Abstract
Enhancer of zeste homolog 2 (EZH2) is the catalitic subunit of polycomb repressive complex 2 and mediates gene silencing. EZH2 is overexpressed in many cancers and correlates with poor prognosis. The role of the gene EZH2 in colorectal cancer survival is uncertainly, the aim of this study is clear this relationship. Relevant literaure was searched from electronic databases. A meta-analysis was performed with elegible studies which quantitatively evaluated the relationship between EZH2 overexpression and survival of patients with colorectal cancer. Survival data were aggregated and quantitatively analyzed. We performed a meta-analysis of 8 studies (n = 1059 patients) that evaluated the correlation between EZH2 overexpression and survival in patients with colorectal cancer. Combined hazard ratios suggested that EZH2 overexpression was associated with better prognosis of overall survival (OS) HR(hazard ratio) = 0.61 95% CI (0.38–0.84) We performed bias analysis according Egger and Begg,s test and we did not find publication bias. EZH2 overexpression indicates a better prognosis for colorectal cancer.
Introduction
Colorectal cancer (CRC) is one of the main causes of death in industrialized countries, coming in third place for incidence and fourth for mortality in the world as a whole1.
Colorectal cancer develops from the progressive accumulation of molecular events, like somatic mutations in oncogenes, or epigenetic mechanisms such as methylation of DNA or post-transcriptional modification of histones2. Over recent years, various studies have focused on the discovery of the molecular changes that participate in the process of tumour development, with the aim of finding biomarkers potentially useful in predicting survival or directing therapeutic strategies3.
One of the mechanisms that regulate histone epigenetic modification is mediated by the polycomb repressive complexes (PcG). PcG are epigenetic modifiers that promote gene repression through modification and compaction of chromatin. Two major complexes, designated as PRC1 and PRC2, perform different functions in cells related to gene silencing4,5. PRC1 includes the sub-units Bmi1, Ring1b, CBX4 and PHC, and induces mono-ubiquitination of the residue of lysine 119 from histone H2. PRC2 is formed by the protein EZH2, a catalytic sub-unit with a methyl-transeferase activity, and the sub-units SUZ12, EED and RbAp48, necessary for maintaining the integrity of the complex6.
It is believed that the EZH2 protein participates in the transcriptional repression of genes through various mechanisms, such as trimethylation of the residue of lysine 27 of histone H3 (H3mek27), or methylation of the CpG islands It also operates as a platform recruiting other enzymes involved in gene silencing, like histone de-acetylases (HDACs)7,8 and methyltransferases (DNMT1, DNMT3A, and DNMT3b)9.
In cancer, EZH2 promotes cell proliferation, invasion, apoptosis, angiogenesis and metastasis, according to the findings of in vitro studies on different cell lines10–13. Moreover, it has been found over-expressed in the tumour tissues of numerous neoplasias affecting the prostate7,14, breast15, bladder16, ovaries17, small-cell18 and non-small-cell lung cancer19, brain tumours20, kidney cancer21, gastric cancer22, and cancer of the oesophagus23, pancreas24, or melanoma25.
A good number of studies suggest that the over-expression of EZH2 may have a prognostic value in some types of cancer, and it has been associated with a worse prognosis and survival rate in breast and prostate tumours7,14,15,26. As regards CRC, its role is less well known, and the mechanisms and routes in which it participates are not clear. A limited number of studies focus on the relationship between the expression de EZH2 and overall and disease free survival or with responses to treatment.
The present work undertakes a systematic review of studies that include the analysis of the expression of EZH2, and attempts to evaluate its influence over prognosis in CRC, through an analysis of the overall survival rates. Additionally, the methodological quality of the selected studies was assessed.
Methods
Search Strategy
A systematic review of the academic literature was performed, taking the expression of EZH2 as exposure variable and the survival from CRC as effect variable. The study was carried out in accordance with recommendations defined in the REMARKS (Reporting Recommendations for Tumour Marker Prognostic Studies) guide for meta-analyses and systematic reviews27,28.
Two researchers (Molina AJ and Vilorio-Marqués L) carried out independently searches for original works in Pubmed, Scopus and WOS (World of Science) among all material published up to August 2016, using the terms “EZH2” or “PCR2” and “Colorectal” or “Colon” and “Cancer” or “tumour” or “Neoplasm” or “Carcinoma”. A second phase incorporated a root search in respect of the papers included in the study, with the aim of detecting pieces of work that had not been netted in the first search.
Selection of Papers
The systematic review included all those articles fulfilling the following criteria: studies done on sets of cases, cases and controls or cohorts of patients histologically diagnosed with CRC, studies determining the expression of EZH2 in human tissues by means of immunohistochemical techniques (IHC) or real-time quantitative polymerase chain reaction (q-PCR). The studies had to contain sufficient information for an estimate of Hazard Ratio (HR) relative to Overall Survival (OS), Disease-Free Survival (DFS), or both, with a confidence interval of 95%.
The review excluded in vitro or ex vivo studies, letters, narrative reviews, conferences summaries, or works related to other pathologies or neoplasias. The initial search was undertaken with no limitations as to language, but full paper reading and later assessment were only completed in works published in English.
Data Collection
Molina AJ and Vilorio-Marqués L reviewed all the articles independently, checking their titles and abstracts, and gathered data from eligible studies. Disagreements were resolved through debate and consensus with a third researcher, Martín V.
The information collected for each study was: name of the first author, name of the journal, year of publication, type of study, number of cases, analysis of EZH2 expression, method employed (IHC and/or qPCR) data on follow-up, overall survival and disease-free survival (OS and DFS). The data selected for the research is summarized in Table 1.
Table 1.
Characteristics of Studies Eligible for the Systematic Review.
| Name of first author | Journal publishing study | Year of publication | Type of study | Method of detecting expression of EZH2 | Number of cases | Survival measure | Methodology score | Included in study |
|---|---|---|---|---|---|---|---|---|
| Kurihara H | Oncotarget | 2016 | Cohort study | IHC | 310 | OS | 90.90 | YES |
| Liu YL | Journal of Cancer Research and Clinical Oncology | 2014 | Case study | q-PCR | 82 | DFS | 40.90 | YES |
| Benard A | Public Library of Science One | 2014 | Cohort study | IHC | 247 | OS/DFS/RFS | 90.90 | YES |
| Jinushi T | Cancer Medicine | 2014 | Case study | ------- | 71 | ------- | ------- | NO |
| Ishikawa S | International Journal of Cancer | 2013 | Case study | ------- | 742 | ------- | ------- | NO |
| Tamagawa H | European Journal of Surgical Oncology. | 2013 | Case study | IHC | 61 | OS | 35.71 | YES |
| Lin Y | The Journal of Pathology | 2013 | Case study | IHC | 129 | OS | 43.18 | YES |
| Takawa M | Cancer Science | 2011 | Case study | IHC | 172 | OS | 68.18 | YES |
| Kogo R | Cancer Research | 2011 | Case study | ------- | 100 | ------- | ------- | NO |
| Kodach LL | Carcinogenesis | 2010 | Case study | IHC | 72 | OS | 40.90 | YES |
| Wang CG | World Journal of Gastroenterology | 2010 | Case study | IHC | 119 | DFS | 63.63 | YES |
| Fluge | British Journal of Cancer | 2009 | Cohort study | IHC | 409 | RFS | 84.09 | YES |
| Mimori K | European Journal of Surgical Oncology | 2005 | Case study | q-PCR | 61 | OS | 35.71 | YES |
Methodological Assessment
In order to evaluate the methods used in the studies, three researchers, (Diez-Tascón C, Martín V and Sevilla F), read each publication independently and scored all of them for methodology and reproducibility in accordance with the REMARKS guidelines. In addition, a second scoring system established in a previous work, the “European Lung Cancer Working Party quality scale for biological prognostic factors for lung cancer” was used29. Four main categories were assessed, with several items per category: scientific design, methods used in the laboratory, generability and analysis of results. Each item was scored as follows: 2 points if well defined, 1 point if unclear or incomplete, and 0 points if undefined. According to the criteria proposed by Chen et al.30, a work was considered of high methodological quality if scored above 80%29,30.
Statistical Analysis
Values for the hazard ratio (HR) and confidence intervals were taken directly from the articles when supplied by the authors, otherwise they were estimated through visual inspection of the Kaplan-Meier survival graphs. Survival data and time to events were extracted from the graphs, and the rate of data censored during follow-up was assumed constant31.
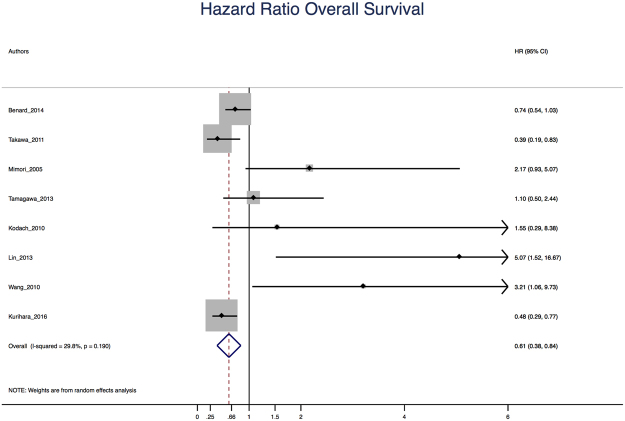
A meta-analysis was performed using the random effects model of DerSimonian and Laird32 to estimate the role of over-expression of EZH2 in survival from CRC, assuming a worse survival when HR > 1 The horizontal lines indicate confidence intervals of 95% and the boxes representing the estimates for HR are proportional to the weighting for the study. Moreover, to assess differences depending on the technique for detecting overexpression, meta-analysis were performed separately for q-PCR and IHC studies.
Additionally, the heterogeneity of the information (I2) was assessed, according to the Mantel Haenszel model. The I2 value measures the degree of inconsistency or incompatibility between studies, so higher values for I2 indicate greater heterogeneity30. An analysis of publication bias was carried out with the methods proposed by Egger et al.33 and Begg & Mazumdar34. For all these analyses, the statistical package Stata 13 was used.
Results
Search Procedure and Identification of Relevant Studies
During the initial search, we identified 384 articles from PubMed, Scopus and Web of Science databases. Of these, 134 duplicated works were eliminated. Subsequently, a first reading of the abstracts allowed to identify those studies that did not meet the defined selection criteria In brief, a total of 237 studies were excluded. Of those, 77 publications were narrative reviews, letters to the editor or conference summaries. 89 articles involved exclusively in vitro studies, 3 covered experimental work with a mouse model, 37 concentrated on other pathologies (malignant neoplasias other than CRC) 24 articles investigated other genes and 6 articles analysed polymorphisms (genetic variations) and not the expression of the gene and 1 study was in Chinese language. Finally, 13 studies resulted eligible for further assessment (Fig. 1).
Figure 1.
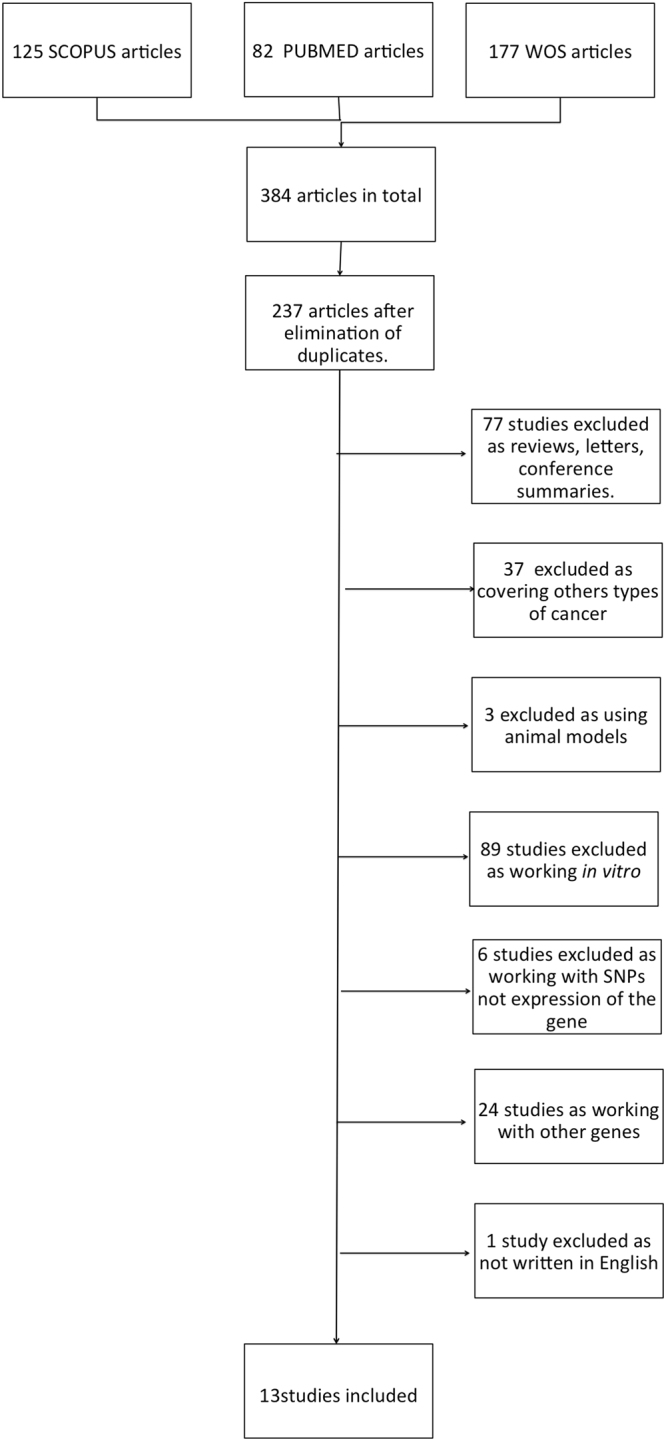
Flowchart for the Systematic Review.
The 13 fully reviewed articles were published in the period from 2005 to 2016. Of these, 6 studies were from Japan, 3 from China, 2 from the Netherlands, 1 from Norway and 1 from Greece. At this point 3 articles were further excluded after full reading, since they focused on the expression of EZH2 versus other markers (Long Non Coding RNA and mi-RNA)35,36, and treatments with statins37 rather than on the association between the expression of EZH2 and survival parameters.
Hence, ten studies resulted finally eligible for the systematic review. Assessment of their methodological quality showed compliance values ranging from 35.7% to 90.9%, with more than half scoring below 50% for quality and just two articles exceeding a quality threshold of 80% (Table 1). The studies looked at different measures for survival, studying OS (8 articles), DFS or RFS (3 articles) (Table 2). Of the ten studies, in six of them the authors found that over-expression of EZH2 tend to be an indicator of poor prognosis for colorectal cancer survival38–43, but only one of them reports statistically significant results38. The four remaining studies found instead that over-expression of EZH2 would be a good prognostic factor44–47, with two of them reporting statistically significant results45,47.
Table 2.
Details of the Studies Included in the Systematic Review. (T = length of time monitored; OS_C = raw overall survival rate; OS_A = adjusted overall survival rate; DFS_C = raw disease-free survival rate; DFS_A = adjusted disease-free survival rate; RFS_C = raw recurrence-free survival rate; RFS_A = adjusted recurrence-free survival rate).
| AUTHOR | YEAR | Technique | Cutoff over-expression | N | T(YEARS) | OS_C 95% CI | OS_A 95% CI | DFS_C/RFS_C 95% CI | DFS_A/ RFS_A 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Kurihara_2016 | 2016 | IHC | − → < 80% Mean nuclear positivity + → ≥ 80% Mean nuclear positivity | 301 | 4.4 | 0.48(0.29–0.77) | |||
| Benard_2014 | 2014 | IHC | − → ≤ median % of nuclear positivity + → > median % of nuclear positivity | 247 | 8.6 | 0.74(0.54–1.03) | 0.84(0.60–1.18) | 0.64(0.42–0.99) | 0.67(0.43–1.05) |
| Liu_2014 | 2014 | q-PCR | − → ≤ median tumor/normal ratio + → > median tumor/normal ratio | 82 | 3 | — | — | 4.18(2.08–8.36) | 2.52(1.10–5.73) |
| Tamagawa_2013 | 2013 | IHC | − → ≤ median value of H-score EZH2 (0–300) + → > median value of H-score EZH2 (0–300) | 54 | 8 | 1.09(0.50–2.44) | — | — | — |
| Lin_2013 | 2013 | IHC | Index value calculated as a product of the grades of the extent and intensity of staining:− → Grades 0–3 + → Grades 4–12 | 33 | 5.8 | 5.07(1.51–16.7) | — | — | — |
| Takawa_2011 | 2011 | IHC | − → No appreciable staining in tumor cells + → Brown staining appreciable in the nucleus of tumor cells | 172 | 5 | 0.39(0.19–0.83) | 0.42(0.18–0.97) | — | — |
| Kodach_2010 | 2010 | IHC | − → < 70% positive tumor cells + → ≥ 70% positive tumor cells | 72 | 6.6 | 1.54 (0.29–8.38) | — | — | — |
| Wang_2010 | 2010 | IHC | − → ≤ 30% positive tumor cells + → > 30% positive tumor cells | 119 | 6.6 | — | 3.21(1.06–9.72) | — | — |
| Fluge_2009 | 2009 | IHC | Index value calculated as a product of the grades of the extent and intensity of staining:− → Grades 0–3 + → Grades 4–9 | 409 | 7 | — | — | 1.17(0.46–2.98) | — |
| Mimori_2005 | 2005 | q-PCR | − → ≤ median tumor/normal ratio + → > median tumor/normal ratio | 61 | 8 | 2.17(0.93–5.07) | — | — | — |
Meta-Analysis
Eight studies that investigated overall survival were included in the meta-analysis. In five of them the HR was reported, while in the remaining three it was estimated from the Kaplan Meier survival graphs. The overall HR obtained was HR = 0.61 CI 95% (0.38–0.84) (Fig. 2). These data suggest that in CRC over-expression of EZH2 is a good prognosis factor for survival. In respect to the heterogeneity of the studies the value for I2 was 29.8%, which is not statistically significant (p = 0.190). In addition, we analyzed and performed the meta analysis specifically for the seven studies using IHC obtaining an HR = 0.58 CI 95% (0.38–0.79).
Figure 2.
Plot for the Meta-Analysis of Raw Overall Survival (OS).
Analysis of Publication Bias
Although the funnel plot (Fig. 3) showed some asymmetry, neither Egger’s test (p = 0.121) nor Begg’s test (p = 0.072) were statistically significant. These results suggest that there is no publication bias for the over-expression of EZH2 in survival from CRC.
Figure 3.
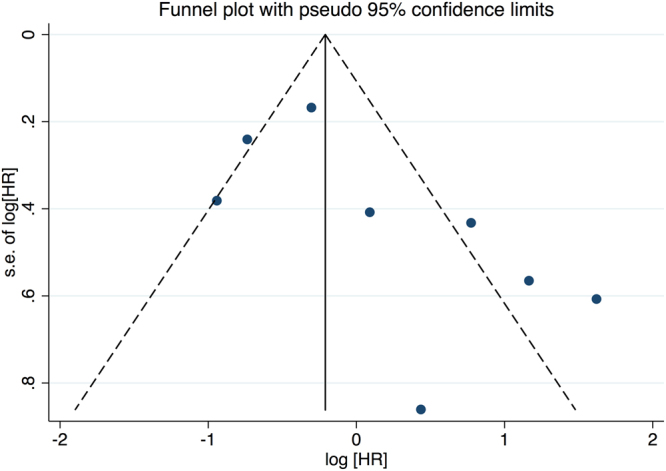
Funnel Plot for the Eight Studies Investigating the Role of EZH2 in Overall Survival from CRC.
Discussion
The systematic review presented in this article identified ten studies evaluating survival from CRC related to the expression of EZH2 in tumour tissue, with OS as a measure of survival in eight of these works. A meta-analysis was performed by merging the eight studies and including a total of 1059 CRC patients. The combination of HR suggested that over-expression of EZH2, is associated with a better prognosis and a better OS with an overall HR = 0.61 CI 95% (0.38–0.84) this result was statistically significant.
These data are consistent with the values obtained by Wang et al.48 and Jiang et al.49, who in two recently published meta-analyses found values for overall HR = 0.91 CI 95% (0.63 to 1.19) and HR = 0.75 CI 95% (0.28 to 1.22) respectively for the relationship between the expression of EZH2 and survival from CRC. On the other hand, another previous meta-analysis30, which covered even more tumour types, that included just two articles for CRC with a total of 291 patients, reported an overall HR = 1.13 CI 95% (0.16 to 8.21), which is not in line with our results. In the present review, we have incorporated more articles referring to CRC that those consider by these previous meta-analysis.
This finding of a protective role of over-expression of EZH2 for CRC is noticeable, because in other solid tumors, such as prostate, breast and lung, the available data support an opposite effect than ours8,26,50. Moreover, it is known that EZH2 is overexpressed in tumour tissue as compared to healthy tissue14,15,18,20,51, and it has been suggested, and supported by convincing mechanicistic data, an oncogenic function of EZH2 related to PRC2 functioning that, through histone methylation, would lead to chromatin condensation repressing the expression of tumour suppressor genes52. This process would be associated with a worse prognosis and lower survival rate4.
Nevertheless, recent studies suggest that EZH2 is a dual-faced molecule which may act as transcripcional repressor, but also as an activator53–55. Post-translational modifications56, variations in its association with other PRC2 subunits, such as SUZ12 and EED57 and the existence of PRC2-independent activity of EZH258,59, and the role of SNPs in their expression or functionality60–62 are some of the proposed reasons for this variability in the role of EZH2 in cancer survival. In addition, other studies have shown that a loss of the trimethylation state of H3k27 is related to lower survival rates in cancers of breast63,64, ovaries, pancreas63 and rectum65, which would be consistent with the results found in the present meta-analysis. Recently, Wassef & Margueron, suggests that taken into account its biological function as an important layer of gene regulation, is not surprising that a lower PRC2 function could be related to an oncogenic effect due the possible activation of genes that would otherwise remain silent55.
Furthermore, these findings would support the hypothesis that there exist methylation patterns of histones, genes and the wide genome so tumour genesis would be associated with hypomethylation in histones and non-coding genome together with hypermethylation at specific loci and in individual CpG islands of specific genes66.
To the best of our knowledge, the present study is the most comprehensive meta-analysis hitherto on the role of EZH2 in survival from colorectal cancer. We performed this systematic review and meta-analysis to yield summary statistics by including more recent related studies and by generally using a more comprehensive search strategy. Screening, study selection, and quality assessment were performed. We also explored heterogeneity and potential publication bias in accordance with published guidelines.
Nevertheless, the limitations of this work include the number of articles included, which restricts interpretation using the methods of Egger and Begg, despite the fact that no publication bias of statistical significance were found67. It should not be forgotten, either that all the publications considered were written in English, and the tendency in the academic world is to publish the most striking results in international journals whereas local journals and grey literature report not so impactful findings.
Moreover, studies may differ with regard to the baseline characteristics of the patients included age, disease stage, duration of follow up, and also in the use of different techniques to assess the expression of EZH2 and the absence of a standarized cut-off to consider overexpression in each of these techniques. All these variability sources could suppose limitations for comparison, as has been previously reported in other meta-analysis30,49.
Another potential source of bias relates to the method of extrapolating HR and the 95% confidence interval, because these were calculated from data provided in articles, or else they were extrapolated from survival curves, when not reported by authors, which necessarily involved assumptions being made about any censoring process.
In conclusion, the present meta-analysis suggest a protective role for over-expression of EZH2 in survival from CRC. Nevertheless, the paucity of available studies limits its potential application as a prognostic biomarker. Therefore, further studies, both retrospective and prospective, are warranted in order to explore the expression of EZH2 as a biomarker for survival and to clarify the molecular mechanisms that involve EZH2 in colorectal cancer.
Acknowledgements
This manuscript has been funded by Grant BIO/LE09/13 from Junta de Castilla y León.
Author Contributions
The manuscript has eight co-authors which contributions were: L.V.M. and A.J.M. directed the study; L.V.M., A.J.M. and V.M. conceived, performed and designed the study: L.V.M. and A.J.M. performed the search strategy, C.D.T., M.F.G.S. and V.M.S. performed the qualitative assessment, A.J.M., T.F.V. and L.V.M. analyzed the data and wrote the manuscript, V.M., C.D.T., E.H. and V.D.B. contributed to the supervision of the study and critical analysis of the article. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ervik, M. et al. Cancer Today. Lyon, France: International Agency for Research on Cancer. (2016). Available at: http://gco.iarc.fr/today, (Accessed: 19th November 2016).
- 2.Khare S, Verma M. Epigenetics of colon cancer. Methods Mol. Biol. 2012;863:177–185. doi: 10.1007/978-1-61779-612-8_10. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues D, Longatto-Filho A, Martins SF. Predictive Biomarkers in Colorectal Cancer: From the Single Therapeutic Target to a Plethora of Options. Biomed Res. Int. 2016;2016:1–12. doi: 10.1155/2016/6896024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon Ja, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat. Rev. Mol. Cell Biol. 2009;10:697–708. doi: 10.1038/nrn2731. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi H, Hung M-C. Regulation and Role of EZH2 in Cancer. Cancer Res. Treat. 2014;46:209–22. doi: 10.4143/crt.2014.46.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan JZ, Yan Y, Wang XX, Jiang Y, Xu HE. EZH2: biology, disease, and structure-based drug discovery. Acta Pharmacol Sin. 2014;35:161–174. doi: 10.1038/aps.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon, J. a & Lange, C. a. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res. 647, 21–9 (2008). [DOI] [PubMed]
- 8.Deb G, Thakur VS, Gupta S. Multifaceted role of EZH2 in breast and prostate tumorigenesis: Epigenetics and beyond. Epigenetics. 2013;8:464–476. doi: 10.4161/epi.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viré E, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 10.Margueron R, et al. Ezh1 and Ezh2 Maintain Repressive Chromatin through Different Mechanisms. Mol. Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L, Cui J, Liang S, Pang Y, Liu P. Update of research on the role of EZH2 in cancer progression. OncoTargets and Therapy. 2013;6:321–324. doi: 10.2147/OTT.S42453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varambally S, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 13.Tan J, et al. Integrative epigenome analysis identifies a Polycomb-targeted differentiation program as a tumor-suppressor event epigenetically inactivated in colorectal cancer. Cell Death Dis. 2014;5:e1324. doi: 10.1038/cddis.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Leenders GJLH, et al. Polycomb-Group Oncogenes EZH2, BMI1, and RING1 Are Overexpressed in Prostate Cancer With Adverse Pathologic and Clinical Features. Eur. Urol. 2007;52:455–463. doi: 10.1016/j.eururo.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Kleer CG, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc. Natl. Acad. Sci. USA. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang, H. et al. Increased EZH2 protein expression is associated with invasive urothelial carcinoma of the bladder. Urol. Oncol. 10.1016/j.urolonc.2010.09.005 (2011). [DOI] [PubMed]
- 17.Rao Z-Y, et al. EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-beta1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis. 2010;31:1576–83. doi: 10.1093/carcin/bgq150. [DOI] [PubMed] [Google Scholar]
- 18.Sato T, et al. PRC2 overexpression and PRC2-target gene repression relating to poorer prognosis in small cell lung cancer. Sci. Rep. 2013;3:1911. doi: 10.1038/srep01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huqun, et al. Enhancer of zeste homolog 2 is a novel prognostic biomarker in nonsmall cell lung cancer. Cancer. 2012;118:1599–1606. doi: 10.1002/cncr.26441. [DOI] [PubMed] [Google Scholar]
- 20.Crea F, Hurt EM, Farrar WL. Clinical significance of Polycomb gene expression in brain tumors. Mol. Cancer. 2010;9:265. doi: 10.1186/1476-4598-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, et al. Overexpression of YB1 and EZH2 are associated with cancer metastasis and poor prognosis in renal cell carcinomas. Tumor Biol. 2015;36:7159–7166. doi: 10.1007/s13277-015-3417-z. [DOI] [PubMed] [Google Scholar]
- 22.He L-J, et al. Prognostic significance of overexpression of EZH2 and H3k27me3 proteins in gastric cancer. Asian Pac. J. Cancer Prev. 2012;13:3173–8. doi: 10.7314/APJCP.2012.13.7.3173. [DOI] [PubMed] [Google Scholar]
- 23.Yamada A, et al. Aberrant expression of EZH2 is associated with a poor outcome and P53 alteration in squamous cell carcinoma of the esophagus. Int. J. Oncol. 2011;38:345–353. doi: 10.3892/ijo.2010.868. [DOI] [PubMed] [Google Scholar]
- 24.Kuroki H, et al. EZH2 Is Associated with Malignant Behavior in Pancreatic IPMN via p27Kip1 Downregulation. PLoS One. 2014;9:e100904. doi: 10.1371/journal.pone.0100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHugh JB, Fullen DR, Ma L, Kleer CG, Su LD. Expression of polycomb group protein EZH2 in nevi and melanoma. J. Cutan. Pathol. 2007;34:597–600. doi: 10.1111/j.1600-0560.2006.00678.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, et al. Clinical and prognostic relevance of EZH2 in breast cancer: A meta-analysis. Biomed. Pharmacother. 2015;75:218–25. doi: 10.1016/j.biopha.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 27.McShane LM, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br. J. Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PLoS Med. 2012;9:e1001216. doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steels E, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur. Respir. J. 2001;18:705–19. doi: 10.1183/09031936.01.00062201. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, et al. Enhancer of zeste homolog 2 as an independent prognostic marker for cancer: a meta-analysis. PLoS One. 2015;10:e0125480. doi: 10.1371/journal.pone.0125480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 35.Jinushi T, et al. Low expression levels of microRNA-124-5p correlated with poor prognosis in colorectal cancer via targeting of SMC4. Cancer Med. 2014;3:1544–52. doi: 10.1002/cam4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kogo R, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa S, et al. Statins inhibit tumor progression via an enhancer of zeste homolog 2-mediated epigenetic alteration in colorectal cancer. Int. J. cancer. 2014;135:2528–36. doi: 10.1002/ijc.28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y-L, et al. Expression and clinicopathological significance of EED, SUZ12 and EZH2 mRNA in colorectal cancer. J. Cancer Res. Clin. Oncol. 2015;141:661–9. doi: 10.1007/s00432-014-1854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamagawa H, et al. Global histone modification of H3K27 correlates with the outcomes in patients with metachronous liver metastasis of colorectal cancer. Eur. J. Surg. Oncol. 2013;39:655–61. doi: 10.1016/j.ejso.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Kodach LL, et al. The role of EZH2 and DNA methylation in the silencing of the tumour suppressor RUNX3 in colorectal cancer. Carcinogenesis. 2010;31:1567–1575. doi: 10.1093/carcin/bgq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CG, et al. EZH2 and STAT6 expression profiles are correlated with colorectal cancer stage and prognosis. World J. Gastroenterol. 2010;16:2421–2427. doi: 10.3748/wjg.v16.i19.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fluge Ø, et al. Expression of EZH2 and Ki-67 in colorectal cancer and associations with treatment response and prognosis. Br. J. Cancer. 2009;101:1282–9. doi: 10.1038/sj.bjc.6605333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mimori K, et al. Clinical significance of enhancer of zeste homolog 2 expression in colorectal cancer cases. Eur. J. Surg. Oncol. 2005;31:376–380. doi: 10.1016/j.ejso.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Benard, A. et al. Prognostic value of polycomb proteins EZH2, BMI1 and SUZ12 and histone modification H3K27me3 in colorectal cancer. PLoS One9 (2014). [DOI] [PMC free article] [PubMed]
- 45.Takawa M, et al. Validation of the histone methyltransferase EZH2 as a therapeutic target for various types of human cancer and as a prognostic marker. Cancer Sci. 2011;102:1298–305. doi: 10.1111/j.1349-7006.2011.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin Y-W, et al. Role of STAT3 and vitamin D receptor in EZH2-mediated invasion of human colorectal cancer. J. Pathol. J Pathol. 2013;230:277–290. doi: 10.1002/path.4179. [DOI] [PubMed] [Google Scholar]
- 47.Kurihara H, et al. The relationship between EZH2 expression and microRNA-31 in colorectal cancer and the role in evolution of the serrated pathway. Oncotarget. 2016;7:12704–17. doi: 10.18632/oncotarget.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W, et al. Prognostic significance of EZH2 expression in patients with digestive cancers: a meta-analysis. Int. J. Clin. Exp. Med. 2015;8:16043–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang T, et al. Prognostic value of high EZH2 expression in patients with different types of cancer: a systematic review with meta-analysis. Oncotarget. 2016;7:4584–97. doi: 10.18632/oncotarget.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, et al. Prognostic Significance of EZH2 Expression in Non-Small Cell Lung Cancer: A Meta-analysis. Sci. Rep. 2016;6:19239. doi: 10.1038/srep19239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bachmann IM, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J. Clin. Oncol. 2006;24:268–73. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 52.Troselj KG, et al. Polycomb repressive complex’s evolutionary conserved function: the role of EZH2 status and cellular background. Clin.Epigenetics. 2016;8:55. doi: 10.1186/s13148-016-0226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deb G, et al. EZH2: Not EZHY (Easy) to deal. Mol. Cancer Res. 2014;12:639–653. doi: 10.1158/1541-7786.MCR-13-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sashida G, Iwama A. Multifaceted role of the polycomb-group gene EZH2 in hematological malignancies. Int. J. Hematol. 2017;105:23–30. doi: 10.1007/s12185-016-2124-x. [DOI] [PubMed] [Google Scholar]
- 55.Wassef, M. & Margueron, R. The multiple facets of PRC2 alterations in cancers. J. Mol. Biol. pii: S0022-2836(16)30427-2 (2016). [DOI] [PubMed]
- 56.Lu H, et al. Regulation and role of post-translational modifications of enhancer of zeste homologue 2 in cancer development. Am J Cancer Res. 2016;6:2737–2754. [PMC free article] [PubMed] [Google Scholar]
- 57.Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7:299–313. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen, Y. et al. Role of EZH2 in cancer stem cells: from biological insight to a therapeutic target. Oncotarget. doi:10.18632/oncotarget.16467 (2017). [DOI] [PMC free article] [PubMed]
- 59.Xu K, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–9. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crea F, et al. An EZH2 polymorphism is associated with clinical outcome in metastatic colorectal cancer patients. Ann. Oncol. 2012;23:1207–1213. doi: 10.1093/annonc/mdr387. [DOI] [PubMed] [Google Scholar]
- 61.Fornaro L, et al. Molecular and pathological characterization of the EZH2 rs3757441 single nucleotide polymorphism in colorectal cancer. BMC Cancer. 2015;15:874. doi: 10.1186/s12885-015-1889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y, et al. EZH2 genetic variants affect risk of gastric cancer in the Chinese Han population. Mol. Carcinog. 2014;53:589–97. doi: 10.1002/mc.21871. [DOI] [PubMed] [Google Scholar]
- 63.Wei Y, et al. Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers. Mol. Carcinog. 2008;47:701–6. doi: 10.1002/mc.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bae WK, et al. The methyltransferase EZH2 is not required for mammary cancer development, although high EZH2 and low H3K27me3 correlate with poor prognosis of ER-positive breast cancers. Mol. Carcinog. 2015;54:1172–80. doi: 10.1002/mc.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benard A, et al. Epigenetic status of LINE-1 predicts clinical outcome in early-stage rectal cancer. Br. J. Cancer. 2013;109:3073–83. doi: 10.1038/bjc.2013.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007;8:286–98. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 67.Palma Pérez S, Delgado Rodríguez M. [Practical considerations on detection of publication bias] Gac. Sanit. 2006;20(Suppl 3):10–6. doi: 10.1157/13101085. [DOI] [PubMed] [Google Scholar]