Abstract
Current clinical practices focus on a small number of biochemical directly related to the pathophysiology with patients and thus only describe a very limited metabolome of a patient and fail to consider the interations of these small molecules. This lack of extended information may prevent clinicians from making the best possible therapeutic interventions in sufficient time to improve patient care. Various post-genomics ‘(’omic)’ approaches have been used for therapeutic interventions previously. Metabolomics now a well-established’omics approach, has been widely adopted as a novel approach for biomarker discovery and in tandem with genomics (especially SNPs and GWAS) has the potential for providing systemic understanding of the underlying causes of pathology. In this review, we discuss the relevance of metabolomics approaches in clinical sciences and its potential for biomarker discovery which may help guide clinical interventions. Although a powerful and potentially high throughput approach for biomarker discovery at the molecular level, true translation of metabolomics into clinics is an extremely slow process. Quicker adaptation of biomarkers discovered using metabolomics can be possible with novel portable and wearable technologies aided by clever data mining, as well as deep learning and artificial intelligence; we shall also discuss this with an eye to the future of precision medicine where metabolomics can be delivered to the masses.
1. Introduction
Central to this review is the role of metabolomics within the clinical sciences and so metabolomics as a discipline is first introduced, along with the role of clinically useful biomarkers (small molecules). Following this we discuss metabolomics approaches for personalised and precision medicine and the future role of delivering metabolomics to the masses.
Whilst there are many definitions of metabolomics we consider that metabolomics is a multidisciplinary science that seeks to define the entire complement of small molecular weight molecules termed metabolites within a biological matrix of interest. Metabolomics has been readily applied to a vast array of biological matrices of pre-clinical and clinical medicine relevance, with perhaps not surprisingly the most common being blood plasma and serum as well as urine. These are not the only samples accessible to the clinician and many studies have also focussed on extending these measurements towards intact tissues. This is particularly important for cancer diagnostics as measuring the pathology directly is likely to yield pathophysiological information about the disease (i.e. the cause) rather than measuring circulating metabolites (i.e. the likely downstream effect). In addition, studies have also shown that it is possible to generate information-rich metabolomes from human saliva, breath, cerebrospinal fluid (CSF), broncho alveolar lavage (BAL), sweat, faeces (as well as other locations in the gastro-intestinal tract), semen, and amniotic fluid. Finally, some research has also cultured primary cells for mammalian cell-based models, which may be particularly important for ADME-Tox (adsorption, distribution, metabolism and excretion-toxicology) studies.
The term metabolomics was first coined in the late 1990s [1] and had its 18th anniversary last year [2]. Metabolomics has increased in popularity and applicability ever since. Metabolomics can no longer be described as a novel concept within the clinical arena and it is now emergent. A simple search of Web of Science (on 7th Feb 2017) for (metabolom* OR metabonom*) AND (clinical OR medicine) within the research topic field returns over 3700 articles. Within the range of ‘omic approaches (i.e. transcriptome, proteome) the metabolome is perhaps the most closely linked to the phenotype of the subject and thus, can report on disease status as well as the effect and response to external stimuli (e.g. drug therapy, nutrition, exercise, etc).
2. The role of biomarkers in clinics
To treat disease GPs, clinicians and health workers require diagnostic indicators of disease, which can be used not only to diagnose said disease but also to assess the applicability of therapeutic interventions. These indicators are referred to as biomarkers and the NIH definition of a biomarker is [3]:
“A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”
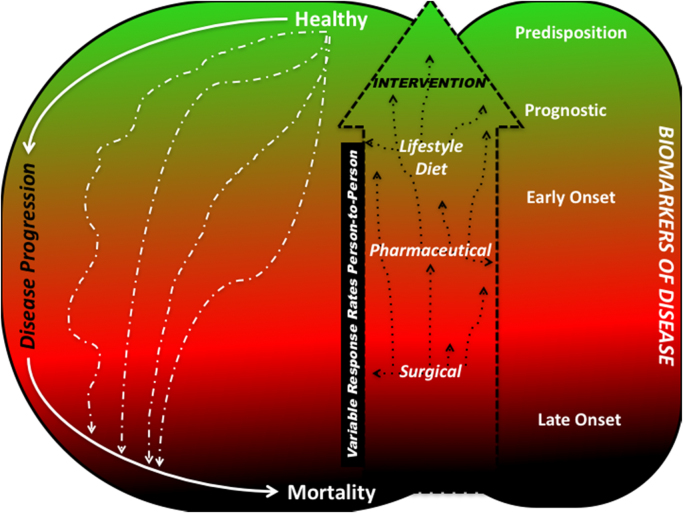
The use of biomarkers to direct therapeutic intervention in terms of whether dietary and lifestyle interventions are necessary, or whether drugs are appropriate or if surgery is needed, is highlighted in Fig. 1.
Fig. 1.
Figure illustrating disease progression (left hand side) along with the role of biomarkers on disease (right hand side) and how these may inform a range of personalised interventions.
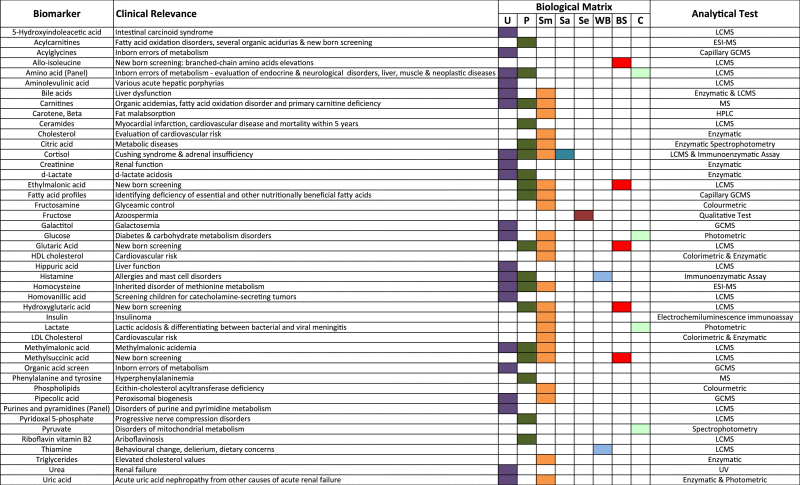
A summary of clinically useful biomarkers used in clinical practice are provided in Table 1 (summarised from a test catalogue provided by Mayo Clinic, US available at http://www.mayomedicallaboratories.com). Whilst these biomarkers are very valuable for diagnosing disease there is an urgent need to have many more that are highly predictive and robust. There are many more biomarkers waiting to be discovered and this is a very active area of research in metabolomics.
Table 1.
A selection of small molecule biomarkers and their clinical relevance; summarised from an available test list at the Mayo Clinic, US. The biological matrix investigated (U- urine, P-plasma, Sm-serum, Sa-saliva, Se-semen, WB-whole blood, BS-dried blood spot & C-CSF) and the analytical method/test applied is detailed.
 |
3. Current positioning of metabolomics
With such popularity and regard, metabolomics as a discipline must look towards the future and begin to anticipate how the field can develop and transition into the modern-day world. As mentioned above metabolomics is now established, and whilst work is on-going in terms of technology and computational improvements, the method is now considered routine. Despite this maturity, the literature is unfortunately saturated with small-scale preliminary-type studies with many suffering from being poor in experimental design and thus any findings are likely to be false as they lack statistical robustness and validity [4]. This is not a unique feature of metabolomics and as nicely exemplified by George Poste [5] in his article entitled “Bring on the biomarkers”, whilst many biomarkers have been described in the academic literature comparatively few (well almost zero) have made it into the clinic. With reference to ‘omics and biomarker discovery one is often reminded of Henry Nix's famous statement [6]:
“Data does not equal information; information does not equal knowledge; and, most importantly of all, knowledge does not equal wisdom. We have oceans of data, rivers of information, small puddles of knowledge, and the odd drop of wisdom.”
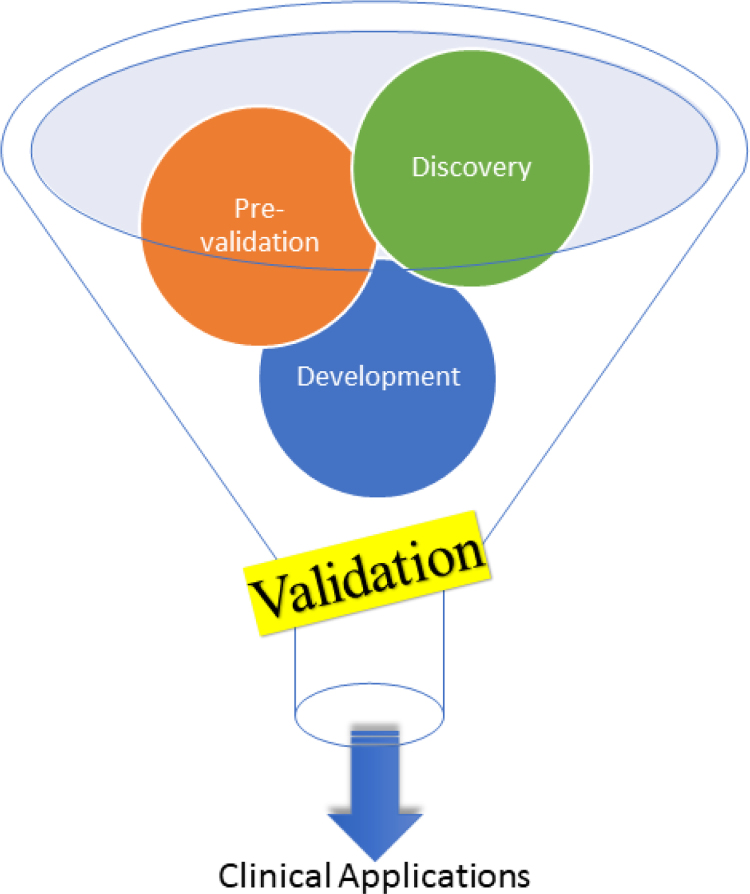
With this in mind we believe that the field must drive forward towards the undertaking of large cohort multi-centre studies to enhance the discovery of biomarkers that have increased prospects of translation into point of care and rapid diagnostics; this biomarker discovery process is highlighted in Fig. 2, and of course is not limited to metabolites but any molecule.
Fig. 2.
Schematic representation of the major steps for metabolomics biomarker discover. This initially starts out with a “Discovery” phase which involves in depth metabolomics assessment in (for example) case-control for disease stratification; this tends to be done on relatively small cohorts (n = 100 s). Following this a “Pre-validation” phase then repeats this untargeted metabolomics assessment in a different patient-control cohort (also of n = 100 s and preferably from a geographically distinct area from the first discovery phase). Following this there is an analytical “Development” phase for the assessment of the biomarker(s) discovered using lower cost technologies: this represents a shift from mass spectrometry or NMR spectroscopy to targeted chromatography or direct measurements using (for example) lateral flow devices. Finally using this faster and cheaper technology there is a “Validation” phase in large patient cohorts (n = 10,000/100,000 s) to assess the robustness of the biomarker(s) discovered.
Table 2 highlights several key metabolomics studies that have been aimed towards identifying biomarker candidates for an array of diseases. This table indicates the target disease of interest and the publication year, which illustrates the attempts made for biomarker discovery using metabolomics approaches, for a specific condition. It also summarises the number of control candidates and the number of diseased patients that were incorporated into the studies. Although these and other authors do not deliberately eschew obfuscation these numbers are often difficult to distinguish clearly within a manuscript. In addition, in some cases longitudinal studies are conducted whereby a patient is their own control. In order to have clarity in what was done within a study and what should be reported the Metabolomics Standards Initiative (MSI) initiated and subsequently published a series of papers on minimum reporting standards [7]. Within Table 2 the biomarker (or biomarker panels) that have been discovered within each study are documented and, we note if an independent validation has occurred within the same study which will of course increase confidence in the validity of said biomarker.
Table 2.
Potential new metabolite biomarkers discovered and reported since 2000. Various sets of biomarkers have been proposed over the years for a number of diseases based on metabolomic investigations. Studies marked with an asterisk (*) indicates a further validation study that was included in the same publication.
| Disease/condition | Year of publication | Control subjects | Test subjects | Proposed biomarkers |
|---|---|---|---|---|
| Abnormal savda | 2008 [84] | 20 | 110 | Glycochenodeoxycholic acid and bilirubin |
| Acute coronary syndrome | 2009 [90] | 10 | 19 | Citric acid, 4-hydroxyproline, aspartic acid, fructose, lactate, urea, glucose and valine |
| Acute kidney injury | 2012 [132] | 17 | 17 | Dimethylarginine, pyroglutamate, lysoPC (selection of), acylcarnitine (selection of), phenylalanine, creatinine, homocysteine, methionine, arginine, tryptophan |
| Advanced liver fibrosis | 2016 [165] | 30 | 27 | Panel inc: choline, glucose, glutamine, cysteine, histidine, citrate, acetoacetate |
| Alzheimer's disease | 2010 [99] | 20 | 20 | Lysophosphocholine, tryptophan, phytosphingosine, dihydrosphingosine, hexadecosphinganine |
| Alzheimer's disease | 2012 [127] | ~52 | ~77 | Desmosterol |
| Alzheimer's disease | 2014 [148] | 57 | 57 | Arachidonic acid, N, N-dimethylglycine, thymine, glutamine, glutamic acid, and cytidine |
| Alzheimer's disease | 2014 [151] | 15 | 15 | Alanine and taurine |
| Alzheimer's disease | 2015 [164] | 218 | 256 | Sphinganine−1-phosphate, ornithine, phenlyllatic acid, inosine, 3-dehydrocarnitine, hypoxanthine |
| Asthma | 2011 [110] | 42 | 20 | Panel inc: Adenosine, alanine, carnitine, formate, fumarate, glucose, histidine, taurine, threonine, succinate |
| Asthma | 2013 [139] | 26 | 39 | methionine, glutamine, histidine |
| Atherosclerosis | 2010 [103] | 28 | 16 | Palmitate, stearate and 1-monolinoleolglycerol |
| Autism* | 2015 [161] | 24 | 22 | Methylguanidine, indoxyl sulfate, glucuronic acid, desaminotyrosine, guanidiosuccinate acid |
| Autism* | 2016 [169] | 63 | 73 | Panel inc: decanoylcarnitine, pregnanetriol, uric acid, 9,10 epoxyoctadecanoic acid, docosahexanoic acid, docosapentanoic acid |
| Bladder cancer* | 2011 [125] | 16 | 28 | Panel of 50+ differential metabolites |
| Bladder cancer | 2014 [146] | 121 | 138 | Succinate, pyruvate, oxoglutarate, carnitine & acylcarnitines, phosphoenolpyruvate |
| Breast cancer | 2010 [97] | 50 | 50 | Five unidentified biomarkers |
| Breast cancer | 2012 [134] | 34 | 80 (40 vs 40) | Palmitic acid, stearic acid, linoleic acid, FFA |
| Cardiovascular diseases | 2014 [145] | / | 67 | Medium-and long-chain acylcarnitines, alanine |
| Chronic heart failure | 2013 [143] | 15 | 39 | Lactate, creatine, glucose, glycoprotein, lipid species and amino acids |
| Chronic Hepatitis B | 2006 [73] | 50 | 37 | Lysophosphatidyl choline and glycochenodeoxycholic acid |
| Chronic kidney disease | 2011 [120] | 13 | 18 | Urinary neutrophil gelatinase-associated lipocalin |
| Chronic widespread musculoskeletal pain | 2015 [160] | 3736 | 1191 | Epiandrosterone sulfate, dehydroisoandrosterone sulfate, androsterone sulfate, 3-(4-hydroxyphenyl) acetate, nonadecanoate |
| Colorectal cancer staging | 2009 [87] | – | 31 | Panel inc: fatty acids, organic acids, sugars, steroid, fatty acid ester and pyrimidine nucleoside. |
| Colorectal cancer* | 2010 [94] | 110 | 112 | Hydroxylated, polyunsaturated ultra-long-chain fatty acids |
| Colorectal cancer | 2011 [117] | 8 | 42 | Free fatty acids and esterified fatty acids |
| Colorectal cancer | 2016 [170] | 254 | 320 (31) | Panel inc: octadecanoic acid, lactic acid, threonic acid, 3-hydroxy butanoic acid, serine, cysteine |
| Coronary artery disease | 2012 [126] | 2023 | Dicarboxylacylcarnitines, medium-chain acylcarnitines, fatty acids | |
| Coronary heart disease | 2009 [88] | 25 | 23 | Saturated fatty acids, trans-fatty acid, n3 and n6 poly unsaturated fatty acids |
| Coronary heart disease* | 2014 [41] | 897 | 131 | LysoPC (18:1), LysoPC (18:2), MG (18:2), SM (28:1) |
| Diabetes | 2010 [106] | 60 | 40 | 3-indoxyl sulfate, glycerophospholipids, free fatty acids and bile acids |
| Diabetic kidney disease | 2012 [128] | 52 (26 vs 26) | Acyl-carnitines, acyl-glycine and metabolites related to tryptophan metabolism | |
| Diabetic mellitus and diabetic nephropathy | 2011 [111] | 30 | 120 | Non-esterified fatty acids and esterified fatty acids |
| Diabetic nephropathy and type 2 diabetes | 2009 [93] | 25 | 41 | Phytospingosine, glycine, lysine, dihydrosphingosine, leucine |
| Disorders of Propionate Metabolism* | 2007 [78] | 10 | 9 | Propionyl carnitine, unsaturated acylcarnitine, γ-butyrobetaine, siovaleryl carnitine |
| Down syndrome | 2015 [159] | 93 | 23 | Progesterone and dihydrouracil |
| Endometrial carcinoma | 2016 [173] | 25 | 25(10) | Porphobilinogen, acetlycysteine, n-acetylserine, urocanic acid, isobutylglycine |
| Gastric cancer | 2016 [166] | 40 | 83 | Sucrose, dimethylamine, 1-methylnicotinamide, 2-furoylglycine, N-acetyl- serotonin, trans-aconitate, alanine, formate, and serotonin |
| Gastrointestinal cancer | 2012 [129] | 12 | 38 | 3-hydroxypropionic acid, pyruvic acid, L-alanine, glucuronolactone, L-glutamine |
| Healthy plasma metabolome | 2008 [81] | 269 | – | 300+ unique compounds |
| Hepatitis B* | 2013 [140] | 11 | 13 | Tyrosinamide, biotin sulfone, hexanoic acid, 1-aminonaphthalene, 7-dehydroxycholesterol, azelaic acid |
| Hepatitis E and Hepatitis B | 2011 [119] | 18 | 32 | Panel inc: L-proline, L-isoleucine, acetone, glycerol, glycine, biopterine, adenosine |
| Hepatocarcinoma | 2011 [121] | 38 | 41 | 1-methyladenosine |
| Hepatocellular carcinoma | 2009 [92] | 20 | 20 | Panel of 18 metabolites inc: glycine, urea, threonine |
| High altitude pulmonary edema* | 2015 [162] | 35 | 35 | Methionine, hypoxanthine, inosine, sphingosine, palmitoyl carnitine, C8 carnitine |
| Human hepatocellular carcinoma | 2011 [116] | 71 | 106 | Bile acids, histidine, inosine, glycochenodeoxychoclic acid, glycocholic acid, taurocholic acid and chenodeoxycholic acid |
| Interstitial cystitis | 2016 [172] | 21 | 42 | Oleic acid, 2-deoxytetronic acid, saccharic acid, phosphate, trehalose, erthronic acid, oxalic acid, sulfuric acid, cystine, lyxitol, lysine, histidine |
| Intestinal fistulas | 2006 [76] | 17 | 40 | Glycochenodeoxycholic acid, glycodeoxycholic acid, taurochenodexycholic acid, taurodeoxycholic acid, lysophosphatidyl choline (C16: 0 and C18:2), phenylalanine, tryptophan and carnitine |
| IVF | 2008 [85] | 17 | 17 | Glutamate and alanine/lactate ratios |
| Lepromatous leprosy | 2011 [118] | 10 | 13 | Eicosapentaenoic acid, docosahexaenoic acid and arachidonic acid |
| Liver cirrhosis | 2011 [113] | 22 | 37 | Lysophosphatidyl cholines, bile acids, hypoxanthine, stearamide, oleamide, myristamide |
| Liver failure due to Hepatitis B | 2010 [104] | 16 | 26 | 1-Lioleoylglycerophosphocholine or 1-linoleoylphosphatidylcholine |
| Lung cancer | 2010 [108] | 12 | 12 | Lysophosphatidylcholines: lyso16:0, sn−2 lysoPC 16:0, sn−1 lysoPC 18:0, sn−1 lysoPC 18:1 and sn−1 lysoPC 18:2 |
| Lung cancer | 2011 [122] | 29 | 33 | A panel of 23 serum metabolites and 48 tissue specific metabolites |
| Lung cancer* | 2014 [149] | 536 | 469 | Creatine riboside, cortisol sulfate, N-acetylneuraminic acid |
| Lung cancer* | 2015 [157] | 20 | 18 | Maltose, ethanolamine, glycerol, palmitic acid, lactic acid, |
| Lung cancer | 2015 [155] | 55 | 41 | Panel inc: trisaccharide phosphate, trihexose, nonanedioic acid, MG (22:2), tetrahexose |
| Lung cancer | 2016 [167] | 34 | 23 (11) | Isobutyl decanoate, putrescine, diethyl glutarate, cysteamine |
| Major depressive disorder | 2012 [135] | 25 | 26 | Tryptophan, GABA and lysine |
| Major depressive disorder* | 2015 [153] | 59 | 60 | Acyl carnitines, lipid metabolism and tryptophan |
| Malignant adrenal tumours | 2011 [124] | 45 | 102 | Panel inc: metabolites from steroid metabolism pathways |
| Malignant Oligodendroglioma* | 2008 [83] | 10 | 24 | Alanine, lipids, valine, the total choline compounds, proline, myoinositol, taurine, glutamine, glutamate, GABA, NAA, acetate, and creatine |
| Melamine-induced nephrolithiasis | 2011 [123] | 74 | 73 | Proline, 5C-aglycone and hypoxanthine |
| Multiple sclerosis | 2014 [150] | 17 | 15 | Choline, myo-inositol, threonate |
| Multiple sclerosis | 2015 [156] | 12 | 13 | LPC (18:1), LPC (18:0), LPI (16:0), Glutamate |
| Muscle respiratory chain deficiencies | 2015 [163] | 13 | 24 | AMP, n-acetyl asparagine, oxoglutaric acid, n-succinyl-L-L2.6 diaminopimelate |
| Nasopharyngeal carcinoma | 2011 [115] | 40 | 37 | Kynurenine, N-acetylglucosaminylamine, N-acetylglucosamine and hydroxyphenylpyruvate |
| Oesophageal cancer | 2013 [141] | 26 | 89 | Formate, acetate, short-chain fatty acids, GABA |
| Oesophageal squamous-cell carcinoma | 2013 [144] | 53 | 53 | Phosphatidylserines, 12-oxo−20-dihydroxy-leukotriene B4, sphinganine 1-phosphate, LysoPC, phosphatidyl ethanolamine, phosphatidyl choline |
| Onchocerciasis* | 2010 [105] | 56 | 76 | Panel of 14 inc: hexacosenoic acid, fatty acids, proteins, sterol lipids and phosphorylated sphingolipids |
| Oral cancer | 2014 [152] | 50 | 30 | Phenylalanine & leucine |
| Oral, breast and pancreatic cancer | 2010 [95] | 87 | 128 | betaine, choline, carnitine, glycerophosphocholine, cadaverine, putrescine, hypoxanthine, ethanolamine, trimethylamine and amino acids |
| Osteoarthritis* | 2010 [98] | 299 | 123 | Valine to histidine ratio and leucine to histidine ratio |
| Ovarian cancer | 2011 [112] | 27 | 57 | 27-nor−5-beta-cholestane−3,7,12,24,25 pentol glucuronide |
| Ovarian cancer | 2011 [114] | 12 | 18 | N-acetylasparate and N-acetyl-aspartyl-glutamate |
| Ovarian cancer* | 2012 [131] | 50 | 50 | 2-piperidinone, L-tryptophan, lysoPC (18:3), lysoPC (14:0) |
| Ovarian endometriosis | 2012 [133] | 52 | 40 | Sphingomyelins and phosphatidylcholines |
| Paediatric acute liver failure | 2009 [89] | 20 | 20 | α-NH2-butyric-acid (Aab) and Aab: leucine ratio |
| Pancreatic cancer | 2016 [168] | 40 | 40 | Panel inc: palmitic acid, 1,2 dioeoyl GLP Na2, lanosterol, lignorceric acid, 1 oleoyl rac GL, chol epoxide, erucic acid |
| Parkinson's disease | 2008 [79] | 25 | 66 | Uric acid and glutathione |
| Parkinson's disease | 2009 [91] | 37 | 43 | Pyruvate |
| Parkinson's disease | 2015 [158] | 104 | 297 | Cortisol, 11-deocycortisol, 21-deoxycortisol, histidine, urocanic acid, imadazoleacetic acid, hydroxyphenylacetic acid |
| Periodontal disease | 2010 [101] | 21 | 18 | Inosine, lysine, putrescine and xanthine |
| Pre-eclampsia | 2005 [72] | 87 | 87 | Three unidentified molecules |
| Pre-eclampsia | 2017 [174] | 20 | 20 | Panel inc: PC (14:0/0:0), proline betaine, proline |
| Premature labour* | 2010 [107] | 16 | 39 | Panel inc: Methyladenine, heptanedioic acid, N-acetylglutamine, glycerol, succinic acid, mannose |
| Prostate cancer | 2010 [96] | 30 | 40 | Acylcarnitine and arachidonoyl amine |
| Prostate cancer | 2013 [138] | 178 | 331 | Panel of 25 metabolites inc top 5: histidine, glycine, alanine, kynurenine, glutamate & glycerol−3-phosphate |
| Psoriasis | 2017 [175] | 15 | 14 | Asparagine, aspartic acid, isoleucine, phenylalanine, ornithine, proline, lactic acid & urea |
| Rectal cancer | 2013 [142] | 43 | 127 | Lactate, threonine, acetate, glutathione, uracil, succinate, serine, formate, lysine and tyrosine |
| Renal cell carcinoma | 2010 [100] | 13 | 32 | Panel inc: acetate, glutamate, glutamine, glucose, tyrosine, histidine, phenylalanine, formic acid, alanine, lactate |
| Rheumatoid arthritis | 2010 [102] | 51 | 47 | Cholesterol, lactate, acetylated glycoprotein and lipids |
| Rheumatoid arthritis | 2011 [109] | 20 | 25 | Panel inc: Glyceric acid, hypoxanthine, histidine, threonic acid, methionine, cholesterol, threonine |
| Rheumatoid arthritis | 2016 [171] | 19 | 46 | Arginine, aspartic acid, glutamic acid, phenylalanine, serine, threonine, methlynicotinamide |
| Schizophrenia | 2006 [74] | 70 | 82 | Citrate, glutamine, acetate, lactate |
| Schizophrenia | 2007 [77] | – | 50 | 50 lipids including triacylglycerols, free fatty acids, phosphatidylethanolamine. |
| Schizophrenia* | 2013 [137] | 62 | 62 | Glycerate, eicosenoic acid, beta-hydroxybutyrate, pyruvate, cysteine |
| Systemic inflammatory response syndrome (SIRS) & Sepsis | 2012 [130] | 143 (74 vs 69) | Acylcarnitines and glycerophosphatidylcholines (C10:1 and PCaaC32:0) | |
| Type 2 diabetes | 2006 [75] | 45 | 78 | Non-esterified and esterified fatty acids in plasma |
| Type 2 diabetes | 2008 [80] | 28 | 23 | 3-hydroxyhippuric acid |
| Type 2 diabetes | 2008 [86] | time course study | 75 | Citrate, IL−8 and methyl-histidine and branched amino acid degradation products |
| Type 2 diabetes (T2DM) and Type 2 diabetic coronary heart diseases (T2DM-CHD) | 2008 [82] | 45 | 71 and 37 for T2DM & T2DM-CHD | Free fatty acid (C16:0, C18:1 n−9 and C18:2 n−6) |
| Type 2 diabetes & impaired fasting glucose | 2013 [136] | 1897 | 115 & 192 respectively | Panel inc: amino acids, lipids, carbohydrates (T2D) & panel of lipids, carbohydrates, amino acid plus urate & erythritol (IFG) |
| Type 2 diabetes mellitus | 2015 [154] | 300 | 300 | Lipids, hexose sugars, purine nucleotide |
| Ulcerative colitis (UC) & Crohn's disease (CD) | 2014 [147] | 17 | 24 UC & 19 CD | Panel inc: N-acetylated glycoprotein, lactate, methanol, mannose, formate |
It is clear from inspecting this Table 2 that there is a broad difference in the number of subjects included in these studies. The community is yet to decide what this number should be, but it should be noted and acknowledged that the availability of patients will greatly vary from disease to disease and equally access to valuable (sometimes very rare) samples will be limited. In this century alone, there have been more than 1600 publications (using a combined search of the above PLUS biomarker* from 2000 to date) that ‘claim’ to have discovered a biomarker using a metabolomics approach, which is nearly half of all papers surveyed! Although there are some exceptions, most of this research fails to acquire enough statistical power due to a limited sample size (<100 subjects in total) and almost none repeat the analysis in a further cohort and thus fail to demonstrate a lack of biomarker utility. We believe that these thwart the potential translation of metabolomics research into clinics. For instance, there is minimal-known translation of metabolomics biomarker discovery into clinics for the top five causes of death in the UK (Table 3) which include: ischaemic heart diseases, dementia and Alzheimer's disease, malignant neoplasms of trachea, bronchus and lung, chronic lower respiratory diseases and cerebrovascular diseases [8]. Malignant neoplasms, respiratory disease and ischaemic heart diseases are also three of the top five leading causes of death across Europe [9].
Table 3.
Top 5 leading causes of death in men and women in England and Wales (2014).
| Men | |
| Ischaemic heart diseases | 36,293 |
| Dementia and Alzheimer's disease | 15,973 |
| Malignant neoplasm of trachea, bronchus and lung | 14,359 |
| Chronic lower respiratory diseases | 13,952 |
| Cerebrovascular diseases | 12,584 |
| Women | |
| Dementia and Alzheimer's disease | 33,153 |
| Ischaemic heart diseases | 24,057 |
| Cerebrovascular diseases | 19,127 |
| Chronic lower respiratory diseases | 14,181 |
| Malignant neoplasm of trachea, bronchus and lung | 11,309 |
Despite the above disease being of obvious importance we note the rapid rise of microorganisms as contributing to world-wide mortality. The obvious ‘culprits’ here being Mycobacterium tuberculosis and HIV, but with the almost meteoric rise in antimicrobial resistance (AMR) many normally harmless opportunistic pathogens will become increasingly important. Indeed it is predicted by 2050 that bacterial infections will kill more humans than cancer and heart disease [10]. Whilst it is accepted that there are many microbial interactions with the host cell microbiome and that man is a true superorganism [11] it is also notable that many common human disease may indeed have a microbial origin [12]. Metabolomics is likely to play a valuable role in understanding AMR and the host-pathogen interaction.
This review seeks to provide an overview of metabolomics in respect to diagnostic applications and demographic screening and present a futuristic perspective on the implementation of the field with novel portable and wearable technologies.
4. Is the future of healthcare simply personalized medicine?
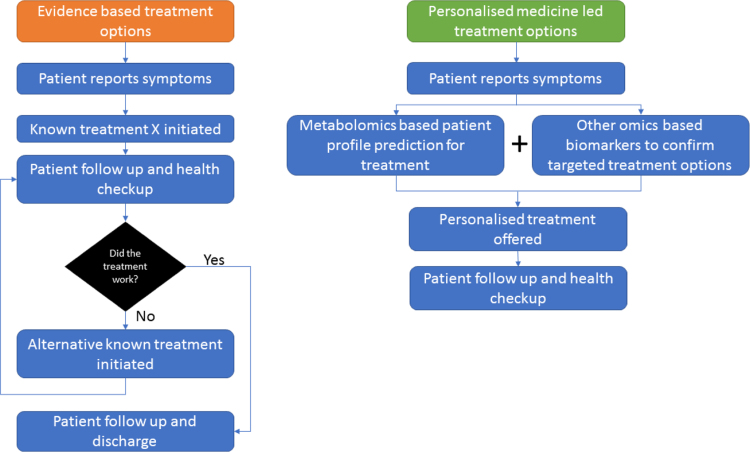
Although personalised medicine is a generic entity relatively new to the field of healthcare research, it has of course been practiced for decades within a so-called evidence-based framework (Fig. 3). In evidence-based medicine an individual is treated for disease largely based on the most popular medicine. After the drug is taken for some time an assessment is made, with the desire to evaluate whether this has relieved symptoms (this may involve the measurement of a clinically useful biomarker (Table 1)). Based on this deterministic assessment the patient may then stay on the same drug, be diagnosed an alternate medicine, or be given a treatment to relieve side effects of the first drug. This process is slow and potentially dangerous to the patient. A much more desirable approach is to use precision medicine and this was brought to the forefront of attention when, during his 2015 State of the Union address President Obama announced that he was launching the Precision Medicine Initiative. This was heralded as a bold new research direction [13] with changing for biobank made available by NIH to support the initiative [14].
Fig. 3.
Flow diagram illustrating personalised medicine and highlighting the differences between Evidence-based versus Precision medicine-based approaches to disease treatment. As is clear the evidence-based approach is imprecise as it relies on the patient reporting progress to therapy. By contrast, precision medicine necessitates analytical measurements on the patient – typically from genetics (viz. SNPs) and metabolomics–and then using these to direct therapy.
Precision medicine involves assessing the genotype (e.g. SNPs) and phenotype (e.g. metabolome) of the patient before they undergo any treatment (Fig. 3) and therefore relies on accurate analytical methods for directing therapy [15]. Biomarkers are needed that can accurately identify the underlying pathology as these may help understand the disease aetiology and thereby result in a precise treatment [16], [17]. Clearly the lack of suitable biomarkers currently holds back the wider implementation of personalised medicine [18]. This is where metabolomics plays a key role as an approach to discover a biomarker, trial its detection within a large diverse population and then translate its detection into cheaper, quicker and reliable methods that could be used by a wider audience [19]. As indicated above the main use of metabolomics as a tool is for biomarker discovery [20], [21], [22], [23], [24]. The closest representation of a disease phenotype is a key-driving factor for the increased use of metabolomics for biomarker discovery to understand disease pathologies and finding methods of cure, and as many diseases result in changes in human metabolism it makes sense to use a method that measures metabolism directly!
However, the focus of biomarker discovery should not only be for pathological cures but also for preventive screening of healthy individuals (Fig. 1), as earlier biomarkers may be useful in directing dietary and lifestyle changes prior to more radical surgical treatment. Within biomarker discovery this raises the tantalising idea that all healthy individuals should undergo some biomarker screen well before any disease is found so that any change in a biomarker(s) level is personalised; for example, someone with an already raised PSA level may not have prostate cancer and this higher PSA levels maybe indicative of an enlarged prostate as one ages [25]. Offering a well-designed screening program at a reasonable cost may not always be possible due to the numerous associated challenges; these include monetary limitations (labour and consumable costs) as well as ethical, legal and social considerations for an opt-in test. The risk-benefit ratio needs to be clearly defined per disease for a successful personalised screening [26]. Encouraging biomarker discoveries from within the plasma metabolome [27], serum metabolome [28], [29], [30], [31], urinary metabolome [32], [33], [34], [35] as well as the volatilome [36], [37], [38] show immense potential of dramatically reducing health risks in fatal health conditions such as cancer, congenital disease, heart diseases and respiratory diseases (Table 3). It should be noted that a key hurdle for translation of these biomarkers into a routine clinical test is the failure to validate. In the absence of a universally accepted procedure for metabolic profiling used for biomarker discovery, different sites use their own optimized procedures. Additionally, even if identical analytical platforms and routines are used, the inherent inter-laboratory variation will play a great role in detracting from the validity of a potential biomarker and there can never be certainty that the entire metabolome has been profiled with that said platform, and in fact it is accepted in the metabolomics community that there is no magic tricorder that measures everything [39]. Thus, there is always a potentially ‘better’ biomarker waiting to be discovered.
Some notable large scale and/or multicentre metabolomics studies have been successfully conducted (Table 2) to map the human serum metabolome [40], to identify biomarkers for incident coronary heart disease [41], and to study the response of Aspergillus nidulans to epigenetic perturbation with a hope to expedite the search for new pharmaceutical leads [42]. A correct balance needs to be considered between large scale vs. small sub-population focused studies where the risk is minimal but with maximum benefits [43], [44]. Due to the higher cost and effort involved in the analysis of samples by a standard metabolomics workflow, it is often tempting (albeit one could say lazy) to use a smaller sample size for biomarker discovery and pre-validation [45]. However, such studies which lack the required statistical power for confident biomarker assessment will entice anyone to start designing specific assays for assessments in large cohorts (Fig. 2).
Like all ‘omics which are data rich, metabolomics on humans is influenced by many confounding factors such as age, gender, ethnicity, diet etc. [40] and thus, large validation studies with suitable control cohorts must be used to remove any potential bias [4], [46]. Certain metabolites that alter with normal physiological changes may also be significantly different in a metabolomics study. By way of an example, citrate has been shown to increase with age [40] even in healthy individuals. A recent metabolomics study indicated amongst other metabolites that citrate was a significantly important biomarker for cancer [47]. However, since an increase in citrate could also be attributed to difference in mean age (17 cancer patients = 70 and 21 healthy controls = 60) rather than altered TCA cycle in cancer, in the absence of closely age matched case-control cohort such results need to be taken with caution before inferring pathological importance of such a biomarker.
Whilst the current perception is that screening large control groups of healthy individuals at the same time as diseased populations is not an option for validation studies, this position must change. Indeed, many people already use wearable technology for the assessment of their exercise levels, heart rate, blood oxygen levels, as well as sleeping patterns, so collecting data on ‘healthy’ individuals is not that maverick.
5. Metabolomics for the masses
With recent technological advancements in the form of affordable hardware (e.g. pedometers which include heart rate monitoring), health apps on smartphones, fitness bands and smart-watches, it is feasible to generate large amounts of useful health-related data even in healthy populations [48], [49]. These measurements are readily available on a personalised level and could be used to complement clinical studies. For example, in treatment regimens which may include nutritional and exercise advice.
The tantalising question is whether metabolomics could be delivered to the masses on a personalised level? Whilst mass spectrometry linked to chromatography is a very power metabolomics platform for biomarker discovery, it is laborious and expensive and therefore unlikely to be suitable for large-scale screening of very large populations (i.e. when n > 10,000, which is of course still small when we consider that the earth's population is estimated to be >7.5×109; http://www.worldometers.info/world-population/). Of course, once a series of biomarkers are discovered and validated the scenario is different where one now knows the measurands and these can be detected and quantified using analytical chemistry. These can include methods based on:
-
•
Lateral flow devices – much like the pregnancy test which is based on antibody detection of the appropriate antigen (viz., human chorionic gonadotropin (hCG));
-
•
Dipstick approaches – for example the detection of nitrite for confirming urinary tract infections;
-
•
Breath measurements for volatiles – for example ethanol detection and quantification using fuel cells for road side testing;
-
•
Electrochemical detection – under skin glucose test is based on this and allows constant assessment of blood glucose that can be linked automatically to insulin injections [50].
With the above in mind emerging technologies in metabolomics provide new platforms for high-throughput, highly sensitive, functional assays, biomarker discovery and offer opportunities for personalised medicine, complementing existing and emerging genomic, proteomic and transcriptomic technologies (Fig. 4). However, personalised medicine in the future could be better served when these biomarkers provide enough knowledge to translate them successfully into one or more types of wearable technologies that are readily available to an end user (as also illustrated in Fig. 4). Biosensors used in wearable technologies like smartphones [51], [52], smart-watches [53] for monitoring heart conditions, health bands, necklaces, glucose monitoring contact lenses [54], [55], headbands etc., are excellent innovations transferring biomarker discovery onto a more individual level. Technological advances translating biochemical changes into physical signals is not something new, but in this age of bionics and biohacking [56], it is putting the technology in the hands of end user and thus able to boost the personalised medicine movement [57]. Although, we recognise that these devices should always be with continued consultation with a clinical practitioner who can advise the wearer; much like home testing for blood pressure is currently practiced.
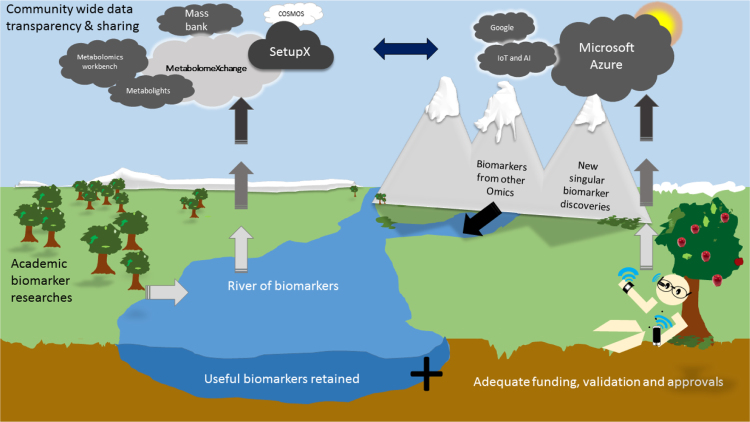
Fig. 4.
The future cycle of metabolomics precision medicine-based research and healthcare where academia, industrial partners, corporate data analytics work with patients’ wearable data collection devices to provide health monitoring solutions.
Metabolomics studies seldom lack information about the studied bio-system, and although this science is described as data rich, as many metabolites are measured, it often lacks a sizable population. Being able to translate a subset of metabolite measurements onto a set of devices that not only validates the results but also provides other useful complementary information about the patient we believe is the next step forward.
Chemometric-based analyses following any metabolomics study can immensely benefit from such wealth of continual metabolite data and metadata obtained from a target cohort. When metabolomics for the masses does occur the data processing may need to depend on large computing power and data storage space and these could be stored securely and privately within an advanced cloud-computing environment [58]. In such a scenario, these measurements from different populations could be linked within the internet (so called internet of things (IoT)) allowing ensemble computation and for example epidemiological assessment of disease progression and spread (Fig. 4). This is not novel as the added benefit of current wearable biosensors is that the collected data are directly synced onto intelligent cloud services like Microsoft Azure or Google Cloud [59]. An individuals (health) data collected over time via portable (a set of wearables) devices has the potential of producing copious amounts of telemetry data that can be computed over the cloud, producing predictions for future health risks. For example the mPower, mobile Parkinson's Disease (PD) study that attempts to research the occurrence, presentation and management of PD symptoms via survey telemetry data using a smartphone app [60]. Another nice example is a smart-phone based application to monitor the association between pain and the weather for people suffering from rheumatoid arthritis [61]. Use of artificial intelligence (AI) for screening, decision making and management whilest not new, can be adapted for risk stratification, prevention and choice of treatment in the healthcare systems [62]. Recently, US Food and Drug Administration (FDA) has approved an AI based machine learning application for the use in clinics for making informed decisions about the health of heart where AI provides accurate measurements of the volume of each ventricle to physicians. This is thought to speed up and improve the decision making for heart surgeries [63]. There is an enormous potential for a happy marriage between metabolomics and AI or machine learning technologies that are driven by data. This is where metabolomics should aim to take personalised medicine to - not only being able to predict a persons current or near future health or globally screen for potential biomarkers - but to link that information to dynamic metadata from patients to predict further risks and disease prognosis (Fig. 1). This approach as opposed to evidence-based medicine (Fig. 3) will enable better health care outcomes instead of trial and error treatment regimes.
A potential future scenario illustrating precision medicine were together the patient and physician are at the centre of the diagnostics is shown in Fig. 5, once the hurdles of costs, barriers to patient inclusion and ease of use are overcome [64]. On the right-hand side of this figure is the expected laboratory-based scenario where metabolomics data are a standalone set of information which may be frequently linked to other ‘omics data. These measurements are detailed and thus slow and usually reserved for the initial diagnostics often when disease is already apparent. This provides useful but limited retrospective information about a population. By contrast the left-hand side illustrates the role of self-testing at home which can occur much more frequently, and for some wearable devices constantly and in real-time. For example, using dipstick tests for diabetes may be a quicker assessment of glucose levels but as is already known by individuals with Type I diabetes lacks real-time prolonged monitoring of patient health. As mentioned above implantable devices are now available for real-time glucose sensing and when combined with a ‘health band’ which reports information on a patients sleep patterns, heart rate, and physical exercise schedules may lead to better management of the disease.
Fig. 5.
A potential future where the patient is at the centre of their own health care. Where research/omics data and clinical data (right sides) are combined with novel future wearable and at home testing to generate more precise and thus precision medicine-based diagnostics. Thus, bucketing patients with similar health profiles would aid clinics to differentiate those that need urgent medical intervention from those that will benefit more from change in lifestyle choices and non-medical aid. This approach can thus help identify subgroup(s) of patients with similar drug responses or disease profiles, enabling affordable care as proposed by the Obama Care Bill without excluding those with pre-existing health conditions (that are not deemed life threatening but manageable) or comorbidities.
6. Conclusions
The future of metabolomics does not stop at personalised medicine itself. For the application of metabolomics in preventive medicine as well as screening, the world is your oyster. Indeed, metabolomics could play not only a crucial role in monitoring life on the Earth but also beyond [65]. NASA's recent famous twin study which was concluded last year will hopefully show a glimpse of how powerful and useful understanding the human metabolome can be [66], [67].
At present metabolomics is very much research laboratory-based and needs to move out of academic laboratories and into the clinic. As a step towards this the UK has established two Phenome centres [68], one in London and the other in Birmingham; time will tell whether these are successful but a real opportunity is presented for the large-scale use of metabolomics for preventive health care, disease diagnosis, disease monitoring as well as finding novel therapeutics on a personalised level, which will account for differences within each individual.
A recently published white paper demonstrates the strengths of metabolomics in shaping precision medicine [69], and we would urge all readers to dip into the text along with the accompanying Topical Issue published in Metabolomics on “Recent advances in Pharmacometabolomics: enabling tools for precision medicine” [70].
As the ancient proverb says:
“Vita brevis, ars longa, occasio praeceps, experimentum periculosum, iudicium difficile” [71]
which translates to:
“Life is short, and art long, opportunity fleeting, experimentations perilous, and judgement difficult.”
Thus, there is an urgent and somewhat imminent need for precision medicine! This will require appropriate infrastructure for metabolomics for (and indeed on) the masses and will require alterations in healthcare practices across the globe. Once delivered this may improve medicine, put the patient at the centre of the analysis, and allow for healthier lifestyles and efficient medication for each and every one of us.
Acknowledgments
D.K.T. and R.G. thank the Cancer Research UK (including Experimental Cancer Medicine Centre award) for funding. RG also thanks the UK Medical Research Council (Grant MRC G1001375/1) and the Wellcome Trust (Grant 202952/Z/16/Z) for funding of metabolomics.
References
- 1.Oliver S.G. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998;16(9):373–378. doi: 10.1016/s0167-7799(98)01214-1. [DOI] [PubMed] [Google Scholar]
- 2.Kell D.B., Oliver S.G. The metabolome 18 years on: a concept comes of age. Metabolomics. 2016;12(9):148. doi: 10.1007/s11306-016-1108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strimbu K., Tavel J.A. What are biomarkers? Curr. Opin. HIV AIDS. 2010;5(6):463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broadhurst D.I., Kell D.B. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics. 2006;2(4):171–196. [Google Scholar]
- 5.Poste G. Bring on the biomarkers. Nature. 2011;469(7329):156–157. doi: 10.1038/469156a. [DOI] [PubMed] [Google Scholar]
- 6.Nix, H., A National Geographic Information System - An Achievable Objective?, in: Keynote address, Aurisa, 1990.
- 7.Sansone S., Goodacre F.T., Griffin R., Wardy J.L., Kaddurah-Daouk N.W., Kristal R., London B.S., Mendes J., Morrison P., Nikolau N., Robertson B., Sumner D., Taylor L.W., van der Werf C., Ommen M., Fiehn O B.V. The metabolomics standards initiative. Nat. Biotechnol. 2007;25(8):846–848. doi: 10.1038/nbt0807-846b. [DOI] [PubMed] [Google Scholar]
- 8.Statistics Of.N. Registered deaths by age, sex, selected underlying causes of death, and the 10 leading causes of death for both males and females. Stat. Bull. 2015 [Google Scholar]
- 9.Commission E. Causes of death - standardised death rate by residence. Eurostat. 2013 [Google Scholar]
- 10.O’Neill J. Review on antimicrobial resistance. Antimicrob. Resist.: Tackling Crisis Health Wealth Nations. 2014 [Google Scholar]
- 11.Goodacre R. Metabolomics of a superorganism. J. Nutr. 2007;137(1):259S–266S. doi: 10.1093/jn/137.1.259S. [DOI] [PubMed] [Google Scholar]
- 12.Potgieter M. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol. Rev. 2015;39(4):567–591. doi: 10.1093/femsre/fuv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman D.J. A survey of US adults' opinions about conduct of a nationwide precision medicine Initiative® cohort study of genes and environment. PLoS One. 2016;11(8):e0160461. doi: 10.1371/journal.pone.0160461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.N.O.O. Communications, NIH funds biobank to support Precision Medicine Initiative Cohort Program, in: NIH News Releases, 2016. [online].
- 15.FDA, Precision Medicine. Science and Research Special Topics, 2016.
- 16.Beger R.D. Metabolomics enables precision medicine: “a white paper, community perspective”. Metabolomics. 2016;12(9):149. doi: 10.1007/s11306-016-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neavin D., Kaddurah-Daouk R., Weinshilboum R. Pharmacometabolomics informs pharmacogenomics. Metabolomics. 2016;12:7. doi: 10.1007/s11306-016-1066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crockett D. In: Going Beyond Genomics in Precision Medicine: What's Next. Crockett D., editor. Healthcare Transformation and Precision Medicine; 2016. https://www.healthcatalyst.com [Google Scholar]
- 19.Clish C.B. Metabolomics: an emerging but powerful tool for precision medicine. Mol. Case Stud. 2015;1(1) doi: 10.1101/mcs.a000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteiro M.S. Metabolomics analysis for biomarker discovery: advances and challenges. Curr. Med. Chem. 2013;20(2):257–271. doi: 10.2174/092986713804806621. [DOI] [PubMed] [Google Scholar]
- 21.Armitage E.G., Barbas C. Metabolomics in cancer biomarker discovery: current trends and future perspectives. J. Pharm. Biomed. Anal. 2014;87:1–11. doi: 10.1016/j.jpba.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 22.Kell D.B. Metabolomic biomarkers: search, discovery and validation. Expert Rev. Mol. Diagn. 2007;7(4):329–333. doi: 10.1586/14737159.7.4.329. [DOI] [PubMed] [Google Scholar]
- 23.Altadill T. Enabling metabolomics based biomarker discovery studies using molecular phenotyping of exosome-like vesicles. PLoS One. 2016;11(3):e0151339. doi: 10.1371/journal.pone.0151339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia J. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013;9(2):280–299. doi: 10.1007/s11306-012-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhanasekaran S.M. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412(6849):822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 26.Pashayan N., Pharoah P. Population-based screening in the era of genomics. Personal. Med. 2012;9(4):451–455. doi: 10.2217/pme.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo L. Plasma metabolomic profiles enhance precision medicine for volunteers of normal health. Proc. Natl. Acad. Sci. USA. 2015;112(35):E4901–E4910. doi: 10.1073/pnas.1508425112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanmassenhove J. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol. Dial. Transplant. 2013;28(2):254–273. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]
- 29.Mathelin C. Serum biomarkers for detection of breast cancers: a prospective study. Breast Cancer Res. Treat. 2006;96(1):83–90. doi: 10.1007/s10549-005-9046-2. [DOI] [PubMed] [Google Scholar]
- 30.Tessitore A. Serum biomarkers identification by mass spectrometry in high-mortality tumors. Int. J. Proteom. 2013;2013:15. doi: 10.1155/2013/125858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung L. Novel serum protein biomarker panel revealed by mass spectrometry and its prognostic value in breast cancer. Breast Cancer Res. 2014;16(3):R63. doi: 10.1186/bcr3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen C. Developing urinary metabolomic signatures as early bladder cancer diagnostic markers. Omics. 2015;19(1):1–11. doi: 10.1089/omi.2014.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim K. Urine metabolomic analysis identifies potential biomarkers and pathogenic pathways in kidney cancer. OMICS: J. Integr. Biol. 2011;15(5):293–303. doi: 10.1089/omi.2010.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trivedi D.K., Iles R.K. Shotgun metabolomic profiles in maternal urine identify potential mass spectral markers of abnormal fetal biochemistry - dihydrouracil and progesterone in the metabolism of Down syndrome. Biomed. Chromatogr. 2015;29(8):1173–1183. doi: 10.1002/bmc.3404. [DOI] [PubMed] [Google Scholar]
- 35.Laiakis E.C. Metabolomic analysis in severe childhood pneumonia in The Gambia, West Africa: findings from a pilot study. PLoS One. 2010;5(9):e12655. doi: 10.1371/journal.pone.0012655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van de Kant K.D.G. Clinical use of exhaled volatile organic compounds in pulmonary diseases: a systematic review. Respir. Res. 2012;13(1) doi: 10.1186/1465-9921-13-117. (117-117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R. Schnabel, et al., Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Scientific Reports. 5, 2015, p. 17179. [DOI] [PMC free article] [PubMed]
- 38.Sethi S., Nanda R., Chakraborty T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin. Microbiol. Rev. 2013;26(3):462–475. doi: 10.1128/CMR.00020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollywood K., Brison D.R., Goodacre R. Metabolomics: current technologies and future trends. Proteomics. 2006;6(17):4716–4723. doi: 10.1002/pmic.200600106. [DOI] [PubMed] [Google Scholar]
- 40.Dunn W.B. Molecular phenotyping of a UK population: defining the human serum metabolome. Metabolomics. 2015;11:9–26. doi: 10.1007/s11306-014-0707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganna A. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014;10(12):e1004801. doi: 10.1371/journal.pgen.1004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albright J.C. Large-scale metabolomics reveals a complex response of aspergillus nidulans to epigenetic perturbation. ACS Chem. Biol. 2015;10(6):1535–1541. doi: 10.1021/acschembio.5b00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris R. Overview of screening: where we are and where we may be headed. Epidemiol. Rev. 2011;33(1):1–6. doi: 10.1093/epirev/mxr006. [DOI] [PubMed] [Google Scholar]
- 44.Khoury M.J., McCabe L.L., McCabe E.R.B. Population screening in the age of genomic medicine. N. Engl. J. Med. 2003;348(1):50–58. doi: 10.1056/NEJMra013182. [DOI] [PubMed] [Google Scholar]
- 45.Munafò M.R. A manifesto for reproducible science. Nat. Hum. Behav. 2017;1:0021. doi: 10.1038/s41562-016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunn W.B. The importance of experimental design and QC samples in large-scale and MS-driven untargeted metabolomic studies of humans. Bioanalysis. 2012;4(18):2249–2264. doi: 10.4155/bio.12.204. [DOI] [PubMed] [Google Scholar]
- 47.Wen H. A new NMR-based metabolomics approach for the diagnosis of biliary tract cancer. J. Hepatol. 2010;52(2):228–233. doi: 10.1016/j.jhep.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Costa e Silva J.A. Personalized medicine in psychiatry: new technologies and approaches. Metabolism. 2013;62(Suppl. 1):S40–S44. doi: 10.1016/j.metabol.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Harrer, S. Measuring Life: Sensors and Analytics for Precision Medicine, 2015.
- 50.Vaddiraju S. Technologies for Continuous Glucose Monitoring: current Problems and Future Promises. J. Diabetes Sci. Technol. 2010;4(6):1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.BinDhim N.F. Depression screening via a smartphone app: cross-country user characteristics and feasibility. J. Am. Med. Inform. Assoc. 2015;22(1):29–34. doi: 10.1136/amiajnl-2014-002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nam H.S. Development of smartphone application that aids stroke screening and identifying nearby acute stroke care hospitals. Yonsei Med. J. 2014;55(1):25–29. doi: 10.3349/ymj.2014.55.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.C. Boletsis, S. McCallum, B.F. Landmark, The use of smartwatches for health monitoring in home-based dementia care, in: Human Aspects of IT for the Aged Population. Design for Everyday Life: First International Conference, ITAP 2015, Held as Part of HCI International 2015, Los Angeles, CA, USA, August 2–7, 2015. Proceedings, Part II, J. Zhou and G. Salvendy, Editors, Cham, Springer International Publishing, 2015, pp. 15–26.
- 54.Badugu R., Lakowicz J.R., Geddes C.D. A glucose-sensing contact lens: from bench top to patient. Curr. Opin. Biotechnol. 2005;16(1):100–107. doi: 10.1016/j.copbio.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu M.X. Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta. 2011;83(3):960–965. doi: 10.1016/j.talanta.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 56.Bodhani A. The connected body. Eng. Technol. 2015;10(4):44–47. [Google Scholar]
- 57.Marschollek M. Wearable sensors in healthcare and sensor-enhanced health information systems: all our tomorrows? Healthc. Inform. Res. 2012;18(2):97–104. doi: 10.4258/hir.2012.18.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar B.K. Big data for secure healthcare system: a conceptual design. Complex Intell. Syst. 2017:1–19. [Google Scholar]
- 59.Park S., Chung K., Jayaraman S. 1 - wearables: fundamentals, advancements, and a roadmap for the future A2 - Sazonov, Edward. In: Neuman M.R., editor. Wearable Sensors. Chapter 1. Academic Press; Oxford: 2014. pp. 1–23. [Google Scholar]
- 60.Bot B.M. The mPower study, Parkinson disease mobile data collected using Research Kit. Sci. Data. 2016;3:160011. doi: 10.1038/sdata.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reade S. Cloudy with a chance of pain: engagement and subsequent attrition of daily data entry in a smartphone pilot study tracking weather, disease severity, and physical activity in patients with rheumatoid arthritis. JMIR Mhealth Uhealth. 2017;5(3):e37. doi: 10.2196/mhealth.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Long E. An artificial intelligence platform for the multihospital collaborative management of congenital cataracts. Nat. Biomed. Eng. 2017;1:0024. [Google Scholar]
- 63.Marr B. First FDA approval for clinical cloud-based deep learning in healthcare. Forbes. 2017 (online) [Google Scholar]
- 64.Bonham V.L., Callier S.L., Royal C.D. Will precision medicine move us beyond race? N. Engl. J. Med. 2016;374(21):2003–2005. doi: 10.1056/NEJMp1511294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt M.A., Goodwin T.J. Personalized medicine in human space flight: using Omics based analyses to develop individualized countermeasures that enhance astronaut safety and performance. Metabolomics. 2013;9(6):1134–1156. doi: 10.1007/s11306-013-0556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.NASA, Metabolomic and Genomic Markers of Atherosclerosis as Related to Oxidative Stress, Inflammation, and Vascular Function in Twin Astronauts. ISS Science for Everyone, 2016.
- 67.NASA, Human Exploration Research Opportunities - Differential Effects on Homozygous Twin Astronauts Associated with Differences in Exposure to Spaceflight Factors ISS Science for Everyone, 2016.
- 68.Lindon J.C., Nicholson J.K. The emergent role of metabolic phenotyping in dynamic patient stratification. Expert Opin. Drug Metab. Toxicol. 2014;10(7):915–919. doi: 10.1517/17425255.2014.922954. [DOI] [PubMed] [Google Scholar]
- 69.Beger R.D. Metabolomics enables precision medicine: “a white paper, community perspective”. Metabolomics. 2016;12(10):1–15. doi: 10.1007/s11306-016-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rattray N.J.W., Daouk R.K. PharmacometabOlomics and Precision Medicine Special Issue Editorial. Metabolomics. 2017;13(5):59. [Google Scholar]
- 71.Hippocrates, Aphorisms, in: The genuine works of Hippocrates: translated from the Greek with a preliminary discourse and annotations, London, Sydenham Society, 1849.
- 72.Kenny L.C. Novel biomarkers for pre-eclampsia detected using metabolomics and machine learning. Metabolomics. 2005;1(3):227. [Google Scholar]
- 73.Yang J. High performance liquid chromatography-mass spectrometry for metabonomics: potential biomarkers for acute deterioration of liver function in chronic hepatitis B. J. Proteome Res. 2006;5(3):554–561. doi: 10.1021/pr050364w. [DOI] [PubMed] [Google Scholar]
- 74.Holmes E. Metabolic profiling of CSF: evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med. 2006;3(8):e327. doi: 10.1371/journal.pmed.0030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yi L.-Z. Plasma fatty acid metabolic profiling and biomarkers of type 2 diabetes mellitus based on GC/MS and PLS-LDA. FEBS Lett. 2006;580(30):6837–6845. doi: 10.1016/j.febslet.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 76.Yin P.Y. Metabonomics study of intestinal fistulas based on ultraperformance liquid chromatography coupled with Q-TOF mass spectrometry (UPLC/Q-TOF MS) J. Proteome Res. 2006;5(9):2135–2143. doi: 10.1021/pr060256p. [DOI] [PubMed] [Google Scholar]
- 77.Kaddurah-Daouk R. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol. Psychiatry. 2007;12(10):934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- 78.Wikoff W.R. Metabolomics identifies perturbations in human disorders of propionate metabolism. Clin. Chem. 2007;53(12):2169–2176. doi: 10.1373/clinchem.2007.089011. [DOI] [PubMed] [Google Scholar]
- 79.Bogdanov M. Metabolomic profiling to develop blood biomarkers for Parkinson's disease. Brain. 2008;131(2):389–396. doi: 10.1093/brain/awm304. [DOI] [PubMed] [Google Scholar]
- 80.Chen J. Practical approach for the identification and isomer elucidation of biomarkers detected in a metabonomic study for the discovery of individuals at risk for diabetes by integrating the chromatographic and mass spectrometric information. Anal. Chem. 2008;80(4):1280–1289. doi: 10.1021/ac702089h. [DOI] [PubMed] [Google Scholar]
- 81.Lawton K.A. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9(4):383–397. doi: 10.2217/14622416.9.4.383. [DOI] [PubMed] [Google Scholar]
- 82.Yi L.Z. Plasma fatty acid metabolic profile coupled with uncorrelated linear discriminant analysis to diagnose and biomarker screening of type 2 diabetes and type 2 diabetic coronary heart diseases. Metabolomics. 2008;4(1):30–38. [Google Scholar]
- 83.Erb G. Toward improved grading of malignancy in oligodendrogliomas using metabolomics. Magn. Reson. Med. 2008;59(5):959–965. doi: 10.1002/mrm.21486. [DOI] [PubMed] [Google Scholar]
- 84.Yin P. Serum metabolic profiling of abnormal savda by liquid chromatography/mass spectrometry. J. Chromatogr. B. 2008;871(2):322–327. doi: 10.1016/j.jchromb.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 85.Seli E. Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic resonance correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil. Steril. 2008;90(6):2183–2189. doi: 10.1016/j.fertnstert.2008.07.1739. [DOI] [PubMed] [Google Scholar]
- 86.Qiu Y. Multivariate classification analysis of metabolomic data for candidate biomarker discovery in type 2 diabetes mellitus. Metabolomics. 2008;4(4):337–346. [Google Scholar]
- 87.Chan E.C.Y. Metabolic profiling of human colorectal cancer using high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and gas chromatography mass spectrometry (GC/MS) J. Proteome Res. 2009;8(1):352–361. doi: 10.1021/pr8006232. [DOI] [PubMed] [Google Scholar]
- 88.Zheng X. Plasma fatty acids metabolic profiling analysis of coronary heart disease based on GC–MS and pattern recognition. J. Pharm. Biomed. Anal. 2009;49(2):481–486. doi: 10.1016/j.jpba.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 89.Rudnick D.A. Serum α-NH(2)-butyric acid may predict spontaneous survival in pediatric acute liver failure. Pediatr. Transplant. 2009;13(2):223–230. doi: 10.1111/j.1399-3046.2008.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vallejo M. Plasma fingerprinting with GC-MS in acute coronary syndrome. Anal. Bioanal. Chem. 2009;394(6):1517–1524. doi: 10.1007/s00216-009-2610-6. [DOI] [PubMed] [Google Scholar]
- 91.Ahmed S.S. Metabolic profiling of Parkinson's disease: evidence of biomarker from gene expression analysis and rapid neural network detection. J. Biomed. Sci. 2009;16(1):63. doi: 10.1186/1423-0127-16-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu H. Metabolomic profiling of human urine in hepatocellular carcinoma patients using gas chromatography/mass spectrometry. Anal. Chim. Acta. 2009;648(1):98–104. doi: 10.1016/j.aca.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 93.Zhang J. Metabonomics research of diabetic nephropathy and type 2 diabetes mellitus based on UPLC–oaTOF-MS system. Anal. Chim. Acta. 2009;650(1):16–22. doi: 10.1016/j.aca.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 94.Ritchie S.A. Reduced levels of hydroxylated, polyunsaturated ultra long-chain fatty acids in the serum of colorectal cancer patients: implications for early screening and detection. BMC Med. 2010;8(1):13. doi: 10.1186/1741-7015-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sugimoto M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010;6(1):78–95. doi: 10.1007/s11306-009-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lokhov P.G. Metabolite profiling of blood plasma of patients with prostate cancer. Metabolomics. 2010;6(1):156–163. [Google Scholar]
- 97.Kim Y. Multivariate classification of urine metabolome profiles for breast cancer diagnosis. BMC Bioinform. 2010;11(2):S4. doi: 10.1186/1471-2105-11-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhai G. Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann. Rheum. Dis. 2010;69(6):1227–1231. doi: 10.1136/ard.2009.120857. [DOI] [PubMed] [Google Scholar]
- 99.Li N.-J. Plasma metabolic profiling of Alzheimer's disease by liquid chromatography/mass spectrometry. Clin. Biochem. 2010;43(12):992–997. doi: 10.1016/j.clinbiochem.2010.04.072. [DOI] [PubMed] [Google Scholar]
- 100.Zira A.N. 1H NMR metabonomic analysis in renal cell carcinoma: a possible diagnostic tool. J. Proteome Res. 2010;9(8):4038–4044. doi: 10.1021/pr100226m. [DOI] [PubMed] [Google Scholar]
- 101.Barnes V.M. Assessment of the effects of dentifrice on periodontal disease biomarkers in gingival crevicular fluid. J. Periodontol. 2010;81(9):1273–1279. doi: 10.1902/jop.2010.100070. [DOI] [PubMed] [Google Scholar]
- 102.Lauridsen M.B. 1H NMR spectroscopy-based interventional metabolic phenotyping: a cohort study of rheumatoid arthritis patients. J. Proteome Res. 2010;9(9):4545–4553. doi: 10.1021/pr1002774. [DOI] [PubMed] [Google Scholar]
- 103.Chen X. Plasma metabolomics reveals biomarkers of the atherosclerosis. J. Sep. Sci. 2010;33(17–18):2776–2783. doi: 10.1002/jssc.201000395. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L. Development and validation of a liquid chromatography–mass spectrometry metabonomic platform in human plasma of liver failure caused by hepatitis B virus. Acta Biochim. Biophys. Sin. 2010;42(10):688–698. doi: 10.1093/abbs/gmq078. [DOI] [PubMed] [Google Scholar]
- 105.Denery J.R. Metabolomics-based discovery of diagnostic biomarkers for onchocerciasis. PLoS Negl. Trop. Dis. 2010;4(10):e834. doi: 10.1371/journal.pntd.0000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Suhre K. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5(11):e13953. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Romero R. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J. Matern. Fetal Neonatal Med. 2010;23(12):1344–1359. doi: 10.3109/14767058.2010.482618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dong J. Lysophosphatidylcholine profiling of plasma: discrimination of isomers and discovery of lung cancer biomarkers. Metabolomics. 2010;6(4):478–488. [Google Scholar]
- 109.Madsen R.K. Diagnostic properties of metabolic perturbations in rheumatoid arthritis. Arthritis Res. Ther. 2011;13(1):R19. doi: 10.1186/ar3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saude E.J. Metabolomic profiling of asthma: diagnostic utility of urine nuclear magnetic resonance spectroscopy. J. Allergy Clin. Immunol. 2011;127(3):757–764. doi: 10.1016/j.jaci.2010.12.1077. (e6) [DOI] [PubMed] [Google Scholar]
- 111.Han L.-D. Plasma esterified and non-esterified fatty acids metabolic profiling using gas chromatography–mass spectrometry and its application in the study of diabetic mellitus and diabetic nephropathy. Anal. Chim. Acta. 2011;689(1):85–91. doi: 10.1016/j.aca.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 112.Chen J. Serum 27-nor-5β-cholestane-3,7,12,24,25 pentol glucuronide discovered by metabolomics as potential diagnostic biomarker for epithelium ovarian cancer. J. Proteome Res. 2011;10(5):2625–2632. doi: 10.1021/pr200173q. [DOI] [PubMed] [Google Scholar]
- 113.Lian J.S. A serum metabonomic study on the difference between alcohol- and HBV-induced liver cirrhosis by ultraperformance liquid chromatography coupled to mass spectrometry plus quadrupole time-of-flight mass spectrometry. Chin. Med. J. 2011;124(9):1367–1373. [PubMed] [Google Scholar]
- 114.Fong M.Y., McDunn J., Kakar S.S. Identification of metabolites in the normal ovary and their transformation in primary and metastatic ovarian cancer. PLoS One. 2011;6(5):e19963. doi: 10.1371/journal.pone.0019963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang F. Novel potential markers of nasopharyngeal carcinoma for diagnosis and therapy. Clin. Biochem. 2011;44(8–9):711–718. doi: 10.1016/j.clinbiochem.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 116.Chen T. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol. Cell. Proteom. 2011;10(7) doi: 10.1074/mcp.M110.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kondo Y. Serum fatty acid profiling of colorectal cancer by gas chromatography/mass spectrometry. Biomark. Med. 2011;5(4):451–460. doi: 10.2217/bmm.11.41. [DOI] [PubMed] [Google Scholar]
- 118.Al-Mubarak R. Serum metabolomics reveals higher levels of polyunsaturated fatty acids in lepromatous leprosy: potential markers for susceptibility and pathogenesis. PLoS Negl. Trop. Dis. 2011;5(9):e1303. doi: 10.1371/journal.pntd.0001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Munshi S.U. Metabonomic analysis of hepatitis E patients shows deregulated metabolic cycles and abnormalities in amino acid metabolism. J. Viral Hepat. 2011;18(10):e591–e602. doi: 10.1111/j.1365-2893.2011.01488.x. [DOI] [PubMed] [Google Scholar]
- 120.Atzori L. Clinical metabolomics and urinary NGAL for the early prediction of chronic kidney disease in healthy adults born ELBW. J. Matern. Fetal Neonatal. Med. 2011;24(sup. 2):40–43. doi: 10.3109/14767058.2011.606678. [DOI] [PubMed] [Google Scholar]
- 121.Chen F. Identification of serum biomarkers of hepatocarcinoma through liquid chromatography/mass spectrometry-based metabonomic method. Anal. Bioanal. Chem. 2011;401(6):1899. doi: 10.1007/s00216-011-5245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hori S. A metabolomic approach to lung cancer. Lung Cancer. 2011;74(2):284–292. doi: 10.1016/j.lungcan.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 123.Duan H. Identification of biomarkers for melamine-induced nephrolithiasis in young children based on ultra high performance liquid chromatography coupled to time-of-flight mass spectrometry (U-HPLC–Q-TOF/MS) J. Chromatogr. B. 2011;879(30):3544–3550. doi: 10.1016/j.jchromb.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 124.Arlt W. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011;96(12):3775–3784. doi: 10.1210/jc.2011-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Putluri N. Metabolomic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Cancer Res. 2011;71(24):7376–7386. doi: 10.1158/0008-5472.CAN-11-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shah S.H. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J. 2012;163(5) doi: 10.1016/j.ahj.2012.02.005. (844-+) [DOI] [PubMed] [Google Scholar]
- 127.Sato Y. Identification of a new plasma biomarker of Alzheimer's disease using metabolomics technology. J. Lipid Res. 2012;53(3):567–576. doi: 10.1194/jlr.M022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van der Kloet F.M. Discovery of early-stage biomarkers for diabetic kidney disease using ms-based metabolomics (FinnDiane study) Metabolomics. 2012;8(1):109–119. doi: 10.1007/s11306-011-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ikeda A. Serum metabolomics as a novel diagnostic approach for gastrointestinal cancer. Biomed. Chromatogr. 2012;26(5):548–558. doi: 10.1002/bmc.1671. [DOI] [PubMed] [Google Scholar]
- 130.Schmerler D. Targeted metabolomics for discrimination of systemic inflammatory disorders in critically ill patients. J. Lipid Res. 2012;53(7):1369–1375. doi: 10.1194/jlr.P023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang T. Discrimination between malignant and benign ovarian tumors by plasma metabolomic profiling using ultra performance liquid chromatography/mass spectrometry. Clin. Chim. Acta. 2012;413(9–10):861–868. doi: 10.1016/j.cca.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 132.Sun J.C. Serum metabolomic profiles from patients with acute kidney injury: a pilot study. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2012;893:107–113. doi: 10.1016/j.jchromb.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vouk K. Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Hum. Reprod. 2012;27(10):2955–2965. doi: 10.1093/humrep/des152. [DOI] [PubMed] [Google Scholar]
- 134.Lv W.W., Yang T.S. Identification of possible biomarkers for breast cancer from free fatty acid profiles determined by GC-MS and multivariate statistical analysis. Clin. Biochem. 2012;45(1–2):127–133. doi: 10.1016/j.clinbiochem.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 135.Xu H.B. Potential clinical utility of plasma amino acid profiling in the detection of major depressive disorder. Psychiatry Res. 2012;200(2–3):1054–1057. doi: 10.1016/j.psychres.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 136.Menni C. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013;62(12):4270–4276. doi: 10.2337/db13-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang J. Potential metabolite markers of schizophrenia. Mol. Psychiatry. 2013;18(1):67–78. doi: 10.1038/mp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McDunn J.E. Metabolomic signatures of aggressive prostate cancer. Prostate. 2013;73(14):1547–1560. doi: 10.1002/pros.22704. [DOI] [PubMed] [Google Scholar]
- 139.Jung J. Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin. Exp. Allergy. 2013;43(4):425–433. doi: 10.1111/cea.12089. [DOI] [PubMed] [Google Scholar]
- 140.Zhang A.H. Urinary metabolic biomarker and pathway study of hepatitis B virus infected patients based on UPLC-MS system. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang L. H-1-NMR based metabonomic profiling of human esophageal cancer tissue. Mol. Cancer. 2013:12. doi: 10.1186/1476-4598-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang H.J. H-1 NMR-based metabolic profiling of human rectal cancer tissue. Mol. Cancer. 2013:12. doi: 10.1186/1476-4598-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang J. Metabolomic identification of diagnostic plasma biomarkers in humans with chronic heart failure. Mol. Biosyst. 2013;9(11):2618–2626. doi: 10.1039/c3mb70227h. [DOI] [PubMed] [Google Scholar]
- 144.Liu R. Identification of plasma metabolomic profiling for diagnosis of esophageal squamous-cell carcinoma using an UPLC/TOF/MS platform. Int. J. Mol. Sci. 2013;14(5):8899–8911. doi: 10.3390/ijms14058899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rizza S. Metabolomics signature improves the prediction of cardiovascular events in elderly subjects. Atherosclerosis. 2014;232(2):260–264. doi: 10.1016/j.atherosclerosis.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 146.Jin X. Diagnosis of bladder cancer and prediction of survival by urinary metabolomics. Oncotarget. 2014;5(6):1635–1645. doi: 10.18632/oncotarget.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dawiskiba T. Serum and urine metabolomic fingerprinting in diagnostics of inflammatory bowel diseases. World J. Gastroenterol. 2014;20(1):163–174. doi: 10.3748/wjg.v20.i1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang G. Plasma metabolite profiles of Alzheimer's disease and mild cognitive impairment. J. Proteome Res. 2014;13(5):2649–2658. doi: 10.1021/pr5000895. [DOI] [PubMed] [Google Scholar]
- 149.Mathe E.A. Noninvasive urinary metabolomic profiling identifies diagnostic and prognostic markers in lung cancer. Cancer Res. 2014;74(12):3259–3270. doi: 10.1158/0008-5472.CAN-14-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reinke S.N. Metabolomic profiling in multiple sclerosis: insights into biomarkers and pathogenesis. Mult. Scler. 2014;20(10):1396–1400. doi: 10.1177/1352458513516528. [DOI] [PubMed] [Google Scholar]
- 151.Graham S.F., Holscher C., Green B.D. Metabolic signatures of human Alzheimer's disease (AD): H-1 NMR analysis of the polar metabolome of post-mortem brain tissue. Metabolomics. 2014;10(4):744–753. [Google Scholar]
- 152.Wang Q. Measurement of salivary metabolite biomarkers for early monitoring of oral cancer with ultra performance liquid chromatography-mass spectrometry. Talanta. 2014;119:299–305. doi: 10.1016/j.talanta.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 153.Liu X. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography-mass spectrometry. J. Proteome Res. 2015;14(5):2322–2330. doi: 10.1021/acs.jproteome.5b00144. [DOI] [PubMed] [Google Scholar]
- 154.Drogan D. Untargeted metabolic profiling identifies altered serum metabolites of type 2 diabetes mellitus in a prospective, nested case control study. Clin. Chem. 2015;61(3):487–497. doi: 10.1373/clinchem.2014.228965. [DOI] [PubMed] [Google Scholar]
- 155.Calderon-Santiago M. Human sweat metabolomics for lung cancer screening. Anal. Bioanal. Chem. 2015;407(18):5381–5392. doi: 10.1007/s00216-015-8700-8. [DOI] [PubMed] [Google Scholar]
- 156.Pieragostino D. An integrated metabolomics approach for the research of new cerebrospinal fluid biomarkers of multiple sclerosis. Mol. Biosyst. 2015;11(6):1563–1572. doi: 10.1039/c4mb00700j. [DOI] [PubMed] [Google Scholar]
- 157.Miyamoto S. Systemic metabolomic changes in blood samples of lung cancer patients identified by gas chromatography time-of-flight mass spectrometry. Metabolites. 2015;5(2):192–210. doi: 10.3390/metabo5020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Luan H. LC-MS-based urinary metabolite signatures in idiopathic Parkinson's disease. J. Proteome Res. 2015;14(1):467–478. doi: 10.1021/pr500807t. [DOI] [PubMed] [Google Scholar]
- 159.Trivedi D.K., Iles R.K. Shotgun metabolomic profiles in maternal urine identify potential mass spectral markers of abnormal fetal biochemistry – dihydrouracil and progesterone in the metabolism of Down syndrome. Biomed. Chromatogr. 2015;29(8):1173–1183. doi: 10.1002/bmc.3404. [DOI] [PubMed] [Google Scholar]
- 160.Livshits G. An omics investigation into chronic widespread musculoskeletal pain reveals epiandrosterone sulfate as a potential biomarker. Pain. 2015;156(10):1845–1851. doi: 10.1097/j.pain.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dieme B. Metabolomics study of urine in autism spectrum disorders using a multiplatform analytical methodology. J. Proteome Res. 2015;14(12):5273–5282. doi: 10.1021/acs.jproteome.5b00699. [DOI] [PubMed] [Google Scholar]
- 162.Guo L. Three plasma metabolite signatures for diagnosing high altitude pulmonary edema. Sci. Rep. 2015;5 doi: 10.1038/srep15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Venter L. Untargeted urine metabolomics reveals a biosignature for muscle respiratory chain deficiencies. Metabolomics. 2015;11(1):111–121. [Google Scholar]
- 164.Liang Q. Metabolomics-based screening of salivary biomarkers for early diagnosis of Alzheimer's disease. RSC Adv. 2015;5(116):96074–96079. [Google Scholar]
- 165.Embade N. Metabolic characterization of advanced liver fibrosis in HCV patients as studied by serum H-1-NMR spectroscopy. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chan A.W. H-1-NMR urinary metabolomic profiling for diagnosis of gastric cancer. Br. J. Cancer. 2016;114(1):59–62. doi: 10.1038/bjc.2015.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cameron S.J.S. The metabolomic detection of lung cancer biomarkers in sputum. Lung Cancer. 2016;94:88–95. doi: 10.1016/j.lungcan.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 168.Di Gangi I.M. Metabolomic profile in pancreatic cancer patients: a consensus-based approach to identify highly discriminating metabolites. Oncotarget. 2016;7(5):5815–5829. doi: 10.18632/oncotarget.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang H. Potential serum biomarkers from a metabolomics study of autism. J. Psychiatry Neurosci. 2016;41(1):27–37. doi: 10.1503/jpn.140009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Farshidfar F. A validated metabolomic signature for colorectal cancer: exploration of the clinical value of metabolomics. Br. J. Cancer. 2016;115(7):848–857. doi: 10.1038/bjc.2016.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smolenska Z., Smolenski R.T., Zdrojewski Z. Plasma concentrations of amino acid and nicotinamide metabolites in rheumatoid arthritis--potential biomarkers of disease activity and drug treatment. Biomarkers. 2016;21(3):218–224. doi: 10.3109/1354750X.2015.1130746. [DOI] [PubMed] [Google Scholar]
- 172.Kind T. Interstitial cystitis-associated urinary metabolites identified by mass-spectrometry based metabolomics analysis. Sci. Rep. 2016;6:39227. doi: 10.1038/srep39227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shao X. Screening and verifying endometrial carcinoma diagnostic biomarkers based on a urine metabolomic profiling study using UPLC-Q-TOF/MS. Clin. Chim. Acta. 2016;463:200–206. doi: 10.1016/j.cca.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 174.Chen T. Biomarker identification and pathway analysis of preeclampsia based on serum metabolomics. Biochem. Biophys. Res. Commun. 2017;485(1):119–125. doi: 10.1016/j.bbrc.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 175.Kang H. Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br. J. Dermatol. 2017;176(3):713–722. doi: 10.1111/bjd.15008. [DOI] [PubMed] [Google Scholar]