Abstract
Coccinia grandis (L.) fruits (CGFs) are commonly used for culinary purposes and has several therapeutic applications in the Southeast Asia. The aim of this work was to evaluate phytochemical profile, aldose reductase inhibitory (ARI), and antioxidant activities of CGF extract. The CGFs were extracted with different solvents including petroleum ether, dichloromethane, acetone, methanol, and water. The highest yield of total extractable compounds (34.82%) and phenolic content (11.7 ± 0.43 mg of GAE/g dried extract) was found in methanol extract, whereas water extract showed the maximum content of total flavonoids (82.8 ± 7.8 mg QE/g dried extract). Gas chromatography–mass spectroscopy (GC–MS) analysis of methanol and water extract revealed the presence of flavonoids, phenolic compounds, alkaloids, and glycosides in the CGFs. Results of the in vitro ARI activity against partially purified bovine lens aldose reductase showed that methanol extract of CGFs exhibited 96.6% ARI activity at IC50 value 6.12 µg/mL followed by water extract 89.1% with the IC50 value 6.50 µg/mL. In addition, methanol and water extracts of CGF showed strong antioxidant activities including ABTS*+ scavenging, DPPH* scavenging, and hydroxyl radical scavenging. Our results suggest that high percentage of both flavonoids and phenolic contents in the CGFs are correlated with the ARI and antioxidant activities. The fruits of C. grandis are thus potential bifunctional agents with ARI and antioxidant activities that can be used for the prevention and management of DM and associated diseases.
Keywords: Coccinia grandis L, GC–MS analysis, Aldose reductase inhibitor, Antioxidant activity, Diabetic complications
Introduction
Diabetes mellitus (DM), commonly known as diabetes, is a series of metabolic disorders characterized by hyperglycemia (high blood glucose) arising from the impaired insulin secretion, insulin action, or both (American Diabetes Association 2010). According to World Health Organization (WHO) report, an estimated 422 million adults were affected by diabetes in 2014 (WHO 2016). The chronic hyperglycemia of diabetes leads to serious damage of body’s systems such as kidney failure, heart attacks, stroke, retinopathy, and cataract formation (WHO 2016; Kador 1988). Aldose reductase (EC 1.1.1.21) is a first and rate-limiting enzyme in the polyol pathway that catalyzes the reduction of glucose to sorbitol, which is then converted to fructose by sorbitol dehydrogenase (Lorenzi 2007). Normally, 3% glucose enters in the polyol pathway (Morrison et al. 1970), but under the hyperglycemic condition, it goes to more than 30%, resulting in several metabolic imbalances of the cell (Gonzalez et al. 1984). Excess accumulation of sorbitol in the lens fibers causes the influx of water and generates osmotic swelling resulting into cataracts and progression of diabetic complications (Kinoshita et al. 1981; Mestry et al. 2016). Advances in understanding the pathological roles of aldose reductase revealed that this enzyme is also involved in inflammatory signaling under both normoglycemic and hyperglycemic conditions. In addition, oxidative stress and free radicals are closely responsible for the induction of diabetic complications (Wiernsperger 2003). Hence, there is growing interest in searching novel and effective ARIs, particularly with antioxidant activities that can be an effective strategy for the prevention and management of DM (Gacche and Dhole 2011).
To date, a great variety of ARIs has been identified including tolrestat, epalrestat, zenarestat, zopolrestat, sorbinil, quercetin, fidarestat, ranirestat, ponalrestat, lidorestat, and resveratrol (Wang et al. 2017; Reddy et al. 2014). However, use of most inhibitors remained limited due to the low in vivo efficacy, or adverse side effects (Miyamoto 2002). Epalrestat is the only drug which showed a good clinical effect and thus used in Japan, China, and India for the treatment of diabetes (Hotta et al. 1996; Iso et al. 2001). Moreover, the use of synthetic antioxidants such as butylated hydroxytoluene is also harmful due to their carcinogenic effects (Wang et al. 2015). Therefore, several studies have been undertaken to search plant products that could effectively inhibit aldose reductase, reduce oxidative stress, and exhibit antioxidant activity. Interestingly, it has been suggested that sufficient consumption of polyphenolics and flavonoid-rich plant foods could help in preventing about 90% of DM cases (Willett 2002).
C. grandis L. (Family: Cucurbitaceae) is a climbing perennial herb, growing throughout India. It has also been found in several Asian and African countries. It is well known for fruiting throughout the year. The fruits of C. grandis are traditionally used for culinary and medicinal purposes. The medicinal uses of Coccinia plant can be traced to an ancient period, where the juice of the roots and leaves was used in the treatment of diabetes, gonorrhoea, and constipation (Rao et al. 2003; Vaishnav et al. 2001). In addition, various parts of the herb (including the leaves, stems, and roots) are traditionally used for the treatment of bronchitis, jaundice, burns, skin eruptions, fever, insect bites, allergy, eye infections, gonorrhoea, syphilis, etc. (Wasantwisut and Viriyapanich 2003). Leaves of C. grandis have been reported to contain a high amount of phenolic and flavonoid compounds and possess antioxidant properties (Umamaheswari and Chatterjee 2008). Pharmacologically, fruits of C. grandis have been screened for anti-glycation and insulinotropic activities (Meenatchi et al. 2017). In particular, the fruits of C. grandis plant used in culinary purposes have become the natural source of herbal medicine. Search for components with ARI and antioxidant activities revealed that C. grandis is a very important herb with recognized biological activities. However, there is a dearth of scientific information on the phytochemical contents of C. grandis fruits and how it impacts ARI and antioxidant activity.
To the best of our knowledge, no ARI activity studies with this plant fruits and identification of its biologically active phytochemicals were carried out, showing the novelty of our work. Therefore, this study reports for the first time, the ARI activity of CGF extract and identification of phytochemical constituents from it. In addition, the antioxidant activities such as ABTS*+ scavenging, DPPH* scavenging, and hydroxyl radical scavenging were also determined. These findings will not only extend our knowledge of ARI and antioxidant activity of C. grandis, but will also provide a valuable information for developing natural drugs that can alleviate diabetes-induced complications.
Materials and methods
Materials
2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Trolox, gallic acid, and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nicotinamide adenine dinucleotide (NADPH), DEAE cellulose, DL glyceraldehyde, ethylenediaminetetraacetic acid (EDTA), and Folin–Ciocalteu′s phenol reagent were procured from HiMedia Laboratories Pvt. Ltd. (Mumbai, MS, India). All high-performance liquid chromatography (HPLC) grade solvents such as petroleum ether, dichloromethane, acetone, and methanol were obtained from Merck (Darmstadt, Germany).
Fruits and extraction preparation
The C. grandis fruits were obtained from food market (Barshi, MS, India) and identified by Professor Prakash Sarwade (SPM RG Shinde Mahavidyalaya, Paranda, MS, India). The CGFs were washed thoroughly with distilled water to remove the adhered dust, cut into small pieces, and dried under shade at a temperature below 40 °C. Dried fruit pieces were ground in a mixer and sieved to obtain a uniform powder. The fruit powder (100 gm) was then extracted with different solvents (500 mL) of increasing polarity v.z. petroleum ether, dichloromethane, acetone, methanol, and water for 12 h with soxhlet extractor. The extracts were filtered (Whatman grade No. 1 filter paper), evaporated under low pressure (Rotavapor®R-300, Buchi, Flawil, Switzerland), concentrated to a powder by freeze dryer (Alpha 1–2 LDplus, Martin Christ, Osterode am Harz, Germany), and weighted. The freeze dried CGF extracts were dissolved in DMSO (1 gm/mL) and used for in vitro studies unless otherwise stated.
Total phenolic and flavonoid contents
The total phenolic content (TPC) of the various CGF extracts was determined using Folin–Ciocalteu′s phenol reagent and external standard calibration with gallic acid (Singleton et al. 1999). Briefly, the extract solution (0.5 mL, 1 gm/mL) prepared in DMSO was mixed with Folin–Ciocalteu′s phenol reagent (0.5 mL). The mixture was shaken for 5 min and neutralized with sodium carbonate (0.5 mL, 20%, w/v). The reaction mixture volume made to 5 mL with distilled water and incubated at room temperature (25 °C) for 90 min with intermittent shaking. The absorbance of blue color formed was measured spectrophotometrically at 760 nm (UV-1700, Shimadzu, Kyoto, Japan). The control reaction contained all reagents except extract. The TPC was calculated as mg of gallic acid equivalent/g dry extract using an equation derived from the gallic acid calibration curve.
The total flavonoids content (TFC) of the various CGF extracts was determined by the aluminum chloride colorimetric method with slight modifications (Chang et al. 2002). For each sample, the extract solution (250 µL, 1 gm/mL) prepared in DMSO was mixed with distilled water (1 mL) and sodium nitrate solution (150 µL, 5%, w/v). After 6 min of incubation, aluminum chloride solution (75 µL, 10%, w/v) was added and the mixture was further allowed to stand for 5 min. Then, NaOH solution (1 mL, 1 mol/l) was added, and the final mixture volume was made to 2.5 mL with distilled water. The mixtures were vigorously mixed, and the absorbance was measured at 510 nm after 15 min of incubation. TFC was expressed as mg of quercetin equivalent/g dry extract using an equation derived from quercetin calibration curve.
GC–MS analysis
The phytochemical evaluation of the methanolic and water extracts of CGF (100 µg/mL, 1 gm/mL chloroform) was performed on a GC–MS instrument (QP2010 model, Shimadzu Corporation, Kyoto, Japan) as per previous report (Ghosh et al. 2015). For MS detection, an electron ionization system with ionization energy of 70 eV was operated. HP-5MS capillary column (30 m × 0.25 i.d., film thickness 0.25 µm) was used. Helium (99.99%) was used as carrier gas at a flow rate of 1 mL/min. The GC injector and MS transfer line temperature were set at 280 and 290 °C, respectively. An injection volume of 1 µL was employed for each extract. The GC was operated in splitless mode and at a range of 50–550 m/z. The phytochemicals of CGF extracts were identified by comparing their retention times and mass fragmentation patterns with the spectrum of known compounds available at Wiley 7.0 library.
ARI assay
The isolation and purification of AR from bovine lens were performed as per the modified method of Hayman and Kinoshita (1965). The ARI activity of varying concentrations (10, 25, 50, and 100 µg/mL in DMSO) of CGF extracts was assayed spectrophotometrically by monitoring the oxidation of NADPH at 340 nm (UV-1700, Shimadzu, Kyoto, Japan). Quercetin was used as a positive control for ARI. The percent inhibition was calculated using the following formula:
The concentration of extracts required for ARI (50%) was calculated from the least-squares regression line of the logarithmic concentrations plotted versus the residual activity.
Determination of ABTS*+ scavenging ability
Radical scavenging activity of the CGF extracts was assessed spectrophotometrically by ABTS*+ cation decolorization assay (Re et al. 1999). Briefly, an aqueous solution of ABTS*+ (7 mM) and potassium persulfate (2.45 mM) was mixed and left at room temperature for 10–12 h under dark until absorbance gets stable. The characteristic of blue–green colored ABTS*+ solution was diluted with ethanol to an absorbance of 0.70 at 734 nm. Then, extract sample (0.1 mL) (10, 25, 50, and 100 μg/mL) and ABTS*+ solution (0.9 mL) were mixed and incubated at 30 °C for 30 min, and the absorbance was taken at 734 nm. Trolox was used as positive control. The ABTS*+ scavenging ability of CGF extracts was determined using the following formula:
Determination of DPPH free radical scavenging ability
Free radical scavenging activity of the various CGF extracts was determined according to the method of Blios (1958). Briefly, 100 µL of different concentrations of CGF extracts in methanol (10, 25, 50, and 100 μg/mL) were added to DPPH* methanolic solution (5 mL, 0.1 mM). The content was homogenized vigorously and left for 20 min at room temperature in dark. Absorbance of the aqueous solution was measured spectrophotometrically at 517 nm against a blank. BHT was used as positive control. The antioxidant activity was expressed as the percent reduction of free radical by the sample and calculated according to the following formula:
Hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity of the methanolic and water extracts of CGF was determined according to the earlier method (Klein et al. 1981). Different concentrations of CGF extracts (25, 50, 100, and 200 μg/mL) in methanol (1 mL) were made and iron–EDTA solution (0.13% ferrous ammonium sulphate and 0.26% EDTA), EDTA (0.5 mL, 0.018% w/v), DMSO (1 mL, 0.85% v/v in 0.1 M phosphate buffer, pH 7.4), and ascorbic acid (0.5 mL, 0.22% w/v) were added. The reaction mixture was incubated in a water bath (80–90 °C) for 15 min, and the reaction was terminated by ice-cold trichloroacetic acid (TCA) (1 mL, 17.5% w/v) addition. To the reaction content, Nash reagent (3 mL) having a composition of ammonium acetate (75.0 g), glacial acetic acid (3 mL), and acetylacetone (2 mL) in distilled water (total volume to 1 L) was mixed and made up the volume to 1 L with distilled water. After incubation for 15 min at room temperature, the absorbance of formed yellow color was measured at 412 nm against a reagent blank. The percent inhibition was calculated using the following formula:
Results and discussion
Extraction yields, TPC, and TFC
Among several methods to recover and isolate phytochemicals from the plant, the solvent extraction has been widely used. In this work, CGFs extraction was carried out using different solvents and the obtained extraction yield is presented in Table 1. The yield of extractable compounds relative to the weight of dried CGFs extract ranged from 10.13% (petroleum ether extraction) to 34.82% (methanol extraction). The highest amount of total extractable compounds was observed in methanol extract, whereas the extraction yield with petroleum ether (10.13%) and acetone (16.24%) was small in comparison with other solvents. However, the dichloromethane (20.87%) and water extract (23.68%) yield percentage were found to be comparable with that of the methanol solvent. Differences in the extraction yield in each solvent may be due to the variety of compounds present in the fruit extract of C. grandis with different relative solubilities. The maximum extraction yields in methanol and water extract suggest the presence of intermediate-to-high polar compounds such as phenolics in the CGFs.
Table 1.
Extraction yield, TPC, and TFC of CGF extracts
| Solvent system | Extraction yield (%) | TPC (mg GAE/g dry extract) | TFC (mg QE/g dry extract) |
|---|---|---|---|
| Petroleum ether | 10.13 | 1.2 ± 0.10 | 32.4 ± 4.2 |
| Dichloromethane | 20.87c | 2.3 ± 0.32c | 42.7 ± 4.3d |
| Acetone | 16.24d | 2.0 ± 0.20d | 53.4 ± 7.8c |
| Methanol | 34.82a | 11.7 ± 0.43a | 78.2 ± 2.1b |
| Water | 23.68b | 8.2 ± 0.21b | 82.8 ± 7.8a |
Results of triplicate experiments were expressed as the mean ± standard deviation (SD). Value in the same column followed by a different letter (a–d) is significantly different (p < 0.05) by one-way analysis of variance (ANOVA) with Tukey–Kramer comparison test. TPC was expressed as milligram of gallic acid equivalent per gram of dry CGFs extract (mg GAE/g dry CGFs). TFC was expressed as milligram of quercetin equivalent per gram of dry CGFs extract (mg QE/g dry CGFs).
Phenolics are the main responsible constituents of plants for antioxidant activity due to their scavenging ability on free radicals conferred by their hydroxyl groups (Yildirim et al. 2000). In addition, phenolic compounds are effective on polyol enzymes, which are involved in the diabetic complications (Aslan and Beydemir 2017). This class of phytochemicals is, therefore, important for the human nutrition and health. TPC of the different solvent extracts of CGF calculated as gallic acid equivalent/g is presented in Table 1. Among the five solvents used in this study, the highest amount of TPC was obtained in methanol extract (11.7 ± 0.43 mg of GAE/g dried extract) followed by water dichloromethane, acetone, and petroleum ether. The very small phenolic content was found in the petroleum ether extract (1.2 ± 0.10 mg of GAE/g dried extract) due to the less polarity aspect. A significant variation in TPC of the different solvent extract was observed which might be attributed to the polarities of compounds present in the fruit extract of C. grandis. These results are in agreement with a previous report which showed that methanol is the most efficient solvent for extraction of phenolic compound (Yen et al. 1995).
Flavonoids are polyphenolic compounds which exhibit excellent scavenging activity against most of the oxidizing molecules such as singlet oxygen and several free radicals involved in many diseases (Bravo 1998). Flavonoids, including flavones, flavonols, and tannins have shown potent antioxidant activity in vitro and demonstrated a wide range of therapeutic uses such as anti-inflammatory, antiviral, anti-carcinogenic, ant-aging, and anti-allergenic (Geetha et al. 2003; Shimoi et al. 1996; Prasad et al. 2009). In addition, certain flavonoids are capable of inhibiting enzyme aldose reductase involved in the diabetics (Chaudhry et al. 1983). Flavonoids are ubiquitously found in vegetables and fruits. The TFC in the different solvent extracts of CGF calculated as quercetin equivalent/g dry extract and given in Table 1. The quantitative analysis of total flavonoids revealed the highest amount in water extract (82.8 ± 7.8 mg QE/g dried extract) followed by methanol (78.2 ± 2.1 mg QE/g dry extract), acetone (53.4 ± 7.8 mg QE/g dry extract), dichloromethane (42.7 ± 4.3 mg QE/g dry extract), and petroleum ether (32.4 ± 4.2 mg QE/g dry extract) (Table 1). These results suggest the presence of a good amount of flavonoids in the fruit extract of C. grandis which increases with solvent polarity. The phytochemical screening for TPC and TFC of the CGFs extract is in good agreement with the report of Meenatchi et al. (2017). Therefore, the presence of both phenolic and flavonoid contents in the fruits of C. grandis could contribute directly to the antioxidant activity by means of free radicals neutralization and inhibition of aldose reductase involved in the polyol pathway of diabetic complications.
Chemical composition of the CGF extracts
The GC–MS analysis was used for the identification of biologically active phytochemicals such as phenolics and flavonoids in the extract obtained from C. grandis fruits. Among different solvent extracts, GC–MS was performed on methanolic and water extracts of CGF only due to its highest content of total phenolic and flavonoid compounds. As shown in Table 2, GC–MS analysis of methanol extract of CGFs resulted in the identification of 17 different chemical compounds representing 99.45% the relative area, with more than 90% similarity with that of the standard mass spectra in the data library. The pharmaceutically important chemical ingredients found in methanol extract were 2(3H)-furanone, 2-methoxy-4-vinylphenol, benzofuranone, 9,12-Octadecadienoic acid, tocopherol, campoesterol, stigmatosterol, and ethisteron. The results of the phytochemical analysis suggest that the methanol extract obtained from C. grandis fruits is characterized by the presence of alkaloids and phenolic compounds. The polyphenols which are regarded as natural antioxidants and some flavonoids are known to be effective in reducing the aldose reductase activity (Hsieh et al. 2010). Many of these identified compounds in the methanol extract of CGFs have already been reported from a number of plants (Song et al. 2015; Li et al. 2012; Gurnani et al. 2016).
Table 2.
Phytochemicals identified in the methanol extract of CGFs by GC–MS
| No. | RT (min) | Name of the compound | Molecular formula | Molecular weight | Peak area (%) |
|---|---|---|---|---|---|
| 1 | 9.580 | 2(3H)-furanone | C4H4O2 | 84.02 | 0.23 |
| 2 | 15.099 | 2-methoxy-4-vinylphenol | C9H10O2 | 150.07 | 0.14 |
| 3 | 17.650 | Phenol-2-methoxy-5(1-propenyl) | C10H12O2 | 164.08 | 0.89 |
| 4 | 18.140 | Undecanol | C11H24O | 172.18 | 0.63 |
| 5 | 18.673 | Phenol, 2.4-bis(1,1-dimethyethyl) | C14H22O | 206.17 | 0.94 |
| 6 | 19.125 | Benzofuranone | C8H6O2 | 134.04 | 0.84 |
| 7 | 24.317 | 3,7,11,15-Tetramethyl-2-hexdecen-1-ol | C20H40O | 296.53 | 0.78 |
| 8 | 25.235 | Hexadecanoic acid methyl ester | C17H34O2 | 270.26 | 0.67 |
| 9 | 29.893 | 9,12-Octadecadienoic acid | C18H32O2 | 280.24 | 0.31 |
| 10 | 40.422 | 2-Methyl-Z,Z-3, 13-Octadecadienol | C19H36O | 280.28 | 0.51 |
| 11 | 41.052 | Tocopherol | C29H50O2 | 430.38 | 10.24 |
| 12 | 41.586 | Beta-sitosterol acetate | C31H52O2 | 456.76 | 9.38 |
| 13 | 43.401 | Campoesterol | C28H48O | 400.69 | 2.36 |
| 14 | 43.413 | Campesterol | C28H48O | 400.69 | 3.14 |
| 15 | 43.812 | Stigmatosterol | C29H48O | 412.69 | 10.13 |
| 16 | 48.010 | Beta-sitosterol | C29H50O | 414.70 | 58.12 |
| 17 | 49.098 | Ethisteron | C21H28O2 | 312.44 | 0.14 |
The GC–MS analysis of the crude water extract obtained from the fruits of C. grandis resulted in the identification of 11 phytochemicals representing 98.18% of the relative area in the aqueous extract. The water extract was predominated by fatty acids including n-pentadecanoic acid (18.20%), hexadecanoic acid (58.73%), linoleic acid (5.78%), oleic acid (1.10%), and α-tocopherol (11.72%) (Table 3). The detection of significantly more fatty acids in the water extract could be due to its higher polarity than methanol. In addition, the presence of polar carboxyl group in fatty acids may be resulted into the appreciable solubility in water and thus observed by GC–MS analysis. It is known that fatty acids such as oleic acid and linoleic acid are essential in human nutrition as they helps to reduce the level of cholesterol and maintenance of nervous system, respectively (Sprecher 1981; Bourre 2006).
Table 3.
Phytochemicals identified in the water extract of CGFs by GC–MS
| No. | RT (min) | Name of the compound | Molecular formula | Molecular weight | Peak area (%) |
|---|---|---|---|---|---|
| 1 | 14.113 | Hexadecanoic acid methyl ester | C17H34O2 | 270.45 | 0.32 |
| 2 | 16.097 | Do-decanedioic acid | C12H22O4 | 230.30 | 0.38 |
| 3 | 16.230 | Isosteviol | C20H30O3 | 318.45 | 0.74 |
| 4 | 16.495 | Biphenyl | C12H10 | 154.21 | 0.51 |
| 5 | 19.389 | α-tocopherol | C29H50O2 | 430.71 | 11.72 |
| 6 | 24.430 | n-pentadecanoic acid | C15H30O2 | 242.39 | 18.20 |
| 7 | 26.795 | Hexadecanoic acid | C16H32O2 | 256.42 | 58.73 |
| 8 | 31.328 | Linoleic acid | C18H32O2 | 280.44 | 5.78 |
| 9 | 32.422 | Oleic acid | C18H34O2 | 282.46 | 1.10 |
| 10 | 41.805 | Lukianol | C25H17NO5 | 411.11 | 0.60 |
| 11 | 45.922 | Benzene | C6H6 | 78.11 | 0.10 |
Two compounds, hexadecanoic acid methyl ester (0.67% in methanol and 0.32% in water) and tocopherol (10.24% in methanol and 11.72% in water) were found in both the extracts of CGFs. Various compounds identified from CGFs water extract have already been reported from other plant fruits and are known to be pharmacologically active (Gurnani et al. 2016; Chen and Zuo 2007, Zuo et al. 2002). For example, hexadecanoic acid and a long-chain unsaturated fatty acid such as linoleic acid are known to have potential antioxidant and antimicrobial properties, respectively (Wei et al. 2011; Chang et al. 2005). The rich content of phenolics, flavonoids, and other phytochemical in the fruits of C. grandis strengthens our study as these are the important bioactive compounds known to inhibit the aldose reductase activity. However, further study on the isolation of individual phytochemical constituent from CGFs and subjecting it to the biological activity will be helpful for antidiabetic drug development.
ARI activity profile
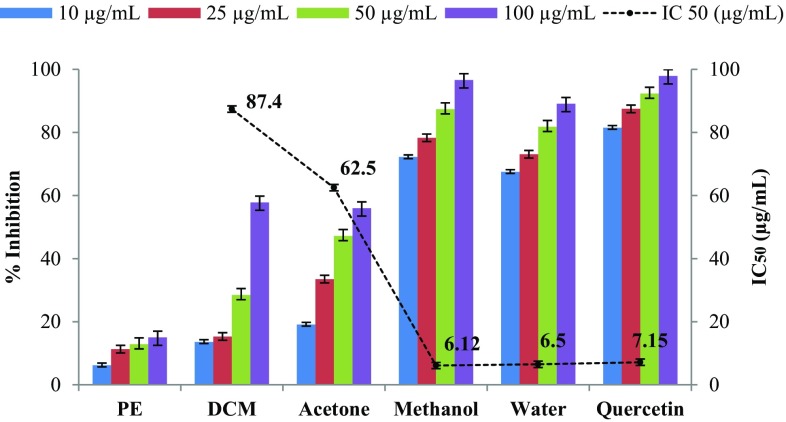
Aldose reductase, the first and rate-limiting enzyme in the polyol pathway that reduces excess glucose into sorbitol with concomitant conversion of NADPH into NADP+ has been involved in the pathogenesis of diabetic complications such as neuropathy, nephropathy, retinopathy, and cataract (Kato et al. 2009). It is suggested that the inhibition of aldose reductase by natural products is a best therapeutic strategy to ameliorate diabetic complications (Karasu et al. 2012). Therefore, in the present study, all the fruit extracts of C. grandis were evaluated in vitro for their ability to inhibit aldose reductase activity with quercetin as a reference. The IC50 values were determined for those CGFs solvent extract only which showed greater than 50% inhibition and the corresponding data, as shown in Fig. 1. Among the five solvent tested, four CGF extracts (100 µg/mL concentration) were found to inhibit the bovine lens aldose reductase activity with IC50 values ranging from 6.50 to 87.40 µg/mL. The methanol and aqueous extracts were more potent in ARI, as indicated by its lower IC50 values (6.12 and 6.50 µg/mL, respectively) in comparison with those of the other extracts. These values are relatively comparable to the standard quercetin which has IC50 value 7.15 µg/mL. Altogether, methanol and water extracts of CGF were found effective at all concentrations including 10, 25, and 50 µg/mL, while the dichloromethane and acetone extract did not produce significant ARI activity at similar concentrations. Among all the extract, petroleum ether extract was found to exhibit the least ARI potential (15.0 ± 0.2%). As methanol and aqueous extracts of CGFs showed potent ARI activity, it could be useful to treat early stage diabetic complications. The ARI effect of plant phytochemicals has been attributed to the presence of phenolics and flavonoids, which are reported to reduce the carbohydrate hydrolyzing enzymes activities (Viswanatha et al. 2010). Such phytochemicals are also capable of reducing the oxidative stress by scavenging reactive oxygen species and prevent the damage to cell (Girija et al. 2011). In addition, the presence of polyphenolic and total flavonoids in the fruit extract has been reported to possess anti-glycan effect (Meenatchi et al. 2017). This strengthens our study, because fruits of C. grandis also contain high amounts of TPC, TFC, and other phytochemicals.
Fig. 1.
ARI activity of different solvent extracts of CGF. Results of triplicate experiments were expressed as the mean ± SD. Value of IC50 followed by methanol, water, and acetone is significantly different (p < 0.05). PE petroleum ether, DCM dichloromethane
Antioxidant ability against ABTS*+ and DPPH*
It has long been recognized that naturally occurring substances in higher plants possess antioxidant activity. Among these substances, the phenolics and flavonoids which are the secondary metabolites of plants have the ability to scavenge a wide range of free radicals and hydroxyl radicals by single electron transfer (Zou et al. 2004). In addition, these molecules are capable of chelating metal ions and, therefore, reduce their prooxidant ability (Pham et al. 2013). Thus, evaluation of antioxidants activity, specifically the free radical scavenging abilities, has been suggested to screen bioactive compounds with regard to phenolics, flavonoids, anthocyanins, and carotenoids for human health benefits (Lee et al. 2013; Denardin et al. 2015). ABTS*+ and DPPH* assays are the most widely accepted tools for antioxidant activity determination in food industries owing to their great reproducibility (Lee et al. 2013; Denardin et al. 2015). Hence, we investigated the scavenging effect of CGFs extract in comparison with those of Trolox (positive control) and BHT (positive controls) and expressed as percentage inhibition against ABTS and DPPH radicals, respectively.
Results of the antioxidant assay revealed that all the five extracts were able to scavenge ABTS*+ at 100 µg/mL concentration. However, the methanol extract exhibited the highest ABTS*+ scavenging ability (95.8%) followed by water (88.4%), dichloromethane (61.0%), acetone (57.4%), and petroleum ether (43.2%) (Table 4). The methanol extract free radical scavenging ability was slightly lower than Trolox (96.4%). Thus, the addition of phenolics rich CGFs to the food can enhance the antioxidant ability of diets. The strong antioxidant properties of phytochemicals are responsible for lowering the risk of developing chronic disorders (Rao et al. 1999). It is known that the effectiveness of phenolic compounds depends on the molecular weight, number of aromatic rings present, and the nature of hydroxyl group’s substitution (Hagerman et al. 1998). In the present study, free radical scavenging activity of CGFs extracts might be due to the presence of high molecular phenolics, such as flavonoids and phenolic acids as revealed by GC–MS analysis.
Table 4.
ABTS*+ and DPPH* scavenging ability of the different solvent extracts of CGF
| Extracts | Radical scavenging ability (%) | |
|---|---|---|
| ABTS*+ | DPPH* | |
| Petroleum ether | 43.2 ± 0.2 | 40.0 ± 0.4 |
| Dichloromethane | 61.0 ± 0.5 | 57.5 ± 0.7 |
| Acetone | 57.4 ± 0.4 | 54.2 ± 0.6 |
| Methanol | 95.8 ± 1.0 | 91.8 ± 1.0 |
| Water | 88.4 ± 0.6 | 84.2 ± 0.8 |
| Positive control | 96.4 ± 1.0 | 92.7 ± 1.0 |
The free radical scavenging activity was further tested by measuring the ability of CGF extract to quench the DPPH*. The DPPH values of the CGF extracts from different solvent differed significantly (Table 4). The highest scavenging effect was observed in the methanol extract (91.8%) followed by water (84.2), dichloromethane (57.5%), acetone (54.2%), and petroleum ether (40.0%). These results suggest that methanol extract which content the highest amount of total phenolics and flavonoids was the potential radical scavenger. A similar trend with methanol extract was observed for DPPH assay in that methanol extract showed strong antioxidant activity (Meenatchi et al. 2017). The results from ABTS*+ and DPPH* assays suggest that antioxidant activity of CGF extracts is strongly related to the nature of phenolic and flavonoid compounds present, thus contributing to their electron transfer hydrogen donating ability.
Results of triplicate experiments were expressed as the mean ± SD. Radical scavenging ability of the different solvent extracts of CGF and positive control was carried out at 100 µg/mL. Positive control: ABTS: Trolox, DPPH: BHT.
Hydroxyl radical scavenging ability
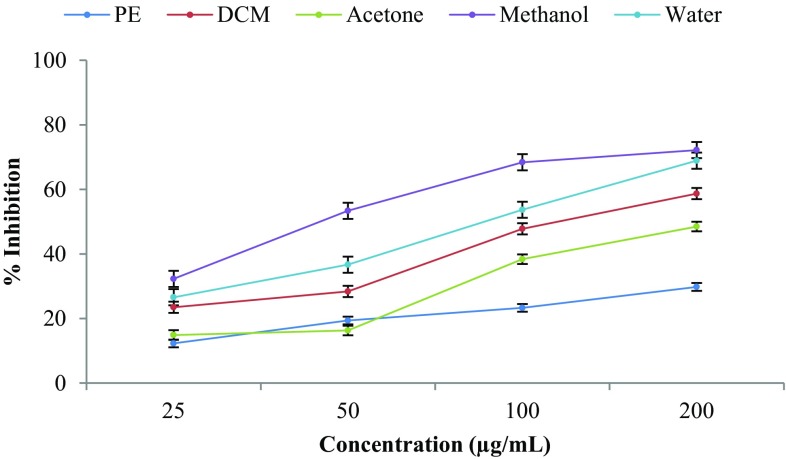
The hydroxyl radicals are often referred as extremely reactive free radical in the biological system. It has been implicated as highly potent oxygen-centred radical capable of tissue damage and cell death in the living systems (Hochstein and Atallah 1998). Hydroxyl radical is very strong reactive oxygen species which react readily with most biomolecules such as proteins, carbohydrates, lipids, and DNA in cells, and leads to carcinogenesis and mutagenesis in humans. There is no any specific enzyme present in the human body to defend against hydroxyl radical, and thus, it is essential to find a substance with good scavenging capacity against such reactive oxygen species. It is known that the hydroxyl radical scavenging capacity of plant extract is directly related to its antioxidant activity (Manian et al. 2008). Result of the scavenging effect of a different solvent extract of CGFs against hydroxyl radicals demonstrated that all the extracts possess good hydroxyl radical scavenging ability (Fig. 2). The methanol and water extract showed significant hydroxyl radical scavenging activity with IC50 values 48.9 and 74.9 µg/mL, respectively. However, petroleum ether and acetone extract did not decrease hydroxyl radical scavenging activity at similar concentrations. The ability of the methanol and water fruit extract to quench hydroxyl radicals appears directly related to the inhibition of propagation of lipid peroxidation. As these extracts were good scavengers of active oxygen species, it will reduce the rate of a chain reaction. Hydrogen peroxide scavenging by CGF extracts may be attributed to their phenolic content, which is known to donate electrons to H2O2 and, therefore, neutralizes it to H2O (Saurav and Kannabiran 2012).
Fig. 2.
Hydroxyl radical scavenging activity of the different solvent extracts of CGF at different concentrations. Results of triplicate experiments were expressed as the mean ± SD
Conclusion
The GC–MS data of CGFs extract demonstrated the presence of biologically active compounds. The methanol and water extracts obtained from the fruits of C. grandis showed potent ARI activity against bovine lens aldose reductase when compared with the standard quercetin. In addition, CGFs extract demonstrated strong antioxidant ability with that of known standards. The correlation between the phytochemical analysis and inhibition of aldose reductase suggests that the high content of flavonoids and phenolics in the CGF extracts could be involved in exerting the ARI and antioxidant activities. The presence of various phytochemicals in the methanol and water extracts as detected by GC–MS supports the use of whole fruit for therapeutic purpose. However, further efforts should be made to isolate the individual bioactive components from the fruits of C. grandis and evaluate its vivo ARI activity in animal models. This will contribute to developing natural drugs for the prevention and management of DM.
Acknowledgements
This paper was supported by the KU Research Professor Program of Konkuk University.
Compliance with ethical standards
Conflict of interest
The authors declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan HE, Beydemir S. Phenolic compounds: the inhibition effect on polyol pathway enzymes. Chem Biol Interact. 2017;266:47–55. doi: 10.1016/j.cbi.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging. 2006;10(5):377–385. [PubMed] [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev. 1998;56(11):317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–182. [Google Scholar]
- Chang JZ, Sung YJ, Gyu LT, Young CH. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005;579(23):5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Chaudhry PS, Cabrera J, Juliani HR, Varma SD. Inhibition of human lens aldose reductase by flavonoids, sulindac and indomethacin. Biochem Pharmacol. 1983;32(13):1995–1998. doi: 10.1016/0006-2952(83)90417-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Zuo Y. Identification of flavonol glycosides in American cranberry fruit. Food Chem. 2007;101(4):1357–1364. doi: 10.1016/j.foodchem.2006.03.041. [DOI] [Google Scholar]
- Denardin CC, Hirsch GE, da Rocha RF, Vizzotto M, Henriques AT, Moreira JCF, Guma FTCR, Emanuelli T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J Food Drug Anal. 2015;23(3):387–398. doi: 10.1016/j.jfda.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacche RN, Dhole NA. Aldose reductase inhibitory, anti-cataract and antioxidant potential of selected medicinal plants from the Marathwada region. India Nat Prod Res. 2011;25(7):760–763. doi: 10.1080/14786419.2010.536951. [DOI] [PubMed] [Google Scholar]
- Geetha S, Sai-Ram M, Mongia SS, Singh V, Ilavazhagan G, Sawhney RC. Evaluation of antioxidant activity of leaf extract of Seabuckthorn (Hippophae rhamnoides L.) on chromium(VI) induced oxidative stress in albino rats. J Ethnopharmacol. 2003;87(2–3):247–251. doi: 10.1016/S0378-8741(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Ghosh G, Panda P, Rath M, Pal A, Sharma T, Das D. GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum leaves. Pharmacognosy Res. 2015;7(1):110–113. doi: 10.4103/0974-8490.147223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girija K, Lakshman K, Chandrika U, Ghosh SS, Divya T. Anti-diabetic and anti-cholesterolemic activity of methanol extracts of three species of Amaranthus. Asian Pac J Trop Biomed. 2011;1(2):133–138. doi: 10.1016/S2221-1691(11)60011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RG, Barnett P, Aguayo J, Cheng HM, Chylack LTJ. Direct measurement of polyol pathway activity in the ocular lens. Diabetes. 1984;33(2):196–199. doi: 10.2337/diab.33.2.196. [DOI] [PubMed] [Google Scholar]
- Gurnani N, Gupta M, Mehta D, Mehta BK. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.) J Taibah Univ Sci. 2016;10(4):462–470. doi: 10.1016/j.jtusci.2015.06.011. [DOI] [Google Scholar]
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW. High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J Agric Food Chem. 1998;46(5):1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Hayman S, Kinoshita JH. Isolation and properties of lens aldose reductase. J Biol Chem. 1965;240(2):877–882. [PubMed] [Google Scholar]
- Hochstein P, Atallah AS. The nature of oxidants and antioxidant systems in the inhibition of mutation and cancer. Mutat Res. 1998;202(2):363–375. doi: 10.1016/0027-5107(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Hotta N, Sakamoto N, Shigeta Y, Kikkawa R, Goto Y. Clinical investigation of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy in Japan: multicenter study. J Diabetes Compl. 1996;10(3):168–172. doi: 10.1016/1056-8727(96)00113-4. [DOI] [PubMed] [Google Scholar]
- Hsieh PC, Huang GJ, Ho YL, Lin YH, Huang SS, Chiang YC, Tseng MC, Chang YS. Activities of antioxidants, α-glucosidase inhibitors and aldose reductase inhibitors of the aqueous extracts of four Flemingia species in Taiwan. Bot Stud. 2010;51(3):293–302. [Google Scholar]
- Iso K, Tada H, Kuboki K, Inokuchi T. Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in type 2 diabetic patients. J Diabetes Complic. 2001;15(5):241–244. doi: 10.1016/S1056-8727(01)00160-X. [DOI] [PubMed] [Google Scholar]
- Kador PF. The role of aldose reductase in the development of diabetic complications. Med Res Rev. 1988;8(3):325–352. doi: 10.1002/med.2610080302. [DOI] [PubMed] [Google Scholar]
- Karasu C, Cumaoğlu A, Gurpinar AR, Kartal M, Kovacikova L, Milackova I, Stefek M. Aldose reductase inhibitory activity and antioxidant capacity of pomegranate extracts. Interdiscip Toxicol. 2012;5(1):15–20. doi: 10.2478/v10102-012-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Yasuko H, Goto H, Hollinshead J, Nash RJ, Adachi I. Inhibitory effect of rhetsinine isolated from Evodia rutaecarpa on aldose reductase activity. Phytomedicine. 2009;16(2–3):258–261. doi: 10.1016/j.phymed.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Kinoshita JH, Kador P, Catiles M. Aldose reductase in diabetic cataract. J Am Med Assoc. 1981;246(3):257–261. doi: 10.1001/jama.1981.03320030049032. [DOI] [PubMed] [Google Scholar]
- Klein SM, Cohen G, Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating system. Biochemistry. 1981;20(21):6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park KH, Lee MH, Kim HT, Seo WD, Kim JY, Baek IY, Jang DS, Ha TJ. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013;136(2):843–852. doi: 10.1016/j.foodchem.2012.08.057. [DOI] [PubMed] [Google Scholar]
- Li J, Tian YZ, Sun BY, Yang D, Chen JP, Men QM. Analysis on volatile constituents in leaves and fruits of Ficus carica by GC-MS. Chin Herb Med. 2012;4(1):63–69. [Google Scholar]
- Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manian R, Anusuya N, Siddhuraju P, Manian S. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 2008;107(3):1000–1007. doi: 10.1016/j.foodchem.2007.09.008. [DOI] [Google Scholar]
- Meenatchi P, Purushothaman A, Maneemegalai S. Antioxidant, antiglycation and insulinotrophic properties of Coccinia grandis (L.) in vitro: possible role in prevention of diabetic complications. J Tradit Complement Med. 2017;7(1):54–64. doi: 10.1016/j.jtcme.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestry SN, Bhatkhande GS, Dhodi JB, Juvekar AR. Aldose reductase inhibitory potential and anti-cataract activity of Punica granatum fruit extract. Curr Ther Res. 2016;78:S2. doi: 10.1016/j.curtheres.2016.05.004. [DOI] [Google Scholar]
- Miyamoto S. Recent advances in aldose reductase inhibitors: potential agents for the treatment of diabetic complications. Expert Opin Ther Patents. 2002;12(5):621–631. doi: 10.1517/13543776.12.5.621. [DOI] [Google Scholar]
- Morrison AD, Clements RS, Jr, Travis SB, Oski F, Winegrad AI. Glucose utilization by the polyol pathway in human erythrocytes. Biochem Biophys Res Commun. 1970;40(1):199–205. doi: 10.1016/0006-291X(70)91066-1. [DOI] [PubMed] [Google Scholar]
- Pham AN, Xing G, Miller CJ, Waite TD. Fenton-like copper redox chemistry revisited: hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J Catal. 2013;301:54–64. doi: 10.1016/j.jcat.2013.01.025. [DOI] [Google Scholar]
- Prasad KN, Yang B, Dong X, Jiang G, Zhang H, Xie H, Jiang Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov Food Sci Emerg Technol. 2009;10(4):627–632. doi: 10.1016/j.ifset.2009.05.009. [DOI] [Google Scholar]
- Rao AV, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: a review. Nutr Res. 1999;19(2):305–323. doi: 10.1016/S0271-5317(98)00193-6. [DOI] [Google Scholar]
- Rao GMM, Vijayakumar M, Rao CV, Rawat AKS, Mehrotra S. Hepatoprotective effect of Coccinia indica against CCl4 induced hepatotoxicity. Nat Prod Sci. 2003;9(1):13–17. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reddy TN, Ravinder M, Bagul P, Ravikanti K, Bagul C, Nanubolu JB, Srinivas K, Banerjee SK, Rao VJ. Synthesis and biological evaluation of new epalrestat analogues as aldose reductase inhibitors (ARIs) Eur J Med Chem. 2014;71:53–66. doi: 10.1016/j.ejmech.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Saurav K, Kannabiran K. Cytotoxicity and antioxidant activity of 5-(2,4-dimethylbenzyl)pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp. Saudi J Biol Sci. 2012;19(1):81–86. doi: 10.1016/j.sjbs.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoi K, Masuda S, Shen B, Furugori M, Kinze N. Radioprotective effects of antioxidative plant flavonoids in mice. Mutat Res Fundam Mol Mech Mutagen. 1996;350(1):153–161. doi: 10.1016/0027-5107(95)00116-6. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthifer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. In: Packer L, editor. Methods in enzymology. Amsterdam: Elsevier; 1999. pp. 152–178. [Google Scholar]
- Song L, Liu H, Wang Y, Wang Y, Liu J, Zhou Z, Chu H, Zhuang P, Zhang Y. Application of GC/MS-based metabonomic profiling in studying the therapeutic effects of Huangbai-Zhimu herb-pair (HZ) extract on streptozotocin-induced type 2 diabetes in mice. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;997:96–104. doi: 10.1016/j.jchromb.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Sprecher H. Biochemistry of essential fatty acids. Prog Lipid Res. 1981;20:13–22. doi: 10.1016/0163-7827(81)90009-6. [DOI] [PubMed] [Google Scholar]
- Umamaheswari M, Chatterjee TK. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr J Tradit Complement Altern Med. 2008;5(1):61–73. [PMC free article] [PubMed] [Google Scholar]
- Vaishnav MM, Jain P, Jogi SR, Gupta KR. Coccinioside-K, triterpenoid saponin from Coccinia indica. Orient J Chem. 2001;17(3):465–468. [Google Scholar]
- Viswanatha GLS, Vaidya SK, Ramesh C, Krishnadas N, Rangappa S. Antioxidant and antimutagenic activities of bark extract of Terminalia arjuna. Asian Pac J Trop Med. 2010;3(12):965–970. doi: 10.1016/S1995-7645(11)60010-2. [DOI] [Google Scholar]
- Wang Z, Hwang SH, Lim SS. Lipophilization of phenolic acids with phytosterols by a chemoenzymatic method to improve their antioxidant activities. Eur J Lipid Sci Tech. 2015;117(7):1037–1048. doi: 10.1002/ejlt.201400597. [DOI] [Google Scholar]
- Wang Z, Hwang SH, Quispe YNG, Arce PHG, Lim SS. Investigation of the antioxidant and aldose reductase inhibitory activities of extracts from Peruvian tea plant infusions. Food Chem. 2017;231:222–230. doi: 10.1016/j.foodchem.2017.03.107. [DOI] [PubMed] [Google Scholar]
- Wasantwisut E, Viriyapanich T. Ivy gourd (Coccinia grandis Voigt, Coccinia cordifolia, Coccinia indica) in human nutrition and traditional applications. World Rev Nutr Diet. 2003;91:60–66. doi: 10.1159/000069929. [DOI] [PubMed] [Google Scholar]
- Wei LS, Wee W, Siong JY, Syamsumir DF. Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Med Iran. 2011;49(10):670–674. [PubMed] [Google Scholar]
- Wiernsperger NF. Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabetes Metab. 2003;29(6):579–585. doi: 10.1016/S1262-3636(07)70072-1. [DOI] [PubMed] [Google Scholar]
- Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296(5568):695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2016) Global report on diabetes. http://www.who.int/diabetes/global-report/en/
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43(1):27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- Yildirim A, Mavi A, Oktay M, Kara AA, Algur OF, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of Tilia (Tilia argentea Desf Ex DC), Sage (Savia triloba L.), and Black Tea (Camellia sinensis) extracts. J Agric Food Chem. 2000;48(10):5030–5034. doi: 10.1021/jf000590k. [DOI] [PubMed] [Google Scholar]
- Zou Y, Lu Y, Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem. 2004;52(16):5032–5039. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Wang C, Zhan J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC–MS. J Agric Food Chem. 2002;50(13):3789–3794. doi: 10.1021/jf020055f. [DOI] [PubMed] [Google Scholar]