Abstract
Interleukin (IL)-26 is abundant in human airways and this cytokine is involved in the local immune response to a bacterial stimulus in vivo. Specifically, local exposure to the toll-like receptor (TLR) 4 agonist endotoxin does increase IL-26 in human airways and this cytokine potentiates chemotactic responses in human neutrophils. In addition to T-helper (Th) 17 cells, alveolar macrophages can produce IL-26, but it remains unknown whether this cytokine can also be produced in the airway mucosa per se in response to a viral stimulus. Here, we evaluated whether this is the case using primary bronchial epithelial cells from the airway epithelium in vitro and explored the signaling mechanisms involved, including the modulatory effects of additional Th17 cytokines. Finally, we assessed IL-26 and its archetype signaling responses in healthy human airways in vivo. We found increased transcription and release of IL-26 protein after stimulation with the viral-related double stranded (ds) RNA polyinosinic-polycytidylic acid (poly-IC) and showed that this IL-26 release involved mitogen-activated protein (MAP) kinases and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). The release of IL-26 in response to a viral stimulus was modulated by additional Th17 cytokines. Moreover, there was transcription of IL26 mRNA and expression of the protein in epithelial cells of bronchial brush and tissue biopsies respectively after harvest in vivo. In addition, the extracellular IL-26 protein concentrations in bronchoalveolar lavage (BAL) samples did correlate with increased epithelial cell transcription of an archetype intracellular signaling molecule downstream of the IL-26-receptor complex, STAT1, in the bronchial brush biopsies. Thus, our study suggests that viral stimulation causes the production of IL-26 in lining epithelial cells of human airways, structural cells that constitute a critical immune barrier and that this production is modulated by Th17 cytokines.
INTRODUCTION
Interleukin (IL)-26 is a member of the IL-10 cytokine family that may be coproduced with archetype T-helper (Th) 17 cytokines including IL-17A and IL-22 (1–3). Although IL-26 was discovered more than a decade ago (4), surprisingly few studies have addressed its involvement in human immunology and pathology. So far, it has been shown that IL-26 is involved in Crohn disease (5), rheumatoid arthritis (6) and chronic hepatitis C virus (HCV) infections (7), disorders that all share chronic inflammation as a common denominator. Moreover, the synovial cell production of IL-26 induces bone mineralization in joint tissue from patients with the chronic inflammatory disease spondyloarthritis (8).
In a recent in vivo study (9), we forwarded evidence that endogenous IL-26 is abundant and involved in the local immune response against a bacterial stimulus, a toll-like receptor (TLR) 4 agonist, in healthy human airways. We also demonstrated that IL-26 enhances the chemotactic response of neutrophils toward a bacterial stimulus and toward IL-8, while at the same time inhibiting the chemokinesis in these innate effector cells (9–11). Moreover, we demonstrated that alveolar macrophages constitute a prominent source of IL-26, in addition to Th17 cells and other lymphocyte subsets. Based upon these findings, we proposed that IL-26 serves to focus neutrophil mobilization toward sites of bacterial infection and inflammation during activation of pulmonary host defense. In line with our proposal, Meller et al. recently forwarded evidence that IL-26 kills extracellular bacteria through pore formations and proposed that IL-26 encompasses characteristics typical for antimicrobial peptides (12).
In addition to their evidence for antibacterial effects, Meller et al. showed that IL-26 triggers the production of the antiviral cytokine interferon (IFN)-α (12,13) in plasmacytoid dendritic cells via activation of TLR9, by forming complexes with DNA from cells or from dying bacteria (12). Furthermore, the previous study by Miot et al. also showed that IL-26 induces the production of the antiviral cytokines IFN-β and IFN-γ in NK cells in association with chronic HCV infections (7). Thus, IL-26 bears the potential to be involved in immune responses to both bacterial and viral stimulation.
Given the abundance of IL-26 protein in the human airway lumen and its referred involvement in the immune response to bacterial (9,10) and viral stimulation (7,12,13), we hypothesized that IL-26 is involved in pulmonary host defense against viruses by being produced in bronchial epithelial cells after stimulation with a viral stimulus and that this production is modulated by additional Th17 cytokines. We also hypothesized that the intracellular signaling pathways leading to IL-26 release involves mitogen-activated protein (MAP) kinases and the nuclear transcription factor NF-κB. To address these matters, we characterized the expression, production and release of IL-26 in primary bronchial epithelial cells from humans in response to polyinosine–polycytidylic acid (poly-IC), a TLR3-agonist mimicking viral double stranded (ds) RNA (14). We also characterized the involvement of the MAP kinases p38, c-Jun N-terminal kinases (JNK1–3) and extracellular signal–regulated kinases (ERK1/2) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in the downstream signaling events. Furthermore, we characterized the modulatory effects of the Th17 cytokines IL-17A and IL-22 on the release of IL-26 caused by poly-IC. Finally, we assessed IL-26 and its archetype signaling responses in bronchial epithelial cells in vivo, using bronchoalveolar lavage (BAL), bronchial brush and tissue biopsy samples from healthy human subjects.
MATERIALS AND METHODS
Cell Culture and Stimulation
Primary bronchial epithelial cells were harvested from human donors and expanded as previously described (15). Semiconfluent cells were stimulated (1 mL of culture media) with different concentrations of the TLR3 agonist poly-IC, as well as additional types of viral stimulation including the TLR7 agonist Imiquimod, and the TLR8 agonist single stranded (ss) RNA (Invivogen®) (14,16) as well as recombinant human (rh) Th17 cytokine proteins including rhIL-17A and rhIL-22 (R&D systems). Briefly, cells were stimulated with poly-IC (1, 5 and 10 μg/mL) or Imiquimod (1 μg/mL) or single stranded (ss) RNA (1 μg/mL). Cells were also stimulated with rhIL-17A (100 ng/mL) or rhIL-22 (100 ng/mL) or rhIL-17A (100 ng/mL) plus rhIL-22 (100 ng/mL) with and without poly-IC (0.05 μg/mL). Cell-free conditioned media were collected after 24 h of incubation (37°C in 5% CO2) for quantification of IL-26 protein using enzyme-linked immunosorbent assay (ELISA) and the cells were separated for real-time polymerase chain reaction (RT-PCR) assay. For the investigation of intracellular signaling mechanisms, the cells were preincubated with a specific TRIF inhibitor (25 μmol/L) or its vehicle in 0.5 mL culture media for TRIF (TIR [Toll/interleukin-1 receptor domain-containing adaptor protein inducing interferon beta, from Invivogen]) during 5 h or in 1 mL of culture media for a variety of MAP kinase inhibitors and NF-κB (all from Selleckchem.com) and their respective vehicles. The MAP kinase inhibitors utilized targeted either p38 (SB203580—0.1, 1 and 10 μmol/L during 1 h) (17), JNK1–3 (SP600125—8 × 10−3, 8 × 10−2 and 0.8 μmol/L during 1 h) (18) or ERK1/2 (AZD6244—2 × 10−3, 2 × 10−2 and 0.2 μmol/L during 1 h) (19). Cells were also preincubated with an inhibitor targeting NF-κB (SC17741—1, 5 and 10 μmol/L during 5 h) and its vehicle (20). After the preincubations with the respective inhibitors, cells were then cocultured with poly-IC (0.05 μg/mL) during 24 h and cell-free conditioned media collected and frozen for quantification of IL-26 protein concentrations using ELISA and the cells were used for RT-PCR assay. For the quantification of activated/phosphorylated (p) p38, pJNK1–3 and pERK1/2 and pNF-κB, cells were stimulated with poly-IC (0.05 and 0.5 μg/mL during 1.5 h) before or after preincubation with the TRIF inhibitor or its vehicle (25 μmol/L during 5 h). Adherent cells were then lysed on the plate using the PhosphoTracer lysis buffer and lysate used during the PhosphoTracer ELISA assays.
PhosphoTracer ELISA
Intracellular activation/phosphorylation of p38, JNK1–3, ERK1/2 and NF-κB was measured according to manufacturer’s instructions (Abcam®) (9). In brief, cell lysates were transferred in duplicates (50 μL/well) to the plates. The specific antibody mixes for each analyte were added (50 μL/well, during 1 h) at room temperature (RT) on a shaker and washed. The substrate was then added (100 μL/well during 10 min [min]), and the fluorescence signal determined (530 nm) using Wallac victor 3V 1420 multilabel plate reader (PerkinElmer LAS Ltd). The data (raw signals) were presented as relative fluorescence units (RFU).
IL-26 and IL-17A Protein ELISA
IL-26 protein concentrations in cell-free conditioned media and BAL fluid were measured according to manufacturer’s instructions (Cusabio Biotech®) (9). In brief, diluted samples and standards were added to the wells in duplicates and incubated (during 2 h) at 37°C in 5% CO2. Biotin-conjugated detection antibody was added (during 1 h) followed by the avidin-conjugated Horseradish-Peroxidase added (during 1 h). Plates were developed using tetramethylbenzidine (TMB) substrate and finally, a stop solution was added to quench the reaction. The optical density was measured (450 nm) using a microplate reader (Model Spectra Max 250, Molecular DevicesTM).
Similarly, IL-17A protein was measured according to the manufacturer’s instructions (MABTECH AB). In brief, high protein binding ELISA plates (Nunc) were precoated with the primary anti-human IL-17A antibody (2 μg/mL in phosphate-buffered saline (PBS)) followed by the secondary antibody (biotinylated monoclonal antibody, 0.5 μg/mL during 1 h at RT). Plates were subsequently incubated (during 1 h at RT) with streptavidin horseradish peroxidase and developed with TMB substrate and finally, a stop solution was added to quench the reaction. The optical density was measured (450 nm) using a micro plate reader.
Intracellular IL-26 Protein Detection Using Western Blot
Primary bronchial epithelial cells were stimulated with poly-IC (1 ug/mL) or rhIL-17A (100 ng/mL). The epithelial cells were also stimulated with poly-IC (1 ug/mL) with or without the pathway inhibitors of TRIF, p38, JNK, ERK and NF-κB (see above) during 24 h. Cells were then treated with ice-cold lysis buffer containing a proteinase and phosphatase inhibitor cocktail as well as ethylenediaminetetraacetic acid (EDTA) (Thermo Scientific) and the total protein concentration quantified using the Bradford assay Comasie kit (Thermo Scientific). After electrophoresis (10% mini-protein precast gels [Biorad]) and transfer (semi dry transfer at 20V for 30 min), the membranes (Immobilon® PVDF, Sigma Aldrich) were air dried (1 h at RT) and then blocked with Odyssey blocking buffer (Li-Cor™) for another 1 h at RT. The primary IL-26 antibody (at 1:200 dilution) (Abcam) and β-actin (at 1:2000 dilution) (Santa Cruz Biotechnology Inc.™) were then added and incubated overnight (at 4°C). The membranes were then washed (3×) and the Odyssey infrared dye-conjugated secondary antibody (Li-Cor) added (1:15000) for 1 h at RT. Finally, the membranes were washed (3×) and scanned using the Odyssey® CLx Imaging System (Li-Cor).
RT-PCR Analyses
The primer sequences were obtained from the literature (9,21), and purchased from Cybergene. RT-PCR was performed as previously described (9). Data was normalized (controls were set to 1) with reference to the house keeping gene (HPRT) according to the CT method (ΔΔ CT) and presented as fold differences.
Bronchoscopy and Collection of Bronchial Biopsies, Bronchoalveolar Lavage Fluid and Bronchial Brush Biopsies
Healthy never-smokers with normal lung function (post-bronchodilator forced expiratory volume (FEV)1/ forced vital capacity (FVC) >0.7 and FEV1 >80% of predicted) were recruited and investigated after oral and written informed consent, in accordance with the Helsinki declaration. Thus, data and samples from 37 control subjects in the Karolinska Institutet COSMIC cohort (ClinicalTrials.gov Identifier: NCT02627872) were utilized and some of the clinical data sets have been utilized in other studies (22,23). The COSMIC cohort study was previously approved by the Regional committee for ethical review in Stockholm, Sweden (Diary No 2006/959-31/1). Briefly, spirometry (Jaeger Masterscope PC, CareFusion) was measured according to European respiratory society (ERS)/American thoracic society (ATS) guidelines (24). Bronchoscopies were performed under local anesthesia as previously described (25) utilizing a flexible video bronchoscope (Olympus Optical Co). The specimens (BAL, bronchial brush and tissue biopsy samples) were then harvested for subsequent analyses.
Immunoreactivity for IL-26 Protein in Tissue Biopsy Specimens
Here, bronchial tissue biopsy specimens from 8 (4 male and 4 female) subjects were randomly selected from the pool of the COSMIC cohort (22,23). The tissue biopsies were then processed and stained as previously described (26) using the primary monoclonal mouse anti-human IL-26 antibody or mouse IgG2b isotype control (Clone 133303, R&D) accordingly (9). In brief, tissue blocks were formalin-fixed, paraffin embedded, deparaffinized and rehydrated through graded alcohols. Endogenous peroxidase was inactivated and tissues incubated with primary monoclonal mouse anti-human IL-26 antibody or mouse IgG2b isotype control (during 3 min). Slides were then rinsed in distilled water, counterstain in Hematoxylin, rinsed in running tap water, dehydrated, cleared and mounted. The average area of the sections was 1–2 mm2, and the whole tissue section was analyzed by immunohistochemistry (IHC).
mRNA Analyses on Epithelial Cells in Bronchial Brush Biopsies and IL-26 Protein Measurement in BAL Fluid
Here, 10 randomly selected bronchial brush biopsy samples from the human (4 male and 6 female) donors were selected from the pool of the COSMIC cohort (22,23). The analyses of mRNA were then performed on the cells in the bronchial brush biopsy samples as previously described (27). Notably, we ensured that epithelial cells constitute more than 90%–95% of the total cells in the bronchial brush biopsies samples before inclusion. In brief, mRNA was extracted according to the manufacturer’s instructions (Macherey–Nagel). The RNA was amplified using the Low Input Quick Amplification Kit (Agilent Technologies) according to the manufacturer’s protocol, and subsequent Cy3-CTP labeling was performed by using one-color labeling kits (Agilent Technologies). Clean-up of the labeled and amplified probe was performed (Zymo Research Corporation). The size distribution and quantity of the amplified product was assessed by Nanodrop. Equal amounts of Cy3-labeled target were hybridized to Agilent human whole-genome 4 × 44K Inkjet arrays containing a total of 41,000 probes corresponding to 19,596 entrez genes. Hybridizations were performed at 65°C (at a rotation of 10 rpm during 17 h). Arrays were scanned by using the Agilent microarray G2565BA scanner (Agilent Technologies) with Scan region: Agilent HD (61 × 21.6) and a resolution of 5 μm, TIFF: 16 bit, XDR: 0.10. Raw signal intensities were extracted with Feature Extraction v10.1 software (Agilent Technologies). Outliers that were identified by the software were not included in any subsequent analyses. We also measured (by ELISA) concentrations of IL-26 protein in the cell-free BAL samples harvested during bronchoscopy as described above and previously (9).
Statistical Analysis
Parametric descriptive and analytical statistics were applied (GraphPad Prism® software) unless otherwise stated. The Student two-tailed and paired t test was utilized to compute comparisons between paired data sets unless otherwise stated. The Mann–Whitney test was used to compute differences between the human samples. Correlation analyses were performed using the Spearman rank correlation test. p < 0.05 were considered statistically significant.
RESULTS
Primary Bronchial Epithelial Cells Produce IL-26 Protein in Response to TLR3 Stimulation
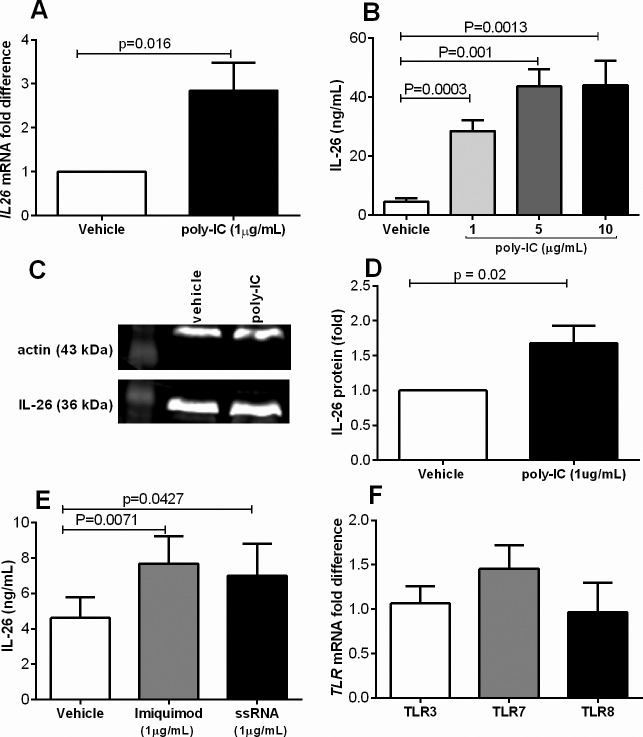
The primary bronchial epithelial cells were exposed (24 h) to viral stimulation and we found that these cells contain the IL26 mRNA that was increased approximately 3 fold after stimulation with poly-IC (1μg/mL) (Figure 1A). Furthermore, we found that increasing concentrations of poly-IC caused a corresponding increase in IL-26 protein release in the cell-free conditioned media (Figure 1B). Using western blot, we found that intracellular IL-26 protein was also increased in response to poly-IC (1 ug/mL) (Figure 1C, D). Notably, we found that only the dimeric form of IL-26 (36kDa) was detectable in the bronchial epithelial cells. Similar to the viral stimulus poly-IC, stimulation with other viral stimuli, the TLR7 agonist Imiquimod (1 μg/mL) and the TLR8 agonist ssRNA (1 μg/mL) (16), also increased IL-26 protein concentrations in the conditioned media (Figure 1E). Moreover, we also verified that the bronchial epithelial cells contain mRNA for TLR3, TLR7 and TLR8 (Figure 1F), here presented as fold differences, with a similar magnitude of transcription.
Figure 1.
Primary bronchial epithelial cells produce IL-26 enhanced by viral-related stimuli. Cells were stimulated (24 h) with different viral stimuli (TLR3 agonist poly-IC, TLR7 agonist imiquimod and TLR8 agonist ssRNA). Extracellular concentrations in cell-free conditioned media as well as intracellular expression of IL-26 protein were measured using ELISA and western blot, respectively, and levels of mRNA using real time. (A) IL26 mRNA levels after stimulation with poly-IC (n = 11). (B) Extracellular concentrations of IL-26 in cell-free conditioned media in response to poly-IC at different concentrations (n = 8). (C) Intracellular IL-26 protein (representative western blot). (D) the average protein expression (fold difference) after stimulation with poly-IC (1ug/mL) during 24 h. (E) Extracellular concentrations of IL-26 in cell-free conditioned media in response to imiquimod or ssRNA (n = 8). (F) TLR3, TLR7 and TLR8 mRNA levels (fold) (n = 5). The p values indicated are according to the Student paired t test. p < 0.05 is considered significant.
The Release of IL-26 Protein in Response to Poly-IC Involves TRIF, MAP Kinases and NF-κB
The adaptor protein TRIF and the MAP kinases p38, JNK1–3 and ERK1/2 and NF-κB are generic molecules involved in signal transduction downstream of TLR3 (28–32). Given the lack of specific knowledge for IL-26 release in this respect, we determined the involvement of these signaling molecules in the release of IL-26. First, TRIF was inhibited (25 μmol/L) in cultures in the presence of a suboptimal concentration of poly-IC (0.05μg/mL) to render any TRIF-inhibitory effect noticeable. We found that IL-26 release was almost completely blocked by the TRIF inhibitor (Figure 2A). Notably, given that an unaltered number of cells were stimulated in half the volume of culture media (0.5 mL), the IL-26 concentrations became higher. We then investigated the intracellular levels of IL-26 at the gene and protein level using RT-PCR and western blot respectively. To induce an optimal mRNA level and protein expression, cells were stimulated with 1 ug/mL poly-IC. We then found that inhibition of TRIF did not alter the IL26 mRNA (Figure 2B) nor did it alter the intracellular protein levels (Figure 2C, D).
Figure 2.
The adaptor protein TRIF is involved in poly-IC-induced release of IL-26. Primary bronchial epithelial cells were preincubated (5 h) with TRIF inhibitor and vehicle (25 μmol/L). Extracellular concentrations of IL-26 in cell-free conditioned media as well as intracellular levels were measured using ELISA and western blot respectively and mRNA levels using real time. (A) Extracellular concentrations of IL-26 in cell-free conditioned in response to poly-IC (0.05 μg/mL) in the presence of the TRIF inhibitor (n = 7). (B) Level of IL26 mRNA in response to poly-IC (1μg/mL) in the presence of the TRIF inhibitor (n = 8). (C) Intracellular IL-26 protein (representative western blot) and (D) the average protein expression (fold difference) in response to poly-IC (1 μg/mL) in the presence of the TRIF inhibitor (n = 4).
Second, we investigated the involvement of p38, JNK1–3 and ERK1/2 and NF-κB by measuring their activation (phosphorylation) levels in response to poly-IC (0.05 μg/mL and 0.5 μg/mL). We also investigated whether this phosphorylation was TRIF-dependent or not and we found that poly-IC at both concentrations induced rapid phosphorylation of p38, JNK1–3, ERK1/2 and NF-κB (Figure 3A–D), which is compatible with increased levels of IL-26 protein in the conditioned media (Figure 1B). Interestingly, inhibition of TRIF also inhibited the phosphorylation of these molecules (Figure 3A, B, D) except for ERK1/2 (Figure 3C), which is also compatible with decreased level of IL-26 (Figure 2A). We noted, however, that the effect on pERK1/2 induced by poly-IC was modest, even though statistically significant. Moreover, we compared inherent levels of these phosphorylated molecules and found that pERK1/2 displayed a higher levels compared with pNF-κB, pp38 and pJNK1–3 (Figure 3E).
Figure 3.

Stimulation with poly-IC induces phosphorylation of MAP kinases and NF-κB and is inhibited by TRIF. Cells were preincubated with the TRIF inhibitor (ihh-TRIF) or vehicle (V-TRIF) (25 μmol/L for 5 h) and/or stimulated with poly-IC (0.05 μg/mL or 0.5 μg/mL) for another 1.5 h. Adherent cells were then lysed and the lysate used to measure phosphorylated levels of p38, JNK1–3, ERK1/2 and NF-κB by phosphorTracer ELISA. Panels (n = 8) show relative fluorescence units (RFU) of (A) Phosphorylated (p) p38, (B) pJNK1–3, (C) pERK1–3, (D) pNF-κB and (E) comparative levels for the different molecules. The p values indicated are according to Student paired t test and a p < 0.05 is considered significant.
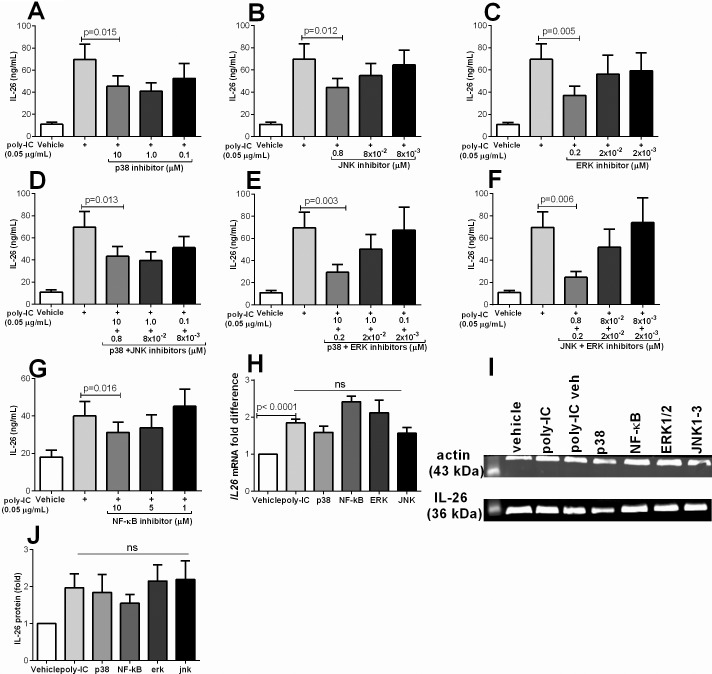
Selective Inhibition of p38, JNK1–3, ERK1/2 and NF-κB Inhibits IL-26 Protein Release
Here, we inhibited the signaling molecules (p38, JNK1–3 and ERK1/2) using specific inhibitors (17–20). We found that inhibition of p38, JNK1–3 and ERK1/2 inhibited the poly-IC-induced release of IL-26 protein (Figure 4A–C). Furthermore, we also found clear cut inhibition of IL-26 release after double inhibition of p38 and JNK1–3, p38 and ERK1/2 or JNK1–3 and ERK1/2 (Figure 4D–F). Moreover, inhibition of NF-κB also inhibited the release of IL-26 protein (Figure 4G). Notably, we found no difference in IL-26 release in cells stimulated with poly-IC and poly-IC plus the relevant vehicle dimethyl sulfoxide (DMSO) (data not shown). We then investigated the intracellular levels of IL-26 at the gene and protein level using RT-PCR and western blot, respectively. We found that inhibition of these MAP kinases or NF-κB did not alter the IL26 mRNA (Figure 4H) and the intracellular protein levels (Figure 4I, J).
Figure 4.
Inhibition of p38, JNK1–3, ERK1/2 and NF-κB attenuates poly-IC induced release of IL-26. Primary bronchial epithelial cells were preincubated (1 h) with MAP kinase inhibitors for p38 (SB203580), JNK1–3 (SP600125), ERK1/2 (AZD6244) as well as combined (p38 plus ERK1/2, p38 plus JNK1–3 and JNK1–3 plus ERK1/2). Cells were preincubated for 5 h with the NF-κB inhibitor (SC17741). Cells were then stimulated with poly-IC (0.05 μg/mL) for another 24 h. Figure panels show the different concentrations of each inhibitor used during poly-IC stimulation. Extracellular concentrations of IL-26 in cell-free conditioned media as well as intracellular levels were measured using ELISA and western blot respectively and mRNA expression by real time. Panels show IL-26 concentrations after the inhibition of (A) p38, (B) JNK1–3, (C) ERK1/2, (D) p38 plus JNK1–3, (E) p38 plus ERK1/2 and (F) JNK1–3 plus ERK1/2 and (G) NF-κB (n = 7 for all panels). (C) Intracellular IL-26 (representative western blot) and (D) the average protein levels (fold difference) in response to poly-IC (1 μg/mL) in the presence of the MAP kinase or NF-κB inhibitors (n = 5). The p values indicated are according to Student paired t test and p < 0.05 is considered significant.
The Release of IL-26 in Response to Poly-IC Is Modulated by Th17 Cytokines
We have shown previously that rhIL-17 is a potent inducer of cytokine responses in transformed human bronchial epithelial cells (33). Here, we investigated the release of IL-26 protein in primary bronchial epithelial cells in response to stimulation with rhIL-22, rhIL-17A and rhIL-22 plus rhIL-17A as well as their costimulations with poly-IC. Thus, the primary bronchial epithelial cells were stimulated with rhIL-17A and/or rhIL-22 (100 ng/mL). In doing so, we found that rhIL-17A increased IL-26 protein concentration (Figure 5A) as well as the level of IL26 mRNA (Figure 5B). However, rhIL-22 did not alter IL-26 concentrations in the conditioned media nor induce any synergistic effects with rhIL-17A (Figure 5A). When the bronchial epithelial cells were then costimulated with poly-IC (0.05μg/mL) and rhIL-17A, we found a synergistic induction of IL-26 release (Figure 5C), but this was not the case for costimulation with rhIL-22 and poly-IC (Figure 5D). Moreover, when cells stimulated with poly-IC were also costimulated with both rhIL-17A and rhIL-22, we found that rhIL-22 induced an additive effect on top of the synergistic effects of poly-IC and rhIL-17A (Figure 5E). Notably, when we tried to quantify the concentrations of IL-17A protein in the cell-free conditioned media after stimulation with poly-IC or rhIL-22 stimulation, we did not detect this cytokine (34) (data not shown). To further confirm the effect of rhIL-17A on the release of IL-26, we investigated the intracellular protein levels of IL-26 using western blot. We then found that rhIL-17A increased the intracellular levels of IL-26 protein (Figure 5E, F).
Figure 5.
Th17 cytokines modulate poly-IC induced release of IL-26. Primary bronchial epithelial cells were stimulated with poly-IC (0.05 μg/mL) in the presence or absence of rhIL-17A (100 ng/mL) or rhIL-22 (100 ng/mL) or rhIL-17A (100 ng/mL) plus rhIL-22 (100 ng/mL) (24 h). Extracellular concentrations of IL-26 in cell-free conditioned media as well as intracellular levels were measured using ELISA and western blot respectively and mRNA level by real time PCR. (A) Extracellular concentrations of IL-26 in cell-free conditioned media in response to rhIL-17A and/or rhIL-22 (n = 14). (B) IL26 mRNA level in response to rhIL-17A (n = 9). (C) Extracellular concentrations of IL-26 in cell-free conditioned media in response to poly-IC plus rhIL-17A (n = 6). (D) Extracellular concentrations of IL-26 in cell-free conditioned media in response to poly-IC plus IL-22 (n = 6). (E) Extracellular concentrations of IL-26 in cell-free conditioned media in response to poly-IC plus rhIL-17A plus rhIL-22. (n = 6). (F) Intracellular IL-26 protein (representative western blot) and (G) the average protein level (fold difference) in response to IL-17A during 24 h (n = 10). The p values indicated are cording to the Student paired t test. p < 0.05 is considered significant
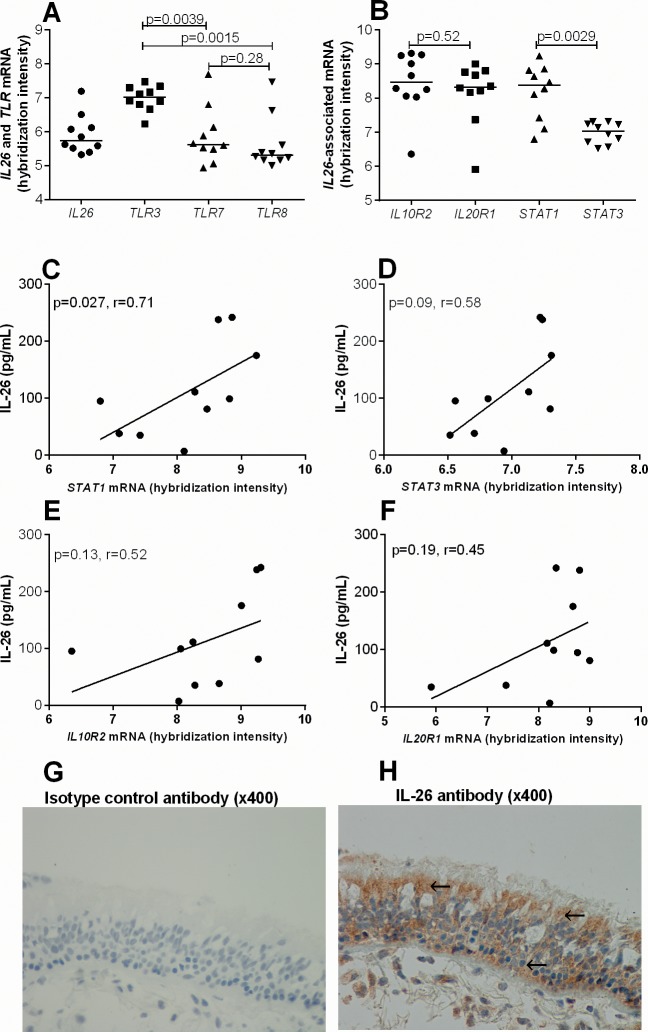
Interleukin-26 and Its Archetype Signaling Responses in Bronchial Epithelial Cells In Vivo
We also investigated the gene expression profile of bronchial epithelial cells in bronchial brush biopsies harvested from healthy subjects by selecting data of interest from a broad mRNA analyses of the COSMIC cohort material (22,23). Here, we found that these bronchial epithelial cells contain mRNA for IL26, TLR3, TLR7 and TLR8 among others (Figure 6A), which are compatible with our finding on primary bronchial epithelial cells in vitro. The level of TLR3 was significantly higher compared with TLR7 and TLR8 (Figure 6A). Still, with reference to IL-26, we found that these cells also contained mRNA for the IL-26 receptor complex IL10R2 and IL20R1 as well as the specific intracellular signaling molecules downstream of the IL-26-receptor-complex STAT1 and STAT3 (Figure 6B), in line with our previous study in vitro (9). Notably, there was no clear difference in the levels of IL10R2 and IL20R1 but the level of STAT1 was higher compared with STAT3. Given that we also observed increased levels of IL10R, IL20R1, STAT1 and STAT3 mRNA after stimulation with rhIL-26 in our previously published study in vitro (9), we here evaluated the corresponding phenomenon in vivo. First, we quantified the concentrations of IL-26 protein in cell-free BAL (67 pg/mL [7–238 pg/mL]) harvested in vivo and correlated these concentrations with the level of the respective mRNAs within the same subject. We then found a positive correlation between IL-26 protein and STAT1 (Figure 6C) and a strong corresponding trend for STAT3 (Figure 6F). However, although there was no correlation between IL-26 protein and IL10R2 as well as IL20R1 mRNA, we did observe weak positive trends between these mRNAs (IL10R2 and IL20R1) and the IL-26 protein (Figure 6D, E). Finally, we confirmed IL-26 protein expression in lining epithelial cells within bronchial tissue biopsies of healthy human subjects using IHC (Figure 6G, H). We detected clear-cut inherent expression of the IL-26 protein both in the granulae and cytoplasm (Figure 6G, H).
Figure 6.
IL-26 in bronchial brush biopsies and bronchial tissue biopsies. The gene expression profile of epithelial cells in bronchial brush biopsies was determined by mRNA analyses. (A) IL26 and TLR3, TLR7 and TLR8 mRNA levels (n = 10). (B) The mRNA level for the IL-26 associated genes IL26, IL10R2, IL20R1, STAT1 and STAT3 (n = 10). (C–F) Correlation between BAL IL-26 protein (67pg/mL [7–238pg/mL]) and mRNA levels for STAT1, STAT3, IL10R2 and IL20R1 respectively (n = 10). Cellular IL-26 protein expression in bronchial tissue biopsies was determined using IHC. (G) Monoclonal IgG2b isotype control antibody and (H) positive staining (brown) with monoclonal specific IL-26 antibody. Arrows show granular and cytoplasmic IL-26 expression. Each panel show a representative staining out of 8 subjects at a magnification of 400×. The p values indicated are according to the Mann–Whitney test (A and B) and Spearman correlation test (C–F) and p < 0.05 is considered significant.
DISCUSSION
It is known that lining epithelial cells in the airways direct viral tropism more than any other cell type in the lungs and, thereby, these cells constitute a critical immune barrier (35). Indeed, viral infections constitute a major risk factor for exacerbations during chronic inflammatory lung disorders including chronic obstructive pulmonary disease (COPD) and severe asthma (36,37). In the current study, we show for the first time that bronchial epithelial cells release IL-26 protein in response to several types of viral stimulation. Primarily, we examined the epithelial cells in response to poly-IC, a TLR3 agonist synonymous to viral dsRNA, and found increased production and release of IL-26 protein. We also obtained evidence that the intracellular signaling molecules TRIF, MAPK and NF-κB are involved in the TLR3-induced release of IL-26 protein, whereas these signaling molecules are not involved in the transcription and translation of IL-26 within the cell. Furthermore, we identified modulatory effects of Th17 cytokines including the original observation that rhIL-17A triggers the production of IL-26 in bronchial epithelial cells and also induces a synergistic release on the effect of poly-IC. We also observed that rhIL-22 causes an additive effect on top of the synergy between rhIL-17A and poly-IC. In addition, we obtained evidence for IL-26 protein interacting with bronchial epithelial cells, as we found positive associations with IL-26 and its receptors and signaling molecules in vivo.
The primary bronchial epithelial cells utilized in our current study constitute a relevant model of lining epithelial cells in the airways, which in turn represent both a structural and immune barrier to inhaled contents of the airway lumen. Our findings that bronchial epithelial cells contain mRNA transcripts for the TLRs (TLR3, 7 and 8) and that triggering via TLRs yields release of IL-26 are in line with descriptions of the presence TLRs on lining epithelial cells in the airways (38). Furthermore, we showed that bronchial epithelial cells harvested from bronchial brush biopsies in vivo also contain mRNA transcripts for these receptors alongside mRNA for the IL-26 receptor sub-units IL10R2 and IL20R1 and the downstream intracellular signaling molecules STAT1 and STAT3. We show that these immune barrier cells release IL-26 in response to not only the TLR3 agonist poly-IC but also to the TLR7 agonist imiquimod and the TLR8 agonist ssRNA. Given that RNA viruses commonly cause infections in healthy subjects as well as in patients with chronic inflammatory lung disorders (35), the release of IL-26 in response to the tested viral stimuli is a strong indication of its involvement in pulmonary host defense against viral infections in the airways. This is particularly true given that IL-26 stimulates the production of antiviral cytokines including IFN-β and IFN-γ in NK cells during chronic HCV infections (7,13), as well as IFN-α in plasmacytoid dendritic cells in complex with DNA from dying bacteria and cells in vitro (12,13). Using IHC, we found a clear-cut expression of IL-26 protein in granulae and the cytoplasm of epithelial cells in bronchial tissue biopsy samples from healthy human subjects (with normal CRP levels). However, we cannot rule out that these subjects and samples have been exposed to viral stimuli without being clinically infected in vivo; even if we have previously shown that inherent IL-26 is abundantly expressed in BAL fluid as well as BAL cells from healthy human subjects (9). Further studies will be required to evaluate the possibility that viral stimuli contribute to the inherent expression of IL-26 in human airways in vivo.
We identified a positive association between the concentrations of IL-26 protein and the levels of STAT1 and a strong corresponding trend with the levels of STAT3 in the epithelial cells in vivo. We think that the facts that IL-26 signals through STAT1 and STAT3 (39) and that the matching mRNAs are increased in responses to rhIL-26 in vitro (9) make our current demonstration of a corresponding association in vivo of particular interest and relevance.
We characterized the signal transduction events after stimulation with poly-IC by examining TRIF, the MAP kinases p38, JNK1–3 and ERK1/2 as well as the nuclear transcription factor NF-κB (28,29,32). In doing so, we proved that the referred molecules are involved in the release of IL-26 protein in bronchial epithelial cells. Indeed, this involvement of generic signaling molecules in the specific release of IL-26 is not only novel—it is also compelling given that these are archetype signaling pathways known to mediate a plethora of immune regulatory events in humans (40–44). Here, the release mechanism involved the rapid increase in the activation of the referred signaling molecules within 1.5 h of stimulation, which was matched by an increased release of IL-26 protein. We also found that this signal activation and release mechanism of IL-26 that is caused by MAP kinases is controlled by the adaptor protein TRIF (located upstream) (28,29). Although the poly-IC-induced activation of ERK1/2 was visibly small, we nonetheless demonstrated that the inherent phosphorylated level of this molecule is very high compared with phosphorylated (p) p38, pJNK1–3 and pNF-κB. Furthermore, the selective inhibition of ERK1/2 suppressed the release of IL-26 in the same magnitude as p38, JNK1–3 and NF-κB. We believe that these mechanistic findings may prove important in human pathologies fueled by the same type of intracellular signaling. Notwithstanding the intracellular signaling events, the TRIF, MAP kinases and NF-κB pathways were not directly involved in the transcription and translation of IL-26 within the epithelial cell per se, given that the level of IL26 mRNA and the intracellular protein levels were not altered after inhibition of these pathways. Here, it can be considered a well-known fact that transcript abundances only partially reflect protein abundances and vice versa (45). Thus, we think that cytokines restricted within the intracellular compartments are unlikely to serve a functional purpose until they are released into the extracellular space where they can bind to receptors and stimulate targets. We therefore think that the respective involvement of TRIF, MAP kinases and NF-κB in the release mechanism of this cytokine is crucial for the effector functions of this cytokine in response to TLR3 stimulation by viral particles.
We also obtained clear-cut evidence that the archetype Th17 cytokine IL-17A induces the production (both transcription and translation) of IL-26 in bronchial epithelial cells, as well as a synergistic effect on top of the poly-IC-induced release of IL-26. It is known that IL-17A acts through the IL-17 receptor complex (IL-17RA and IL-17RC) (46) and in a previous study, we showed that rhIL-17A induces cytokine release in transformed human bronchial epithelial cells via MAP kinases (33). Thus, it can be speculated that the rhIL-17A-induced release of IL-26 in primary bronchial epithelial cells occurs via MAP kinases and, therefore, their simultaneous stimulation by poly-IC and rhIL-17A may contribute to the observed synergistic effects. In addition, we observed that rhIL-22 alone has neither an effect on the release of IL-26 protein nor a synergistic effect on top of rhIL-17A or poly-IC. In contrast, we found that rhIL-22 causes a substantial additive effect on top of the rhIL-17A-poly-IC synergy. This particular finding may thus be receptor-dependent whereby the presence of poly-IC and rhIL-17A enhances receptor expression or increase downstream signaling leading to increased IL-26 induction. Thus, our finding supports the idea that, in situ, the two Th17 cytokines IL-22 and IL-17A may act together to boost antiviral immune responses by inducing the production of antimicrobial peptides in epithelial cells (47,48), but also by stimulating the production of IL-26 in epithelial cells. We have previously shown that rhIL-26 itself acts on primary bronchial epithelial cells to inhibit cytokine release (9). It is therefore possible that a dsRNA- or IL-17A-induced release of IL-26 (in vivo) in turn exerts autocrine effects on the same cells by inhibiting the continuous release of IL-26.
CONCLUSION
This study on primary bronchial epithelial cells, brush and tissues biopsy cells from human airways forwards the first original evidence that lining epithelial cells in human airways produce and release IL-26 in response to viral stimulation via several TLRs. This release of IL-26 is the result of “classic” intracellular signaling, involving TRIF, MAP kinases and NF-κB. Notably, the archetype Th17 cytokine IL-17A is also a prominent inducer of IL-26 production per se and on top of poly-IC stimulation, the latter being further enhanced by IL-22, yet another Th17 cytokine. Given the fact that IL-26 causes the release of antiviral cytokines (IFN) (7,12,13) and is also produced by alveolar macrophages in response to a bacteria stimulus (9), the current demonstration of a viral-related response in bronchial epithelial cells suggests that IL-26 is actually produced by two critical immune barrier cells in the airways. In this way, IL-26 may contribute to pulmonary host defenses against pathogens. Tentatively, IL-26 may be a more generic and important mediator in pulmonary host defense than previously recognized, one that also bears potential for the development of novel therapy.
ACKNOWLEDGMENTS
We thank Jie Ji, M.Sc., Institute of Environmental Medicine, Karolinska Institutet, for assisting with primary bronchial epithelial cells as well as Max Vikström, Unit for Cardiovascular Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, for assisting with the statistics. We also thank Emma Åkerlund, Unit for Biochemical Toxicology, Institute of Environmental Medicine, Karolinska Institutet for assistance with the western blot assay.
Project funding was obtained from the Swedish Heart-Lung Foundation (No. 20150303), the Swedish Research Council (No. 2016-01653), King Gustav V’s and Queen Victoria’s Freemason Research Foundation (ALF No. 20140309). In addition, federal funding was obtained from Karolinska Institutet and, through the Regional ALF Agreement. Project funding was also obtained from Foundation of the Finnish Anti-Tuberculosis Association. No funding was obtained from the tobacco industry.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Che KF, et al. (2017) Interleukin-26 production in human primary bronchial epithelial cells in response to viral stimulation: Modulation by TH17 cytokines. Mol. Med. 23:247–57.
REFERENCES
- 1.Donnelly RP, et al. Interleukin-26: an IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev. 2010;21:393–401. doi: 10.1016/j.cytogfr.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fickenscher H, Pirzer H. Interleukin-26. Int Immunopharmacol. 2004;4:609–13. doi: 10.1016/j.intimp.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 4.Knappe A, Hor S, Wittmann S, Fickenscher H. Induction of a novel cellular homolog of interleukin-10, AK155, by transformation of T lymphocytes with herpesvirus saimiri. J Virol. 2000;74:3881–7. doi: 10.1128/jvi.74.8.3881-3887.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dambacher J, et al. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut. 2009;58:1207–17. doi: 10.1136/gut.2007.130112. [DOI] [PubMed] [Google Scholar]
- 6.Corvaisier M, et al. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol. 2012;10:e1001395. doi: 10.1371/journal.pbio.1001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miot C, et al. IL-26 is overexpressed in chronically HCV-infected patients and enhances TRAIL-mediated cytotoxicity and interferon production by human NK cells. Gut. 2015;64:1466–1475. doi: 10.1136/gutjnl-2013-306604. [DOI] [PubMed] [Google Scholar]
- 8.Heftdal LD, et al. Synovial cell production of IL-26 induces bone mineralization in spondyloarthritis. J Mol Med. 2017;95:779–87. doi: 10.1007/s00109-017-1528-2. [DOI] [PubMed] [Google Scholar]
- 9.Che KF, et al. Interleukin-26 in antibacterial host defense of human lungs. Effects on neutrophil mobilization. Am J Respir Crit Care Med. 2014;190:1022–31. doi: 10.1164/rccm.201404-0689OC. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths KL, Khader SA. Bringing in the cavalry: IL-26 mediates neutrophil recruitment to the lungs. Am J Respir Crit Care Med. 2014;190:1079–80. doi: 10.1164/rccm.201410-1870ED. [DOI] [PubMed] [Google Scholar]
- 11.Tengvall S, Che KF, Linden A. Interleukin-26: an emerging player in host defense and inflammation. J Innate Immun. 2016;8:15–22. doi: 10.1159/000434646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meller S, et al. T(H)17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat Immunol. 2015;16:970–9. doi: 10.1038/ni.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol. 2015;15:231–42. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 14.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 15.Strandberg K, Palmberg L, Larsson K. Effect of formoterol and salmeterol on IL-6 and IL-8 release in airway epithelial cells. Respir Med. 2007;101:1132–9. doi: 10.1016/j.rmed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Jurk M, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 17.Saleh A, Shan L, Halayko AJ, Kung S, Gounni AS. Critical role for STAT3 in IL-17A-mediated CCL11 expression in human airway smooth muscle cells. J Immunol. 2009;182:3357–65. doi: 10.4049/jimmunol.0801882. [DOI] [PubMed] [Google Scholar]
- 18.Bennett BL, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase; Proc Natl Acad Sci U S A; 2001. pp. 13681–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh H, Soo KC, Chow PK, Tran E. Targeted inhibition of the extracellular signal-regulated kinase kinase pathway with AZD6244 (ARRY-142886) in the treatment of hepatocellular carcinoma. Mol Cancer Ther. 2007;6:138–46. doi: 10.1158/1535-7163.MCT-06-0436. [DOI] [PubMed] [Google Scholar]
- 20.Ehrhardt C, et al. The NF-kappaB inhibitor SC75741 efficiently blocks influenza virus propagation and confers a high barrier for development of viral resistance. Cell Microbiol. 2013;15:1198–211. doi: 10.1111/cmi.12108. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, et al. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol. 2011;226:1265–73. doi: 10.1002/jcp.22454. [DOI] [PubMed] [Google Scholar]
- 22.Forsslund H, et al. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest. 2014;145:711–22. doi: 10.1378/chest.13-0873. [DOI] [PubMed] [Google Scholar]
- 23.Kohler M, et al. Gender differences in the bronchoalveolar lavage cell proteome of patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131:743–51. doi: 10.1016/j.jaci.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Karimi R, Tornling G, Grunewald J, Eklund A, Skold CM. Cell recovery in bronchoalveolar lavage fluid in smokers is dependent on cumulative smoking history. PloS One. 2012;7:e34232. doi: 10.1371/journal.pone.0034232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karvonen HM, et al. Myofibroblast expression in airways and alveoli is affected by smoking and COPD. Respir Res. 2013;14:84. doi: 10.1186/1465-9921-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levanen B, et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131:894–903. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 29.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4:a006049. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawyer TK, et al. Protein phosphorylation and signal transduction modulation: chemistry perspectives for small-molecule drug discovery. Med Chem. 2005;1:293–319. doi: 10.2174/1573406053765486. [DOI] [PubMed] [Google Scholar]
- 31.Cai C, Chen L, Jiang X, Lu X. Modeling signal transduction from protein phosphorylation to gene expression. Cancer Inform. 2014;13:59–67. doi: 10.4137/CIN.S13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Laan M, Lotvall J, Chung KF, Linden A. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol. 2001;133:200–6. doi: 10.1038/sj.bjp.0704063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013;2:e60. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–34. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 39.Hor S, et al. The T-cell lymphokine interleukin-26 targets epithelial cells through the interleukin-20 receptor 1 and interleukin-10 receptor 2 chains. J Biol Chem. 2004;279:33343–33351. doi: 10.1074/jbc.M405000200. [DOI] [PubMed] [Google Scholar]
- 40.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Dev A, Iyer S, Razani B, Cheng G. NF-kappaB and innate immunity. Curr Top Microbiol Immunol. 2011;349:115–43. doi: 10.1007/82_2010_102. [DOI] [PubMed] [Google Scholar]
- 42.Liang Y, Zhou Y, Shen P. NF-kappaB and its regulation on the immune system. Cell Mol Immunol. 2004;1:343–50. [PubMed] [Google Scholar]
- 43.Baltimore D. NF-kappaB is 25. Nat Immunol. 2011;12:683–85. doi: 10.1038/ni.2072. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 45.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–26. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toy D, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–9. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang W, Valdez P. IL-22 in mucosal immunity. Mucosal Immunol. 2008;1:335–8. doi: 10.1038/mi.2008.26. [DOI] [PubMed] [Google Scholar]
- 48.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]