Abstract
Response surface optimization was applied for the extraction of antibacterial substances from Rhubarb (ASR) against aquatic pathogenic Vibrio harveyi. Based on the experimental results of single factors, the optimal extraction conditions were determined by Box–Behnken design combined with response surface methodology with conditions: 100% ethanol as extraction solvent, liquid/material ratio of 29 mL/g and extraction temperature at 88 °C for 148 min. The factual value of inhibition zones can reach 21.31 ± 0.95 mm and is not different from the predicted value (21.74 mm), which showed that the response surface methodology applied to the extraction optimization of antibacterial substances from Rhubarb against V. harveyi is feasible. Moreover, the yield of ASR was 30.29 ± 2.27%. Five compounds, namely, aloe-emodin, rhein, emodin, chrysophanol and physcion, were identified in ASR by comparing the HPLC chromatogram of the reference mixtures and the sample. Contents of the five compounds were 0.68 ± 0.02, 0.24 ± 0.05, 0.78 ± 0.07, 6.68 ± 0.97 and 0.58 ± 0.15%, respectively. The minimal inhibitory concentration (MIC) values of ASR, aloe-emodin, rhein, emodin, chrysophanol and physcion were 0.625, 0.125, 0.015, > 1, > 1, and > 1 mg/mL, respectively, which indicated that aloe-emodin and rhein are the main antibacterial compounds of Rhubarb.
Keywords: Rhubarb, Antibacterial substances, Vibrio harveyi, Extraction process, Chemical identification
Introduction
Vibrio harveyi is a gram-negative and luminescent bacterium that is widely distributed in the mariculture environment. V. harveyi is one of the important pathogens that induce animal mortality during early larval stages, resulting in huge losses in the production and marketing of aquatic animals, especially on farmed and wild shrimp (Thompson et al. 2010; Morya et al. 2014). Over the years, antibiotics have played an irreplaceable role in preventing and treating bacterial diseases of aquatic animals. However, the long-term application or abuse of antibiotics can lead to drug-resistant strains, ecological imbalance and weakened immune systems. It can result in drug residues in aquatic products that are harmful to the human body (Harikrishnan et al. 2010; Cao et al. 2011). Thus an increasing demand exists for the prevention and control of V. harveyi in aquaculture. The alternative sources instead of antibiotics are the use of essential oils (Randrianarivelo et al. 2010), plant extracts (Turker and Yildirim 2015), probiotics (Kesarcodi-Watson et al. 2008; Morya et al. 2014) and microbial metabolites (Guo et al. 2016a; Xu et al. 2014; Yu et al. 2012), which have been used in vivo as antibacterial agents to control bacterial infections.
Chinese herbal medicines have broad application prospects in the prevention and control of aquatic diseases because of their low drug resistance, low drug residue and low toxic effects. Rhubarb (Da Huang) is a commonly used Chinese herbal medicine. It is the dried root and rhizome of Rheum palmatum L., Rheum offcinale Baill., and Rheum tanguticum Maxim. Ex Balf. according to the Chinese Pharmacopoeia (Zhao et al. 2011). Rhubarb contains several chemical components, such as anthraquinones, anthrones, saccharides, stilbenes, and tannins. (Lin et al. 2006), which contributes to the pharmacological properties of anti-inflammatory, antitumor, antimicrobial, purgation, cardiovascular protection, hepatoprotectiom, choleretic and anti-ageing effects, as well as other bioactivities (Chen and Wang 2009; Fu et al. 2011; Hsu et al. 2013). Rhubarb has been approved for use in the prevention and control of aquatic bacterial diseases by the Chinese ministry of agriculture. However, to our best knowledge, no reports towards identification of antibacterial active compounds of Rhubarb and evaluation of their pharmacology in vivo have been conducted.
To obtain an agent with high purity and to identify antibacterial active substances, in the present study, Box–Behnken design (BBD) and response surface methodology (RSM) were adopted to optimize the extraction conditions for antibacterial substances from Rhubarb (ASR) against V. harveyi. Subsequently, five compounds were identified by HPLC method from ASR, the content and antibacterial activities against V. harveyi of the five compounds were appraised.
Materials and methods
Materials and chemicals
Rhubarb was purchased from Anhui Traditional Chinese Medicinal Factory (Baozhou, Anhui Province, China). The raw materials were air dried, ground into fine powder and passed through a 40-mesh sieve. V. harveyi were provided by Dr. Zhenxia Su and kept at the Marine Microbial Natural Products Chemistry Laboratory of Huaihai Institute of Technology (Lianyungang, China). Aloe-emodin, rhein and chrysophanol were purchased from Shanghai Yuanye Biotech Co., Ltd (Shanghai, China). Emodin and physcion were purchased from Shanghai Jinsui Biotech Co., Ltd (Shanghai, China). Methanol (HPLC grade) and all other chemicals (analytical grade) were purchased from Sinapharm Chemical Reagent Co., Ltd (Shanghai, China).
Apparatus
QJ32W1000A high speed disintegrator (Tianjing TST Equipment Co., Tianjing, China), HH-4 thermostatic water bath boiler (Jiangnan Equipment Co., Jintan, China), RE-52A rotary evaporator (Shanghai Yarong Biochem Equipment Co., Shanghai, China), APX-250B biochemical inculator (Shanghai Boxun Medical Biological instrument Co., Shanghai, China), ultimate 3000 high performance liquid chromatography (Thermo Fisher Scientific, USA)
Primary extraction experiments
Rhubarb powder (1 g) was accurately weighed and used for each experiment. The single factor experiments were set as described below: Firstly, the effect of extraction solvent on the antibacterial activity of the extracts was studied. Rhubarb powder (1 g) was placed into a 100 mL round-bottom flask and 20 mL of different extraction solvents (distilled water, methanol, 50% methanol, ethanol and 50% ethanol) were added. Extraction was conducted in HH-4 thermostatic water bath boiler for 120 min at 80 °C. Secondly, the effect of different ethanol concentrations on the antibacterial activity of the extracts was studied. One gram of Rhubarb powder was put into a 100 mL round-bottom flask, 20 mL different concentration of ethanol (60–100%) was added and the extraction was conducted for 120 min at 80 °C. Thirdly, the influence of extraction temperature on the antibacterial activity of the extracts was investigated. One gram of Rhubarb powder was put into a 100 mL round-bottom flask, 20 mL ethanol was added and extraction was performed at different temperatures (60–100 °C) for 120 min. Fourthly, the effect of extraction time on the antibacterial activity of the extracts was studied. 20 mL ethanol was added in Rhubarb powder (1 g). The extraction performed at 90 °C for different times (60–180 min). Finally, the effect of liquid to material ratio on the extraction was studied. Different volumes of ethanol (10–50 mL) were added in 1 g of Rhubarb powder and the extraction was performed at 90 °C for 150 min. Extracts were centrifuged at 5000 rpm/min for 10 min. The supernatant was diluted or concentrated to 20 mL for the determination of antibacterial activity against V. harveyi.
Optimization of the extraction process
BBD combined with RSM was selected to optimize the extraction conditions. Data analysis and model building were carried out using Design Expert 7.0.0 (Stat-Ease, Minneapolis, USA) (Guo et al. 2016b). BBD consisting of 12 factorial points and 5 central points, the dependent variable (Y, mm) was the diameter of inhibition zone against V. harveyi, whereas the extraction temperature (X 1), extraction time (X 2) and liquid/material ratio (X 3) were chosen as independent factors. Ranges and levels of three independent variables are presented in Table 1. By this software, the optimization objective of the extraction process was to achieve the maximum diameter of the inhibition zone of the extracts. The pattern of the system was evaluated by the following second-order polynomial equation:
| 1 |
where Y represents the predicted response, β 0, β i, β ii and β ij are constant coefficients, while X i and X j are the independent variables.
Table 1.
Coded (actual) levels of the operational parameters and observed values of Box–Behnken design
| No. | X 1 | X 2 | X 3 | Response |
|---|---|---|---|---|
| Temperature (°C) | Time (min) | L/M (mL/g) | Inhibition zone (mm) | |
| 1 | − 1 (80) | − 1 (90) | 0 (20) | 17.91 |
| 2 | + 1 (100) | − 1 (90) | 0 (20) | 18.89 |
| 3 | − 1 (80) | + 1 (150) | 0 (20) | 20.31 |
| 4 | + 1 (100) | + 1 (150) | 0 (20) | 18.04 |
| 5 | − 1 (80) | 0 (120) | − 1 (10) | 18.56 |
| 6 | + 1 (100) | 0 (120) | − 1 (10) | 16.96 |
| 7 | − 1 (80) | 0 (120) | + 1 (30) | 19.59 |
| 8 | + 1 (100) | 0 (120) | + 1 (30) | 19.30 |
| 9 | 0 (90) | − 1 (90) | − 1 (10) | 19.27 |
| 10 | 0 (90) | + 1 (150) | − 1 (10) | 20.08 |
| 11 | 0 (90) | − 1 (90) | + 1 (30) | 21.71 |
| 12 | 0 (90) | + 1 (150) | + 1 (30) | 21.72 |
| 13 | 0 (90) | 0 (120) | 0 (20) | 20.46 |
| 14 | 0 (90) | 0 (120) | 0 (20) | 20.52 |
| 15 | 0 (90) | 0 (120) | 0 (20) | 20.93 |
| 16 | 0 (90) | 0 (120) | 0 (20) | 21.16 |
| 17 | 0 (90) | 0 (120) | 0 (20) | 20.96 |
Identification and quantification of chemical components of ASR
Identification and quantification of chemical components of ASR were performed by HPLC method and used standard curves (Zhao et al. 2016). The standard curve between the peak areas and the concentrations of reference samples (aloe-emodin, rhein, emodin, chrysophanol and physcion) were established by HPLC on an ODS column (Shim-Pack CLC-ODS, 6.0 × 150 mm, 5 μm, 1 mL/min) using standard solutions over the concentration ranging from 5.0 to 20.0 μg/mL and UV detection at 280 nm. The mobile phase consisted of a gradient elution of methanol and water. The gradient program was as follows: 0–5 min 50% (v/v) CH3OH, 5.1–20 min 50–100% CH3OH, 20–22.5 min 100% CH3OH, 22.5–25 min 100–50% CH3OH and 25–30 min 50% CH3OH.
Determination of antibacterial activity
Antibacterial activities against V. harveyi of the active extracts were estimated by the Oxford cup method (Guo et al. 2016a). Beef extract peptone medium (BP) composed of 0.3% beef extract, 1% peptone, 0.5% NaCl and 1.5% agar was used, and the pH was adjusted to 7.0 with NaOH. These cups were placed on plates previously inoculated with the pathogen V. harveyi. Each extract (200 µL) was added in Oxford cups and incubated at 37 °C for 12 h. The experiments were carried out in triplicate and the average diameter of inhibition zones (mm) was recorded as the antibacterial activity of the extracts.
Compounds were dissolved and diluted into different concentrations with dichloromethane/methanol (1:1) using the continuous two-fold dilution method. These solutions were directly used to determine their antibacterial activities against V. harveyi using the above method. Minimum inhibitory concentration (MIC) was defined as the lowest concentration of compounds that produced the inhibition zone against V. harveyi (Guo et al. 2016a). Experiments were performed in duplicate with full agreement between both results.
Results and discussion
Optimization of the extraction parameters of ASR
In this study, the antibacterial substances against aquatic pathogenic V. harveyi from Chinese herbal medicine Rhubarb were studied. Before the optimized experiments, the key factors independently affecting the antibacterial activity of the extracts were investigated as extraction solvent, concentration of solvent, extraction temperature, extraction time and liquid to material ratio.
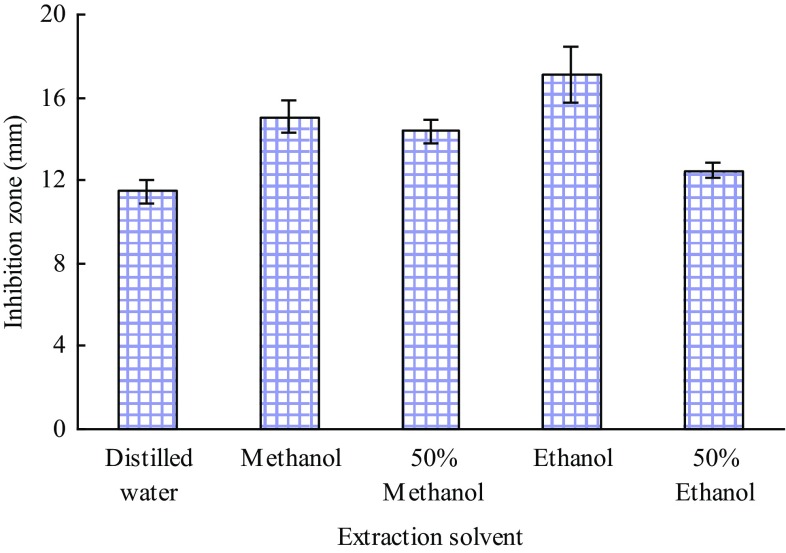
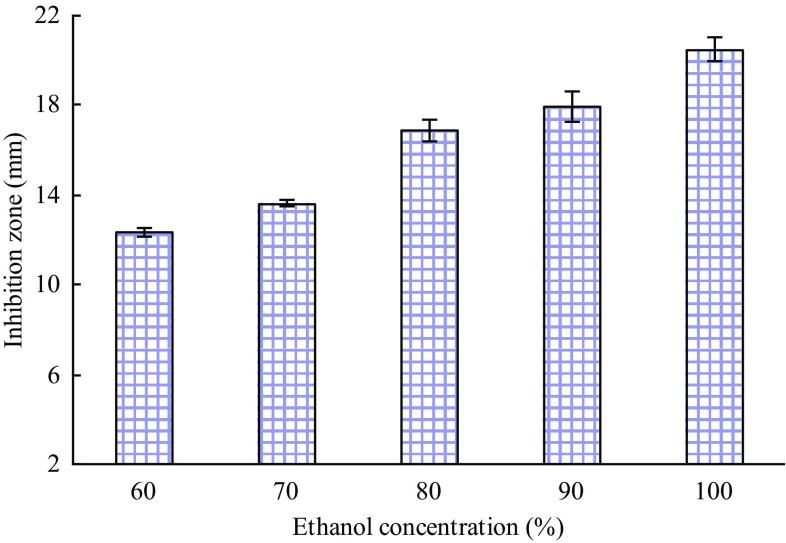
Figure 1 shows the effects of extraction solvents (distilled water, methanol, 50% methanol, ethanol and 50% ethanol) on the inhibition zone of the extracts, with ethanol being the best solvent. Subsequently, the effects of different ethanol concentrations on the antibacterial activity of the extracts were studied. Figure 2 presents an increased inhibition zone diameter with increasing ethanol concentration, which indicated that the antibacterial substances are hydrophobic. Thus, 100% ethanol was selected as the extraction solvent.
Fig. 1.
Effect of extraction solvent on the inhibitory activity of the extracts
Fig. 2.
Effect of ethanol concentration on the inhibitory activity of the extracts
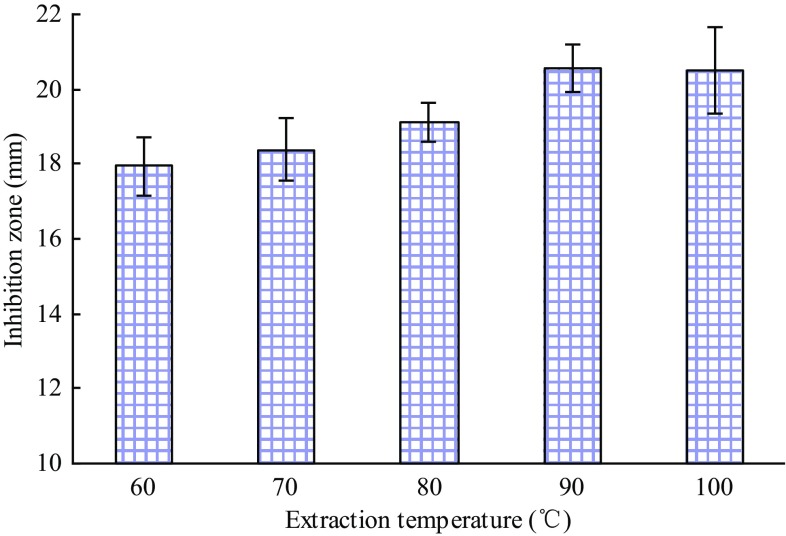
Figure 3 reveals the increased inhibition zone diameters as the temperature increased from 60 to 90 °C. The increase in temperature may have contributed to the extraction of ASR by reducing the viscosity, enhancing the coefficient of diffusion and increasing the solubility of active substances (Guo et al. 2016b). Thus, 90 °C was selected as the centre point for the RSM.
Fig. 3.
Effect of extraction temperature on the inhibitory activity of the extracts
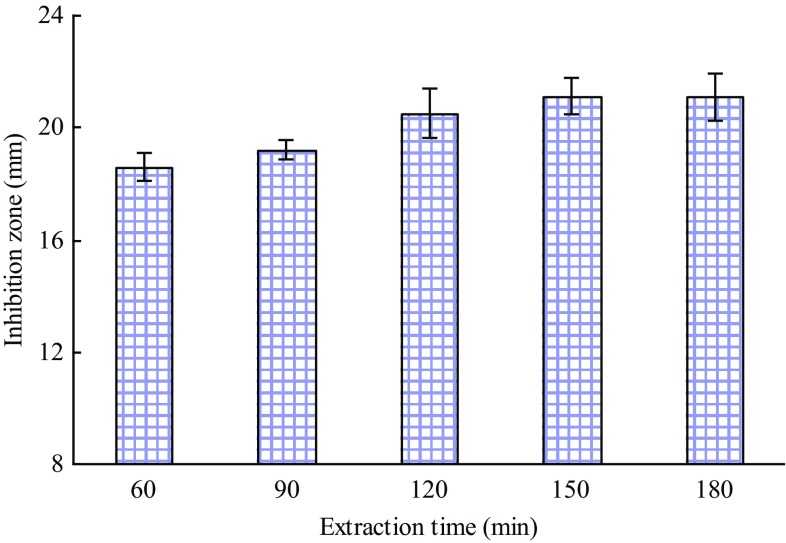
Figure 4 shows a comparatively prompt increase in the diameters of inhibition zone with the extraction time from 60 to 150 min; thereafter, the increase was no longer significant in the inhibition zone diameters. Hence, extraction time of 120 min was chosen as the centre point for the RSM.
Fig. 4.
Effect of extraction time on the inhibitory activity of the extracts
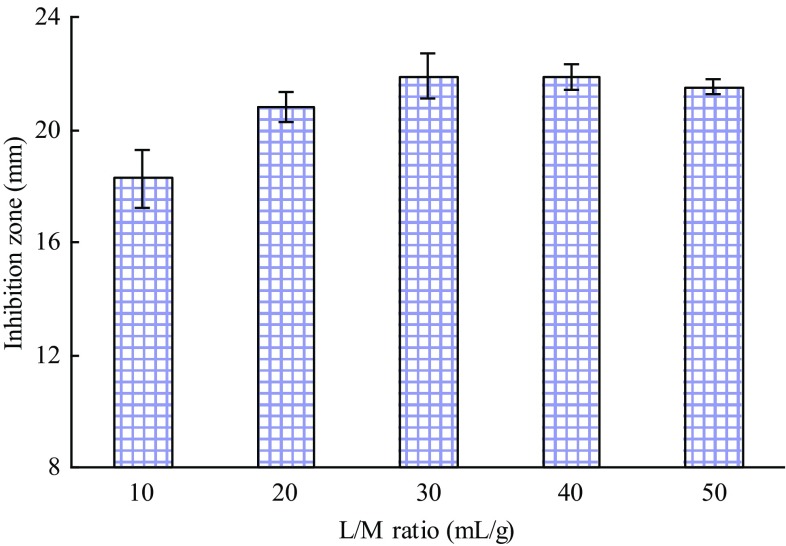
Figure 5 demonstrates the diameters of inhibition zone being elevated as the liquid/material ratio varied from 10:1 to 30:1 mL/g. However, the inhibition zone diameter did not increase when the ratio increased continuously. Therefore, a liquid to material ratio of 20:1 mL/g was chosen as the centre point for the RSM.
Fig. 5.
Effect of liquid to material ratio on the inhibitory activity of the extracts
Based on the results of single factors trial, extraction temperature (X 1), extraction time (X 2) and liquid to material ratio (X 3) were chosen for further optimization in the trial of BBD. The matrixs of BBD is shown in Table 1. Based on the parameter estimates, the application of response surface methodology can offer an empirical relationship between the response variable and test variables. By applying multiple regression analysis on the experimental data, the equation for the diameters of inhibition zone was built as:
| 2 |
Table 2 shows the analysis of variance (ANOVA) for the experimental results of BBD. The value of determination R 2 (0.9824) for Eq. (2) is fairly approximate to 1, which revealed that the regression model sufficiently defined the true behaviour of the system (Guo et al. 2014). The P value < 0.0001 indicated that the fitness of the model was significant. In addition, the lack of fit value of the model was 0.6299, which did not have significant difference (Guo et al. 2016b). The results also indicated that the linear effects of extraction temperature, extraction exttime and liquid to material ratio are significant (P < 0.05). The intercept effect of extraction temperature and time, and quadratic effect of temperature (P < 0.05) were also significant.
Table 2.
ANOVA for the effect of temperature, time and liquid to material ratio on inhibition zone of the extracts using the quadratic response surface model
| Source | Sum of Squares | df | Mean square | F value | P value | Sig. |
|---|---|---|---|---|---|---|
| Model | 30.23 | 9 | 3.36 | 43.50 | < 0.0001 | ** |
| X 1 | 1.26 | 1 | 1.26 | 16.37 | 0.0049 | ** |
| X 2 | 0.70 | 1 | 0.70 | 9.09 | 0.0195 | * |
| X 3 | 6.94 | 1 | 6.94 | 89.84 | < 0.0001 | ** |
| X 1 X 2 | 2.64 | 1 | 2.64 | 34.20 | 0.0006 | ** |
| X 1 X 3 | 0.43 | 1 | 0.43 | 5.56 | 0.0506 | |
| X 2 X 3 | 0.16 | 1 | 0.16 | 2.07 | 0.1932 | |
| X 21 | 17.79 | 1 | 17.79 | 230.38 | < 0.0001 | ** |
| X 22 | 0.01 | 1 | 0.01 | 0.07 | 0.7926 | |
| X 23 | 0.09 | 1 | 0.09 | 1.19 | 0.3106 | |
| Lack of fit | 0.17 | 3 | 0.06 | 0.64 | 0.6299 |
* 0.01 < P < 0.05, ** P < 0.01
Interactions between three variables (extraction temperature, extraction time and liquid to material ratio) and the inhibition zone were exhibited by the response surface and contour plots (Fig. 6). Effects of extraction temperature interaction with each of the two other variables on the diameter of the inhibition zone are presented in Fig. 6a, b. The diameter of inhibition zone increased to a high value with increasing temperature from 80 to 88 °C, but then the inhibition zone decreased with increasing temperature, which indicated that high temperature may destroy the antibacterial active substances. The effects of extraction time interaction with each of the two other variables on the diameter of inhibition zone are presented in Fig. 6a, c, and the inhibition zone increased to a definite value with the extension of time and subsequently maintained stable. A similar phenomenon is observed in Fig. 6b, c with liquid/material ratio and the other two factors, the increase in liquid/material ratio enhanced the diameter of inhibition zone.
Fig. 6.
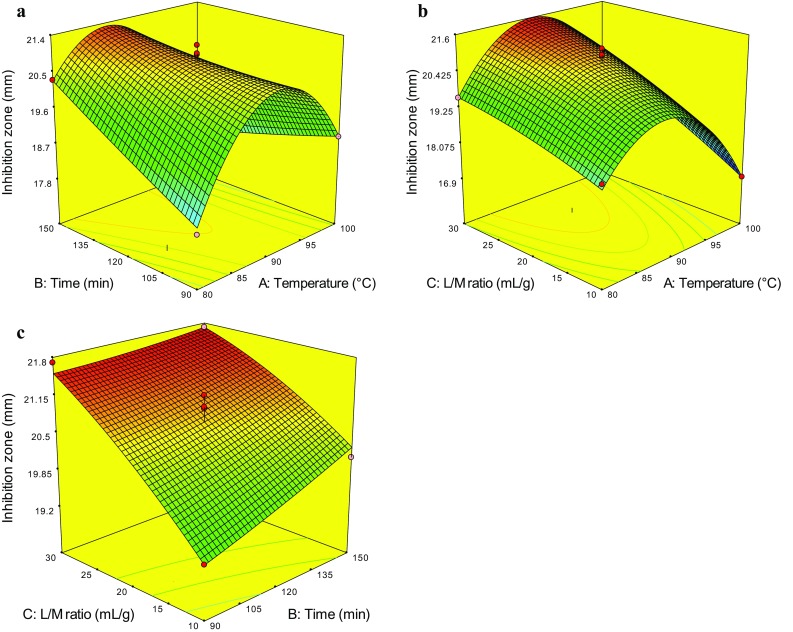
Response surface plots for the effects of temperature and time (a), temperature and liquid to material ratio (b), time and liquid to material ratio (c) on inhibition zone of the extracts. Missing value of in each plot kept at the center point
The predicted optimal parameters were obtained: X 1 = 88 °C, X 2 = 148 min, and X 3 = 29 mL/g by applying the regression analysis to Eq. (2). The corresponding maximal diameter of the inhibition zone was 21.74 mm. The proof test was implemented using the above optimized conditions, and the average value of inhibition zone was 21.31 ± 0.95 with no conspicuous difference to the predicted value. This revealed the good feasibility of RSM for the extraction optimization of ASR. Subsequently, the extracts were vacuum evaporated and freeze-dried to achieve the solid ASR. After finishing the above procedures, the yield of solid ASR was 30.29 ± 2.27% (n = 3). The solid ASR was applied to the upcoming determination of chemical components and analysis of antibacterial activities.
Identification, quantification and antibacterial activities of chemical constituents in ASR
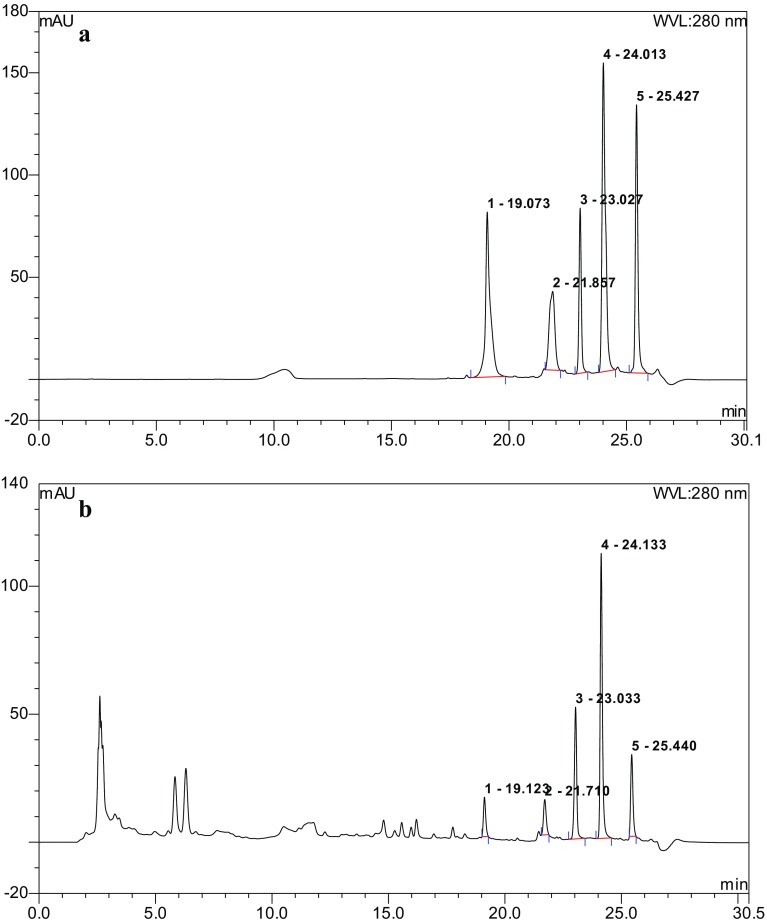
Five reference compounds, namely, aloe-emodin, rhein, emodin, chrysophanol and physcion, were used to identify the chemical substances of ASR by HPLC, and their retention times (t R) were 19.073, 21.857, 23.027, 24.013 and 25.427 min, respectively (Fig. 7a). At the same operational conditions, the above five compounds, aloe-emodin, rhein, emodin, chrysophanol and physcion, were identified in ASR, and their retention times (t R) were 19.123, 21.710, 23.033, 24.133 and 25.440 min, respectively (Fig. 7b). The linear regression equations were achieved and are shown in Table 3, where Y is the peak area (mAu) and X is the concentration of the reference compound (Table 3). The linear relationships of these curves are ideal for the measurement of the above five compounds. The percentages of aloe-emodin, rhein, emodin, chrysophanol and physcion were 0.68 ± 0.02, 0.24 ± 0.05, 0.78 ± 0.07, 6.68 ± 0.97 and 0.58 ± 0.15%, respectively. MIC values of ASR, aloe-emodin, rhein, emodin, chrysophanol and physcion were 0.625, 0.125, 0.015, > 1, > 1, > 1 mg/mL, respectively, which indicated that aloe-emodin and rhein are the main antibacterial compounds of ASR (Table 4).
Fig. 7.
HPLC chromatograms of standard solution of five anthraquinones (a) and antibacerial substances of Rhubarb (ASR, b): 1 aloe-emodin, 2 rhein, 3 emodin, 4 chrysophanol, 5 physcion
Table 3.
Linear relationship of five reference compounds and content in ASR
| Compounds | Linear regression equations | R 2 | Percentages (X ± SD, %) |
|---|---|---|---|
| Aloe-emodin | Y = 32.213X − 2.5027 | 0.9935 | 0.68 ± 0.02 |
| Rhein | Y = 26.927X + 2.1962 | 0.9660 | 0.24 ± 0.05 |
| Emodin | Y = 58.806X − 1.7350 | 0.9781 | 0.78 ± 0.07 |
| Chrysophanol | Y = 9.5589X + 2.4501 | 0.9935 | 6.68 ± 0.97 |
| Physcion | Y = 131.49X − 10.569 | 0.9817 | 0.58 ± 0.15 |
Table 4.
Antibacterial activities against V. harveyi of ASR and its five chemical components
| Category | ASR | Aloe-emodin | Rhein | Emodin | Chrysophanol | Physcion |
|---|---|---|---|---|---|---|
| MIC (mg/mL) | 0.625 | 0.125 | 0.015 | > 1 | > 1 | > 1 |
Aloe-emodin is one of the major active components of Rhubarb and can be separated from other herbal plants such as Aloe and Cassia (Dong et al. 2017). Recent studies have demonstrated that aloe-emodin exhibits various bioactivities such as antifungal (Agarwal et al. 2000), antibacterial (Liu et al. 2015; Smolarz et al. 2013; Wang et al. 2010), anti-inflammatory (Hu et al. 2014), antiviral (Li et al. 2014), antitumor (Dong et al. 2017), anti aggregatory (Furkan et al. 2017), antileishmanial (Dalimi et al. 2015) and other effects. Rhein, a well-known natural compound, is another important bioactive anthraquinone isolated from several traditional Chinese medicines, including R. palmatum L., Aloe barbadensis Miller, Cassia angustifolia Vahl. and Polygonum multiflorum Thunb (Sun et al. 2016). In recent years, rhein has been reported to have antitumor (Cho et al. 2017), antibacterial (Azelmat et al. 2015), anti-inflammatory (Ge et al. 2017), antiviral, antioxidative, antifibrosis, hepatoprotective and nephroprotective effects (Sun et al. 2016). Previous several studies have revealed that aloe-emodin and rhein exhibit antimicrobial effects, but no studies have reported on the mechanism of their antimicrobial action. Thus, work on the mechanisms of antimicrobial action of ASR, aloe-emodin and rhein against the aquatic pathogen V. harveyi would provide a favourable prospect in aquatic applications.
Conclusion
In conclusion, BBD combined with RSM was carried out to optimize the extraction parameters of ASR as follows: 100% ethanol as extraction solvent, liquid/material ratio of 29 mL/g and extraction temperature at 88 °C for 148 min. Yield of ASR was 30.29 ± 2.27%, and the five compounds, namely, aloe-emodin, rhein, emodin, chrysophanol and physcion, were identified in ASR by comparing HPLC chromatograms of the reference substances and sample. Among them, aloe-emodin and rhein are the main antibacterial compounds of Rhubarb. The results provide the basis for the development and application of Rhubarb in the prevention and control of aquatic animal diseases.
Acknowledgements
This work financially supported by the Natural Science Foundation of Jiangsu Province (BK20151283), Technical Plan Project of Lianyungang (CG1612), 521 Talented Project of Lianyungang (KKC17001) and Priority Academic Program Development of Jiangsu Higher Education Institutions (No. 5511201401X).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agarwal SK, Singh SS, Verma S, Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol. 2000;72:43–46. doi: 10.1016/S0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Azelmat J, Larente JF, Grenier D. The anthraquinone rhein exhibits synergistic antibacterial activity in association with metronidazole or natural compounds and attenuates virulence gene expression in Porphyromonas gingivalis. Arch Oral Biol. 2015;60:342–346. doi: 10.1016/j.archoralbio.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Cao H, He S, Wei R, Diong M, Lu L. Bacillus amyloliquefaciens G1: a potential antagonistic bacterium against eel-pathogenic Aeromonas hydrophila. Evid Based Complement Alternat Med. 2011;2011:824104. doi: 10.1155/2011/824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DC, Wang L. Mechanisms of therapeutic effects of rhubarb on gut origin sepsis. Chin J Traumatol. 2009;12:365–369. [PubMed] [Google Scholar]
- Cho JH, Chae JI, Shim JH. Rhein exhibits antitumorigenic effects by interfering with the interaction between prolyl isomerase Pin1 and c-Jun. Oncol Rep. 2017;37:1865–1872. doi: 10.3892/or.2017.5434. [DOI] [PubMed] [Google Scholar]
- Dalimi A, Delavari M, Ghaffarifar F, Sadraei J. In vitro and in vivo antileishmanial effects of aloe-emodin on Leishmania major. J Tradit Complement Med. 2015;5:96–99. doi: 10.1016/j.jtcme.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Fu J, Yin X, Qu C, Yang C, He H, Ni J. Induction of apoptosis in HepaRG cell line by aloe-emodin through generation of reactive oxygen species and the mitochondrial pathway. Cell Physiol Biochem. 2017;42:685–696. doi: 10.1159/000477886. [DOI] [PubMed] [Google Scholar]
- Fu XS, Chen F, Liu XH, Xu H, Zhou YZ. Progress in research of chemical constituents and pharmacological actions of Rhubarb. Chin J New Drugs. 2011;20:1534–1538. [Google Scholar]
- Furkan M, Alam MT, Rizvi A, Khan K, Ali A, Shamsuzzaman Naeem A. Aloe emodin, an anthroquinone from Aloe vera acts as an anti aggregatory agent to the thermally aggregated hemoglobin. Spectrochim Acta A Mol Biomol Spectrosc. 2017;179:188–193. doi: 10.1016/j.saa.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Ge H, Tang H, Liang Y, Wu J, Yang Q, Zeng L, Ma Z. Rhein attenuates inflammation through inhibition of NF-κB and NALP3 inflammasome in vivo and in vitro. Drug Des Dev Ther. 2017;11:1663–1671. doi: 10.2147/DDDT.S133069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhu W, Xu F, Liu M, Xie Y, Zhang J. Optimized ultrasonic-assisted extraction of polysaccharides from Cyclina sinensis and evaluation of antioxidant activities in vitro. CyTA J Food. 2014;12:32–39. doi: 10.1080/19476337.2013.785982. [DOI] [Google Scholar]
- Guo L, Wang C, Zhu W, Xu F. Bioassay-guided fractionation and identification of active substances from the fungus Aspergillus tubingensis against Vibrio anguillarum. Biotechnol Biotechnol Equ. 2016;30:602–606. doi: 10.1080/13102818.2016.1146635. [DOI] [Google Scholar]
- Guo L, Guo J, Zhu W, Jiang X. Optimized synchronous extraction process of tea polyphenols and polysaccharides from Huaguoshan Yunwu tea and their antioxidant activities. Food Bioprod Process. 2016;100:303–310. doi: 10.1016/j.fbp.2016.08.001. [DOI] [Google Scholar]
- Harikrishnan R, Balasundaram C, Heo MS. Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV) Fish Shellfish Immunol. 2010;29:868–874. doi: 10.1016/j.fsi.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Hsu CM, Yu SC, Tsai FJ, Tsai Y. Enhancement of rhubarb extract solubility and bioactivity by 2-hydroxypropyl-β-cyclodextrin. Carbohydr Polym. 2013;98:1422–1429. doi: 10.1016/j.carbpol.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Hu B, Zhang H, Meng X, Wang F, Wang P. Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. J Ethnopharmacol. 2014;53:846–853. doi: 10.1016/j.jep.2014.03.059. [DOI] [PubMed] [Google Scholar]
- Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L. Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture. 2008;274:1–14. doi: 10.1016/j.aquaculture.2007.11.019. [DOI] [Google Scholar]
- Li SW, Yang TC, Lai CC, Huang SH, Liao JM, Wan L, Lin YJ, Lin CW. Antiviral activity of aloe-emodin against influenze A virus via galectin-3 up-regulation. Eur J Pharmacol. 2014;738:125–132. doi: 10.1016/j.ejphar.2014.05.028. [DOI] [PubMed] [Google Scholar]
- Lin CC, Cl Wu, Lin TC, Sheu SJ. Determination of 19 rhubarb constituents by high-performance liquid chromatography-ultraviolet-mass spectrometry. J Sep Sci. 2006;29:2584–2593. doi: 10.1002/jssc.200500307. [DOI] [PubMed] [Google Scholar]
- Liu J, Wu F, Chen C. Design and synthesis of aloe-emodin derivatives as potent anti-tyrosinase, antibacterial and anti-inflammatory agents. Bioorg Med Chem Lett. 2015;25:5142–5146. doi: 10.1016/j.bmcl.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Morya VK, Choi W, Kim EK. Isolation and characterization of Pseudoalteromonas sp. from fermented Korean food, as an antagonist to Vibrio harveyi. Appl Microbiol Biotechnol. 2014;198:1389–1395. doi: 10.1007/s00253-013-4937-3. [DOI] [PubMed] [Google Scholar]
- Randrianarivelo R, Danthu P, Benoit C, Ruez P, Raherimandimby M, Sarter S. Novel alternative to antibiotics in shrimp hatchery: effects of the essential oil of Cinnamosma fragrans on survival and bacterial concentration of Penaeus monodon larvae. J Appl Microbiol. 2010;109:642–650. doi: 10.1111/j.1365-2672.2010.04694.x. [DOI] [PubMed] [Google Scholar]
- Smolarz HD, Swatko-Ossor M, Ginalska G, Medyńska E. Antimycobacterial effect of extract and its components from Rheum rhaponticum. J AOAC Int. 2013;96:155–160. doi: 10.5740/jaoacint.12-010. [DOI] [PubMed] [Google Scholar]
- Sun H, Luo G, Chen D, Xiang Z. A comprehensive and system review for the pharmacological mechanism of action of rhein, an active anthraquinone ingredient. Front Pharmacol. 2016;7:247. doi: 10.3389/fphar.2016.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Gregory S, Plummer S, Shields RJ, Rowley AF. An in vitro and in vivo assessment of the potential of Vibrio spp. as probiotics for the Pacific white shrimp, Litopenaeusvannamei. J Appl Microbiol. 2010;109:1177–1187. doi: 10.1111/j.1365-2672.2010.04743.x. [DOI] [PubMed] [Google Scholar]
- Turker H, Yıldırım AB. Screening for antibacterial activity of some Turkish plants against fish pathogens: a possible alternative in the treatment of bacterial infections. Biotechnol Biotechnol Equ. 2015;29:281–288. doi: 10.1080/13102818.2015.1006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhao H, Kong W, Jin C, Zhao Y, Qu Y, Xiao X. Microcalorimetric assay on the antimicrobial property of five hydroxyanthraquinone derivatives in rhubarb (Rheum palmatum L.) to Bifidobacteriym adolescentis. Phytomedicine. 2010;17:684–689. doi: 10.1016/j.phymed.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Xu HM, Rong YJ, Zhao MX, Song B, Chi ZM. Antibacterial activity of the lipopetides produced by Bacillus amyloliquefaciens M1 against multidrug-resistant Vibrio spp. isolated from diseased marine animals. Appl Microbiol Biotechnol. 2014;98:127–136. doi: 10.1007/s00253-013-5291-1. [DOI] [PubMed] [Google Scholar]
- Yu M, Wang J, Tang K, Shi X, Wang S, Zhu WM, Zhang XH. Purification and characterization of antibacterial compounds of Pseudoalteromonas flavipulchra JG1. Microbiology. 2012;158:835–842. doi: 10.1099/mic.0.055970-0. [DOI] [PubMed] [Google Scholar]
- Zhao LC, Liang J, Li W, Cheng KM, Xia X, Deng X, Yang GL. The use of response surface methodology to optimize the ultrasound-assisted extraction of five anthraquinones from Rheum palmatum L. Molecules. 2011;16:5928–5937. doi: 10.3390/molecules16075928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Guo L, Wang L, Zhu G, Zhu W. Improving the yield of (+)-terrein from the salt-tolerant Aspergillus terreus PT06-2. World J Microbiol Biotechnol. 2016;32:77. doi: 10.1007/s11274-016-2029-0. [DOI] [PubMed] [Google Scholar]