Abstract
Episodic ataxia (EA) is a rare neurological condition characterized by recurrent spells of truncal ataxia and incoordination. Five genes (KCNA1, CACNA1A, CACNB4, SLC1A3, and UBR4) have been linked to EA. Despite extensive efforts to genetically diagnose EA, many patients remain still undiagnosed. Whole-exome sequencing was carried out in 39 Korean patients with EA to identify pathogenic mutations of the five known EA genes. We also evaluated 40 candidate genes that cause EA as a secondary phenotype or cerebellar ataxia. Eighteen patients (46%) revealed genetic information useful for establishing a molecular diagnosis of EA. In 11 patients, 16 pathogenic mutations were detected in three EA genes. These included nine mutations in CACNA1A, three in SLC1A3, and four in UBR4. Three patients had mutations in two genes, either CACNA1A and SLC1A3 or CACNA1A and UBR4, suggesting that SLC1A3 and UBR4 may act as genetic modifiers with synergic effects on the abnormal presynaptic activity caused by CACNA1A mutations. In seven patients with negative results for screening of EA genes, potential pathogenic mutations were identified in the candidate genes ATP1A2, SCN1A, TTBK2, TGM6, FGF14, and KCND3. This study demonstrates the genetic heterogeneity of Korean EA, and indicates that whole-exome sequencing may be useful for molecular genetic diagnosis of EA.
Introduction
Episodic ataxia (EA) is a clinically heterogeneous group characterized by recurrent spells of truncal ataxia and incoordination1–3. Incidence is likely less than 1/100,000. Most cases have autosomal dominant inheritance, although some sporadic cases have been reported. There are eight subtypes according to clinical features and genetic etiologies. The most common subtypes are EA1 and EA2, caused by mutations involving potassium (KCNA1) and calcium (CACNA1A) channel genes, respectively. They have well-defined clinical features and have been reported in ethnically different families. EA1 is characterized by brief episodes of ataxia and constant myokymia, whereas EA2 usually presents with longer episodes of ataxia with interictal nystagmus. Onset is typically in early childhood, and episodes are commonly triggered by physical and emotional stress. In EA2, acetazolamide may reduce frequency and severity of attacks. Mutations in CACNB4 and SLC1A3 cause EA5 and EA6, respectively, but have been reported in only one or two families4–6. In the remaining three subtypes, known EA genes were excluded (EA4) or distinct disease loci were mapped (EA3 and EA7)7–9. A recent whole-exome sequencing (WES) study revealed the association of UBR4 with EA810.
To date, more than 100 different mutations have been described in all EA genes, but molecular diagnostic rates range from 13% to 40%11–13. A significant proportion of EA patients do not have mutations in the known EA genes. This suggests a genetic heterogeneity of EA and presence of additional causative genes. EA belongs to a group of neurological channelopathies manifesting as various episodic neurological symptoms and signs1. So far, the majority of genetically-confirmed EA have been identified in Caucasian families11,14–16. Only a few Asians have been reported, probably due to limited availability of commercial genetic tests1,17,18. However, recent advances in next-generation sequencing techniques, such as WES, will facilitate identification of new mutations associated with EA.
In this study, we conducted next-generation sequencing in Korean EA patients to identify pathogenic mutations of five known EA genes (KCNA1, CACNA1A, CACNB4, SLC1A3, and UBR4), and explored candidate genes that cause EA as a secondary phenotype or cerebellar ataxia.
Results
After variant filtering, annotation, and interpretation, 204 different variants were identified in the protein-coding regions of five EA genes and 40 candidate genes (Supplementary Table S1).
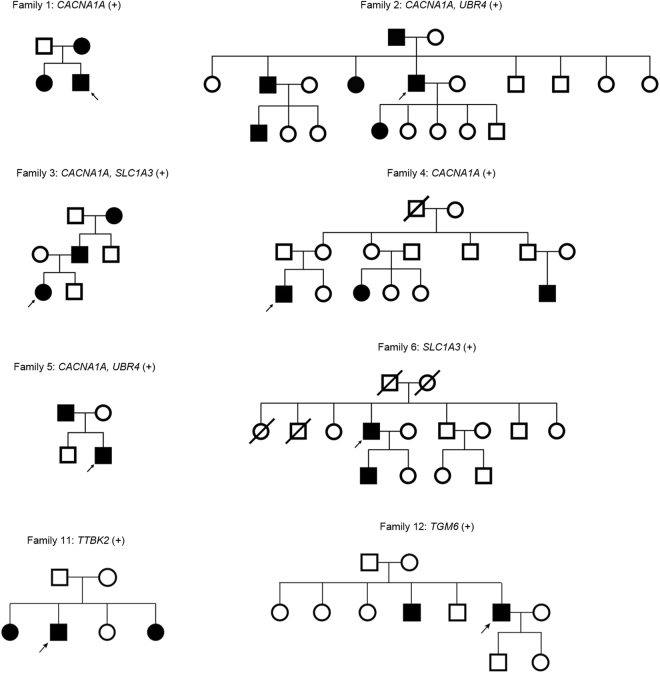
We used a stratified approach to identify disease-causing variants. Variants were regarded as probable pathogenic mutation when 1) variants were previously reported as a disease-causing mutation, 2) protein truncation was caused via a frameshift or stop codon, or 3) nonsynonymous missense variants were predicted as damaging by at least three of four prediction tools, were present below MAF 0.001, involved a conserved nucleotide position based on a positive GERP score, and segregated with at least one additional affected family member. Nonsynonymous missense variants that met two or more of these criteria, but not all, were considered as possible pathogenic mutations. Using this method, we identified 23 pathogenic mutations in 18 of 39 patients (46%, Tables 1 and 2). All variants were absent in 150 unrelated in-house controls. Thirteen of 23 identified mutations were also absent from the inspected exome or genome database. The other 10 were present with a MAF ≤0.0002. Sixteen mutations were found in three EA genes and another seven in three candidate genes. Eight patients had a family history of EA (Fig. 1), while the remaining 10 were sporadic.
Table 1.
Clinical characteristics of the patients with genetically-confirmed EA.
| Patient No | Sex/age | Age of onset | Genetic variants | Duration | Ictal symptoms | Interictal nystagmus | Additional features | Response to acetazolamide |
|---|---|---|---|---|---|---|---|---|
| Family Hx. (+) | ||||||||
| 1 | M/27 | 19 | CACNA1A | minutes-hours | ataxia, vertigo, dysarthria, diplopia | DB | (−) | (+) |
| 2 | M/48 | teenage | CACNA1A UBR4 | hours | ataxia, dysarthria | DB | cerebellar atrophy | NP |
| 3 | F/4 | 3 | CACNA1A SLC1A3 | hours | ataxia, dizziness | GEN | seizure | (+) |
| 4 | M/21 | 12 | CACNA1A | hours | ataxia, vertigo, headache | DB | migraine | (+) |
| 5 | M/41 | 9 | CACNA1A UBR4 | hours | ataxia, dizziness, dysarthria | GEN | migraine | (+) |
| 6 | M/62 | 55 | SLC1A3 | hours | ataxia, dizziness, dysarthria | DB | mild truncal ataxia | (+) |
| 11 | M/68 | 53 | TTBK2 | hours | ataxia, vertigo | (−) | (−) | (−) |
| 12 | M/56 | 54 | TGM6 | hours | ataxia, vertigo, dysarthria | GEN | (−) | (−) |
| Family Hx. (−) | ||||||||
| 15 | M/39 | 34 | CACNA1A | seconds-minutes | dizziness | GEN | (−) | (−) |
| 16 | M/33 | 33 | CACNA1A | hours | ataxia, vertigo, headache, tinnitus | GEN | (−) | (−) |
| 19 | F/49 | 47 | ATP1A2 | days | ataxia, vertigo, dysarthria, weakness | (−) | intermittent LOC, migraine | (+) |
| 22 | F/37 | 30 | SCN1A | hours | dizziness, headache | DB | migraine | (−) |
| 23 | M/45 | 38 | SLC1A3 | hours | ataxia, dizziness, dysarthria | GEN | rebound upbeat nystagmus, cognitive impairment | (−) |
| 26 | F/54 | 53 | UBR4 | seconds-minutes | dizziness | GEN | (−) | NP |
| 28 | F/52 | 49 | UBR4 | hours | ataxia, vertigo, tinnitus | (−) | (−) | NP |
| 30 | F/62 | 60 | SCN1A | hours | vertigo, diplopia | GEN | (−) | (−) |
| 32 | M/46 | 39 | FGF14 | hours | dizziness, headache | DB | (−) | (+) |
| 33 | F/17 | 17 | KCND3 | minutes-hours | ataxia, vertigo, dysarthria | GEN | earfullness | NP |
M = male; F = female; GEN = gaze-evoked nystagmus; DB = downbeat nystagmus; LOC = loss of consciousness; NP = not performed.
Table 2.
Potentially pathogenic variants identified by whole-exome sequencing in 18 EA patients.
| Patient No | Family History | Gene | Exon | mRNA | Protein | Variant effect | SIFT | LRT | Polyphen | Mutation taster | GERP | MAF | Pathogenic |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | CACNA1A | 16 | c.2030 G > A | p.Gly677Glu | missense | D | D | B | D | 4.58 | — | possible |
| 2 | + | CACNA1A | 40 | c.5956 C > T | p.Gln1986* | nonsense | — | — | — | — | — | — | probable |
| UBR4 | 100 | c.14630 A > G | p.Tyr4877Cys | missense | D | D | D | D | 5.75 | — | possible | ||
| 3 | + | CACNA1A | 23 | c.3871_3873delGAG | p.Glu1294 DEL | deletion | — | — | — | — | — | — | probable |
| SLC1A3 | 7 | c.985 G > A | p.Ala329Thr | missense | D | D | P | D | 5.71 | 0.0003† | probable | ||
| 4 | + | CACNA1A | 5 | c.742 T > A | p.Tyr248Asn | missense | D | D | D | D | 5.55 | — | possible |
| 5 | + | CACNA1A | 32 | c.5005 C > T | p.Arg1669* | nonsense | — | — | — | — | — | — | probable |
| CACNA1A | 19 | c.2992 G > C | p.Glu998Gln | missense | T | N | B | D | 1.91 | — | possible | ||
| UBR4 | 83 | c.12332 G > A | p.Arg4111His | missense | T | D | P | D | 5.25 | 0.0001† | possible | ||
| 6 | + | SLC1A3 | 8 | c.1177 G > A | p.Val393Ile | missense | T | D | P | D | 5.8 | — | probable |
| 11 | + | TTBK2 | 15 | c.3467 G > A | p.Arg1156Gln | missense | D | D | D | D | 5.13 | 0.00002† | possible |
| 12 | + | TGM6 | 10 | c.1478 C > T | p.Pro493Leu | missense | T | N | D | D | 4.67 | 0.0001† | possible |
| 15 | − | CACNA1A | 20 | c.3321_3322insC | p.Gly1108Argfs40* | insertion | — | — | — | — | — | — | probable |
| CACNA1A | 46 | c.6605_6616delACC AGGAGCGGG | p.Asp2202_Arg2205DEL | deletion | — | — | — | — | — | 0.0005† | possible | ||
| 16 | − | CACNA1A | 29 | c.4645 C > T | p.Arg1549* | nonsense | — | — | — | — | — | — | probable |
| 19 | − | ATP1A2 | 6 | c.586 C > T | p.Arg196Cys | missense | D | D | D | D | 5.11 | 0.00003† | possible |
| 22 | − | SCN1A | 26 | c.5516 T > G | p.Ile1839Ser | missense | D | D | D | D | 5.6 | 0.000008† | possible |
| 23 | − | SLC1A3 | 7 | c.952 A > G | p.Thr318Ala | missense | T | D | B | D | 6.07 | 0.0002† | possible |
| 26 | − | UBR4 | 103 | c.15125 C > T | p.Ala5042Val | missense | T | D | D | D | 5.39 | 0.0002‡ | possible |
| 28 | − | UBR4 | 52 | c.7742 C > T | p.Ala2581Val | missense | D | D | D | D | 5.95 | — | possible |
| 30 | − | SCN1A | 11 | c.1688T > A | p.Leu563Gln | missense | D | D | D | D | 5.59 | — | possible |
| 32 | − | FGF14 | 1 | c.31 A > G | p.Thr11Ala | missense | D | N | B | D | 4.73 | — | possible |
| 33 | − | KCND3 | 4 | c.1291 C > T | p.Arg431Cys | missense | D | D | D | D | 5.25 | 0.000009† | possible |
Transcript ID: ATP1A2, NM_000702.3 (NP_000693.1); CACNA1A, NM_023035.2 (NP_075461.2); FGF14, NM_175959.2 (NP_787125.1); KCND3, NM_004890.4(NP_004971.2); SCN1A, NM_006920.4 (NP_008851.3); SLC1A3, NM_004172.4 (NP_00416.3); TGM6, NM_198994.2 (NP_945345.2); TTBK2, NM_173500.3 (NP_775771.3); UBR4, NM_020765.2 (NP_065816.2). SIFT- D (damaging), T (tolerated); LRT- D (deleterious), N (neutral); Polyphen- D (probably damaging), P (possibly damaging), B (benign); MutationTaster- D (disease_causing). †MAF based on the Exome Aggregation Consortium (ExAC), ‡MAF based on the 1000 Genomes Project.
Figure 1.
Pedigree of eight families with pathogenic mutations. Solid symbols (squares = males, circles = females) indicate clinically affected individuals; open symbols, unaffected individuals; and slashed symbols, deceased individuals. Probands are indicated by arrows.
Pathogenic mutations identified in previously known EA genes
Of the previously known EA genes, seven probable and nine possible pathogenic mutations were identified in six families and five sporadic cases (Table 2). These included nine mutations in CACNA1A (five probable and four possible; Fig. 2A), three in SLC1A3 (two probable and one possible; Fig. 2B), and four in UBR4 (all possible; Fig. 2C). Three patients (patient 2, 3 and 5) had mutations in two genes, either CACNA1A and SLC1A3 or CACNA1A and UBR4.
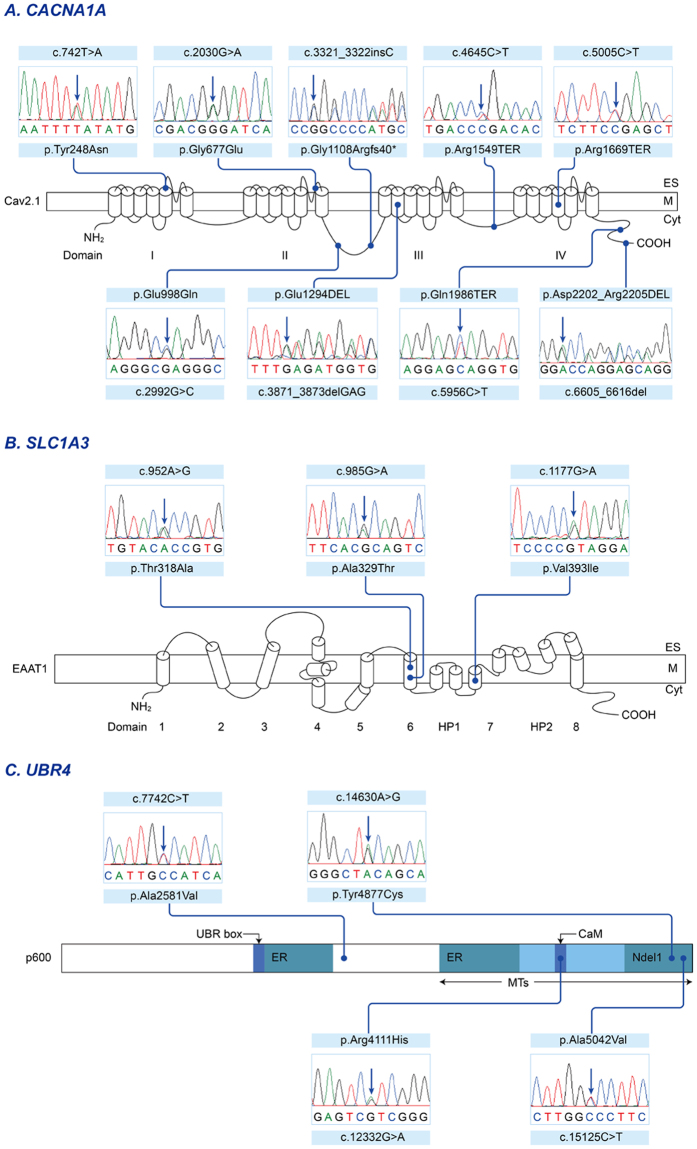
Figure 2.
Sequencing results and localization of the mutations in EA genes. (A) The Cav2.1 encoded by CACNA1A has four homologous domains (I-IV), each consisting of six transmembrane segments (S1-S6) and an additional pore loop located between S5 and S6. Three non-sense mutations (p.Arg1549TER of patient 16, p.Arg1669TER of patient 5 and p.Gln1986TER of patient 2) are predicted premature termination in the S4 of Domain IV or the C-terminal domain. A heterozygous insertion mutation is located in the intracellular linker connecting Domain II and III, and lead to a frameshift and premature termination (p.Gly1108Argfs40TER of patient 15). Two missense mutations (p.Gly677Glu of patient 1 and p.Tyr248Asn of patient 4) involve the pore region (S5-S6) of Domain II and I, respectively. The other missense mutation (p.Glu998Gln of patient 5) is located in the intracellular linker. The in-frame deletions without truncation of the protein (p.Glu1294DEL of patient 3 and p.Asp2202_Arg2205DEL of patient 15) are in the S2 of Domain III and the C-terminal domain, respectively. (B) The EAAT1 encoded by SLC1A3 is composed of eight alpha-helical transmembrane domains (TMDs) and re-entrant hairpin loops (HP) 1 and 2 flanking TMD7. The first sixth TMDs form a scaffold that surrounds a C-terminal core domain comprising HP1, TMD7, HP2, and TMD8. Two missense mutations (p.Ala329Thr of patient 3 and p.Thr318Ala of patient 23) are located in TMD6, while the other mutation (p.Val393Ile of patient 6) is located in TMD7, the critical binding site for glutamate and various coupled ions, Na+, H+ and K+. (C) The p600 protein encoded by UBR4 contains several identified functional domains including UBR box, microtubule (MT)-binding domains, and calmodulin (CaM)-binding domain. The two endoplasmic reticulum (ER)-binding regions are located near the center of the protein and within the MT-binding domain. The Ndel1-binding region overlaps with the MT-binding domain. Three missense mutations (p.Arg4111His of patient 5, p.Tyr4877Cys of patient 2, and p.Ala5042Val of patient 26) are located within the MT-binding domain, and one (p.Arg4111His) involves the CaM-binding domain interacting with calmodulin. The other one (p.Ala2581Val of patient 28) is situated in the functionally-unknown region.
In patient 5, two CACNA1A and one UBR4 mutations were detected. One was a nonsense mutation in CACNA1A (exon 32, c.5005 C > T, p.Arg1669TER), predicted to encode a truncated protein within Domain IV of the Cav2.1 α1 subunit. The remaining two missense mutations (CACNA1A exon 19, c.2992 G > C, p.Glu998Gln; UBR4 exon 83, c.12332 G > A, p.Arg4111His) were either absent in dbSNP147 or had a MAF of 0.0001 in the ExAC Browser, and so were designated as possible pathogenic mutations. Patient 2 also had a nonsense mutation in CACNA1A (exon 40, c.5956 C > T, p.Gln1986TER) that predicted a premature termination within the C-terminal domain. The patient had an additional novel missense mutation in UBR4 (exon 100, c.14630 A > G, p.Tyr4877Cys). Patient 3 had probable pathogenic mutations in CACNA1A and SLC1A3. One was an in-frame deletion of one amino acid due to a three-nucleotide deletion in CACNA1A (exon 23, c.3871_3873del GAG, p.Glu1294DEL) and the other was a SLC1A3 missense mutation in highly conserved amino acid (exon 7, c.985 G > A, p.Ala329Thr). Both mutations were detected in the affected father, but not in the unaffected mother. Patient 1 and 4 had a novel missense mutation in CACNA1A (exon 16, c.2030 G > A, p.Gly677Glu; exon 5, c.742 T > A, p.Tyr248Asn). Mutations involved the pore loop region of Domain II and Domain I, respectively. They changed the highly conserved amino acid and were predicted to be pathogenic with at least three of the prediction tools. However, both were regarded as possible pathogenic mutations because allelic segregation could not be confirmed due to limited availability of additional family members. Patient 15 had two CACNA1A mutations. One was a heterozygous insertion mutation that led to frameshift and premature termination (exon 20, c.3321_3322insC, p.Gly1108Argfs40TER). The other was an in-frame deletion of four amino acid (exon 46, c.6605_6616delACCAGGAGCGGG, p.Asp2202_Arg2205DEL) that has been reported as a rare variant19. Patient 16 had the previously reported disease-causing mutation in CACNA1A (exon 29, c.4645 C > T, p.Arg1549TER)20.
Two SLC1A3 mutations were detected in one family (patient 6) and sporadic case (patient 23). A missense mutation (exon 8, c.1177 G > A, p.Val393Ile) of patient 6 was designated as a probable pathogenic mutation based on segregation within an affected family member, absence in public databases, positive GERP score, and deleterious effect by three prediction tools. Patient 23 had another missense mutation (exon 7, c.952 A > G, p.Thr318Ala) in a highly conserved residue with a MAF of 0.0002 in the ExAC Browser, and was regarded as a possible pathogenic mutation.
Two UBR4 missense mutations were found in two sporadic cases: exon 103, c.15125 C > T, p.Ala5042Val in patient 26; exon 52, c.7742 C > T, p.Ala2581Val in patient 28. Both were either absent in dbSNP147 or had a MAF of 0.0002 in the 1,000 Genomes. They changed a highly conserved amino acid and were predicted to be pathogenic with at least three of the prediction tools, but were regarded as possible pathogenic mutations because we could not confirm they arose de novo.
Pathogenic mutations identified in candidate genes
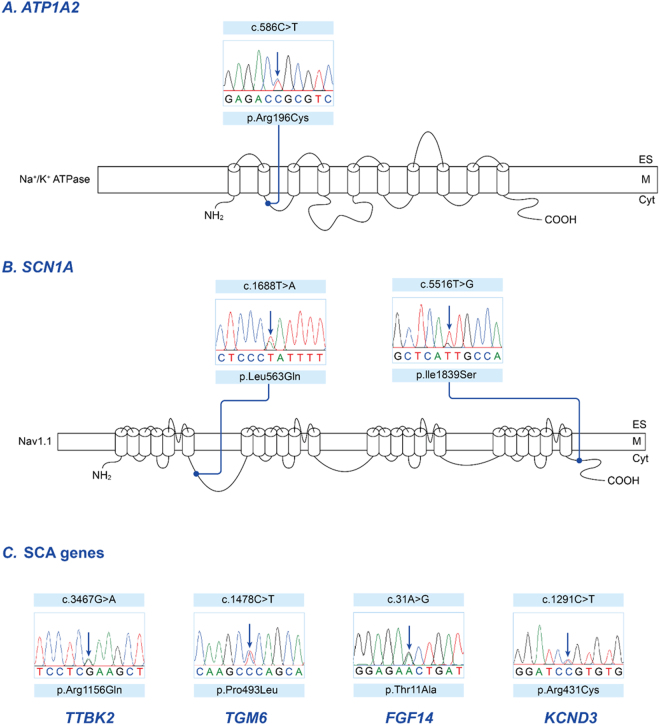
Of 28 patients for whom pathogenic mutations could not be identified in the known EA genes, seven had possible pathogenic mutations in candidate genes (Table 2). Two had a family history, and the remaining five were sporadic cases. Three patients had a missense mutations in ATP1A2 (exon 6, c586C > T, p.Arg196Cys in patient 19; Fig. 3A) or SCN1A (exon 26, c.5516 T > G, p.Ile1839Ser in patient 22; exon 11, c.1688T > A, p.Leu563Gln in patient 30; Fig. 3B), which are genes associated with FHM. All were predicted to be pathogenic by four prediction tools, and were either absent or displayed a MAF < 0.0001 in the ExAC Browser. Four patients had missense mutation in genes associated with spinocerebellar ataxia, such as TTBK2 (exon 15, c.3467 G > A, p.Arg1156Gln in patient 11), TGM6 (exon 10, c.1478 C > T, p.Pro493Leu in patient 12), FGF14 (exon 1, c.31 A > G, p.Thr11Ala in patient 32), KCND3 (exon 4, c.1291 C > T, p.Arg431Cys in patient 33; Fig. 3C). These mutations were either absent in public database or had a MAF < 0.0001.
Figure 3.
Sequencing results and localization of the mutations in candidate genes. (A) The α2 subunit of the Na+/K+-ATPase encoded by ATP1A2, consists of 10 transmembrane segments (M1-M10) with a large cytoplasmic loop between M4 and M5. The missense mutation (p.Arg196Cys of patient 19) is located in the intracellular loop between M2 and M3. This region is associated with A- (actuator) domain responsible for the binding and hydrolysis of ATP. (B) The α1 subunit of the Na+ channel encoded by SCN1A, has four homologous domains (I-IV), each consisting of six transmembrane segments (S1-S6) and an additional pore loop located between S5 and S6. Two missense mutations (p.Ile1839Ser of patient 22 and p.Leu563Gln of patient 30) are located in the C-terminal domain and the intracellular linker connecting Domain I and II, respectively. (C) Missense mutations of genes associated spinocerebellar ataxia in four patients with episodic ataxia: TTBK2 (exon 15, c.3467 G > A, p.Arg1156Gln in patient 11), TGM6 (exon 10, c.1478 C > T, p.Pro493Leu in patient 12), FGF14 (exon 1, c.31 A > G, p.Thr11Ala in patient 32), KCND3 (exon 4, c.1291 C > T, p.Arg431Cys in patient 33).
Additionally, we analyzed rare variants of all genes in the remaining 21 patients to search for putative genes. There were some genes that shared rare variants between individuals; these included MUC6, KCNJ12, SIRPA, CELA1, and DNAH17 (Supplementary Data). However, they could not be considered as new candidate genes associated with EA due to the lack of potential contribution to cerebellar ataxia and the unknown functions of genes.
Clinical characteristics of patients with pathogenic mutations
Clinical characteristics of the 18 patients with pathogenic mutations were presented in Table 1. They included nine men and seven women with mean age of 42.3 ± 16 years.
Five patients with CACNA1A mutation (patients 1, 2, 3, 4, and 5) revealed typical clinical features of EA2 including early age of onset, a positive family history, recurrent ataxia for several hours, interictal nystagmus, and response to acetazolamide. However, the remaining two patients with CACNA1A mutation (patients 15 and 16) were sporadic cases and revealed unusual findings including late-onset of age, brief episodes of dizziness, and poor response to acetazolamide. Two patients (patients 4 and 5) had migraine, and one (patient 3) had a history of febrile convulsion. Although three patients had an additional mutation in SLC1A3 (patient 3) or UBR4 (patients 2 and 5), there were no distinctive symptoms during attacks.
Two patients with SLC1A3 mutations (patients 6 and 23) also exhibited typical EA2-like symptoms including recurrent ataxia, slurred speech lasting several hours and interictal nystagmus, but had late-onset in the fourth or sixth decades. Patient 6 showed a mildly progressive baseline ataxia and good response to acetazolamide or 3,4- diaminopyridine. Patient 23 showed mild cognitive impairments and rebound upbeat nystagmus, which developed on resuming the neutral position after leftward or downward gaze. Attacks did not respond to acetazolamide. Two patients with UBR4 mutation (patients 26 and 28) presented with recurrent vertigo/dizziness and imbalance lasting several hours with interictal nystagmus beginning in their fifth or sixth decades.
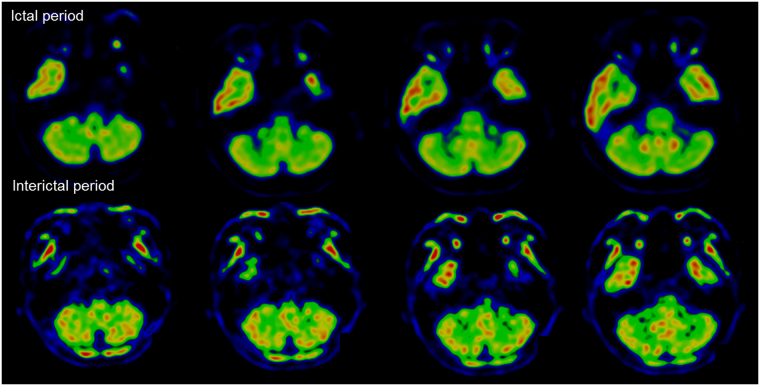
Seven patients with mutations in candidate genes presented with recurrent ataxia, vertigo/dizziness, and interictal nystagmus with variable ages at onset. Two of three patients with FHM gene mutations had a history of migraine. Among them, patient 19 with ATP1A2 mutations had distinctive clinical features. The patient was a 49-year-old woman with recurrent ataxia, dysarthria, vertigo, and limbs weakness provoked by emotional and physical stress since she was 47. She had a history of migraine without visual aura. Between attacks, neurological examinations were unremarkable. During attacks, she showed severe truncal ataxia, dysarthria, quadriparesis of MRC grade 4, and horizontal gaze-evoked nystagmus. Occasionally, she developed decreased consciousness without evidence of seizure on an electroencephalogram. Attacks usually lasted for several days and were resolved in two weeks. Brain magnetic resonance imaging scans were unremarkable, but 18-fluorodeoxyglucose positron emission tomography (FDG-PET) disclosed hypometabolism in both cerebellar hemispheres during attacks, which normalized after one-month (Fig. 4). The attacks were prevented by acetazolamide.
Figure 4.
Ictal and interictal cerebellar metabolism in patient 19 with ATP1A2 mutation. Compared with interictal PET (low panels), ictal images (upper panels) show significant hypometabolism in bilateral cerebellar hemispheres.
Discussion
This is the largest genetic analysis study for Korean patients with EA. Using WES, we identified 11 patients with pathogenic mutations in known EA genes and an additional seven with variants of potential pathogenicity in candidate genes. Overall, 46% of patients revealed genetic information useful for establishing molecular diagnosis. These results indicate that WES may be useful for molecular genetic diagnosis of EA, and should be considered in the routine genetic evaluation of patients with EA.
To date, five genes have been linked to EA1–3. All except UBR4 encode ion channel proteins located on the neuronal or glial membrane, and play critical roles in excitatory neurotransmission. In this study, 28% of patients had pathogenic mutations in three EA genes (CACNA1A, SLC1A3, UBR4). This is consistent with a molecular diagnosis rate of WES in a large cohort of patients referred for evaluation of suspected genetic diseases21,22. Rates varied with clinical phenotype, and the highest was for the specific neurological group22. Nevertheless, approximately 70% of patients do not have mutation in the known EA genes, suggesting a genetic heterogeneity of EA and presence of additional causative EA genes.
EA2 is the most common subtype of EA. It is caused by mutations in CACNA1A that encodes Cav2.1, the α1 subunit of the P/Q-type voltage-gated Ca2+ channel. The α1 subunit is composed of four homologous domains (I-IV), each of which contains six transmembrane segments (S1-S6) and an additional pore loop located between S5 and S623. This channel is widely expressed in Purkinje and granule cells of the cerebellum, and mediates Ca2+ entry into cells and regulates the precision of pace-making. Thus, CACNA1A mutations lead to decreased Ca2+ entry through Cav2.1 and the irregular firing of Purkinje cells. Most mutations in EA2 predict premature truncation of the protein, but some non-truncating mutations have also been reported12,14,24,25. Presently, we identified nine mutations in CACNA1A. Two of them were previously reported as a disease-causing mutation (p.Arg1549TER) and rare variant (p.Asp2202_Arg2205DEL) respectively19,20. The remaining seven were novel mutations, and a missense mutation identified in patient 4 (p.Tyr248Asn) was remarkable because a different missense mutation at the same position had been previously reported (p.Tyr248Cys)26. Two patients (patient 5 and 15) had truncating and non-truncating mutation, but each truncating mutation may be strongly associated with loss of channel function. Indeed, the in-frame deletion of patient 15 has been suggested as a potential genetic modifiers in subjects with Dravet syndrome19.
Our CACNA1A mutations mostly involve critical regions for channel activity. Typically, most CACNA1A mutations are widely scattered along the entire gene, but missense mutations usually involve S5-S6 linkers and their borders of each domain. S5-S6 linkers with S5 and S6 form the pore region of the channels, and play a critical role in ion selectivity and permeation of the Ca2+ channel23. Our two missense mutations (p.Gly677Glu, p.Tyr248Asn) also involved the pore region (S5-S6) of Domain II and I, respectively, that may interrupt channel gating activity. Another two mutations (p.Glu998Gln, p.Gly1108Argfs40TER) were located in the intracellular linker connecting Domain II and III. This loop interacts with SNARE proteins that are essential for docking of synaptic vesicles with the presynaptic membrane23. Three nonsense mutations (p.Arg1549TER, p.Arg1669TER, p.Gln1986TER) were localized behind of Domain III and predicted a premature termination within Domain IV or C-terminal domain. The C-terminal domain is a critical region for the Ca2+ channel function by G protein regulation and binding of Ca2+/Calmodulin, so a truncation of C-terminal may lead to loss of Ca2+ channel function. Most truncating mutations including those of this study are preferentially placed in the second half of the α1 subunit, and this suggests the genetic defect may cause loss of function by dominant negative effect2,11,18,27. However, the haploinsufficiency hypothesis was also proposed because large deletion or shortest truncating mutation with only Domain I intact cannot have a dominant negative effect on normal allele11,28.
EA6 is caused by mutations in SLC1A3, that encode excitatory amino acid transporter 1 (EAAT1), a glial glutamate transporter. Since EAAT1 is responsible for glutamate uptake in synapses, the SLC1A3 mutation leads to excessive extracellular accumulation of glutamate and neurotoxic insults29. UBR4 was recently identified as a candidate gene of EA810. It encodes an ubiquitin-protein ligase that interacts with Ca2+ bound calmodulin in the cytoplasm30. It may also regulate the release of internal Ca2+ stores via an inositol triphosphate receptor associated with SCA 15 and 2930. Thus, the UBR4 mutation may result in abnormal Ca2+ signaling within the neuron and development of ataxia. Nevertheless, both EA6 and EA8 have been reported in only a few patients because they have not been widely explored as a usual target for EA screening5,6,10. This study identified three SLC1A3 and four UBR4 mutations, and most of them involve functional domains of each protein. Interestingly, three were detected along with CACNA1A mutation in identical patients. It should be considered that one mutation of the two genes may be a non-pathogenic variant. However, it is also possible that SLC1A3 and UBR4 may contribute primarily to the development of EA and act as genetic modifiers with synergic effects on the abnormal presynaptic activity caused by CACNA1A mutations. This effect may influence the phenotypic differences or variable penetrances31. Roles of modifier genes have been described in several disorders, such as spinal muscular atrophy, retinitis pigmentosa, and familial amyotrophic lateral sclerosis31. In this study, patients with only SLC1A3 or UBR4 mutations showed typical EA2-like symptoms, but had late-onset of age compared to those with the additional CACNA1A mutation. The roles of SLC1A3 and UBR4 as modifier genes in EA may require further investigation.
Of the 28 patients without mutations in five EA genes, we additionally detected three variants with potential pathogenicity in FHM genes, such as ATP1A2 and SCN1A. FHM is a rare subtype of migraine with aura. Attacks are associated with transient hemiparesis due to mutations in three genes: CACNA1A (FHM1), ATP1A2 (FHM2), and SCN1A (FHM3)32. ATP1A2 encodes the α2 subunit of the Na+/K+-ATPase33, while SCN1A encodes the α1 subunit of the Na+ channel34. Along with other EA genes, they are involved in modulation of ion fluxes in glutamatergic presynaptic terminals, and regulate the release of glutamate in the synapse. The result is a significant clinical overlap between EA and FHM, and many patients with FHM show cerebellar symptoms and signs. EA2 and FHM1 are allelic disorders caused by mutations in CACNA1A 35. Furthermore, clinical spectrums of ATP1A2 or SCN1A mutations have been expanded to alternating hemiplegia of childhood, basilar migraine, childhood epilepsy, and progressive hearing loss with migraine36–40. Recently, a new missense mutation in ATP1A2 was identified in a patient with highly similar symptoms to EA215. Our patients who had ATP1A2 or SCN1A mutations also showed recurrent ataxia, vertigo/dizziness, and interictal nystagmus without hemiplegic migraine. Especially, one patient with ATP1A2 mutation had mainly cerebellar symptoms and signs with bilateral cerebellar hypometabolism on FDG-PET during attacks. These findings lead to potential clinical confusion in differential diagnosis of EA from FHM based on genetic study. Thus, screening of FHM genes may be a clinically valuable approach to establish a genetic confirmation of EA.
In addition, we identified rare variants in the genes associated with SCAs; TTBK2 (SCA 11), KCND3 (SCA 19), FGF14 (SCA 27), and TGM6 (SCA 35). Although SCAs are characterized by progressive cerebellar ataxia, some subtypes may present with fluctuating ataxia like EA. Some patients with SCA6 can show the classic features of EA2 because SCA6 and EA2 are allelic disorders caused by mutation in CACNA1A 1,2. Furthermore, different phenotypes ranging from SCA6 to EA2 can co-occur within the same family41,42. Mutations in FGF14 have been also reported in autosomal dominant EA and fever-triggered EA43,44. Besides SCA genes, many genes encoding the ion channels, transporters, or synaptic proteins play crucial roles in regulating neural excitability in the central nervous system. They are often associated with episodic neurological disorders, such as epilepsy, migraine, and paroxysmal movement disorders. In these disorders, several overlapping syndromes have been described, of which EA has been reported as one of various phenotypes1. Recently, late-onset EA has been proposed as the spectrum of SCN2A-associated phenotypes45,46. In addition, other genes including ATP1A3, NALCN, DARS2, SLC2A1, and PRRT2 have been also reported as causative genes associated with EA47–51. Therefore, extensive searches for these genes may help define novel candidate genes and identify new mutations associated with EA.
This study had several limitations. A selection bias should be considered in interpreting results. Since many patients showed the recurrent episodes lasting several hours, we could not detect KCNA1 mutation resulting in EA1 characterized by brief episodes of ataxia. We also did not perform a functional study determining pathogenicity of our mutations. Despite the rarity and putative pathogenicity of variants, establishing pathogenicity may be difficult without functional study, especially in sporadic cases. For this reason, possible pathogenic mutations were more common in our study. We were unable to identify new casual genes because trio-based exome sequencing could not be performed due to a refusal of inspection in additional family members.
In conclusion, we have identified genetic variants associated with EA in approximately 46% of patients. Our results reveal the significant diagnostic yield of WES to detect mutations in genes causing EA. Since there are still many EA patients without genetic confirmations, further assessments for candidate genes are needed.
Methods
Subjects
We recruited 39 unrelated patients with clinically-diagnosed EA from 2012 to 2016 at seven hospitals in Korea. Inclusion criteria were the repetitive spells of ataxia or vertigo/dizziness of variable duration. All patients had no expansions of coding CAG repeats in genes that underline spinocerebellar ataxia 1, 2, 3, 6, 7, 8, and 17. Patients included 19 men and 20 women with age ranging from 4 to 69 years (mean age 41.7 ± 16.0 years). Age of onset varied and ranged from early childhood to the seventh decades (mean age of onset 32.9 ± 17.1 years). Fourteen had a significant family history of EA. All showed recurrent episodes of ataxia or vertigo/dizziness with a clear onset and resolution of symptoms. Episodes usually lasted for several minutes to hours, and were commonly triggered by physical and emotional stress. Twenty-nine had interictal nystagmus, such as either gaze-evoked nystagmus and/or downbeat nystagmus. Of 29 patients treated with acetazolamide, 20 showed decrease in frequency and severity of episodes.
All experiments followed the tenets of the Declaration of Helsinki, and informed consent was obtained after the nature and possible consequences of this study had been explained to the participants. This study was approved by the institutional review boards of Pusan National University Yangsan Hospital. Because the present study included all consecutive patients during the research period, one family previously reported was included in this report52.
Molecular analysis
Genomic DNA was extracted from the blood sample of each patient. Quality and quantity of extracted DNA were measured using NanoDrop® (Invitrogen Life Technologies; Milan, Italy) and Qubit® (Invitrogen Life Technologies) platform. WES was conducted using the Ion Torrent platform (Life Technologies), according to the manufacturer’s specifications. Briefly, 20ng of purified genomic DNA were used for library construction with the Ion AmpliSeq Exome Panel (Life Technologies). Emulsion PCR was conducted with the OneTouch DL system (Life Technologies). Quality of the obtained library was evaluated by qPCR Quantitation (Corbett: QIAGEN). Sequencing was run on the Ion Torrent Proton (Life Technologies) loaded with a P1 chip as per the manufacturer’s protocol. Data analysis including alignment to the hg19 human reference genome and variant calling was conducted using the Torrent Suite Software v.4.2 (Life Technologies). Filtered variants were annotated using both the Ion Reporter software v4.2 (Life Technologies). Variants causing non-synonymous amino acid changes, stop codons, in-frame insertions/deletions in coding regions, or changes to splice site sequences in exon/intron boundaries were identified. Common variants with minor allele frequency (MAF) > 0.001 that represented in dbSNP147, the Exome Aggregation Consortium (ExAC), the 1000 Genomes Project, and the NHLBI GO Exome Sequencing Project (ESP) 6500 were excluded.
Mutation screening and identification
We adopted a stepwise approach to identify genetic mutation responsible for EA (Supplementary Table). First, we screened for presence of pathogenic variants in EA genes registered on Online Mendelian Inheritance in Man (OMIM). These included KCNA1 (EA1, OMIM 176260), CACNA1A (EA2, OMIM 601011), CACNB4 (EA5, OMIM 601949), SLC1A3 (EA6, OMIM 600111), and UBR4 (EA8, OMIM 609890). If no pathogenic variants were identified in EA genes, we next explored genes known or suggested to cause EA as a part of phenotype in the literature. These included SCN2A 45,46, ATP1A3 47, NALCN 48, DARS2 49, SLC2A1 50, FGF14 43,44, and PRRT2 51. Typically, mutations in these genes lead to epilepsy, neurodegenerative disorders, or paroxysmal dyskinesia as the primary phenotype. We also considered genes associated with familial hemiplegic migraine (FHM) as candidates, because ATP1A2 (FHM2, OMIM 182340) and SCN1A (FHM3, OMIM 182389) play a significant role in excitatory neurotransmission with EA genes within the neuronal and glial membranes, and there are significant clinical overlaps between EA and FHM1,2. Finally, we screened all known genes associated with cerebellar ataxia based on previous literature and OMIM records53. All variants detected by the above process were annotated for previously reported disease-causing variants using the Human Gene Mutation Database (HGMD) and Korean Personal Genome Project (KPGP) information. Pathogenicity of non-synonymous variants was analyzed using the following predictive software: Sorting Intolerant From Tolerant (SIFT), Likelihood Ratio Test (LRT), Polyphen2, and MutationTaster. Conservation at the base position was evaluated using Genomic Evolutionary Rate Profiling (GERP). All variants were further confirmed by PCR-based direct sequencing, and were screened in 150 Korean controls.
Electronic supplementary material
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2013R1A1A2006640, NRF-2015R1D1A1A01060559). We thank the patients and their family for participating in our study.
Author Contributions
K.-D. Choi, as the first authors, contributed to the interpretation of the data, analysis of the data, and drafting the manuscript. J.-H. Choi, as corresponding author, contributed to the design and conceptualization of the study, and revising the manuscript. J.-S. Kim, H.-J. Kim, I. Jung, S.-H. Jeong, S.-H. Lee, D.-U. Kim, S.-H. Kim, S.-Y. Choi, D.-S. Kim and K.-P. Park, contributed to acquisition of the data. J.-H. Shin and H.-S. Kim perfomed the experiment.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14254-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi KD, Choi JH. Episodic Ataxias: Clinical and Genetic Features. J Mov Disord. 2016;9:129–135. doi: 10.14802/jmd.16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jen JC, et al. CINCH investigators. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain. 2007;130:2484–2493. doi: 10.1093/brain/awm126. [DOI] [PubMed] [Google Scholar]
- 3.Tomlinson SE, Hanna MG, Kullmann DM, Tan SV, Burke D. Clinical neurophysiology of the episodic ataxias: insights into ion channel dysfunction in vivo. Clin Neurophysiol. 2009;120:1768–1776. doi: 10.1016/j.clinph.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Escayg A, et al. Coding and noncoding variation of the human calcium-channel beta4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am J Hum Genet. 2000;66:1531–1539. doi: 10.1086/302909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jen JC, Wan J, Palos TP, Howard BD, Baloh RW. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology. 2005;65:529–534. doi: 10.1212/01.WNL.0000172638.58172.5a. [DOI] [PubMed] [Google Scholar]
- 6.de Vries B, et al. Episodic ataxia associated with EAAT1 mutation C186S affecting glutamate reuptake. Arch Neurol. 2009;66:97–101. doi: 10.1001/archneurol.2008.535. [DOI] [PubMed] [Google Scholar]
- 7.Damji KF, et al. Periodic vestibulocerebellar ataxia, an autosomal dominant ataxia with defective smooth pursuit, is genetically distinct from other autosomal dominant ataxias. Arch Neurol. 1996;53:338–344. doi: 10.1001/archneur.1996.00550040074016. [DOI] [PubMed] [Google Scholar]
- 8.Steckley JL, Ebers GC, Cader MZ, McLachlan RS. An autosomal dominant disorder with episodic ataxia, vertigo, and tinnitus. Neurology. 2001;57:1499–1502. doi: 10.1212/WNL.57.8.1499. [DOI] [PubMed] [Google Scholar]
- 9.Kerber KA, Jen JC, Lee H, Nelson SF, Baloh RW. A new episodic ataxia syndrome with linkage to chromosome 19q13. Arch Neurol. 2007;64:749–752. doi: 10.1001/archneur.64.5.749. [DOI] [PubMed] [Google Scholar]
- 10.Conroy J, et al. A novel locus for episodic ataxia: UBR4 the likely candidate. Eur J Hum Genet. 2014;22:505–510. doi: 10.1038/ejhg.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantuano E, et al. Identification of novel and recurrent CACNA1A gene mutations in fifteen patients with episodic ataxia type 2. J Neurol Sci. 2010;291:30–36. doi: 10.1016/j.jns.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Denier C, et al. High prevalence of CACNA1A truncations and broader clinical spectrum in episodic ataxia type 2. Neurology. 1999;52:1816–1821. doi: 10.1212/WNL.52.9.1816. [DOI] [PubMed] [Google Scholar]
- 13.Eunson LH, Graves TD, Hanna MG. New calcium channel mutations predict aberrant RNA splicing in episodic ataxia. Neurology. 2005;65:308–310. doi: 10.1212/01.wnl.0000169020.82223.dd. [DOI] [PubMed] [Google Scholar]
- 14.Jen J, Kim GW, Baloh RW. Clinical spectrum of episodic ataxia type 2. Neurology. 2004;62:17–22. doi: 10.1212/01.WNL.0000101675.61074.50. [DOI] [PubMed] [Google Scholar]
- 15.Maksemous N, Roy B, Smith RA, Griffiths LR. Next-generation sequencing identifies novel CACNA1A gene mutations in episodic ataxia type 2. Mol Genet Genomic Med. 2016;4:211–222. doi: 10.1002/mgg3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sintas C, et al. Mutation spectrum in the CACNA1A gene in 49 patients with episodic ataxia. Sci Rep. 2017;7:25142017. doi: 10.1038/s41598-017-02554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JM, Kim JS, Ki CS, Jeon BS. Episodic ataxia type 2 due to a deletion mutation in the CACNA1A gene in a Korean Family. J Clin Neurol. 2006;2:268–271. doi: 10.3988/jcn.2006.2.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Jiang H, Wang Q, Xie Z, Pan S. Identification of a novel nonsense mutation p.Tyr1957Ter of CACNA1A in a Chinese family with episodic ataxia 2. PLoS One. 2013;8:e56362. doi: 10.1371/journal.pone.0056362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohmori I, et al. CACNA1A variants may modify the epileptic phenotype of Dravet syndrome. Neurobiol Dis. 2013;50:209–217. doi: 10.1016/j.nbd.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Jen J, et al. A novel nonsense mutation in CACNA1A causes episodic ataxia and hemiplegia. Neurology. 1999;13:53:34–37. doi: 10.1212/wnl.53.1.34. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, et al. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 24.Mantuano E, et al. Clusters of non-truncating mutations of P/Q type Ca2+ channel subunit Ca(v)2.1 causing episodic ataxia 2. J Med Genet. 2004;41:e82. doi: 10.1136/jmg.2003.015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imbrici P, et al. Late-onset episodic ataxia type 2 due to an in-frame insertion in CACNA1A. Neurology. 2005;65:944–946. doi: 10.1212/01.wnl.0000176069.64200.28. [DOI] [PubMed] [Google Scholar]
- 26.Zafeiriou DI, et al. Episodic ataxia type 2 showing ictal hyperhidrosis with hypothermia and interictal chronic diarrhea due to a novel CACNA1A mutation. Eur J Paediatr Neurol. 2009;13:191–193. doi: 10.1016/j.ejpn.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Page KM, et al. N terminus is key to the dominant negative suppression of Ca(V)2calcium channels: implications for episodic ataxia type 2. J Biol Chem. 2010;285:835–844. doi: 10.1074/jbc.M109.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan J, et al. Large Genomic Deletions in CACNA1A Cause Episodic Ataxia Type 2. Front Neurol. 2011;2:51. doi: 10.3389/fneur.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang J, Amara SG. New views of glutamate transporter structure and function: advances and challenges. Neuropharmacology. 2011;60:172–181. doi: 10.1016/j.neuropharm.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons K, Nakatani Y, Nguyen MD. p600/UBR4 in the central nervous system. Cell Mol Life Sci. 2015;72:1149–1160. doi: 10.1007/s00018-014-1788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kousi M, Katsanis N. Genetic modifiers and oligogenic inheritance. Cold Spring Harb Perspect Med. 5 (2015). [DOI] [PMC free article] [PubMed]
- 32.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 33.De Fusco M, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003;33:192–196. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- 34.Dichgans M, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005;366:371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- 35.Ducros A, et al. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med. 2001;345:17–24. doi: 10.1056/NEJM200107053450103. [DOI] [PubMed] [Google Scholar]
- 36.Vanmolkot KR, et al. Severe episodic neurological deficits and permanent mental retardation in a child with a novel FHM2 ATP1A2 mutation. Ann Neurol. 2006;59:310–314. doi: 10.1002/ana.20760. [DOI] [PubMed] [Google Scholar]
- 37.Al-Bulushi B, Al-Hashem A, Tabarki B. A wide clinical phenotype spectrum in patients with ATP1A2 mutations. J Child Neurol. 2014;29:265–268. doi: 10.1177/0883073813504623. [DOI] [PubMed] [Google Scholar]
- 38.Ambrosini A, et al. Familial basilar migraine associated with a new mutation in the ATP1A2 gene. Neurology. 2005;65:1826–1828. doi: 10.1212/01.wnl.0000187072.71931.c0. [DOI] [PubMed] [Google Scholar]
- 39.Oh SK, et al. A missense variant of the ATP1A2 gene is associated with a novel phenotype of progressive sensorineural hearing loss associated with migraine. Eur J Hum Genet. 2015;23:639–645. doi: 10.1038/ejhg.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harkin LA, et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007;130:843–852. doi: 10.1093/brain/awm002. [DOI] [PubMed] [Google Scholar]
- 41.Pradotto L, et al. Episodic ataxia and SCA6 within the same family due to the D302N CACNA1A gene mutation. J Neurol Sci. 2016;371:81–84. doi: 10.1016/j.jns.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 42.Romaniello R, et al. A wide spectrum of clinical, neurophysiological and neuroradiological abnormalities in a family with a novel CACNA1A mutation. J. Neurol Neurosurg Psychiatry. 2010;81:840–843. doi: 10.1136/jnnp.2008.163402. [DOI] [PubMed] [Google Scholar]
- 43.Choquet K, La Piana R, Brais B. A novel frameshift mutation in FGF14 causes an autosomal dominant episodic ataxia. Neurogenetics. 2015;16:233–236. doi: 10.1007/s10048-014-0436-7. [DOI] [PubMed] [Google Scholar]
- 44.Coebergh JA, et al. A new variable phenotype in spinocerebellar ataxia 27 (SCA 27) caused by a deletion in the FGF14 gene. Eur J Paediatr Neurol. 2014;18:413–415. doi: 10.1016/j.ejpn.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Liao Y, et al. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology. 2010;75:1454–1458. doi: 10.1212/WNL.0b013e3181f8812e. [DOI] [PubMed] [Google Scholar]
- 46.Schwarz N, et al. Mutations in the sodium channel gene SCN2A cause neonatal epilepsy with late-onset episodic ataxia. J Neurol. 2016;263:334–343. doi: 10.1007/s00415-015-7984-0. [DOI] [PubMed] [Google Scholar]
- 47.Heimer G, et al. CAOS-Episodic Cerebellar Ataxia, Areflexia, Optic Atrophy, and Sensorineural Hearing Loss: A Third Allelic Disorder of the ATP1A3 Gene. J Child Neurol. 2015;30:1749–1756. doi: 10.1177/0883073815579708. [DOI] [PubMed] [Google Scholar]
- 48.Aoyagi K, et al. A Gain-of-Function Mutation in NALCN in a Child with Intellectual Disability, Ataxia, and Arthrogryposis. Hum Mutat. 2015;36:753–757. doi: 10.1002/humu.22797. [DOI] [PubMed] [Google Scholar]
- 49.Synofzik M, et al. Acetazolamide-responsive exercise-induced episodic ataxia associated with a novel homozygous DARS2 mutation. J Med Genet. 2011;48:713–715. doi: 10.1136/jmg.2011.090282. [DOI] [PubMed] [Google Scholar]
- 50.Ohshiro-Sasaki A, Shimbo H, Takano K, Wada T, Osaka H. A three-year-old boy with glucose transporter type 1 deficiency syndrome presenting with episodic ataxia. Pediatr Neurol. 2014;50:99–100. doi: 10.1016/j.pediatrneurol.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Gardiner AR, et al. PRRT2 gene mutations: from paroxysmal dyskinesia to episodic ataxia and hemiplegic migraine. Neurology. 2012;79:2115–2121. doi: 10.1212/WNL.0b013e3182752c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi KD, et al. Late-onset episodic ataxia associated with SLC1A3 mutation. J Hum Genet. 2017;62:443–446. doi: 10.1038/jhg.2016.137. [DOI] [PubMed] [Google Scholar]
- 53.Kim JS, Cho JW. Hereditary Cerebellar Ataxias: A Korean Perspective. J. Mov Disord. 2015;8:67–75. doi: 10.14802/jmd.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.