Abstract
The effects of a two-meal feeding sequence on production performance and milk lipid profile were investigated. Sixty pregnant sows (d 85 of gestation) were assigned to 3 groups: 2 C group (fed a control crude protein [CP] diet at 0600 and 1500 daily), LH group (fed a low CP diet and a high CP diet at 0600 and 1500), or HL group (fed a high CP diet and a low CP diet at 0600 and 1500). Reproductive performance of sows, and lipid profiles of plasma and milk were measured. Results showed that the HL feeding sequence dramatically increased average piglet weight/litter, average daily gain of piglet/litter, and milk production of sows. LH feeding sequence increased milk fat proportion, and HL feeding sequence significantly increased the proportion of milk MUFA on d 14 and 21 of lactation. Interestingly, the HL feeding sequence also reduced the ratio of C18:1cis/C18:1trans in milk, which may account for the greater milk production of sows and growth performance of piglets during lactation. These findings indicated that both the maternal two-meal feeding sequences with varying crude protein improved milk production and milk lipid profiles of sows, which might contribute to improving growth performance of piglets.
Introduction
Feeding sows during the transition from late gestation to lactation is important for the production of colostrum and milk, which is related to the lactation performance of sows and growth rates of piglets1. Milk is the sole source of nutrients for the neonates of most mammalian species2. As the number of nursing piglets per sows has increased in recent years, lactating sows have not provided enough milk to satisfy the needs of rapidly growing piglets3. Consequently, improving milk production and quality are important for the productivity performance of sows. Current feeding systems related to phase-feeding programs and feeds are usually formulated to optimise the performance of the total pig population, despite the fact that in such evaluations, most of the pigs receive more nutrients than they actually need for optimal growth4. Recent studies showed that nutritional outcomes were affected by the timing of food intake, even when the same type of food and the same amount of calories were consumed, as circadian clocks and energy metabolism interacted with each other5,6. Therefore, optimised feeding systems linking dietary formulation and nutritional management techniques to circadian clocks would significantly improve animal husbandry.
Previous studies have reported that feeding time affects body weight and the risk of obesity in humans7,8. Our previous study showed that a daily meal sequence with different dietary crude protein (CP) affected the metabolism and growth performance in growing pigs9, and improved muscle quality characteristics10. As one of the most important parts of milk, fat or fatty acid is important for offspring growth11. However, little is known about how a maternal two-meal feeding sequence affects the milk composition of sows and growth rates of piglets.
In this study, swine models were chosen for their physiological and gastrointestinal similarities to humans. Moreover, sows and neonatal piglets are more similar in size to human mothers and infants than other animal models, such as mice12. Our previous study demonstrated that feeding a high protein meal in the morning and a gradually reduced CP content in meals during the day affected lipid metabolism in barrows10. In addition, we found that long chain fatty acid contents in plasma and liver both exhibited diurnal rhythms in growing pigs13. Therefore, this study investigated the effects of different feeding sequences on milk production, the lipid profiles of sows, and growth performance of their offspring. We hypothesised that the maternal HL two-meal sequence with varying CP would increase the growth performance of neonatal piglets through the mother-to-newborn transfer of milk.
Results
Reproductive performance of sows
Descriptive data on the reproductive performance of sows are presented in Table 1. On average, piglet weights from the 2 C group at d 14 (P = 0.04) and d 21 (P = 0.024) were lower than those from sows in the LH or HL groups; however, there were no significant differences in piglet birth weights among the three groups (P = 0.15). Moreover, both the HL and LH feeding sequences significantly increased the average daily gain of piglets/litter during d 0–21 of lactation (P = 0.034), and by 14.22% and 10.22% during d 14–21 of lactation (P = 0.17), respectively. Additionally, the average daily gain of piglets/litter also showed an increasing trend in the HL group compared with the LH and 2 C groups (P = 0.054). However, no significant difference was observed between groups in the total number of newborn piglets/litter, stillbirth number/litter, or litter size at weaning (P > 0.10).
Table 1.
Reproductive performance of sows according to feeding sequence1,2.
| Item | Feeding sequence | SEM | P-value | ||
|---|---|---|---|---|---|
| 2 C | LH | HL | |||
| Total number of newborn piglets/litter | 13.21 | 12.58 | 12.67 | 0.376 | 0.77 |
| Stillbirth number/litter | 1.100 | 1.105 | 0.952 | 0.135 | 0.87 |
| Litter size at weaning | 11.47 | 11.16 | 11.05 | 0.340 | 0.87 |
| Average piglet’s weight (kg)/litter | |||||
| Birth weight | 1.358 | 1.454 | 1.447 | 0.022 | 0.15 |
| On d 14 | 4.031b | 4.157ab | 4.575a | 0.094 | 0.04 |
| On d 21 | 5.604b | 5.894ab | 6.376a | 0.121 | 0.024 |
| Average daily gain of piglets (kg)/litter | |||||
| d 0–14 | 0.191b | 0.194b | 0.223a | 0.006 | 0.054 |
| d 14–21 | 0.225 | 0.248 | 0.257 | 0.007 | 0.17 |
| d 0–21 | 0.202b | 0.212ab | 0.235a | 0.005 | 0.034 |
a–bValues with different letters within the same row are different (P < 0.05).
12C = fed control diet at 0600 h and 1500 h a day, respectively, LH = fed low protein diet at 0600 h and high protein diet at 1500 h a day, HL = fed high protein diet at 0600 h and low protein diet at 1500 h a day, respectively.
2n = 20.
Milk production
Based on the growth rates of piglets/litter (per sow), we found that the HL feeding sequence increased milk production per sow during d 14–21 (P = 0.049) and d 0–21 (P = 0.007) of lactation, whereas no significant difference was observed among the three groups in milk production during d 0–14 of lactation (P = 0.16) (Fig. 1).
Figure 1.
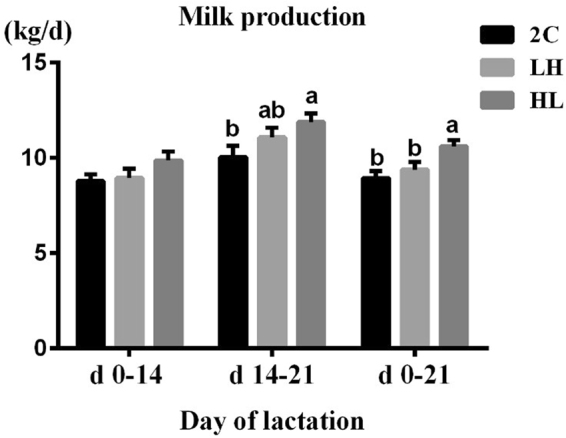
Milk production of sows according to 2 C, LH and HL feeding sequences. Values are means + SEM, and a, b were used to indicate a statistically significant difference (P < 0.05, one-way ANOVA method).
Plasma lipid profiles of sows
As shown in Table 2, the plasma H-DLC/total cholesterol (CHO) ratio in sows in the HL group was greater than that in sows in the LH and 2 C groups (P < 0.05). However, other plasma lipid parameters were not significantly affected by feeding sequences. After 14 days of lactation, plasma H-DLC (P = 0.014), L-DLC (P = 0.038), and total CHO (P = 0.031) declined in the HL group compared with those in the 2 C group. Additionally, the ratio of both H-DLC/L-DLC (P = 0.012) and H-DLC/total CHO (P = 0.003) in the LH group declined, but the HL group was not affected compared with the 2 C group (P > 0.10). Plasma triglyceride in sows was not significantly affected by feeding sequences during late gestation or lactation (P > 0.10).
Table 2.
Plasma lipid profiles in sows according to feeding sequence1,2.
| Item | Feeding sequence | SEM | P-value | ||
|---|---|---|---|---|---|
| 2 C | LH | HL | |||
| Plasma of sows during farrowing | |||||
| H-DLC (mmol/l) | 1.026 | 1.078 | 0.990 | 0.052 | 0.802 |
| L-DLC (mmol/l) | 0.611 | 0.746 | 0.590 | 0.037 | 0.169 |
| Total CHO (mmol/l) | 1.578 | 1.773 | 1.465 | 0.070 | 0.200 |
| Triglyceride (mmol/l) | 0.256 | 0.300 | 0.243 | 0.015 | 0.300 |
| H-DLC/L-DLC | 1.685 | 1.463 | 1.770 | 0.077 | 0.256 |
| H-DLC/total CHO | 0.641ab | 0.607b | 0.710a | 0.017 | 0.036 |
| L-DLC/total CHO | 0.391 | 0.417 | 0.399 | 0.011 | 0.615 |
| Plasma of sows on d 14 of lactation | |||||
| H-DLC (mmol/l) | 1.168a | 0.866b | 0.881b | 0.052 | 0.014 |
| L-DLC (mmol/l) | 1.114a | 1.068a | 0.860b | 0.045 | 0.038 |
| Total CHO (mmol/l) | 2.293a | 1.976ab | 1.695b | 0.101 | 0.031 |
| Triglyceride (mmol/l) | 0.178 | 0.224 | 0.220 | 0.014 | 0.299 |
| H-DLC/L-DLC | 1.053a | 0.810b | 1.014a | 0.036 | 0.012 |
| H-DLC/total CHO | 0.509a | 0.438b | 0.515a | 0.011 | 0.003 |
| L-DLC/total CHO | 0.491 | 0.542 | 0.508 | 0.010 | 0.135 |
a–bValues with different letters within the same row are different (P < 0.05).
12C = fed control diet at 0600 h and 1500 h a day, respectively, LH = fed low protein diet at 0600 h and high protein diet at 1500 h a day, HL = fed high protein diet at 0600 h and low protein diet at 1500 h a day, respectively.
2n = 10.
Proportion of fat and fatty acids profiles in milk
The proportions of fat and fatty acids profiles in milk on d 7 are listed in Table 3. Total proportion of MUFA (P = 0.003), C22:6 (DHA) (P = 0.011), C22:2 (P < 0.0001), C20:3 (P = 0.034), C20:2 (P = 0.025), and C18:1trans (P < 0.0001) in milk increased, but C16:0 (P = 0.025), C17:0 (P = 0.005), C18:1cis (P < 0.001), C18:2trans (P = 0.004), total SFA (P = 0.021), and C18:1cis/C18:1trans ratio (P < 0.0001) in milk decreased in the HL feeding group compared with those in the 2 C and LH groups. The LH feeding sequence significantly reduced the proportions of C18:3 (P < 0.001) and n-3 (P = 0.004), and increased the ratio of n-6/n-3 (P < 0.001). However, the proportion of fat in milk was not affected by the different feeding sequences.
Table 3.
Medium-long-chain fatty acids profile and fat proportion in milk according to feeding sequence on d 71,2,3.
| Item | Feeding sequence | SEM | P-value | ||
|---|---|---|---|---|---|
| 2 C | LH | HL | |||
| Fat and fatty acid value (%) | |||||
| C10:0 | 0.342a | 0.323ab | 0.293b | 0.010 | 0.103 |
| C12:0 | 0.415 | 0.411 | 0.402 | 0.010 | 0.814 |
| C14:0 | 3.954 | 3.882 | 3.897 | 0.070 | 0.932 |
| C14:1 | 0.276 | 0.283 | 0.272 | 0.010 | 0.910 |
| C15:0 | 0.113 | 0.126 | 0.122 | 0.005 | 0.525 |
| C15:1 | 0.314a | 0.297a | 0.115b | 0.040 | 0.044 |
| C16:0 | 32.33a | 31.67ab | 29.94b | 0.370 | 0.025 |
| C16:1 | 11.09 | 11.03 | 10.33 | 0.250 | 0.385 |
| C17:0 | 0.499a | 0.512a | 0.282b | 0.030 | 0.005 |
| C17:1 | 0.108b | 0.153a | 0.102b | 0.010 | 0.045 |
| C18:0 | 3.998 | 3.937 | 3.546 | 0.130 | 0.218 |
| C18:1 cis | 5.340a | 4.923a | 3.520b | 0.220 | <0.001 |
| C18:1 trans | 18.99b | 21.11b | 24.69a | 0.550 | <0.0001 |
| C18:2 cis, n − 6 | 18.77 | 17.13 | 18.70 | 0.440 | 0.210 |
| C18:2 trans, n − 6 | 0.148a | 0.148a | 0.085b | 0.010 | 0.004 |
| C18:3, n − 3 | 1.338a | 1.089c | 1.222b | 0.030 | <0.001 |
| C18:3, n − 6 | 0.135 | 0.153 | 0.143 | 0.010 | 0.753 |
| C20:0 | 0.222 | 1.255 | 0.158 | 0.320 | 0.292 |
| C20:1 | 0.086a | 0.056ab | 0.051b | 0.007 | 0.091 |
| C20:2 | 0.218b | 0.233b | 0.326a | 0.020 | 0.025 |
| C20:3, n − 3 | 0.037 | 0.034 | 0.042 | 0.001 | 0.119 |
| C20:3, n − 6 | 0.082b | 0.089ab | 0.101a | 0.003 | 0.034 |
| C20:4, n − 6 | 0.526 | 0.487 | 0.557 | 0.015 | 0.122 |
| C21:0 | 0.051 | 0.063 | 0.060 | 0.007 | 0.782 |
| C22:0 | 0.035 | 0.046 | 0.044 | 0.005 | 0.640 |
| C22:2 | 0.064b | 0.070b | 0.093a | 0.003 | <0.0001 |
| C22:6, n − 3 | 0.268b | 0.283b | 0.339a | 0.010 | 0.011 |
| MUFA | 36.27b | 37.92a | 39.14a | 0.343 | 0.003 |
| PUFA | 21.59 | 20.71 | 21.61 | 0.332 | 0.440 |
| SFA | 42.26a | 42.53a | 39.16b | 0.583 | 0.021 |
| n − 6 | 19.66 | 18.99 | 19.59 | 0.300 | 0.610 |
| n − 3 | 1.643a | 1.406b | 1.604a | 0.033 | 0.004 |
| Fat | 7.430 | 7.713 | 7.474 | 0.174 | 0.790 |
| The ratio of fatty acids | |||||
| C18:1cis/C18:1trans | 0.293a | 0.239a | 0.142b | 0.014 | <0.0001 |
| n − 6/n−3 | 12.12b | 13.62a | 12.27b | 0.190 | <0.001 |
a − cValues with different letters within the same row are different (P < 0.05).
12 C = fed control diet at 0600 h and 1500 h a day, respectively, LH = fed low protein diet at 0600 h and high protein diet at 1500 h a day, HL = fed high protein diet at 0600 h and low protein diet at 1500 h a day, respectively.
2The percentage values are proportions of each individual fatty acid relative to the total detectable lipid in the sample.
3n = 10.
Similar trends in MUFA (P < 0.0001), C16:0 (P < 0.001), C17:0 (P = 0.008), C20:3 (P < 0.001), C20:2 (P < 0.0001), C18:1cis (P < 0.001), C18:1trans (P < 0.001), C18:1cis/C18:1trans (P < 0.0001), and total SFA (P = 0.002) were observed on day 14 (Table 4 ). Additionally, both HL and LH feeding sequences increased the proportions of C18:3, n-6 (P < 0.001), C11:0 (P < 0.0001), and C17:1 (P < 0.001) in milk compared with those in 2 C, whereas the proportion of C20:0 (P = 0.022) in the LH group was greater than that in the other two groups. Compared with the 2 C group, the proportion of n-6 in the HL and LH groups presented an increasing trend (0.05 < P < 0.10), n-3 was decreased (P < 0.0001), and the n-6/n-3 ratio was significantly increased (P = 0.004). Notably, the proportion of fat was highest in the LH group (P = 0.0005).
Table 4.
Medium-long-chain fatty acids profile and fat proportion in milk according to feeding sequence on d 141–3.
| Item | Feeding sequence | SEM | P-value | ||
|---|---|---|---|---|---|
| 2 C | LH | HL | |||
| Fat and fatty acid value (%) | |||||
| C10:0 | 0.374 | 0.343 | 0.373 | 0.012 | 0.496 |
| C11:0 | 0.049b | 0.161a | 0.173a | 0.014 | <0.0001 |
| C12:0 | 0.470 | 0.464 | 0.464 | 0.007 | 0.939 |
| C14:0 | 4.340 | 4.097 | 4.091 | 0.057 | 0.150 |
| C14:1 | 0.346 | 0.327 | 0.362 | 0.012 | 0.486 |
| C15:0 | 0.117 | 0.109 | 0.121 | 0.005 | 0.528 |
| C16:0 | 33.64a | 31.20b | 29.96b | 0.404 | <0.001 |
| C16:1 | 12.02 | 10.94 | 11.01 | 0.303 | 0.312 |
| C17:0 | 0.312a | 0.198b | 0.142b | 0.023 | 0.008 |
| C17:1 | 0.102b | 0.163a | 0.207a | 0.011 | <0.001 |
| C18:0 | 3.954 | 4.090 | 3.711 | 0.082 | 0.174 |
| C18:1cis | 2.937a | 2.362b | 2.307b | 0.079 | <0.001 |
| C18:1trans | 20.66b | 23.84a | 25.68a | 0.552 | <0.001 |
| C18:2cis, n − 6 | 17.04b | 18.01a | 17.47ab | 0.186 | 0.113 |
| C18:3, n − 3 | 1.321 | 1.191 | 1.242 | 0.045 | 0.448 |
| C18:3, n − 6 | 0.088b | 0.114a | 0.125a | 0.004 | <0.001 |
| C20:0 | 0.105ab | 0.120a | 0.079b | 0.006 | 0.022 |
| C20:1 | 0.033c | 0.143b | 0.245a | 0.016 | <0.0001 |
| C20:2 | 0.122b | 0.299a | 0.351a | 0.022 | <0.0001 |
| C20:3, n − 6 | 0.069c | 0.120a | 0.097b | 0.006 | <0.001 |
| C20:4, n − 6 | 0.392b | 0.448ab | 0.475a | 0.012 | 0.020 |
| C22:6, n − 3 | 0.340a | 0.289b | 0.221c | 0.012 | 0.0001 |
| MUFA | 36.10c | 37.77b | 39.82a | 0.367 | <0.0001 |
| PUFA | 19.37b | 20.47a | 19.98ab | 0.215 | 0.127 |
| SFA | 43.74a | 41.39b | 39.88b | 0.455 | 0.002 |
| n − 6 | 17.58b | 18.69a | 18.16ab | 0.193 | 0.074 |
| n − 3 | 1.839a | 1.481b | 1.464b | 0.038 | <0.0001 |
| Fat | 6.988b | 7.976a | 6.854b | 0.148 | 0.003 |
| The ratio of fatty acids | |||||
| C18:1cis/C18:1trans | 0.147a | 0.099b | 0.090b | 0.005 | <0.0001 |
| n − 6/n − 3 | 9.653b | 13.46a | 12.45a | 0.497 | 0.004 |
a–cValues with different letters within the same row are different (P < 0.05).
12C = fed control diet at 0600 h and 1500 h a day, respectively, LH = fed low protein diet at 0600 h and high protein diet at 1500 h a day, HL = fed high protein diet at 0600 h and low protein diet at 1500 h a day, respectively.
2The percentage values are proportions of each individual fatty acid relative to the total detectable lipid in the sample.
3n = 10.
The proportion of fat and fatty acids profiles in milk on day 21 are listed in Table 5. MUFA (P < 0.0001), n-3 (P < 0.0001), C18:1trans (P < 0.0001), the ratio of n-6/n-3 (P < 0.0001), and C18:1cis/C18:1trans (0.05 < P < 0.10) presented similar quantities as observed on days 7 and 14. Compared with the 2 C group, the proportion of C22:6 (P < 0.0001), C18:3, n-3 (P < 0.0001), and C14:1 (P < 0.0001) were decreased in the HL group. However, C15:0 (P < 0.0001) and C20:2 (P = 0.023) were increased in the HL and LH groups, respectively. The proportion of fat was also increased in the LH group (P = 0.012) compared with that in the 2 C and HL groups. However, the proportion of n-6 in milk on d 7, 14, and 21 was not affected by different feeding sequences.
Table 5.
Medium-long-chain fatty acids profile and fat proportion in milk according to feeding sequence on day 211–3.
| Item | Feeding sequence | SEM | P-value | ||
|---|---|---|---|---|---|
| 2 C | LH | HL | |||
| Fat and fatty acid value (%) | |||||
| C10:0 | 0.365a | 0.342ab | 0.313b | 0.009 | 0.081 |
| C12:0 | 0.480 | 0.479 | 0.483 | 0.007 | 0.968 |
| C14:0 | 4.183 | 4.126 | 4.234 | 0.061 | 0.780 |
| C14:1 | 0.359a | 0.293a | 0.135b | 0.020 | <0.0001 |
| C15:0 | 0.111b | 0.113b | 0.294a | 0.018 | <0.0001 |
| C16:0 | 32.75 | 31.68 | 31.24 | 0.414 | 0.334 |
| C16:1 | 12.12 | 11.56 | 11.34 | 0.271 | 0.495 |
| C17:1 | 0.101 | 0.108 | 0.117 | 0.013 | 0.876 |
| C18:0 | 1.475 | 0.925 | 1.548 | 0.018 | 0.338 |
| C18:1cis | 2.892b | 3.512a | 3.185ab | 0.414 | 0.005 |
| C18:1trans | 21.96b | 26.95a | 27.44a | 0.271 | <0.0001 |
| C18:2cis, n − 6 | 17.68 | 17.90 | 18.14 | 0.013 | 0.757 |
| C18:3, n − 3 | 1.664a | 1.343b | 1.437b | 0.032 | <0.0001 |
| C20:2 | 0.201b | 0.285a | 0.210b | 0.017 | 0.023 |
| C22:6, n − 3 | 0.232a | 0.148b | 0.161b | 0.007 | <0.0001 |
| MUFA | 38.45b | 42.42a | 42.21a | 0.374 | <0.0001 |
| PUFA | 20.27 | 19.67 | 19.95 | 0.223 | 0.590 |
| SFA | 40.09 | 37.76 | 38.19 | 0.505 | 0.137 |
| n − 6 | 18.12 | 17.90 | 18.14 | 0.199 | 0.877 |
| n − 3 | 1.941a | 1.491b | 1.598b | 0.036 | <0.0001 |
| Fat | 6.484b | 7.633a | 6.755b | 0.167 | 0.012 |
| The ratio of fatty acids | |||||
| C18:1cis/C18:1trans | 0.131 | 0.132 | 0.117 | 0.003 | 0.091 |
| n − 6/n − 3 | 9.386c | 12.07a | 11.37b | 0.178 | <0.0001 |
a–cValues with different letters within the same row are different (P < 0.05).
12C = fed control diet at 0600 h and 1500 h a day, respectively, LH = fed low protein diet at 0600 h and high protein diet at 1500 h a day, HL = fed high protein diet at 0600 h and low protein diet at 1500 h a day, respectively.
2The percentage values are proportions of each individual fatty acid relative to the total detectable lipid in the sample.
3n = 10.
Discussion
Energy metabolism and circadian clocks interaction as well as nutritional outcomes are affected by the timing of food intake, even when the same type of food and the same amount of calories are consumed14. A previous study reported that plasma triglycerides show diurnal variation15. Our results also showed that the plasma lipid profiles of sows, especially on d 14 of lactation, was influenced by HL and LH feeding patterns, whereas the plasma triglyceride levels in sows did not show a significant difference among different groups. Moreover, changes in plasma CHO levels in sows suggested that a daily two-meal feeding sequence with different dietary CP, especially the HL feeding sequence, reduced the uptake of CHO or de novo synthesis of CHO in the liver. Notably, the plasma lipid profiles of sows at farrowing represented the late gestation period to some extent, and did not show significant differences between the three groups compared with those during lactation. These findings may be explained by the more vigorous lipid metabolism during lactation than gestation, but further study is needed to verify this.
A previous study reported that neither the volume of milk production nor the total milk lipid concentration was related to the variation in composition or energy content of the maternal diet16, but was strongly influenced by litter size, piglet weight, and suckling interval17,18. Milk production will increase if piglets suckle more frequently. In this study, the HL feeding sequence resulted in the maximum daily milk production/sow determined by litter size and average daily gain of piglets, although we did not evaluate the suckling intervals of piglets/litter. Growth rates of piglets were partially determined by the higher daily milk performance of sows. Normally, piglets that are heavier at birth grow faster during the suckling period because they are able to massage the teats more strongly and obtain more milk at each suckling19. These findings suggested that the HL feeding sequence was the most vigorous in stimulating a greater milk flow, which then helped to improve the growth rates of piglets.
Nutrients are supplied to the newborn through milk, and fat is particularly important not only for energy, but also for supplying specific fatty acids that are essential for optimal organ development20. Fat-free diets fed to infants increased energy requirements and reduced growth21, which indicated that milk fat was of great significance in the development of offspring. Some studies reported that rats and sows fed high-lipid diets during pregnancy and lactation had higher daily outputs of total lipid in milk and their offspring had higher growth rates22,23. It is reported higher piglet growth due to a higher milk fat content24. Our results indicated that the LH feeding sequence may resolve the problems associated with low milk fat content in sows, which also provides information for maternal nutrition in humans, cows, and other mammals. However, in this study, the increase in milk fat in sows from the LH group was not sufficient to account for the maximum growth rates of piglets.
Maternal nutrition has little or no effect on many nutrients in human milk, except that fatty acids show extreme sensitivity to maternal nutrition and are implicated in neurological development25. The utilisation of energy reserves is reflected in the content of milk fat26, fatty acids profile, and the mutual ratio between individual fatty acid proportions in milk27. Recently, dietary PUFA in feeds, specifically the n-3 and n-6 fatty acids, have been studied to improve sow and piglet performance. Moreover, it has been demonstrated that animal performance was influenced by the ratio of n-6/n-3 in pigs28. Conversely, it is reported that changing the n-6/n-3 fatty acid ratio in sow diets changed colostrum and milk fatty acids profiles, but had minimal impact on reproductive performance29. In this study, we found that both the LH and HL feeding sequences affected the proportion of n-3 fatty acids and the ratio of n-6/n-3 in milk. However, these changes did not decrease the growth performance of piglets, which was consistent with the previous study, although robust evidence supporting a direct link between the proportion of milk n-3 fatty acids, n-6/n-3 ratio, and growth performance of suckling piglets is limited.
One study showed that altering the conformation of a C18:1 double bond from cis to trans (oleic acid to elaidic acid) in in vitro cellular models decreased cholecystokinin secretion, a satiety hormone involved in appetite regulation30. In this study, the LH and HL feeding sequences significantly increased the proportion of C18:1trans in milk on days 7, 14, and 21 of lactation, but decreased the proportion of C18:1cis and the ratio of C18:1cis/C18:1trans, which is related to the appetite of suckling piglets. Again, this result indicated that milk production of sows could be increased via the two-meal feeding sequence, especially the HL sequence, to improve the appetite of suckling piglets and milk production of sows, although the latter point needs more studies of suckling behaviour to verify.
Besides C18:1 MUFA, the LH and HL feeding sequences also resulted in a greater total MUFA and lower proportion of SFA in milk. Although there is no conclusive evidence that high levels of SFA in the diet are directly linked to cardiovascular disease, it has been demonstrated that diets with high MUFA could protect against this disease31. Furthermore, it is found that a diet containing a high proportion of SFA reduced feed intake more than one rich in unsaturated fatty acids32. In agreement with a previous study, changes in SFA, C18:1 MUFA, and total MUFA, which are involved in appetite regulation, may explain the optimal milk composition in the HL group. Sow milk composition and production are important factors in determining mortality and growth rates of pre-weaning piglets2. In other words, our data indicated that the HL feeding sequence may improve the growth rates of piglets via altering conformation of C18:1 and SFA proportions in milk that regulate the appetites of piglets, and in turn improve the milk production of sows. Interestingly, the proportion of DHA in milk on d 7 of lactation increased in the HL group, but significantly decreased on days 14 and 21 in the LH and HL groups. The possible reasons for this variation need further study.
Milk fat comes from two sources: biosynthesis of fatty acids within the mammary glands (de novo synthesis) and uptake from the plasma by the mammary glands22, both of which are influenced by maternal nutrition33,34. Milk fatty acids profiles are dependent on maternal dietary lipid intake35,36. In contrast to a former study20, the daily two-meal feeding sequence with varying CP (HL or LH) affected the milk fat content and fatty acids profiles of sows despite constant dietary lipid content. These findings indicated that the growth performance of piglets could be improved without using additives in sow feeds, but by applying a maternal dynamic feeding sequence (i.e., HL or LH) compared with conventional feeding, i.e., the 2 C feeding sequence. Consequently, we suggest that the LH feeding sequence could improve the biosynthesis of fatty acids or absorption by the mammary glands in sows. However, to further understand its modulation by maternal dietary protein, further studies are needed.
A previous study demonstrated that the chemical composition of human milk is modified according to pregnancy maturation (pre- and post-partum) and milking time (night versus day time), and that these differences are adapted to the nutritional needs of the infant37, which was consistent with the results of milk fatty acids profiles in sows on d 7, 14 and 21 of lactation.
Conclusions
Compared to the conventional feeding sequence (2 C), dynamic maternal nutrition of a maternal two-meal with varying CP modulated milk production and lipid profiles in milk and plasma of sows. In turn, this contributed to the greater growth performance of piglets. These findings may be useful for maximising milk production of sows without increasing feed costs, and providing information for mother-infant nutrition in humans.
Materials and Methods
This study was conducted according to the guidelines for the treatment of animal subjects as approved by the Animal Care Committee of the Institute of Subtropical Agriculture, Chinese Academy of Science.
Animals, experimental design, and diets
The diets for sows in the experiment included a control diet (2 C), high-protein diet (HL), and a low protein diet (LH). A total of 60 pregnant sows (Landrace × Large Yorkshire) with a similar parity (3–6) were randomly assigned to one of 3 groups (20 replicates/group): 2 C (sows were fed a basal diet with 19.68% crude protein [CP] at 0600 h and 1500 h daily), LH (sows were fed a basal diet with 18.09% CP and 21.28% CP at 0600 h and 1500 h daily, respectively), and HL (sows were fed a basal diet with 21.28% CP and 18.09% CP at 0600 h and 1500 h daily, respectively).
Gestating sows were fed with the 2 C CP diet until 4 weeks pre-farrowing (d 85 ± 2 of gestation), when they began consuming their assigned treatment diet until weaning. Sows in the HL and LH groups were fed with the same quantity of diets at 0600 h and 1500 h throughout the experiment to ensure they all consumed equal amounts of feed with the same CP content daily.
During gestation, sows were housed in 8 pens, each with 6 individuals, walk in/lock in stalls. On d 110 of gestation, 20 sows were moved from the gestation facility to a farrowing room equipped with 20 individual farrowing crates. Each crate was 183 cm wide and 244 cm long, and had an adjustable sow space and a piglet creep area. All sows were housed individually in an environmentally controlled nursery and had free access to fresh water throughout the experiment. The piglets were suckled only by the sows and received no additional feed until weaning (aged 21 d).
In this study, the nutrients in feed were considered to be adequate for sows and met the NRC-recommended requirements within the appropriate weight range (NRC, 2012). The compositions of the diets are given in Table 6.
Table 6.
Composition of the sow diets (as-fed basis).
| Ingredient (%) | Diets | ||
|---|---|---|---|
| Control | High CP | Low CP | |
| Corn | 30.0 | 26.5 | 33.5 |
| Wheat | 29.9 | 29.9 | 29.9 |
| Wheat bran | 6.0 | 5.0 | 7.0 |
| DDGS | 5.0 | 5.0 | 5.0 |
| Soybean meal, CP 46% | 19.94 | 22.94 | 16.94 |
| Fish meal, CP 65% | 2.5 | 3.5 | 1.5 |
| Chocolate powder | 2.0 | 2.0 | 2.0 |
| Soy oil | 1.0 | 1.5 | 0.5 |
| Salt | 0.3 | 0.3 | 0.3 |
| CaCO3 | 0.82 | 0.82 | 0.82 |
| CaHPO4, 23/17 | 0.97 | 0.97 | 0.97 |
| Limestone | 0.26 | 0.26 | 0.26 |
| Lys, 78.8% | 0.05 | 0.05 | 0.05 |
| Choline | 0.21 | 0.21 | 0.21 |
| Flavoring agent1 | 0.05 | 0.05 | 0.05 |
| Vitamin-mineral Premix2 | 1 | 1 | 1 |
| Nutrient composition | |||
| CP, % | 19.68 | 21.28 | 18.09 |
| ME, MJ/kg | 13.50 | 13.65 | 13.35 |
| Crude Fat | 4.03 | 4.48 | 3.58 |
| Crude fibre | 3.061 | 3.060 | 3.062 |
| Crude ash | 5.38 | 5.58 | 5.19 |
| Salt | 0.34 | 0.35 | 0.33 |
| Ca | 0.79 | 0.82 | 0.75 |
| Available P | 0.46 | 0.49 | 0.43 |
| Lys | 0.97 | 1.09 | 0.85 |
| Met | 0.34 | 0.37 | 0.32 |
| Cys | 0.31 | 0.33 | 0.29 |
| Thr | 0.694 | 0.759 | 0.628 |
| Trp | 0.211 | 0.231 | 0.191 |
1Flavoring agent means flavor and sweetener.
2Premix provided the following per kg of diet: Fe (FeSO4·H2O), 80 mg; Mn (MnSO4·5H2O), 45 mg; Zn (ZnO), 100 mg; Cu (CuSO4·5H2O), 20 mg; I (KI), 0.70 mg; Se (Na2SeO3·H2O), 0.25 mg; vitamin A, 10,000 IU; vitamin D3, 2,500 IU; vitamin E, 100 IU; vitamin K, 10IU; vitamin B2, 10 mg; vitamin B6, 1 mg; vitamin B12 50ug; biotin, 80ug; folic acid, 5 mg; nicotinic acid, 15 mg; choline chloride 1500 mg.
Reproductive performance
After farrowing, the total number of newborn piglets/litter, stillbirth piglets/litter, and litter sizes at weaning were recorded. In addition, the average piglet birth weight/litter before suckling, and on d 14 and 21 (weaning) were recorded, and average daily gain of piglets/litter during d 0–14, 14–21, and 0–21 were recorded. Cross-fostering within diet groups was conducted within 24 h of birth to ensure even numbers of piglets per sow.
Sample collection
A 5 mL blood sample was collected from the marginal ear veins of each sow at farrowing and on d 14 of lactation. Plasma samples were then obtained by centrifugation at 3,000 × g for 10 min at 4 °C and immediately stored at −80 °C for subsequent analysis.
Milk samples were manually collected from 4–6 mammary glands on d 7, 14, and 21 of lactation, following an intramuscular injection of oxytocin to induce milk let down. All the milk samples were stored at −80 °C until analysis. Average milk production per sow during d 0–14, 14–21, and 0–21 of lactation was calculated using the average daily gain per piglet multiplied by the litter size and constant “4”38,39.
Plasma parameters
HDL-C, LDL-C, total CHO, and triglyceride in plasma were measured using an instrument (Biochemical Analytical Instrument, Beckman CX4, Beckman Coulter Inc., Brea, CA) and commercial kits (Sino-German Beijing Leadman Biotech Ltd., Beijing, China).
Determination of lipid profiles in milk
The proportion of fat in milk was analysed using a MilkoScan FT120 infrared automatic analyzer (Foss, Hillerød, Denmark).
Extraction and lipid methylation in milk were performed in duplicate from 200 μL samples, according to the method of Lepage and Roy40, which recommends treatment with 2 mL methanol: toluene 4:1 (v/v) solution, and transmethylation with boron trifluoride (BF3) and methanolic KOH41. Fatty acids profiles were determined using gas chromatography (Agilent 6890, Boston, MA) equipped with a 100 m × 0.25 mm × 0.2 μm film-fused silica capillary column (SP1233, Supelco Inc., Bellefonte, PA, USA) and a flame ionisation detector. Injector and detector temperatures were 280 °C. The column temperature was maintained at 140 °C for 5 min, then increased at a rate of 3 °C/min to 220 °C and held for 40 min. Individual fatty acid peaks were identified by comparison with known reference methyl esters. All fatty acid values were expressed as a proportion of total fatty acids.
Statistical analysis
All results are expressed as means and standard errors. Statistical analyses were conducted using SAS 8.2 (SAS Institute, Inc.). Normality of the data and homoscedasticity were checked using standard tests. All data were analysed using one-way analysis of variance (ANOVA), and feeding sequence was used as the independent variable. Differences were considered statistically significant at P < 0.05.
Implications
Maternal two-meal feeding sequences with varying crude protein reduced the plasma cholesterol levels, increased milk production, and improved milk lipid profiles of sows, which contributed to the health of sows and played a key role in increasing piglet growth performance.
Acknowledgements
This paper was jointly supported by grants from the national key research and development program of China (2016YFD0500504), the earmarked fund for China Agriculture Research System (CARS-35), Major projects of Henan Province and Major Project of Hunan Province (2015NK1002).
Author Contributions
C.Y. Xie, X. Wu, X.Y. Guo, C.M. Long, T.Y. Zhang, T.Z. Gao, and Y.L. Yin, no conflicts of interest. X.W. and Y.L.Y. designed the research; C.Y.X., X.Y.G., C.M.L., T.Y.Z. and T.Z.G. conducted research and animal experiment; C.Y.X. and X.W. analyzed the data and wrote the paper; C.Y.X., X.W. and Y.L.Y. had primary responsibility for final content. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chunyan Xie, Email: xiechunyan@hunau.edu.cn.
Yulong Yin, Email: yinyulong@isa.ac.cn.
References
- 1.Hansen AV, Strathe AB, Kebreab E, France J, Theil PK. Predicting milk yield and composition in lactating sows: A Bayesian approach. J Anim Sci. 2012;90:2285–2298. doi: 10.2527/jas.2011-4788. [DOI] [PubMed] [Google Scholar]
- 2.Farmer C, Palin MF, Theil PK, Sorensen MT, Devillers N. Milk production in sows from a teat in second parity is influenced by whether it was suckled in first parity. J Anim Sci. 2012;90:3743–3751. doi: 10.2527/jas.2012-5127. [DOI] [PubMed] [Google Scholar]
- 3.Tummaruk P, Sumransap P, Jiebna N. Fat and whey supplementation influence milk composition, backfat loss, and reproductive performance in lactating sows. Tropical animal health and production. 2014;46:753–758. doi: 10.1007/s11250-014-0559-8. [DOI] [PubMed] [Google Scholar]
- 4.Hauschild L, Lovatto PA, Pomar J, Pomar C. Development of sustainable precision farming systems for swine: Estimating real-time individual amino acid requirements in growing-finishing pigs. J Anim Sci. 2012;90:2255–2263. doi: 10.2527/jas.2011-4252. [DOI] [PubMed] [Google Scholar]
- 5.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. 2015;277:513–527. doi: 10.1111/joim.12347. [DOI] [PubMed] [Google Scholar]
- 7.Garaulet M, et al. Timing of food intake predicts weight loss effectiveness. Int J Obesity. 2013;37:604–611. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JB, et al. Timing of energy intake during the day is associated with the risk of obesity in adults. Journal of Human Nutrition and Dietetics. 2014;27:255–262. doi: 10.1111/jhn.12141. [DOI] [PubMed] [Google Scholar]
- 9.Xie C, et al. Effects of the Sequence of Isocaloric Meals with Different Protein Contents on Plasma Biochemical Indexes in Pigs. PLoS One. 2015;10:e0125640. doi: 10.1371/journal.pone.0125640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu, X. et al. Effects of a daily 3-meal pattern with different dietary protein contents on pig growth performance, carcass and muscle quality traits. Journal of the science of food and agriculture, doi:10.1002/jsfa.8467 (2017). [DOI] [PubMed]
- 11.Eisenberg SW, et al. Diurnal differences in milk composition and its influence on in vitro growth of Staphylococcus aureus and Escherichia coli in bovine quarter milk. Journal of dairy science. 2016;99:5690–5700. doi: 10.3168/jds.2015-10757. [DOI] [PubMed] [Google Scholar]
- 12.Heying EK, Grahn M, Pixley KV, Rocheford T, Tanumihardjo SA. High-provitamin A carotenoid (Orange) maize increases hepatic vitamin A reserves of offspring in a vitamin A-depleted sow-piglet model during lactation. J Nutr. 2013;143:1141–1146. doi: 10.3945/jn.113.175679. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, et al. Diurnal variations in polyunsaturated fatty acid contents and expression of genes involved in their de novo synthesis in pigs. Biochemical and biophysical research communications. 2017;483:430–434. doi: 10.1016/j.bbrc.2016.12.126. [DOI] [PubMed] [Google Scholar]
- 14.Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. Journal of internal medicine. 2015;277:513–527. doi: 10.1111/joim.12347. [DOI] [PubMed] [Google Scholar]
- 15.Pan X, Hussain MM. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. The Journal of biological chemistry. 2007;282:24707–24719. doi: 10.1074/jbc.M701305200. [DOI] [PubMed] [Google Scholar]
- 16.Ross AC, Davila ME, Cleary MP. Fatty-Acids and Retinyl Esters of Rat Milk - Effects of Diet and Duration of Lactation. J Nutr. 1985;115:1488–1497. doi: 10.1093/jn/115.11.1488. [DOI] [PubMed] [Google Scholar]
- 17.Auldist DE, Carlson D, Morrish L, Wakeford CM, King RH. The influence of suckling interval on milk production of sows. J Anim Sci. 2000;78:2026–2031. doi: 10.2527/2000.7882026x. [DOI] [PubMed] [Google Scholar]
- 18.Spinka M, Illmann G, Algers B, Stetkova Z. The role of nursing frequency in milk production in domestic pigs. J Anim Sci. 1997;75:1223–1228. doi: 10.2527/1997.7551223x. [DOI] [PubMed] [Google Scholar]
- 19.Campbell RG, Dunkin AC. The Effect of Birth-Weight on the Estimated Milk Intake, Growth and Body-Composition of Sow-Reared Piglets. Animal Production. 1982;35:193–197. doi: 10.1017/S0003356100027355. [DOI] [Google Scholar]
- 20.Lauridsen C, Jensen SK. Lipid composition of lactational diets influences the fatty acid profile of the progeny before and after suckling. Animal. 2007;1:952–962. doi: 10.1017/S175173110700033X. [DOI] [PubMed] [Google Scholar]
- 21.Hansen HS. The Essential Nature of Linoleic-Acid in Mammals. Trends Biochem Sci. 1986;11:263–265. doi: 10.1016/0968-0004(86)90191-X. [DOI] [Google Scholar]
- 22.Del Prado M, Villalpando S, Gordillo J, Hernandez-Montes H. A high dietary lipid intake during pregnancy and lactation enhances mammary gland lipid uptake and lipoprotein lipase activity in rats. J Nutr. 1999;129:1574–1578. doi: 10.1093/jn/129.8.1574. [DOI] [PubMed] [Google Scholar]
- 23.Lauridsen C, Danielsen V. Lactational dietary fat levels and sources influence milk composition and performance of sows and their progeny. Livest Prod Sci. 2004;91:95–105. doi: 10.1016/j.livprodsci.2004.07.014. [DOI] [Google Scholar]
- 24.van den Brand H, Heetkamp MJW, Soede NM, Schrama JW, Kemp B. Energy balance of lactating primiparous sows as affected by feeding level and dietary energy source. J Anim Sci. 2000;78:1520–1528. doi: 10.2527/2000.7861520x. [DOI] [PubMed] [Google Scholar]
- 25.Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr. 2014;99:734s–741s. doi: 10.3945/ajcn.113.072595. [DOI] [PubMed] [Google Scholar]
- 26.Bauman DE, Mather IH, Wall RJ, Lock AL. Major advances associated with the biosynthesis of milk. Journal of dairy science. 2006;89:1235–1243. doi: 10.3168/jds.S0022-0302(06)72192-0. [DOI] [PubMed] [Google Scholar]
- 27.Duchacek J, et al. Effect of Cow Energy Status on the Hypercholesterolaemic Fatty Acid Proportion in Raw Milk. Czech Journal of Food Sciences. 2014;32:273–279. [Google Scholar]
- 28.Yao, W. et al. Effects of dietary ratio of n − 6 to n − 3 polyunsaturated fatty acids on immunoglobulins, cytokines, fatty acid composition, and performance of lactating sows and suckling piglets. Journal of Animal Science and Biotechnology 3, doi:Artn 43 10.1186/2049-1891-3-43 (2012). [DOI] [PMC free article] [PubMed]
- 29.Eastwood L, Leterme P, Beaulieu AD. Changing the omega-6 to omega-3 fatty acid ratio in sow diets alters serum, colostrum, and milk fatty acid profiles, but has minimal impact on reproductive performance. J Anim Sci. 2014;92:5567–5582. doi: 10.2527/jas.2014-7836. [DOI] [PubMed] [Google Scholar]
- 30.Hand KV, Bruen CM, O’Halloran F, Giblin L, Green BD. Acute and chronic effects of dietary fatty acids on cholecystokinin expression, storage and secretion in enteroendocrine STC-1 cells. Mol Nutr Food Res. 2010;54:S93–S103. doi: 10.1002/mnfr.200900343. [DOI] [PubMed] [Google Scholar]
- 31.Willett WC, et al. Mediterranean Diet Pyramid - a Cultural Model for Healthy Eating. Am J Clin Nutr. 1995;61:1402s–1406s. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 32.Petit HV, Palin MF, Doepel L. Hepatic lipid metabolism in transition dairy cows fed flaxseed. Journal of dairy science. 2007;90:4780–4792. doi: 10.3168/jds.2007-0066. [DOI] [PubMed] [Google Scholar]
- 33.Innis SMHuman-Milk. and Formula Fatty-Acids. J Pediatr-Us. 1992;120:S56–S61. doi: 10.1016/S0022-3476(05)81237-5. [DOI] [PubMed] [Google Scholar]
- 34.Innis S. Maternal nutrition, genetics and human milk lipids. Curr Nutr Rep. 2013;2:151–158. doi: 10.1007/s13668-013-0048-0. [DOI] [Google Scholar]
- 35.Del Prado M, Delgado G, Villalpando S. Maternal lipid intake during pregnancy and lactation alters milk composition and production and litter growth in rats. J Nutr. 1997;127:458–462. doi: 10.1093/jn/127.3.458. [DOI] [PubMed] [Google Scholar]
- 36.Grigor MR, Allan J, Carne A, Carrington JM, Geursen A. Milk composition of rats feeding restricted litters. The Biochemical journal. 1986;233:917–919. doi: 10.1042/bj2330917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franca EL, Nicomedes TD, Calderon IDP, Franca ACH. Time-dependent alterations of soluble and cellular components in human milk. Biol Rhythm Res. 2010;41:333–347. doi: 10.1080/09291010903407441. [DOI] [Google Scholar]
- 38.Porcelets D, Calculating SOW. milk yields. Pig International. 2001;31:35–37. [Google Scholar]
- 39.Tilton SL, Miller PS, Lewis AJ, Reese DE, Ermer PM. Addition of fat to the diets of lactating sows: I. Effects on milk production and composition and carcass composition of the litter at weaning. J Anim Sci. 1999;77:2491–2500. doi: 10.2527/1999.7792491x. [DOI] [PubMed] [Google Scholar]
- 40.G, L. & CC, R. Direct transesterification of all classes of lipid in on step reaction. J Lipid Res, 114–120 (1986). [PubMed]
- 41.Morrison WR, Smith LM. Preparation of Fatty Acid Methyl Esters + Dimethylacetals from Lipids with Boron Fluoride-Methanol. Journal of Lipid Research. 1964;5:600–&. [PubMed] [Google Scholar]


