Abstract
Objective
To conduct a systematic review and meta-analysis investigating the efficacy and safety of medical expulsive therapy (MET) in low risk of bias (RoB) randomised controlled trials (RCTs).
Methods
A Cochrane style systematic review was conducted on published literature from 1990 to 2016, to include low RoB and a power calculation. A pooled meta-analysis was conducted.
Results
The MET group included 1387 vs 1381 patients in the control group. The analysis reveals α-blockers increased stone expulsion rates (78% vs 74%) (P < 0.001), whilst calcium channel blockers (CCBs) had no effect compared to controls (79% vs 75%) (P = 0.38). In the subgroup analysis, α-blockers had a shorter time to stone expulsion vs the control group (P < 0.001). There were no significant differences in expulsion rates between the treatment groups and control group for stones <5 mm in size (P = 0.48), proximal or mid-ureteric stones (P = 0.63 and P = 0.22, respectively). However, α-blockers increased stone expulsion in stones >5 mm (P = 0.02), as well as distal ureteric stones (P < 0.001). The α-blocker group developed more side-effects (6.6% of patients; P < 0.001). The numbers needed to treat for α-blockers was one in 14, for stones >5 mm one in eight, and for distal stones one in 10.
Conclusion
The primary findings show a small overall benefit for α-blockers as MET for ureteric stones but no benefit with CCBs. α-blockers show a greater benefit for large (>5 mm) ureteric stones and those located in the distal ureter, but no benefit for smaller or more proximal stones. α-blockers are associated with a greater risk of side-effects compared to placebo or CCBs.
Abbreviations: ARR, absolute risk reduction; CCB, calcium channel blocker; MD, mean difference; MeSH, medical subject headings; NNT, numbers needed to treat; MET, medical expulsive therapy; RCT, randomised controlled trial; RoB, risk of bias; RR, risk ratio
Keywords: Ureteric stones, Urinary stones, α-Blockers, Calcium channel blockers, Medical expulsive therapy (MET)
Introduction
Urinary tract stones affect 1–15% of the general population and the incidence is on the rise [1], [2]. Most of these stones will pass spontaneously. Stone passage depends on two main points, the size of the stone and the stone location in the urinary system. Rates of stone passage vary depending on these factors, for stones sized <5 mm passage ranges from 40% to 98% [3], [4], [5], [6], whilst stones that are >5 mm have a passage rate ranging between 55% and 50% [5], [6], [7].
It has been established that the urinary smooth muscles contain α-adrenergic receptors that facilitate contraction, leading to renal colic and ureteric spasms in the presence of a stone [8], [9], [10]. Stones lead to spasmodic contraction of these smooth muscles, which prevent expulsion due to dis-coordinated contractions and eventually stasis. Furthermore, reactive inflammation caused by the stone will promote mucosal oedema, increasing stasis, which may lead to obstruction [8], [9], [10].
The α-adrenergic receptors are found in high density in the lower ureter as opposed to the mid or upper ureter [10]. The use of an α-blocker inhibits basal smooth muscle tone, peristaltic frequency and amplitude, whilst maintaining tonic propulsive contractions, leading to a reduction in the intra-ureteric pressure and increase in fluid transport [8], [9], [10], [11], [12], [13], [14], [15]. Furthermore, on the simple basis that smooth muscle contraction/relaxation is mitigated by calcium levels, the use of calcium channel blockers (CCBs), can lead to similar effect as the α-blockers in reducing intra-ureteric pressure and increasing fluid transport [4], [8], [9], [11], [16], [17], [18], [19], [20], [21].
This has led to a plethora of research into the field aiming at finding a medication that will increase stone passage, shorten time to passage, and alleviate pain [4], [18], [19], [20], [21]. However, despite the multitude of trials published, the debate remains, as most of the research is riddled with bias and confounding factors. At the turn of the century, more robust trials have emerged to address the risk of bias (RoB). Interestingly, even these reported different end outcomes, with studies showing benefit for medical expulsive therapy (MET), whilst others reported no benefit [4], [17], [18], [19], [20], [21], [22]. Furthermore, previous meta-analyses were in favour of MET; however, these were based on the aforementioned high RoB trials, most of which were not sufficiently powered [4], [17], [18], [19], [20], [21], [22].
The debate back and forth for MET has always been one where the cons side would challenge these high RoB trials, whilst the pros side would defend the sub-analysis sections of the meta-analyses and that practice should not change as a result of a few trials reporting negative findings compared to many others showing positive results [23].
Therefore, we aimed to conduct a systematic review of the literature looking at double-blinded randomised controlled trials (RCTs) with low RoB based on Cochrane Risk of Bias Assessment. To increase the level of evidence we only included trials that had powered the trial.
The primary aim of the present study was to evaluate the efficacy of MET, defined as stone expulsion rate, with either α-blockers or a CCB compared to a control group. The secondary aim was to assess the safety of MET, compare between MET and a control regarding time to stone expulsion, difference in stone expulsion depending on the size of the stone (<5 and >5 mm), and on location of the stone in the ureter.
Methods
Search strategy
The systematic review was performed using Cochrane guidelines [24]. The search strategy included the following databases, to identify relevant articles: The USA National Library of Medicine’s life science database (MEDLINE; 1980 to November 2016), EMBASE (1980 to November 2016), Cochrane Central Register of Controlled Trials (CENTRAL, in The Cochrane Library to 2016), CINAHL (1980 to November 2016), Clinicaltrials.gov, Google Scholar and Individual urological journals.
Search terms used in conjunction with each other included: ‘alpha blocker’, ‘tamsulosin’, ‘terazosin’, ‘doxazosin’, ‘alfuzosin’, ‘silodosin’, ‘calcium-channel-blocker’, ‘nifedipine’, ‘medical therapy’, ‘urolithiasis’, ‘urinary calculi’, ‘renal calculi’, ‘ureteric calculi’, ‘urinary stones’, ‘Randomized controlled trial’ and ‘medical expulsive therapy’.
Medical subject headings (MeSH) phrases included:
-
-
(“Adrenergic Alpha-Antagonists”[Mesh]) AND “Randomized Controlled Trial” [Publication Type])
-
-
(“Calcium Channel Blockers”[Mesh]) AND “Randomized Controlled Trial” [Publication Type])
-
-
(“Adrenergic Alpha-Antagonists”[Mesh]) AND “Urinary Calculi”[Mesh]) AND “Randomized Controlled Trial” [Publication Type]))
-
-
(“Calcium Channel Blockers”[Mesh]) AND “Urinary Calculi”[Mesh]) AND “Randomized Controlled Trial” [Publication Type]))
-
-
Same MeSH phrases as above, but replacing the class of medication with the individual drug name.
Study selection
All languages were included if data were extractable, also references of searched papers were evaluated for further studies for potential inclusion. Authors were contacted wherever the data were not available or not clear, to be able to adequately assess inclusion of their study. If data were not extractable, provided or clarified, the study was excluded.
Three reviewers (O.M.A., A.G., and T.A.) identified studies that appeared to fit the inclusion criteria for full review. Four reviewers (O.M.A., T.A., M.M., and A.G.) independently selected studies for inclusion. Disagreement between the authors in study inclusion was resolved by consensus of all authors.
Data extraction
Data of each included study were independently extracted initially by five authors (T.A., B.O., M.M., A.J., and A.G.) after which a senior author (O.M.A.) extracted the data independently and cross checked each data extraction to ensure quality assurance of data across the board. Discrepancy of the data extraction was resolved by consensus by all extracting authors.
Only published RCTs on adult patients were included, comparing either an α-blocker or a CCB to a control group. Only studies using either a placebo or the country’s/hospital’s protocol for conservative management (i.e. analgesics, antispasmodics, hydration) serving as controls were included. Studies on MET after treatments such as shockwave lithotripsy or ureteroscopy were only included if there were the control and experimental arms who had not undergone any other treatment for their stones. Studies comparing other medication to either α-blockers or a CCBs were not included, as we are aiming to establish the α-blocker and CCB efficacy for MET, not that of other medications.
The data of each study were grouped into a meta-analysis, in an intention-to-treat basis.
Statistical analysis and quality assessment
We used the Review manager (RevMan) v.5.2 program (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) to conduct the analysis. For continuous data, a Mantel–Haenszel chi-square test was used and expressed as the mean difference (MD) with 95% CI and for dichotomous data an inverse variance was used and expressed as risk ratio (RR) with 95% CI. A P < 0.05 was considered statistically significant [23], [24]. For the numbers needed to treat (NNT) or harm, we used the GraphPad software (GraphPad Software, Inc., La Jolla, CA, USA).
Heterogeneity was analysed using the chi-squared test on N-1 degrees of freedom, with an α of 0.05 used for statistical significance and with the I2 test. I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity [24]. A fixed-effect model was used unless statistically significant high heterogeneity (I2 > 75% was considered as significantly high heterogeneity) existed between studies. A random-effects model was used if heterogeneity existed [24].
An assessment of the methodological quality of the studies was conducted consistent with the Cochrane handbook [24]. For quality assessment the selection bias, performance bias, detection bias, attrition bias, and reporting bias were assessed in each of the included studies. We sub-analysed studies based on whether or not the trial conducted a power calculation of the sample size, as this point is usually a criticism when debating between the RCTs regarding robustness.
We kept the two groups (α-blockers and CCBs) separate as they are in their own right different classes of medication; however, we did group all sub-types of α-blockers together, as well as analysing them separately.
Results
Literature search
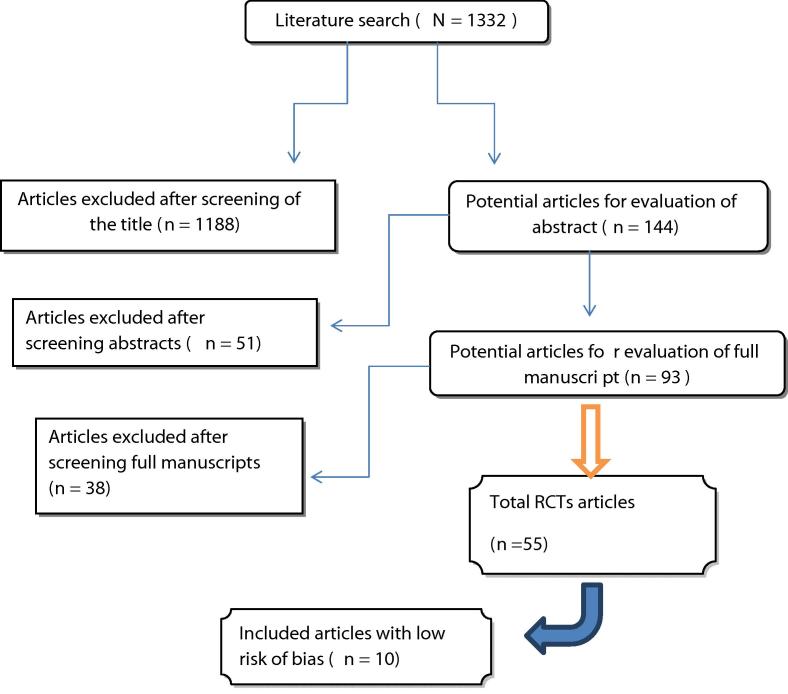
The literature search identified 1332 studies, of which 1188 were excluded due to irrelevance based on titles and 51 excluded due to irrelevance based on the abstracts (Fig. 1). Full manuscripts were evaluated in 93 studies, of which 38 studies were excluded due to not meeting inclusion criteria. The 55 RCTs remaining were scrutinised for RoB and a final 10 studies were included into the meta-analysis [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], all of which were double-blinded RCTs that powered their study and were deemed to have a low RoB based on Cochrane guidelines for assessing RoB [24].
Fig. 1.
Flowchart for article selection process of the review.
Characteristics of the included studies
The trials were all published within the last 8 years (2008–2016). There was a total of 2768 patients, 1387 in the MET group (α-blockers group: 973; CCB group: 414) and 1381 (controlled group vs α-blockers: 967; controlled group vs CCB: 414) in the control groups. The age range was 23–72 years.
Table 1, Table 2 [25], [26], [27], [28], [29], [30], [31], [32], [33], [34] depict the RCT patient and stone demographics and which trial reported on the primary and secondary outcomes.
Table 1.
Demographics of the 10 included studies.
| Reference | Power calculation | Treatment vs control | Age, years, mean (SD) or median (IQR) | Gender, M:F, n | Stone location and size, mm | Stone size, mm, mean (SD) or median (IQR) |
|---|---|---|---|---|---|---|
| Al-Ansari et al. [25] | Yes | Tamsulosin | 37.18 (9.38) | 32:18 | Distal ureter | 5.88 (2.39) |
| Control | 36.13 (9.32) | 35:15 | <10 | 6.04 (2.5) | ||
| Furyk et al. [26] | Yes | Tamsulosin | 45.5 (35–55) | 156:42 | Distal ureter | n/a |
| Control | 46 (37–55) | 164:31 | <10 | |||
| Hermanns et al. [27] | Yes | Tamsulosin | 36 (30–44) | 39:6 | Distal ureter | 4.1 (3.5–4.9) |
| Placebo | 41 (33–54) | 36:9 | <7 | 3.8 (3.4–4.3) | ||
| Ochoa-Gomez et al. [28] | Yes | Tamsulosin | 38.5 (11.3) | 15:17 | Distal ureter | 5.3 (0.55) |
| Control | 38.2 (12.4) | 21:12 | <10 | 5.2 (0.39) | ||
| Pedro et al. [29] | Yes | Alfuzosin | 36.69 (13.06) | 28: 6 | Distal ureter | 3.83 (0.94) |
| Control | 42.03 (12.85) | 27: 8 | <8 | 4.07 (1.13) | ||
| Pickard et al. [30] | Yes | Tamsulosin | 43.1 (11.5) | 310:68 | Pan ureter | 4.6 (1.6) |
| Nifedipine | 42.3 (11.0) | 313:66 | <10 | 4.5 (1.6) | ||
| Control | 42.8 (12.3) | 294:85 | 4.5 (1.7) | |||
| Sameer et al. [31] | Yes | Alfuzosin | 30.82 (7.85) | 26:9 | Distal ureter | 6.26 (1.85) |
| Nifedipine | 32.74 (9.58) | 19:16 | <10 | 6.5 (1.78) | ||
| Control | 33.06 (8.76) | 23:12 | 6.37 (1.85) | |||
| Sur et al. [32] | Yes | Silodosin | 47 (13) | 72:42 | Pan-ureter | 5.4 (1.4) |
| Control | 47 (15) | 80:38 | 4–10 | 5.5 (1.6) | ||
| Vincendeau et al. [33] | Yes | Tamsulosin | 38.9 (13.4) | 43:18 | Distal ureter | 2.9 (1) |
| Placebo | 39.0 (11.4) | 52:9 | 2–7 | 3.2 (1.2) | ||
| Wang et al. [34] | Yes | Silodosin | 51.42 (8.68) | 40:22 | Distal ureter | 6.47 (1.39) |
| Placebo | 51.51 (10.03) | 43:18 | 4–10 | 6.46 (1.31) |
IQR, interquartile range; n/a, not available.
Table 2.
Results of the 10 included studies.
| Reference | Treatment vs control | Expulsion rate, n/N | Expulsion time, days, mean (SD) unless otherwise specified | Standard therapy | Re-hospitalisation, n/N | Side-effects |
|---|---|---|---|---|---|---|
| Al-Ansari et al. [25] | Tamsulosin | 41/50 | 6.4 (2.77) | Diclofenac | 1 retrograde ejaculation and 1 hypotension in tamsulosin group. Dizziness and headache 2 each in each group. 1 rhinitis in tamsulosin group. Fatigue 2 in tamsulosin group and 1 in control group | |
| Control | 28/46 | 9.87 (5.4) | ||||
| Furyk et al. [26] | Tamsulosin | 140/161 | Median 7 | Indomethacin, oxycodone | 20/198 23/193 |
Dizziness, tamsulosin 46, control 36; Palpitations, tamsulosin 13, control 14; Collapse, tamsulosin 3, control 2; Sexual dysfunction, tamsulosin 13, control 5; Headache, tamsulosin 50, control 56; Fatigue, tamsulosin 55, control 47; Nausea, tamsulosin 53, control 55; Vomiting, tamsulosin 14, control 18; Diarrhoea, tamsulosin 23, control 22; Constipation tamsulosin 36, control 28 |
| Control | 127/155 | Median 11 | ||||
| Hermanns et al. [27] | Tamsulosin | 39/45 | Median 7 | Diclofenac, metamizole | 6/45 2/45 |
Tamsulosin group: 1 diarrhoea, 1 cutaneous reaction, 2 retrograde ejaculation Placebo group: 1 dizziness |
| Control | 40/45 | Median 10 | ||||
| Ochoa-Gomez et al. [28] | Tamsulosin | 22/32 | 22 (6.77) | Not specified | Tamsulosin group: 2 dizziness 2 retrograde ejaculation | |
| Placebo | 23/33 | 23 (6.36) | ||||
| Pedro et al. [29] | Alfuzosin | 25/34 | 5.2 (4.82) | None | Alfuzosin 4 patients with dizziness and hypotension and stopped treatment. Nil in placebo | |
| Control | 27/35 | 8.5 (6.99) | ||||
| Pickard et al. [30] | Tamsulosin | 307/378 | 16.5 (12.6), 79 patients | Not clear | Nifedipine 1 with diarrhoea, pain and vomiting, 1 with malaise, headache and chest pain. 1 with chest pain. Placebo 1 with headache, pain, light-headedness |
|
| Nifedipine | 304/379 | 16.2 (14.5), 74 patients | ||||
| Control | 303/379 | 15.9 (11.3), 84 patients | ||||
| Control | 22/30 | – | ||||
| Sameer et al. [31] | Alfuzosin | 30/35 | 12 (6.67) | Diclofenac, tramadol | 5/35 11/35 27/35 |
Alfuzosin 3 retrograde ejaculation Nifedipine 1 hypotension |
| Nifedipine | 21/35 | 12 (6.69) | ||||
| Control | 7/35 | 12.29 (9.46) | ||||
| Sur et al. [32] | Silodosin | 60/115 | – | Oxycodone | Retrograde ejaculation, silodosin 11, control 1; Nausea, silodosin 9, control 2; Dizziness, silodosin 8, control 2; Headache, silodosin 4, control 0; Vomiting, silodosin 4, control 4; Constipation, silodosin 3, control 2; Nasal congestion, silodosin 3, control 0; Diarrhoea, silodosin 2, control 3 | |
| Control | 52/117 | – | ||||
| Vincendeau et al. [33] | Tamsulosin | 47/61 | 10.1 (10) | Ketoprofen and phloroglucinol | 4/61 6/61 |
Headache, placebo 7 tamsulosin 7; Asthenia, placebo 18 tamsulosin 21; Orthostatic hypotension, placebo 3 tamsulosin 6; Palpitation, placebo 1 tamsulosin 3; Nausea or vomiting, placebo 7 tamsulosin 12; Other gastrointestinal disorder, placebo 10 tamsulosin 16; Retrograde ejaculation, tamsulosin 4; Skin reaction placebo 1 |
| Placebo | 43/61 | 9.6 (9.8) | ||||
| Wang et al. [34] | Silodosin | 48/62 | 6.31 (2.13) | Ketoroalic and buprenorphine | 0/62 0/61 |
Silodosin 10, placebo 2: asthenia; hypotension; palpitation; syncope |
| Placebo | 33/61 | 9.73 (2.76) |
Meta-analysis results
None of the RCTs reported any difference between the MET and control groups regarding patients and stone demographics, a meta-analysis of the demographics confirms no significant difference: Age (P = 0.72; MD −0.22, 95% CI −1.33, 0.93); sex (P = 0.75; RR −0.01, 95% CI −0.04, 0.03); or stone size (P = 0.95; MD 0.00, 95% CI −0.13, 0.12).
MET efficacy
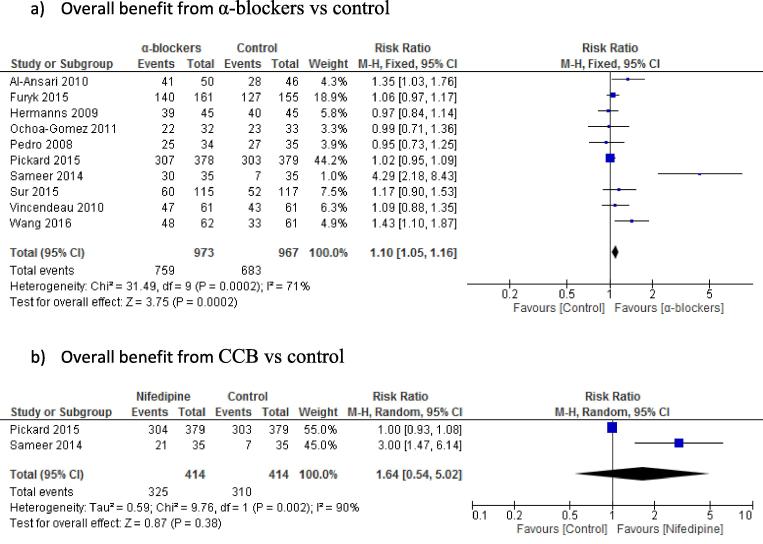
Primary outcome (Fig. 2[25], [26], [27], [28], [29], [30], [31], [32], [33], [34])
Fig. 2.
MET overall expulsion rates.
For MET efficacy measured by stone expulsion, there was statistically significant difference for α-blockers vs control, favouring α-blockers for overall benefit (10 studies, 1940 patients) (78% vs 70.6%; P < 0.001; RR 1.10, 95% CI 1.05, 1.16); however, there was no significant difference between the CCB and control groups (two studies: 828 patients) (78.5% vs 74.9%; P = 0.38; RR 1.64, 95% CI 0.54, 5.02) (Fig. 2).
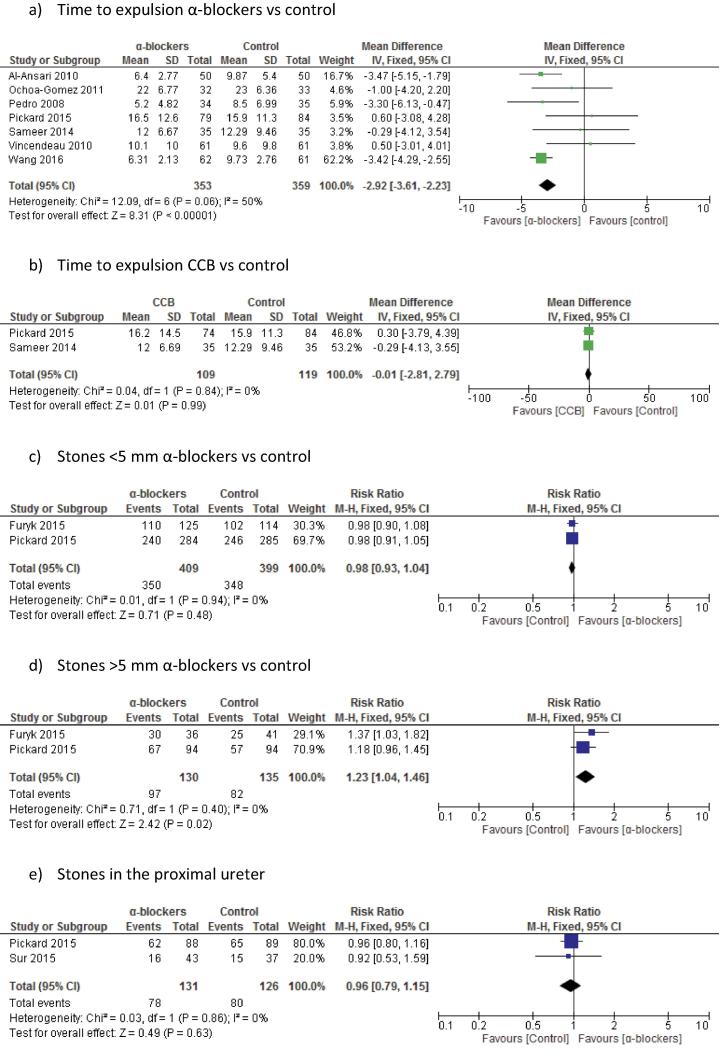
Secondary outcomes (Fig. 3[25], [26], [27], [28], [29], [30], [31], [32], [33], [34])
Fig. 3.
MET secondary outcomes.
There was a statistically significantly shorter time to expulsion with α-blockers compared to controls (seven studies, 712 patients), at a range of 5.2–22 vs 8.5–23 days (P < 0.001; MD −2.92, 95% CI −3.61, −2.23; Fig. 3). Whereas there was no statistically significant difference between CCBs and controls (two studies, 228 patients), at a range of 12–16.2 vs 12.2–15.9 days (P = 0.99; MD −0.01, 95% CI −2.81, 2.79).
There was no statistically significant difference in the stone expulsion rate between the α-blocker and control groups for stones <5 mm (two studies, 808 patients), at 85.6% vs 87.2% (P = 0.48; RR 0.98, 95% CI 0.93, 1.04; Fig. 3). However, there was statistical significance favouring α-blocker for stones >5 mm (two studies, 265 patients), at 74.6% vs 60.7% (P = 0.02; RR 1.23, 95% CI 1.04, 1.46; Fig. 3).
Only one trial on CCB, measured stone size as outcome measure and found no difference between the CCB and control groups for stones sized either <5 or >5 mm.
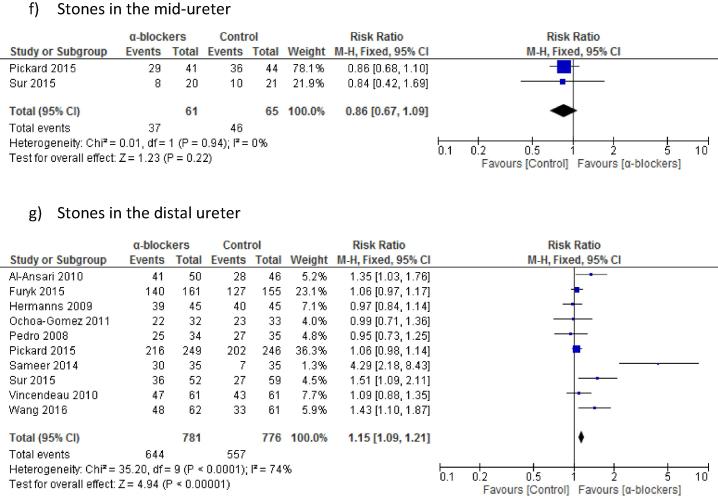
Regarding locality, for proximal ureteric stones there was no statistically significant difference in the stone expulsion rate for α-blockers compared to controls (two studies, 257 patients), at 59.5% vs 63.5% (P = 0.63; RR 0.96, 95% CI 0.79, 1.15; Fig. 3). There was also no difference between the α-blocker and control groups for mid-ureteric stones (two studies, 126 patients), at 60.7% vs 70.8% (P = 0.22; RR 0.86, 95% CI 0.67, 1.09; Fig. 3). However, there was a statistically significant difference favouring α-blocker for distal ureteric stones (10 studies, 1557 patients), at 82.5% vs 71.7% (P < 0.001; RR 1.15, 95% CI 1.09, 1.21).
MET safety
There was statistically significantly more side-effects in the α-blocker group compared to the control group (seven studies, 1268 patients), at 6.6% vs 1.4% (P < 0.001; RR 3.94, 95% CI 2.09, 7.43). Whilst, there was no significant difference regarding side-effects for CCB compared to controls (two studies: 828 patients), at 0.9% vs 0.2% (P = 0.24; RR 3, 95% CI 0.48, 18.87).
There was no statistically significant difference in re-hospitalisation rates in the α-blocker group compared to the control group (five studies, 798 patients), at 8.7% vs 14.6% (P = 0.44; RR 0.67, 95% CI 0.24, 1.84). However, there was significantly more (∼45%) re-hospitalisations in the control group compared to the CCB group (one study, 35 patients in each group), at 77.1% vs 31.4% (P < 0.001; RR 0.41, 95% CI 0.24, 0.69).
Numbers needed to treat (NNT)
We calculated the NNT to establish a better understanding of each subcategory. For α-blockers the NNT was 14, with an absolute risk reduction (ARR) of 7.4% (95% CI 3.5, 11.25%). For nifedipine the NNT was 28, with an ARR of 3.6% (95% CI −2.1, 9.4%).
For stones <5 mm, the NNT was 61, with an ARR of 1.6% (95% CI −3.1, 6.4%). As the 95% CI for the ARR extends from a negative number, there is a risk to do harm with treatment. For stones >5 mm, the NNT was eight, with an ARR of 14% (95% CI 2.8, 25%). For proximal stones, the NNT was 26, with an ARR of 4% (95% CI −8, 15.8%). For mid-ureteric stones, the NNH was one in 10, with an ARR of 10% (95% CI −6.4, 26.6%). As the 95% CI for the ARR extends from a negative number, there is a risk to do harm with treatment. For distal ureteric stones, the NNT was one in 10, with an ARR of 10.7% (95% CI 6.5, 14.8%).
Methodological quality assessment
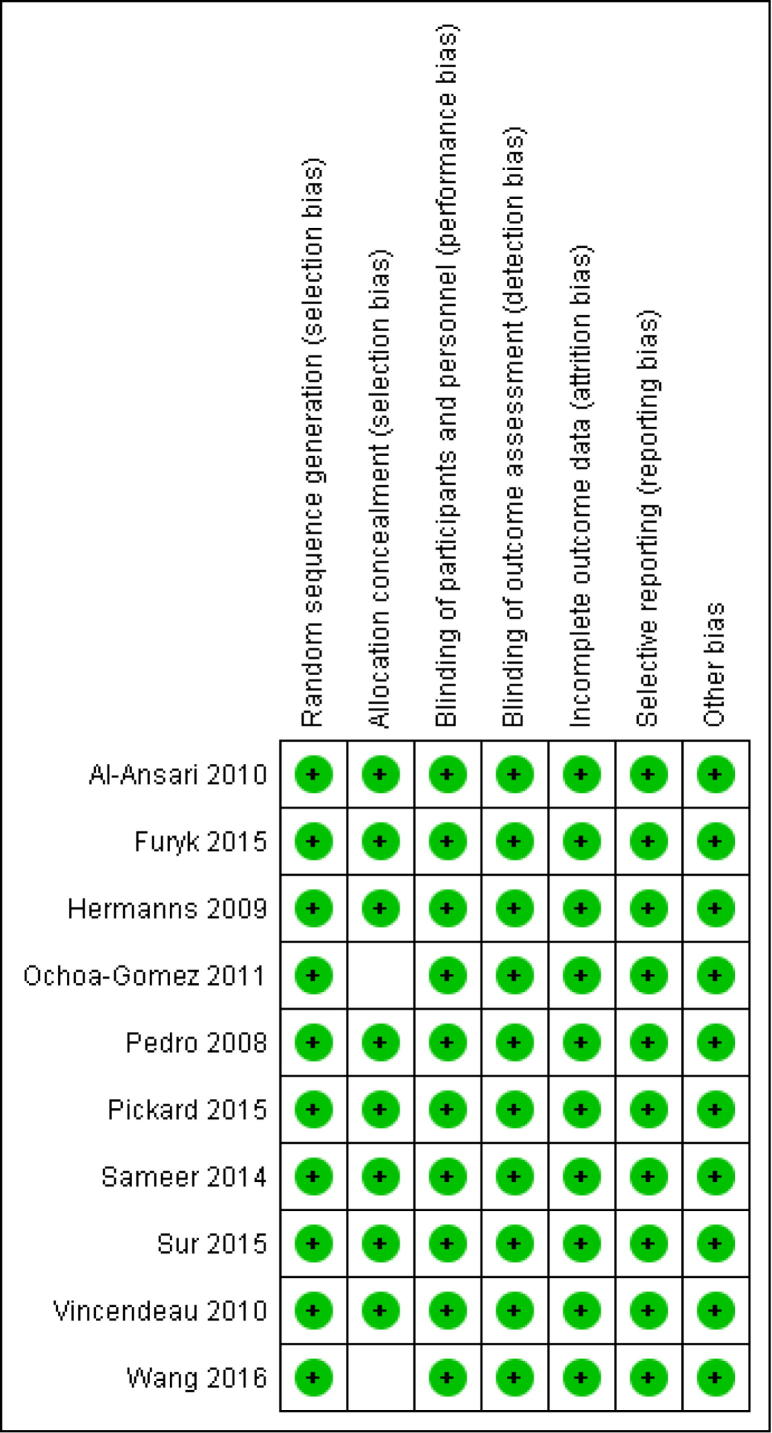
All the studies were powered double-blinded RCTs, ergo considered high-quality studies. Fig. 4 [25], [26], [27], [28], [29], [30], [31], [32], [33], [34] depicts the summary of the quality assessment based on the reviewing author’s judgement of RoB for each included study. All the studies had a low RoB and had powered their studies. We contacted the corresponding authors of trials for clarity if inconsistency arose. Two studies did not mention the allocation concealment method and we were unable to contact them [28], [34]. However, as no reporting, attrition, or any other bias was detected, an ‘unclear’ judgement for concealment was given. Deeming all studies to be of ‘low risk of bias’.
Fig. 4.
RoB table.
Discussion
Summary of meta-analysis
As the main goal of the present review was to establish the efficacy of MET, we analysed all RCTs comparing α-blockers and CCBs with either a placebo or a control group who had pooled their trial and were considered to have a low RoB, which came to only 18% (10/56) of all the RCTs on the subject [25], [26], [27], [28], [29], [30], [31], [32], [33], [34].
Pooled analysis would suggest that α-blockers do have a role in MET, whilst CCBs do not (Table 3). Although, the pooled analysis did find statistical significance favouring α-blockers, the analysis had heterogeneity of 71%. Although not deemed significantly high (I2 > 75% was considered as significantly high heterogeneity), we investigated why heterogeneity was up to 71% (Fig. 2). We found that this was due to one study only, Sameer et al. [31], as their study had a large discrepancy between their α-blocker and control groups stone expulsion rates (α-blockers 30/35 vs control 7/35, i.e. significantly more patients in the control groups failed stone expulsion). Sub-analysing without this trial brought the heterogeneity down to a lower heterogeneity mark (36%); however, the analysis has still shown an overall benefit from α-blockers for MET vs control (nine studies, 1870 patients), at 77.8% vs 72.5% (P = 0.009; RR 1.07, 95% CI 1.00, 1.13). Ergo, there still remains an overall benefit from α-blockers when taking into consideration pooled studies with low heterogeneity papers.
Table 3.
Outcomes of the meta-analysis.
| Primary outcome measures |
| α-Blockers increase expulsion rates for ureteric stones |
| No benefit of CCBs in expulsion rates for ureteric stones |
| Secondary outcome measures |
| α-Blockers led to shorter time to expulsion of stones |
| α-Blockers had increased expulsion rates for ureteric stones of >5 mm |
| α-Blockers lead to increased expulsion of stones in the distal ureter |
| No benefit for α-blockers for proximal ureteric stones |
| No benefit for α-blockers for mid-ureteric stones |
| No benefit for α-blockers for ureteric stones of <5 mm |
| CCBs did not shorten time to expulsion of stones |
| No benefit for CCBs for ureteric stones <5 or >5 mm |
| α-Blockers are associated with increased risk of side-effects |
| No increase in side effects with CCBs use |
| No benefit for α-blockers to prevent re-hospitalisation |
Analysis for our secondary goals has shown that both medication groups had shorter time to expulsion of stones, whilst the α-blockers were beneficial for distally located stones, as well as stones sized >5 mm. By contrast, smaller stones and more proximally located stones showed no benefit. Furthermore, α-blockers were associated with more side-effects.
Interestingly, although six of the 10 powered low-RoB trials found no benefit of MET in expulsion rates overall, pooling these results demonstrated a benefit for MET [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]. Whilst most of these studies did report similar findings with regards to the secondary outcomes as found by the meta-analysis.
The present review has shown that MET increased the expulsion rate of stones >5 mm as opposed to those <5 mm, where no benefit was found, in addition to a reduction in the time to stone expulsion. Lastly, stone expulsion rates were higher in the MET groups in the distal ureter, whereas no difference was found in the mid or proximal ureter compared to control groups [10].
We theorise that the reason for discrepancy between RCTs in finding benefit or negating it for the use of MET, is that the RCTs that found no difference between the two groups had the final results include mixed stone sizes, including small stones <5 mm, in addition to the mid and proximally located stones, where MET has no role for these categories based on our present review findings; hence, diluting the cohort of patients giving a false or inaccurate end result. Furthermore, RCTs that had small (<5 mm) and distal ureteric stones have a high spontaneous expulsion rate as shown by the placebo arm in most trials, and hence the value of MET was perhaps not beneficial, or even harmful, for these stones, as the likelihood of spontaneous passage was high anyway. Nonetheless, pooling studies with a low RoB and who powered their trials, we found a benefit for MET. The sub-analyses dependant on stone size and location, provide further results favouring MET for a select group.
There were no significant side-effects or deaths reported in any of the studies. However, pooled meta-analysis of patients who developed side-effects has shown that more patients developed side-effects in the α-blockers group compared to the controlled groups (6.6% vs 1.4%), whilst no difference was found between the CCB and control groups (0.9% vs 0.2%).
Difference compared to other systematic reviews and strengths and limitations of the present review
Although six journal reviews and a Cochrane review (seven meta-analyses) have been published within the last 10 years, the reviews had methodological flaws, leading to questionability of their end results [4], [17], [18], [19], [20], [21], [22]. The main flaw found, which can significantly introduce bias into a meta-analysis, is that all of the reviews included RCTs with high-RoB into their pooled analyses [4], [17], [18], [19], [20], [21], [22]. This has been a significant criticism of these reviews, which seems to be continually repeated even in the more recent (2014–2016) reviews [17], [18], [19], [22]. Furthermore, six of the meta-analyses included abstract publications in their analysis, which whilst increasing the number of the cohort, makes it difficult to accept as a published trial result [4], [17], [18], [20], [21], [22]. Furthermore, some of these reviews have missed out RCTs found in their search, which should have been included in their analysis [22]. Whilst the Cochrane review, theoretically being the highest form of evidence, also included abstract publications as well as high-RoB trials into their pooled analyses [17]. Furthermore, they had incorrectly extracted data results from trials which were included in their main pooled analysis [17]. Finally, they had excluded trials on shockwave lithotripsy patients; however, they also had a MET arm compared to a placebo arm [17].
We conducted an analysis to include only low-RoB trials, to ensure a ‘low risk of bias’ assessment, where any uncertainty presented, an attempt in contacting the authors to clarify the point was made. To further establish evidence from only the highest quality RCTs, we only included trials that had a power calculation of their sample size.
The main strength of the present review is that it was conducted to Cochrane research methodology standards, in addition, unlike the previous reviews mentioned, we only included truly ‘high quality’ trials with low RoB and a powered calculation, making the present review the first confidently level A evidence.
However, a limitation found was that we included trials with varying stone sizes (Table 1) as a part of their inclusion criteria. We considered this acceptable as we aimed to test the efficacy of α-blockers and CCBs in MET with stones <10 mm. Furthermore, neither the pooled meta-analysis nor the individual trials found significant difference between the MET and control/placebo groups regarding mean stone size.
Implications for research and practice
Based on the results of this meta-analysis, we found that MET does increase stone expulsion rates. Furthermore, there is a selected role for MET in shortening expulsion time and increasing expulsion rates for stones >5 mm or stones in the distal ureter. This benefit was reflected in the NNT calculations for overall benefit, whereby the NNT was 14, the benefit (ARR) was only 7.4%, with no benefit for the CCBs. The NNT for stones >5 mm was eight with a benefit (ARR) of 14%, and for distal ureteric stones the NNT was 10 with a benefit (ARR) of 10.7%. This has to be weighed against a likely side-effect developing in up to 6.6% of patients, in addition to the fact that there was only a marginal clinical benefit for MET (7.4%) despite reaching statistical significance.
Therefore, further research on MET will need to be focused on these secondary outcomes, as it is now evident there is a benefit of MET for increased expulsion rates; however, with a slight chance (6.6%) of developing side-effects.
Conclusion
Pooled analysis of powered RCTs with low RoB would suggest, MET with the use of an α-blocker does increase stone expulsion rates (α-blockers 78% vs 71% control). Furthermore, their role is more significant for larger (>5 mm) stones (α-blockers 75% vs 61% control) and stones in the lower ureter (α-blockers 83% vs 72% control). However, MET was associated with side-effects, albeit not severe.
Conflicts of interest
None.
Acknowledgement
Mr Omar M. Aboumarzouk would like to acknowledge Dr Isra Ashi for her help and support.
Stones/Endourology
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Pak C.Y. Kidney stones. Lancet. 1998;351:1797–1801. doi: 10.1016/S0140-6736(98)01295-1. [DOI] [PubMed] [Google Scholar]
- 2.Ramello A., Vitale C., Marangella M. Epidemiology of nephrolithiasis. J Nephrol. 2000;13(Suppl. 3):S45–S50. [PubMed] [Google Scholar]
- 3.Ahmad H., Azim W., Akmal M., Murtaza B., Mahmood A., Nadim A. Medical expulsive treatment of distal ureteral stone using tamsulosin. J Ayub Med Coll Abbottabad. 2015;27:48–50. [PubMed] [Google Scholar]
- 4.Hollingsworth J.M., Rogers M.A., Kaufman S.R., Bradford T.J., Saint S., Wei J.T. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171–1179. doi: 10.1016/S0140-6736(06)69474-9. [DOI] [PubMed] [Google Scholar]
- 5.Miller O.F., Kane C.J. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162:688–691. doi: 10.1097/00005392-199909010-00014. [DOI] [PubMed] [Google Scholar]
- 6.Preminger G.M., Tiselius H.G., Assimos D.G., Alken P., Buck A.C., Gallucci M. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610–1631. doi: 10.1016/j.eururo.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 7.Dellabella M., Milanese G., Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol. 2005;174:167–172. doi: 10.1097/01.ju.0000161600.54732.86. [DOI] [PubMed] [Google Scholar]
- 8.Davenport K., Timoney A.G., Keeley F.X. A comparative in vitro study to determine the beneficial effect of calcium-channel and alpha(1)-adrenoceptor antagonism on human ureteric activity. BJU Int. 2006;98:651–655. doi: 10.1111/j.1464-410X.2006.06346.x. [DOI] [PubMed] [Google Scholar]
- 9.Davenport K., Timoney A.G., Keeley F.X., Jr. Effect of smooth muscle relaxant drugs on proximal human ureteric activity in vivo: a pilot study. Urol Res. 2007;35:207–213. doi: 10.1007/s00240-007-0100-x. [DOI] [PubMed] [Google Scholar]
- 10.Nakada S.Y. Tamsulosin: ureteric motility. BJU Int. 2008;101:1061–1062. doi: 10.1111/j.1464-410X.2008.07552.x. [DOI] [PubMed] [Google Scholar]
- 11.Maggi C.A., Giuliani S. A pharmacological analysis of calcium channels involved in phasic and tonic responses of the guinea-pig ureter to high potassium. J Auton Pharmacol. 1995;15:55–64. doi: 10.1111/j.1474-8673.1995.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 12.Malin J.M., Jr, Deane R.F., Boyarsky S. Characterisation of adrenergic receptors in human ureter. Br J Urol. 1970;42:171–174. doi: 10.1111/j.1464-410x.1970.tb10018.x. [DOI] [PubMed] [Google Scholar]
- 13.Morita T., Wada I., Saeki H., Tsuchida S., Weiss R.M. Ureteral urine transport: changes in bolus volume, peristaltic frequency, intraluminal pressure and volume of flow resulting from autonomic drugs. J Urol. 1987;137:132–135. doi: 10.1016/s0022-5347(17)43904-8. [DOI] [PubMed] [Google Scholar]
- 14.Richardson C.D., Donatucci C.F., Page S.O., Wilson K.H., Schwinn D.A. Pharmacology of tamsulosin: saturation-binding isotherms and competition analysis using cloned alpha 1-adrenergic receptor subtypes. Prostate. 1997;33:55–59. doi: 10.1002/(sici)1097-0045(19970915)33:1<55::aid-pros9>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Weiss R.M., Bassett A.L., Hoffman B.F. Adrenergic innervation of the ureter. Invest Urol. 1978;16:123–127. [PubMed] [Google Scholar]
- 16.Andersson K.E., Forman A. Effects of calcium channel blockers on urinary tract smooth muscle. Acta Pharmacol Toxicol (Copenh) 1986;58(Suppl. 2):193–200. doi: 10.1111/j.1600-0773.1986.tb02536.x. [DOI] [PubMed] [Google Scholar]
- 17.Campschroer T., Zhu Y., Duijvesz D., Grobbee D.E., Lock M.T. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509. doi: 10.1002/14651858.CD008509.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Huang W., Xue P., Zong H., Zhang Y. Efficacy and safety of silodosin in the medical expulsion therapy for distal ureteral calculi: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:13–22. doi: 10.1111/bcp.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C., Zeng G., Kang R., Wu W., Li J., Chen K. Efficacy and safety of alfuzosin as medical expulsive therapy for ureteral stones: a systematic review and meta-analysis. PLoS One. 2015;10:e0134589. doi: 10.1371/journal.pone.0134589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seitz C., Liatsikos E., Porpiglia F., Tiselius H.G., Zwergel U. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455–471. doi: 10.1016/j.eururo.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Singh A., Alter H.J., Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552–563. doi: 10.1016/j.annemergmed.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth J.M., Canales B.K., Rogers M.A., Sukumar S., Yan P., Kuntz G.M. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112. doi: 10.1136/bmj.i6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somani B.K., Aboumarzouk O., Traxer O., Baard J., Kamphuis G., de la Rosette J. Medical expulsive therapy for ureteral stones: where do we go from here? Nat Rev Urol. 2016;13:608–612. doi: 10.1038/nrurol.2016.146. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org. Accessed March 2017.
- 25.Al-Ansari A., Al-Naimi A., Alobaidy A., Assadiq K., Azmi M.D., Shokeir A.A. Efficacy of tamsulosin in the management of lower ureteral stones: a randomized double-blind placebo-controlled study of 100 patients. Urology. 2010;75:4–7. doi: 10.1016/j.urology.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 26.Furyk J.S., Chu K., Banks C., Greenslade J., Keijzers G., Thom O. Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med. 2016;67(86–95):e2. doi: 10.1016/j.annemergmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Hermanns T., Sauermann P., Rufibach K., Frauenfelder T., Sulser T., Strebel R.T. Is there a role for tamsulosin in the treatment of distal ureteral stones of 7 mm or less? Results of a randomised, double-blind, placebo-controlled trial. Eur Urol. 2009;56:407–412. doi: 10.1016/j.eururo.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 28.Ochoa-Gomez R., Prieto-Diaz-Chavez E., Trujillo-Hernandez B., Vasquez C. Tamsulosin does not have greater efficacy than conventional treatment for distal ureteral stone expulsion in Mexican patients. Urol Res. 2011;39:491–495. doi: 10.1007/s00240-011-0380-z. [DOI] [PubMed] [Google Scholar]
- 29.Pedro R.N., Hinck B., Hendlin K., Feia K., Canales B.K., Monga M. Alfuzosin stone expulsion therapy for distal ureteral calculi: a double-blind, placebo controlled study. J Urol. 2008;179:2244–2247. doi: 10.1016/j.juro.2008.01.141. [DOI] [PubMed] [Google Scholar]
- 30.Pickard R., Starr K., MacLennan G., Lam T., Thomas R., Burr J. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341–349. doi: 10.1016/S0140-6736(15)60933-3. [DOI] [PubMed] [Google Scholar]
- 31.Sameer, Lal S., Charak K.S., Chakravarti S., Kohli S., Ahmad S. Efficacy of nifedipine and alfuzosin in the management of distal ureteric stones: A randomized, controlled study. Indian J Urol. 2014;30:387–391. doi: 10.4103/0970-1591.139572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sur R.L., Shore N., L'Esperance J., Knudsen B., Gupta M., Olsen S. Silodosin to facilitate passage of ureteral stones: a multi-institutional, randomized, double-blinded, placebo-controlled trial. Eur Urol. 2015;67:959–964. doi: 10.1016/j.eururo.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 33.Vincendeau S., Bellissant E., Houlgatte A., Dore B., Bruyere F., Renault A. Tamsulosin hydrochloride vs placebo for management of distal ureteral stones: a multicentric, randomized, double-blind trial. Arch Intern Med. 2010;170:2021–2027. doi: 10.1001/archinternmed.2010.447. [DOI] [PubMed] [Google Scholar]
- 34.Wang C.J., Tsai P.C., Chang C.H. Efficacy of silodosin in expulsive therapy for distal ureteral stones: a randomized double-blinded controlled trial. Urol J. 2016;13:2666–2671. [PubMed] [Google Scholar]