Abstract
Objectives
To study the relationship between sperm DNA fragmentation (SDF) and reactive oxygen species (ROS) levels in infertile patients with varicocele, and to examine the beneficial effect of varicocelectomy and elucidate predictors of improvement after repair.
Patients, subjects and methods
We prospectively studied 60 patients with varicocele and abnormal semen variables who attended the outpatient clinic complaining of infertility for ≥12 months. In all, 25 patients (41.7%) had bilateral varicoceles and 35 (58.3%) had left varicoceles. The DNA fragmentation index (DFI%, percentage of sperm with denatured nuclei), ROS and total non-enzymatic antioxidant capacity (TAC) were measured. Inguinal varicocelectomy was performed in all patients. At 3–6 months postoperatively, all measurements were repeated. A control group, comprised of 20 normozoospermic fertile men, was included. Regression analysis was used to examine predictors of improvement.
Results
The mean (SD) DFI% in the 60 infertile patients with varicocele was 29.9 (8.3) and 7.56 (2.84)% in the controls; ROS levels were 4.49 (0.9) in patients and 2.62 (0.8) photons/min in controls; and the TAC was 0.97 (0.4) in patients and 1.5 (0.5) mM in controls; with highly significant differences between the patients and controls. The DFI% showed a positive correlation with ROS levels, whilst the total motile sperm count (TMSC) had a significant negative correlation with DFI%, ROS levels and grade of varicocele, whilst there was significant positive correlation with TAC. The grade of varicocele and duration of infertility were related to the presence of higher levels of ROS and increased of DFI%. Postoperatively, improvement (measured as a >50% increase in TMSC) occurred in 40 of 55 (73%) patients available at follow-up, with a significant reduction in the mean (SD) DFI% from 29.49 (8.58) to 18.78 (7.23)%, ROS levels from 4.49 (0.88) to 3.27 (1.3) photons/min (both P < 0.001), and a significant increase in the mean (SD) TAC from 1.01 (0.44) to 2.05 (0.51) mM (P < 0.001). Responders had a shorter infertility duration and lower preoperative DFI% and ROS levels. Regression analysis showed that DFI% is a predictor of improvement after varicocelectomy.
Conclusion
SDF was shown to have a negative impact on improvement after varicocelectomy. Hence, DFI% could be recommended as a prognostic test in infertile patients with varicocele to help decision-making as regards the necessity and the anticipated outcome of varicocelectomy in patients with infertility.
Abbreviations: AO, acridine orange; DFI%, DNA fragmentation index, percentage of sperm with denatured nuclei; LH, luteinising hormone; NO, nitric oxide; PBS, phosphate-buffered saline; ROS, reactive oxygen species; SCSA, sperm chromatin structure assay; SDF, sperm DNA fragmentation; TAC, total antioxidant capacity; TMSC, total motile sperm count
Keywords: Sperm DNA damage, Varicocele, Oxidative stress, Infertility
Introduction
Varicocele is one of the major causes of infertility in men, occurring in 15–20% of the general male population [1]. In men with a normal semen analysis varicocele is found in 11.7%, whilst in those with abnormal seminal variables varicocele is found in 25.4% [2]. The pathogenesis of testicular dysfunction and infertility in association with varicocele is probably due to multiple factors, e.g. venous stasis leading to testicular hypoxia, elevation of testicular temperature, reflux of toxic metabolites from adrenal or renal origin, and impairment of the hypothalamic–pituitary–gonadal axis [3], [4]. Reactive oxygen species (ROS) are reactive molecules or free radicals generated as a by-product of normal aerobic metabolism by the reduction of oxygen [5]. Their expression at physiological levels has roles in sperm capacitation and cellular differentiation, whilst oxygen-free radicals at concentrations beyond physiological limits result in oxidative stress [6]. Previous reports have shown that infertile men with clinically diagnosed varicocele have high levels of seminal oxidative stress, as evidenced by increased levels of ROS and reduced total antioxidant capacity (TAC), suggesting that sperm dysfunction in patients with varicocele may be in part related to oxidative stress [7], [8], [9]. DNA integrity with the presence of single- and double-strand DNA breaks was found in the ejaculates of infertile men as consequence of oxidative stress [10]. Sperm DNA integrity is one of the essential determinants of normal fertilisation and embryo growth in natural and assisted conception [11], [12], [13]. Currently, the sperm chromatin structure assay (SCSA) is a clinically applicable method for calculating the sperm DNA fragmentation index (DFI%, percentage of sperm with denatured nuclei) to determine the susceptibility of sperm DNA to denaturation [14]. There is no conclusive evidence that a varicocelectomy improves spontaneous pregnancy rates; however, its beneficial effect on various sperm variables [15], improvement of sperm DNA damage [16], and improvement of the testicular microcirculation has been evaluated [17]. Varicocele repair also has a beneficial effect in reducing seminal oxidative stress [18]. Recently, Esteves et al. [19] hypothesised that a decrease in the DFI% after varicocele repair could serve as an indicator of oxidative stress alleviation, and recommended further evaluation of varicocele repair on DFI%. Considering the above-mentioned findings, the aim of the present study was to investigate the relationship between sperm nuclear DNA damage and ROS levels in ejaculated spermatozoa of infertile patients with varicocele and to examine the beneficial effect, if any, of varicocelectomy and to elucidate predictors of improvement after repair.
Patients, subjects and methods
Study design and patient population
A prospective open-label study conducted at the Department of Urology, Department Dermatology Venereology and Andrology and Department of Clinical and Chemical Pathology at Benha University between November 2013 and May 2015, included 60 infertile men complaining of inability to conceive for ≥12 months of unprotected intercourse and abnormal seminal variables (reduced sperm concentration, motility and morphology on two or more semen samples) associated with unilateral or bilateral clinical varicocele (Grade 1–3). A control group, comprised of 20 normozoospermic healthy fertile men with normal standard semen variables according to WHO criteria [20], was also included. Patients with subclinical varicocele, azoospermia, systemic or endocrine disease, male accessory gland infection, cryptorchidism, testicular atrophy, cigarette smoking, alcohol or drug abuse, or recent hormonal treatment for fertility were excluded from the study. Other exclusion criteria were patients with all other factors that could affect sperm DNA fragmentation (SDF) such as obesity, patients with leucocytospermia or exposed to gonadotoxins, radiochemotherapy, and patients with cancer. The study was approved by the Ethics Committee of the Faculty of Medicine, Benha University. Informed consents for participation were obtained from all patients and the procedure and possible risks were explained thoroughly, according to the Declaration of Helsinki. Patients with palpable varicoceles on physical examination were further confirmed by scrotal ultrasonography. The patients were examined by grey-scale and duplex colour Doppler ultrasonography using a 7.5-MHz probe (Diagnostic Ultrasound Equipment, model SSA-350A, Toshiba Corporation, Japan), during normal respiration and during the Valsalva manoeuvre. The criteria adopted were: >2 mm diameter of a vein of the pampiniform plexus, and flow reversal duration on Valsalva manoeuvre of >1 s with an increase in vein diameter of >3 mm. Grading of varicocele was as follows: grade 0, no dilated vein; Grade 1, dilated veins of <2.5 mm in diameter, with no flow reversal after a Valsalva manoeuvre; Grade 2, dilated tortuous veins of 2.5–3.5 mm in diameter and flow reversal after a Valsalva manoeuvre; and Grade 3, dilated tortuous veins of >3.5 mm in diameter and flow reversal after a Valsalva manoeuvre [21], [22]. All ultrasonographic studies were performed by one experienced examiner (A.M.A.), blinded to the findings of the physical examination, to prevent bias.
Sperm collection and semen analysis
Semen samples were obtained after 3–4 days of sexual abstinence starting <4 weeks before intervention and standard semen analysis was performed within 1 h of collection according to the WHO guidelines [20]. Aliquots of raw semen (containing 1 × 106 spermatozoa) were routinely frozen and stored at −80 °C for later assessment of sperm DNA damage. The semen samples were all analysed by the same clinical pathologist (J.S.). The total motile sperm count (TMSC) was calculated using the following formula: ejaculate volume (mL) × concentration (millions/mL) × motility (%) [23]. Sperm parameters were considered normal when levels equal to or exceeding the 5th percentile as accepted reference lower limits, these being; volume 1.5 mL, concentration 15 × 106/mL, total count 39 × 106 per ejaculate, motility 40% (progressive and non-progressive), and normal morphological forms 4% [20]. At ≥3 months after varicocelectomy another semen sample was obtained and compared to the values measured before surgery.
Measurement of SDF
Sperm DNA damage was measured using the SCSA. The assay measures the susceptibility of sperm nuclear DNA to in situ acid-induced DNA denaturation. As previously described [24], frozen seminal samples containing 1 × 106 spermatozoa were thawed and treated immediately with detergent solution (pH 1.2) containing 0.1% Triton X-100, 0.15 M NaCl and 0.08 M HCl. Spermatozoa were stained after 30 s with a staining solution containing 6 µg/mL acridine orange (AO) in a phosphate citrate (pH 6) buffer. A BD FACScalibur™ flow cytometer (Becton, Dickinson and company, BD Bioscience, San Jose, CA 95131 USA) was used to analyse stained cells. For each measurement, 50,000 events were accumulated and a light source of 488 nm was used to excite cells leading to intercalation of AO to double-stranded DNA that gives green fluorescence, whilst intercalation with single-stranded (denatured) DNA that gives red fluorescence. The raw data of the intensity value of coordinates of red and green fluorescence for every sperm are plotted on a scatter gram using standard Becton Dickinson software. The percentage of spermatozoa with abnormal chromatin structure is represented by the DFI%, which was calculated as the ratio of red to the total of red and green fluorescence. All reagents used in the SCSA were provided by Sigma Diagnostics, St Louis, MO, USA. The SCSA is a clinically applicable test for calculating sperm the DFI% [14].
Measurement of ROS
Levels of ROS were measured using a chemiluminescence assay consuming luminol as a probe (supplied by Sigma). A sperm pellet was obtained by centrifuging the liquefied seminal sample at 300g for 10 min, then re-suspending in phosphate-buffered saline (PBS) at pH 7.4. Luminol (10 µL; prepared as a 5 mM stock in dimethylsulphoxide) was added to 500 µL sperm suspension. A negative control was generated by adding 10 µL luminol to 500 µL PBS. A luminometer (Berthold LB 9505; Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany) measuring the luminol-dependent chemiluminescence was used to assess the ROS levels and the results were expressed as counted photons/min [25].
TAC
Non-enzymatic TAC in the seminal plasma was measured using a TAC colorimetric assay kit (BioVision, Milpitas, CA, USA). The assay measures the antioxidants present in the seminal plasma sample. The Cu2+ ion is converted to Cu+ by antioxidants in presence of a protein mask. The reduced Cu+ ion is chelated with a colorimetric probe giving a broad absorbance peak at 570 nm, proportional to total antioxidant capacity. According to the manufacturer’s recommendations, semen samples were centrifuged at 300g for 10 min, seminal plasma was aliquoted and stored at −80 °C. Samples were tested immediately after thawing at room temperature. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), was used as a reference standard, results are expressed as antioxidant concentration (mM).
Serum hormones measurement
FSH (normal range 2.1–18.6 mIU/mL), luteinising hormone (LH, normal range 1.7–11.2 mIU/mL), and total testosterone (normal range 9.1–30.2 nmol/L) were measured in serum samples using a two-site immunoenzymometric assay (FSH and LH) and competitive enzyme immunoassay (testosterone) performed entirely in the ST AIA-PACK test cups. The TOSOH AIA system analysers read the rate of fluorescence produced and convert it to a concentration (Tosoh Corp., Shiba Minato-Ku, Tokyo, Japan).
Surgical intervention
All patients underwent testicular artery- and lymphatic-sparing inguinal varicocelectomy using ×3 loupe magnification by two surgeons (S.A.) and (A.M.A) under regional anaesthesia using a virtually identical surgical technique. No patients were offered varicocelectomy based on the levels of sperm DNA damage or ROS levels.
Outcome measurement
The primary outcome measurement was semen analysis that was obtained at ≥3 months after varicocelectomy and compared to the values before surgery. Significant improvement in seminal variables after varicocelectomy was considered when there was a ≥50% increase in TMSC from the baseline value. DFI%, TAC, and ROS levels were measured in the seminal samples after surgery and compared with the preoperative values. The secondary endpoint was to elucidate predictors of improvement after repair by a retrospective analysis of the improved cases after surgery.
Statistical analysis
Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS®) statistical software (IBM Corp., Armonk, NY, USA). Results are expressed as the mean (SD, range). The Kolmogorov–Smirnov test was used to assess normality of data. The Mann–Whitney U-Test was used to compare continuous data between the controls and patients. Spearman’s ρ and the Pearson correlation coefficient were used to assess any correlation between DFI%, ROS levels, TAC, and the preoperative TMSC, as appropriate. The Wilcoxon signed-rank test was used to compare pre- and postoperative data. Binary logistic regression analysis was used to identify significant predictors that could affect the outcome of varicocelectomy.
Results
The present prospective controlled study included 60 infertile men with clinical varicocele, 25 (41.7%) had bilateral and 35 (58.3%) had a left varicocele. Their median (range) age was 31 (23–49) years. In addition, 20 normal control subjects were evaluated and had a mean (SD, range) age of was 31.9 (6, 23–41) years. Of the 60 patients included in this study, 41 (68.3%) had primary infertility and 19 (31.7%) had secondary infertility. The mean (SD, range) duration of infertility was 35.6 (13.6, 12–75) months.
Table 1 shows the baseline characteristics of the control subjects and 60 patients with varicocele, the hormonal levels of all patients were within normal limits. In addition to the significant difference in seminal variables as evidence by the TMSC, there was a significantly higher DFI% and ROS levels and a significantly lower in TAC in patients with varicocele vs control subjects. Fig. 1 shows the highly significant positive correlation between DFI% and ROS levels in the 60 infertile patients with clinical varicocele, markers were set according to the subsequent postoperative improvement highlighting that improved cases had a lower DFI% and lower ROS levels preoperatively. From Table 2, the TMSC (as a product of volume, count, and total motility) in patients with varicocele was significantly negatively correlated with DFI%, ROS levels, and grade of varicocele, whilst there was a significant positive correlation with TAC. Although the TMSC diminished with increasing duration of infertility, this was statistically non-significant. The DFI% had a significant positive correlation with ROS levels (Fig. 1), grade of varicocele and duration of infertility, and a significant negative correlation with TAC. Levels of ROS had a significant negative correlation with TAC, and a significant positive correlation with grade of varicocele and duration of infertility. From these correlations, a higher grade of varicocele and longer duration of infertility were related to the presence of higher levels of ROS and a higher DFI%.
Table 1.
Comparison between the control group and the 60 infertile patients with varicocele for the studied variables.
| Variable | Control n = 20 |
Infertile with varicocele n = 60 |
P* |
|---|---|---|---|
| Age, years, median (range) | 31 (23–41) | 33(23–49) | 0.525 |
| Mean (SD, range): | |||
| FSH, IU/L | 5.13 (1.04, 3.45–6.9) | 5.39 (1.1, 3.55–6.95) | 0.325 |
| LH, IU/L | 5.51 (1.6, 2.1–7.42) | 5.02 (1.4, 2.1–7.56) | 0.136 |
| Testosterone, nmol/L | 14.86 (3.5, 10.23–22.31) | 13.78 (2.7, 10.1–19.1) | 0.264 |
| Seminal volume, mL | 2.7 (0.8, 1.5–4) | 2.4 (0.8, 1.5–4.5) | 0.078 |
| Sperm density, millions/mL | 46.8 (7.2, 41–75) | 11 (3, 5.2–17) | <0.001 |
| Total motility (PR + NP),% | 50.5 (11.3,40–80) | 35.3 (10.9, 10–60) | <0.001 |
| TMSC, ×106/ejaculate | 63.98 (30.9, 34.5–157.5) | 8.85 (3.9, 2–19.62) | <0.001 |
| PR,% | 30.7 (5.2, 25–42) | 10.8 (4.5, 4–21) | <0.001 |
| Normal morphology (Kruger’s),% | 3.6 (0.5, 3–4) | 2.3 (0.7, 1–3) | <0.001 |
| DFI% | 7.56 (2.84, 4.1–16.8) | 29.9 (8.3, 16.2–56.2) | <0.001 |
| ROS level [Log(ROS + 1)], photons/min | 2.62 (0.8, 1.2–4.5) | 4.49 (0.9, 2.3–6.1) | <0.001 |
| TAC, mM | 1.5 (0.5, 0.95–2.3) | 0.97 (0.4, 0.4–2.5) | <0.001 |
Mann–Whitney U-test. PR, progressive motility; NP, non-progressive motility.
Fig. 1.
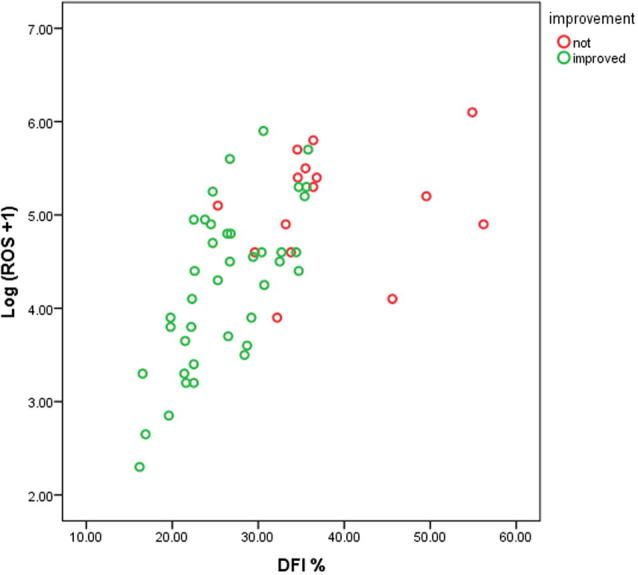
Correlation between preoperative DFI% and levels of ROS. Markers were set according to subsequent postoperative improvement.
Table 2.
Spearman’s rho correlation of seminal parameters with DFI%, ROS levels, TAC, grade of varicocele and duration of infertility in 60 infertile patients.
| DFI% | ROS | TAC | Varicocele grade | Duration of infertility | ||
|---|---|---|---|---|---|---|
| TMSC | rho | −0.350 | −0.312 | 0.350 | −0.463 | −0.240 |
| P | 0.006 | 0.015 | 0.006 | <0.001 | 0.065 | |
| DFI% | rho | 0.654 | −0.669 | 0.379 | 0.475 | |
| P | <0.001 | <0.001 | 0.003 | <0.001 | ||
| ROS | rho | −0.791 | 0.304 | 0.441 | ||
| P | <0.001 | 0.018 | <0.001 | |||
In the 55 patients available at follow up (Table 3), there were significant improvements in various seminal variables apart from seminal volume. In addition, there was a significant increase in the TAC, decrease in ROS levels, and a reduction in the DFI%. In all, 40 of the 55 patients (73%) available at follow-up had improved (measured as a ≥50% increase in TMSC), Table 4 compares between responders and non-responders after varicocelectomy for preoperative age, duration of infertility, laterality and the highest grade of varicocele present. Responders to varicocelectomy were relatively young, although this was statistically non-significant. The duration of infertility was significantly shorter in improved cases. Although higher success rates were noticed after bilateral varicocelectomy than after unilateral and with higher grades of varicocele, this was statistically non-significant. Improved cases were characterised by the presence of preoperatively lower levels of ROS and DFI% and higher TAC levels than cases that did not improve. The FSH, LH and total testosterone levels did not change significantly after varicocelectomy.
Table 3.
Effect of varicocelectomy on various studied parameters in 55 patients.
| Variable | Mean (SD) value |
P* | |
|---|---|---|---|
| Preoperative | Postoperative | ||
| Seminal volume, mL | 2.4 (0.8) | 2.3 (0.6) | 0.378 |
| Sperm density, millions/mL | 10.9 (2.8) | 21.04 (8.9) | <0.001 |
| Total motility (PR + NP),% | 36.4 (10.7) | 53.6 (18.9) | <0.001 |
| TMSC, ×106/ejaculate | 9.18 (3.9) | 26.08 (16.9) | <0.001 |
| PR,% | 10.8 (4.6) | 19.1 (8.1) | <0.001 |
| Normal morphology (Kruger’s),% | 2.3 (0.7) | 2.7 (0.6) | <0.001 |
| DFI% | 29.49 (8.58) | 18.78 (7.23) | <0.001 |
| ROS level [Log(ROS + 1)], photons/min | 4.49 (0.88) | 3.27 (1.3) | <0.001 |
| TAC, mM | 1.01 (0.44) | 2.05 (0.51) | <0.001 |
Wilcoxon signed-rank test. PR, progressive motility; NP, non-progressive motility.
Table 4.
Univariate analysis of different postoperative variables in responders and non-responders.
| Variable | Responders n = 40 |
Non-responders n = 15 |
P |
|---|---|---|---|
| Mean (SD, range) | |||
| Age, years | 34.3 (11.4) | 36.9 (17 | 0.124a |
| Duration of infertility, months | 39.9 (15.4, 13–84) | 53.2 (11.1, 24–72) | 0.005a |
| Postoperative TMSC, ×106/ejaculate | 31.75 (16.3, 6.18–68.64) | 10.94 (5.4, 3.44–22.75) | <0.001a |
| Volume, mL | 2.4 (0.7, 1.5–4) | 1.9 (0.4, 1.5–3) | 0.010a |
| Sperm density, millions/mL | 23.3 (8, 11.5–36.5) | 11.4 (3, 6.9–16) | <0.001a |
| Total motility (PR + NP),% | 61.7 (13.9, 20–80) | 35.1 (15.7, 14–66) | <0.001a |
| PR,% | 40.7 (17.5, 14–75) | 17.3 (8.9, 5–36) | <0.001a |
| Normal morphology Kruger’s),% | 2.9 (0.7, 2–4) | 2.7 (0.5, 2–3) | 0.390a |
| DFI% | 26.18 (5.6, 16.2–35.8) | 38.3 (9.1, 25.3–56.2) | <0.001a |
| ROS levels [Log(ROS + 1)], photons/min | 4.26 (0.9, 2.3–5.9) | 5.1 (0.6, 3.9–6.1) | 0.001a |
| TAC, mM | 1.1 (0.5, 0.4–2.5) | 0.76 (0.2, 0.45–1.25) | 0.002a |
| N (%): | |||
| Grade I (n = 8) | 5 (62.5) | 10 (37.5) | 0.597b |
| Grade II (n = 27) | 19 (70) | 8 (30) | |
| Grade III (n = 20) | 16 (80) | 4 (20) | |
| Unilateral | 20 (67) | 10 (33) | 0.366c |
| Bilateral | 20 (80) | 5 (20) | |
PR, progressive motility; NP, non-progressive motility.
Mann–Whitney U-test.
Chi-square test.
Fisher’s exact test.
Logistic regression analysis was used to predict the probability that a patient would improve after varicocelectomy. The predictor variables entered were those found to be significantly different between responders and non-responders, namely preoperative level of ROS, DFI%, and duration of infertility. The TAC was not used as it represents or is indicative of ROS levels in the opposite direction. A test of the full model vs a model with intercept only was statistically significant (N = 55; chi-square = 28.074; P < 0.001). The model was able correctly to classify 90% of those who improved and 66.7% of those who did not, for an overall success rate of 83.6%. Table 5 shows the logistic regression coefficient (B), Wald test, significance and odds ratio for each of the predictors with 95% CI. Using a 0.05 criterion of statistical significance, DFI% was found to be a significant predictor and had a negative impact, and by inverting the odds for DFI% (0.723) for easier interpretation, every one-point increase in DFI% decreased the chance of improvement by a multiplicative factor of 1.4.
Table 5.
Binary logistic regression analysis for predictors of improvement.
| Variables in the Equation | B | Wald | P | Odds ratio (95% CI) | |
|---|---|---|---|---|---|
| Step 1a | Duration | 0.025 | 0.338 | 0.561 | 1.025 (0.943–1.114) |
| DFI% | −0.324 | 5.424 | 0.020 | 0.723 (0.550–0.950) | |
| ROS | −0.661 | 0.788 | 0.375 | 0.517 (0.120–2.221) | |
Variable(s) entered on step 1: DFI%, ROS, and duration of infertility.
Discussion
Varicocele epidemiology remains incompletely understood and generally reported to be present in 15% of the general male population, in 35% of men with primary infertility, and in up to 80% of men with secondary infertility [26], [27], [2]. DNA damage has been found in association with varicocele, which could be secondary to varicocele-mediated oxidative stress [28]. The aim of the present study was to assess the relationship of sperm nuclear DNA damage and ROS levels in ejaculated spermatozoa of infertile patients with varicocele and to evaluate the beneficial effect of varicocelectomy and elucidate predictors of improvement after repair. In the present study, comparing control subjects with infertile patients with varicocele, apart from the significant difference in seminal variables, there was highly significant difference in the DFI%, ROS levels and TAC (Table 1). These observations further confirm the previous findings of increased SDF in patients with varicocele compared to controls [28], [29].
Evenson et al. [24], who first described the SCSA, suggested that a threshold of 0–15% DFI% correlated to a high fertility potential, whilst 16–29% and a >30% DFI%, correlated to moderate, and low fertility potential, respectively.
In our present cohort of patients with varicocele, the TMSC had a significant negative correlation with DFI%, ROS levels and grade of varicocele, and a positive correlation with TAC signifying the deleterious effect of varicocele on the seminal variables, which increase with increasing grade of varicocele. Increased oxidative stress as evidenced by a low TAC and high ROS levels and DFI% were found with high grades of varicocele and long duration of infertility (Table 2). Scrotal hyperthermia and generation of ROS could be potential mechanisms of varicocele-mediated sperm dysfunction and DNA damage [30]. Presence of spermatozoa with damaged DNA may be indicative of apoptosis, which is an ongoing physiological phenomenon, when deregulated in several stress conditions such as varicocele, has been documented to play a role in male infertility. Markers of apoptosis found in ejaculated human spermatozoa include DFI%, caspase activation, externalisation of phosphatidylserine, and alteration of mitochondrial membrane potential [31], [32], [33]. The results of the present study are in agreement with the findings of a recent study on seminal samples from 60 patients with clinical varicocele and 90 infertile men without varicocele, the authors found high levels of DNA damage in the spermatozoa of patients with varicocele in association with abnormal motility [34]. Another study that investigated the role of seminal oxidative stress in mediating DNA damage showed that a significant increase in DNA damage was induced by exposing spermatozoa to artificially produced ROS causing modification of all bases and producing base-free sites, deletions, frame shifts, and DNA cross-links [35]. In agreement with another study [7], we found that the ROS concentration was higher in semen samples from men with varicocele, the authors suggested that sperm dysfunction in varicocele is in part related to oxidative stress and reduced antioxidant capacity in semen. There are many potential sources of ROS production in semen such as immature sperm and peroxidase-positive leucocytes [36]. Several clinical situations have been implicated as a cause of oxidative stress in semen, e.g. varicocele [37], cigarette smoking [38], [39], and spinal cord injury [40]. The oxidative stress could be due to an increase in nitric oxide (NO) and the release of NO synthase and xanthine oxidase in dilated spermatic veins of men affected with varicocele, NO in high concentration has deleterious effects on human sperm function [39], [41]. In addition, in the present study, we assessed the status of the TAC in seminal plasma for controls and patients with clinical varicocele. We found decreased TAC levels in association with increased ROS levels and DFI%. This agrees with many previous studies suggesting an association between decreased TAC and male infertility [42], [43]. Whereas a more recent study [44] reported no significant difference between the TAC levels in patients with varicocele and controls, which was explained as increasing ROS on its own and not due to decreased TAC levels. Whilst others claimed that normal TAC levels could be associated with increased levels of ROS production in patients examined for infertility [10].
As for the impact of varicocelectomy, it was followed by significant improvement in various seminal variables manifest by the improved TMSC in association with a significant reduction in DFI% and ROS levels. Our present results agree with a recent prospective trial [45] carried out on 29 infertile men with clinical varicocele, the authors found that varicocelectomy was associated with a significant improvement in sperm chromatin compaction and DNA integrity, using three different assays. In another similar study in 92 patients, Kadioglu et al. [46] reported a large decrease in DFI% from a preoperative mean of 42.6% to a postoperative mean of 20.5% (P < 0.001) and concluded that varicocelectomy can improve seminal variables and sperm DNA damage in infertile men with varicocele. In another recent study, the DFI% decreased significantly in 49 infertile patients after varicocelectomy from 35.2% to 30.2% (P = 0.019) with significant improvement in seminal variables [47]. Contrary to the previously mentioned recent studies and the present study, there was an earlier study carried out on 37 men who underwent microsurgical varicocelectomy, in which the authors reported a significant reduction in SDF, but the improvement in sperm concentration, motility and morphology after varicocele repair did not reach statistical significance [48].
To establish which factors contributed to improvement after varicocele repair, a retrospective univariate analysis was used to compare the preoperative characteristics of the 55 cases available at follow-up and we found that cases with high preoperative ROS levels and a greater DFI% with a low TAC and longer duration of infertility had poorer responses to varicocelectomy. In addition, although the improvement was more pronounced in the bilateral group and with higher grades of varicocele, the difference was statistically non-significant. Cayan et al. [49] noticed a poor response regarding TMSC in men with varicocele and genetic lesions (Y-chromosome microdeletion or abnormal karyotypes) after varicocele repair compared to men without coexisting genetic lesions. Our present results are in agreement with many studies regarding the laterality of varicocelectomy and the response in different grades of varicocele. Many studies have reported a statistically non-significant difference between bilateral and unilateral repair with similar seminograms [16], [17], [50]. In addition, some authors found no clear correlation between ligated vein size (individual or cumulative) and improvements in seminal variables [51]; whilst others have found a significant difference in favour of bilateral repair [49% (54/110) vs 36% (53/146), P < 0.05] [52].
For predicting the variables that could affect the outcome of varicocelectomy and considering amelioration of seminal variables as a sign of improvement, the studied variables that showed a significant difference between responders and non-responders were duration of infertility, preoperative levels of ROS, and DFI%, which were entered in a logistic regression equation and preoperative DFI% was found to be the only significant predictor and had a negative impact on response or capacity to improve after repair. A factor that could explain this may be the assumption that in the presence of high oxidative stress and denatured DNA spermatogenesis may be impaired. Other factors that may be essential for spermatogenesis and that were previously found to be significant predictors of success after repair are a good preoperative testicular blood supply and normal Sertoli cell and Leydig cell secretory function [17]. Whilst in another study, preoperative serum FSH and testosterone concentrations were predictors of improvement after varicocelectomy [53].
There are some limitations to the present study. The spontaneous pregnancy rate was not followed-up after varicocele repair. In addition, the return of the DFI% to control levels may require longer observation to assess if it can return to normal after varicocelectomy.
We can conclude that SDF has a negative impact on improvement after varicocelectomy. Hence, the DFI% could be recommended as a prognostic test in infertile patients with varicocele to aid decision-making as regard the necessity and the anticipated outcome of varicocelectomy in patients with infertility.
Conflict of interest
No conflict of interest.
Andrology/Sexual Medicine
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Kursh E.D. What is the incidence of varicocele in a fertile population? Fertil Steril. 1987;48:510–511. doi: 10.1016/s0015-0282(16)59432-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 1992;57:1289–1293. [PubMed] [Google Scholar]
- 3.Ficarra V., Crestani A., Novara G., Mirone V. Varicocele repair for infertility: what is the evidence? Curr Opin Urol. 2012;22:489–494. doi: 10.1097/MOU.0b013e328358e115. [DOI] [PubMed] [Google Scholar]
- 4.Dabaja A., Wosnitzer M., Goldstein M. Varicocele and hypogonadism. Curr Opin Urol. 2013;14:309–314. doi: 10.1007/s11934-013-0339-4. [DOI] [PubMed] [Google Scholar]
- 5.Turrens J.F. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lamirande E., Hon J., Armand Z., Hideya K., Claude G. Reactive oxygen species and sperm physiology. Rev Reprod. 1997;2:48–54. doi: 10.1530/ror.0.0020048. [DOI] [PubMed] [Google Scholar]
- 7.Hendin B., Kolettis P., Sharma R.K., Thomas A.J., Jr., Agarwal A. Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999;161:1831–1834. [PubMed] [Google Scholar]
- 8.Agarwal A., Saleh R.A., Bedaiwy M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 9.Meucci E., Milardi D., Mordente A., Martorana G.E., Giacchi E., De Marinis L., Mancini A. Total antioxidant capacity in patients with varicoceles. Fertil Steril. 2003;79(Suppl. 3):1577–1583. doi: 10.1016/s0015-0282(03)00404-7. [DOI] [PubMed] [Google Scholar]
- 10.Moustafa M.H., Sharma R.K., Thornton J., Mascha E., Abdel-Hafez M.A., Thomas A.J., Jr Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod. 2004;19:129–138. doi: 10.1093/humrep/deh024. [DOI] [PubMed] [Google Scholar]
- 11.De Jonge C. The clinical value of sperm nuclear DNA assessment. Hum Fertil (Camb) 2002;5:51–53. doi: 10.1080/1464727022000198922. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A., Said T.M. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331–345. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 13.Tavalaee M., Razavi S., Nasr-Esfahani M.H. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91:1119–1126. doi: 10.1016/j.fertnstert.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 14.Ni K., Steger K., Yang H., Wang H., Hu K., Zhang T. Comprehensive investigation of sperm DNA damage and oxidative stress injury in infertile patients with subclinical, normozoospermic, and astheno/oligozoospermic clinical varicocoele. Andrology. 2016;4:816–824. doi: 10.1111/andr.12210. [DOI] [PubMed] [Google Scholar]
- 15.Baazeem A., Belzile E., Ciampi A., Dohle G., Jarvi K., Salonia A. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Zini A., Azhar R., Baazeem A., Gabriel M.S. Effect of microsurgical varicocelectomy on human sperm chromatin and DNA integrity: a prospective trial. Int J Androl. 2011;34:14–19. doi: 10.1111/j.1365-2605.2009.01048.x. [DOI] [PubMed] [Google Scholar]
- 17.Al-Adl A.M., El-Karamany T., Issa H., Zaazaa M. The influence of antisperm antibodies, intratesticular hemodynamics and surgical approach to varicocelectomy on seminal variables. Arab J Urol. 2014;12:309–317. doi: 10.1016/j.aju.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamada A., Esteves S.C., Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 19.Esteves S.C., Gosálvez J., López-Fernández C., Núñez-Calonge R., Caballero P., Agarwal A. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol. 2015;47:1471–1477. doi: 10.1007/s11255-015-1053-6. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . fifth ed. WHO; Geneva, Switzerland: 2010. WHO laboratory manual for the Examination and processing of human semen. [Google Scholar]
- 21.Liguori G., Trombetta C., Garaffa G., Bucci S., Gattuccio I., Salame L., Belgrano E. Color doppler ultrasound investigation of varicocele. World J Urol. 2004;22:378–381. doi: 10.1007/s00345-004-0421-0. [DOI] [PubMed] [Google Scholar]
- 22.Hoekstra T., Witt M.A. The correlation of internal spermatic vein palpability with ultrasonographic diameter and reversal of venous flow. J Urol. 1995;153:82–84. doi: 10.1097/00005392-199501000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda T., Miyake H., Enatsu N., Matsushita K., Fujisawa M. Assessment of time-dependent changes in semen parameters in infertile men after microsurgical varicocelectomy. Urology. 2015;86:48–51. doi: 10.1016/j.urology.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Evenson D.P., Larson K.L., Jost L.K. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23:25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H., Gil-Guzman E., Mahran A.M., Rakesh Nelson D.R., Thomas A.J., Jr., Agarwal A. Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J Androl. 2001;22:568–574. [PubMed] [Google Scholar]
- 26.Alsaikhan B., Alrabeeah K., Delouya G., Zini A. Epidemiology of varicocele. Asian J Androl. 2016;18:179–181. doi: 10.4103/1008-682X.172640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke B.G. Incidence of varicocele in normal men and among men of different ages. JAMA. 1966;198:1121–1122. [PubMed] [Google Scholar]
- 28.Zini A., Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril. 2011;96:1283–1287. doi: 10.1016/j.fertnstert.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 29.La Vignera S., Condorelli R., Vicari E., D'Agata R., Calogero A.E. Effect of varicocelectomy on sperm DNA fragmentation, mitochondrial function, chromatin condensation and apoptosis. J Androl. 2012;33:389–397. doi: 10.2164/jandrol.111.013433. [DOI] [PubMed] [Google Scholar]
- 30.Saleh R.A., Agarwa A., Sharma P.K., Said T.M., Sikka S.C., Thomas A.J., Jr. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80:1431–1436. doi: 10.1016/s0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 31.Sakkas D., Moffatt O., Manicardi G.C., Mariethoz E., Tarozzi N., Bizzaro D. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod. 2002;66:1061–1067. doi: 10.1095/biolreprod66.4.1061. [DOI] [PubMed] [Google Scholar]
- 32.Chen C.H., Lee S.S., Chen D.C., Chien H.H., Chen I.C., Chu Y.N. Apoptosis and kinematics of ejaculated spermatozoa in patients with varicocele. J Androl. 2004;25:348–353. doi: 10.1002/j.1939-4640.2004.tb02799.x. [DOI] [PubMed] [Google Scholar]
- 33.Said T.M., Gaglani A., Agarwal A. Implication of apoptosis in sperm cryoinjury. Reprod Biomed Online. 2010;21:456–462. doi: 10.1016/j.rbmo.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Peluso G., Palmieri A., Cozza P.P., Morrone G., Verze P., Longo N. The study of spermatic DNA fragmentation and sperm motility in infertile subjects. Arch Ital Urol Androl. 2013;85:8–13. doi: 10.4081/aiua.2013.1.8. [DOI] [PubMed] [Google Scholar]
- 35.Duru N.K., Morshedi M., Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000;74:1200–1207. doi: 10.1016/s0015-0282(00)01591-0. [DOI] [PubMed] [Google Scholar]
- 36.Plante M., de Lamirande E., Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa are sufficient to affect sperm motility. Fertil Steril. 1994;62:387–393. doi: 10.1016/s0015-0282(16)56895-2. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal A., Prabakaran S., Allamaneni S. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online. 2006;12:630–633. doi: 10.1016/s1472-6483(10)61190-x. [DOI] [PubMed] [Google Scholar]
- 38.Saleh R., Agarwal A., Sharma R.K., Nelson D.R., Thomas A.J., Jr. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril. 2002;78:491–499. doi: 10.1016/s0015-0282(02)03294-6. [DOI] [PubMed] [Google Scholar]
- 39.Yousefniapasha Y., Jorsaraei G., Gholinezhadchari M., Mahjoub S., Hajiahmadi M., Farsi M. Nitric oxide levels and total antioxidant capacity in the seminal plasma of infertile smoking men. Cell J. 2015;17:129–136. doi: 10.22074/cellj.2015.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padron O.F., Brackett N.L., Sharma R.K., Lynne C.M., Thomas A.J., Agarwal A. Seminal reactive oxygen species, sperm motility and morphology in men with spinal cord injury. Fertil Steril. 1997;67:1115–1120. doi: 10.1016/s0015-0282(97)81448-3. [DOI] [PubMed] [Google Scholar]
- 41.Romeo C., Ientile R., Impellizzeri P., Turiaco N., Teletta M., Antonuccio P. Preliminary report on nitric oxide-mediated oxidative damage in adolescent varicocele. Hum Reprod. 2003;18:26–29. doi: 10.1093/humrep/deg004. [DOI] [PubMed] [Google Scholar]
- 42.Smith R., Vantman D., Ponce J., Escobar J., Lissi E. Total antioxidant capacity of human seminal plasma. Hum Reprod. 1996;11:1655–1660. doi: 10.1093/oxfordjournals.humrep.a019465. [DOI] [PubMed] [Google Scholar]
- 43.Pasqualotto F.F., Sharma R.K., Nelson D.R., Thomas A.J., Agarwal A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril. 2000;73:459–464. doi: 10.1016/s0015-0282(99)00567-1. [DOI] [PubMed] [Google Scholar]
- 44.Smith R., Kaune H., Parodi D., Madariaga M., Rios R., Morales I. Increased sperm DNA damage in patients with varicocele relationship with seminal oxidative stress. Hum Reprod. 2006;21:986–993. doi: 10.1093/humrep/dei429. [DOI] [PubMed] [Google Scholar]
- 45.Alhathal N., San Gabriel M., Zini A. Beneficial effects of microsurgical varicocelectomy on sperm maturation, DNA fragmentation, and nuclear sulfhydryl groups: a prospective trial. Andrology. 2016;4:1204–1208. doi: 10.1111/andr.12256. [DOI] [PubMed] [Google Scholar]
- 46.Kadioglu T.C., Aliyev E., Celtik M. Microscopic varicocelectomy significantly decreases the sperm DNA fragmentation index in patients with infertility. Biomed Res Int. 2014;2014:695713. doi: 10.1155/2014/695713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smit M., Romijn J.C., Wildhagen M.F., Veldhoven J.L., Weber R.F., Dohle G.R. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2013;189(Suppl.):S146–S150. doi: 10.1016/j.juro.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 48.Zini A., Blumenfeld A., Libman J., Willis J. Beneficial effect of microsurgical varicocelectomy on human sperm DNA integrity. Hum Reprod. 2005;20:1018–1021. doi: 10.1093/humrep/deh701. [DOI] [PubMed] [Google Scholar]
- 49.Cayan S., Lee D., Black L.D., Reijo Pera R.A., Turek P.J. Response to varicocelectomy in oligospermic men with and without defined genetic infertility. Urology. 2001;57:530–535. doi: 10.1016/s0090-4295(00)01015-3. [DOI] [PubMed] [Google Scholar]
- 50.Fujisawa M., Ishikawa T., Takenaka A. The efficacy of bilateral varicocelectomy in patients with palpable bilateral varicoceles: comparative study with unilateral varicocele. Urol Res. 2003;31:407–409. doi: 10.1007/s00240-003-0361-y. [DOI] [PubMed] [Google Scholar]
- 51.Shindel A.W., Yan Y., Naughton C.K. Does the number and size of veins ligated at left-sided microsurgical subinguinal varicocelectomy affect semen analysis outcomes? Urology. 2007;69:1176–1180. doi: 10.1016/j.urology.2007.01.086. [DOI] [PubMed] [Google Scholar]
- 52.Libman J., Jarvi K., Lo K., Zini A. Beneficial effect of microsurgical varicocelectomy is superior for men with bilateral versus unilateral repair. J Urol. 2006;176:2602–2605. doi: 10.1016/j.juro.2006.07.161. [DOI] [PubMed] [Google Scholar]
- 53.Kondo Y., Ishikawa T., Yamaguchi K., Fujisawa M. Predictors of improved seminal characteristics by varicocele repair. Andrologia. 2009;41:20–23. doi: 10.1111/j.1439-0272.2008.00882.x. [DOI] [PubMed] [Google Scholar]


