) | |
| PR | |||
| Positive | 42 (63, 6 %) | 24 (36, 4 %) | 0, 507 |
| Negative | 14 (63, 6 %) | 8 (36, 4 %) | |
| Her2/neu | |||
| Positive | 20 (64, 5 %) | 11 (35, 5 %) | 0, 684 |
| Negative | 30 (60 %) | 20 (40 %) | |
| P53 | |||
| Positive | 21 (67, 7 %) | 10 (32, 3 %) | 0,503 |
| Negative | 16 (59, 3 %) | 11 (40, 7 %) | |
There was not any correlation between tumor biology and SLN positivity. Micrometastatic deposits in sentinel lymph nodes were detected in six patients, five with ductal carcinoma and one with mixed type. This patient group was accepted to be SLNB positive and underwent axillary dissection.
Discussion
Today, most of the women diagnosed with breast cancer have a similar quality of life with other women within the same age range. Early diagnosis, the biological behavior of the tumor and the availability of effective adjuvant therapeutic methods provide satisfactory outcomes for patients. Recent studies demonstrate that mastectomy or breast-preserving surgery performed in stage I and stage II breast cancer patients do not cause a significant difference between the disease-free survival and overall survival times of patients [4, 5]. Today, sentinel lymph node biopsy (SLNB) is accepted as the main surgical method for clinical axillary nodal staging in axillary lymph node-negative breast cancer patients [6–8]. Thus far, various predictive factors including the age, body mass index (BMI), tumor localization, and stage and the method of determining the sentinel lymph node were indicated to be effective on the SLN positivity [9, 10]. In their 2004 study, Viale et al. investigated the predictive factors affecting the sentinel lymph node metastatic status in 4333 female and 7 male patients treated at the European Institute of Oncology and University of Milan between 1996 and 2003 [11]. They detected a marked correlation between SLN positivity and various factors such as the patient age, tumor size, grade, multifocality, the presence of peri-tumoral vascular invasion, high proliferation, and PR negativity. Investigating the patients by age group, the increasing age was observed to be inversely proportional to SLN positivity.
The definition of LVI is the presence of an invasion of cancer cells into the blood vessels or lymphatic channels. Positive LVI is correlated with aggressive tumor behavior and metastatic ability [12]. LVI has been consistently shown to be predictive of ALNM in many studies [12–16]. The LVI also increases the incidence of non-sentinel lymph node metastases and isolated tumor cells in the sentinel lymph node [15, 17]. The presence of LVI as the most important predictor is well accepted.
In a retrospective study of 256 patients by Elezoglu, increase in SLN metastasis was detected to be associated with the vascular invasion of the primary tumor, the presence of lymphatic invasion, increased primary tumor diameter, and advanced age [18]. In a study by Ozmen et al., a tumor size larger than 2 cm (comparison of the T1 and T3 tumors) and the LVI presence were associated with SLN positivity [19]. In a 2009 study by Capdet et al., as the patient age increased, the SLN positivity was also reported to increase [20]. Again, in the same trial, the tumors located in the inner quadrants were observed to have a lower SLN positivity relative to outer quadrant tumors. In contrast, the study by Ozmen et al. failed to confirm such a correlation. In line with the results from the study by Ozmen et al., we also showed no effect of tumor localization on the SLN positivity. In addition, no statistically significant correlation was detected between the patient age and the primary tumor diameter and the increased SLN metastasis, and the presence of lymph vascular invasion was observed to statistically affect the SLN positivity.
Reviewing the previous studies, the association between the hormonal receptors (ER/PR, HER/neu2) and the SLN positivity was assessed [18–20]. In a trial by Viale et al., a higher rate of SLN metastasis was detected in PR negative patients; however, in other studies, as is the case in our trial, no such correlation was detected [11, 15, 17].
According to American Society of Clinical Oncology (ASCO) [21], until further clinical studies on the importance of the Isolated Tumor Cell or micro metastases will not be finalized, the guidelines recommend the axillary dissection for patients with micrometastases in SLN, whereas there are no indications for the treatment of ITC. More extensive studies are needed in this subject. We treated our patients with micrometastases as SLNB positive.
Conclusion
LVI is an important predictive factor for the lymph node metastasis, and LVI presence is a high risk for positive lymph nodes.
With a model based only on clinical routine pathologic parameters obtained from the primary tumor, it is possible to predict SLN status. Based on the current data, it may be possible to perform therapeutic and diagnostic procedures in the axilla in breast cancer patients without affecting the patient survival and by increasing the quality of life. In the future, upon introduction of new biomarkers and with relevant studies, it may be possible to predict the SLNB status of patients at an adequately high accuracy and a low risk.
Acknowledgments
The authors thank all the general surgery staff for their cooperation. All the authors read and approved the paper.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer: a multicenter validation study. N Engl J Med. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 2.Schrenk P, Shamiyeh A, Wayand W. Sentinel lymph-node biopsy compared to axillary lymph-node dissection for axillary staging in breast cancer patients. Eur J Surg Oncol. 2001;27:378–382. doi: 10.1053/ejso.2001.1139. [DOI] [PubMed] [Google Scholar]
- 3.Weiser MR, Montgomery LL, Tan LK, et al. Lymphovascular invasion enhances the prediction of non-sentinel node metastases in breast cancer patients with positive sentinel nodes. Ann Surg Oncol. 2001;8:145–149. doi: 10.1007/s10434-001-0145-y. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi M. Sentinel lymph node biopsy as an alternative to routine axillary lymph node dissection in breast cancer patients. J Surg Oncol. 2001;76:144–156. doi: 10.1002/1096-9098(200102)76:2<144::AID-JSO1028>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Galimberti V, Mariani L, et al. Sentinel node biopsy in breast cancer: early results in 953 patients with negative sentinel node biopsy and no axillary dissection. Eur J Cancer. 2005;41:231–237. doi: 10.1016/j.ejca.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Intra M, Rotmensz N, Mattar D, et al. Unnecessary axillary node dissections in the sentinel lymph node era. Eur J Cancer. 2007;43:2664–2668. doi: 10.1016/j.ejca.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Cox CE, Dupont E, Whitehead GF, et al. Age and body mass index may increase the chance of failure in sentinel lymph node biopsy for women with breast cancer. Breast J. 2002;8:88–91. doi: 10.1046/j.1524-4741.2002.08203.x. [DOI] [PubMed] [Google Scholar]
- 10.Goyal A, Newcombe RG, Chhabra A, ALMANAC Trialists Group et al. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer: results of the ALMANAC validation phase. Breast Cancer Res Treat. 2006;99:203–208. doi: 10.1007/s10549-006-9192-1. [DOI] [PubMed] [Google Scholar]
- 11.Viale G, Zurrida S, Malorano E, et al. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer. 2005;103:492–500. doi: 10.1002/cncr.20809. [DOI] [PubMed] [Google Scholar]
- 12.Gurleyik G, Gurleyik E, Aker F, et al. Lymphovascular invasion, as a prognostic marker in patients with invasive breast cancer. Acta Chir Belg. 2007;107:284–287. doi: 10.1080/00015458.2007.11680057. [DOI] [PubMed] [Google Scholar]
- 13.Nos C, Harding-MacKean C, Freneaux P, et al. Prediction of tumour involvement in remaining axillary lymph nodes when the sentinel node in a woman with breast cancer contains metastases. Br J Surg. 2003;90:1354–1360. doi: 10.1002/bjs.4325. [DOI] [PubMed] [Google Scholar]
- 14.Tan YY, Wu CT, Fan YG, et al. Primary tumor characteristics predict sentinel lymph node macrometastasis in breast cancer. Breast J. 2005;11:338–343. doi: 10.1111/j.1075-122X.2005.00043.x. [DOI] [PubMed] [Google Scholar]
- 15.Hwang RF, Krishnamurthy S, Hunt KK, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10:248–254. doi: 10.1245/ASO.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Wong JS, O’Neill A, Recht A, et al. The relationship between lymphatic vessell invasion, tumor size, and pathologic nodal status: can we predict who can avoid a third field in the absence of axillary dissection? Int J Radiat Oncol Biol Phys. 2000;48:133–137. doi: 10.1016/S0360-3016(00)00605-2. [DOI] [PubMed] [Google Scholar]
- 17.Mittendorf EA, Sahin AA, Tucker SL, et al. Lymphovascular invasion and lobular histology are associated with increased incidence of isolated tumor cells in sentinel lymph nodes from early-stage breast cancer patients. Ann Surg Oncol. 2008;15:3369–3377. doi: 10.1245/s10434-008-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elezoglu B, Tolunay Ş, Tasdelen İ, et al. Histopathologic characteristics of sentinel lymph node biopsy in breast carcinoma: Uludağ University Faculty of Medicine Experience. Turk Klin J Med Sci. 2011;31:1324–1329. [Google Scholar]
- 19.Ozmen V, Karanlik H, Cabioglu N, et al. Factors predicting the sentinel and non-sentinel lymph node metastases in breast cancer. Breast Cancer Res Treat. 2006;95:1–6. doi: 10.1007/s10549-005-9007-9. [DOI] [PubMed] [Google Scholar]
- 20.Capdet J, Martel P, Charitansky H, et al. Factors predicting the sentinel node metastases in T1 breast cancer tumor: an analysis of 1416 cases. EJSO. 2009;35:1245–1249. doi: 10.1016/j.ejso.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Lyman Gary H, Giuliano Armando E, et al. American society of clinical oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]