Abstract
With increased usage of cardiovascular drugs (CVDs) for treating cardiovascular diseases, it is important to analyze CVD-associated adverse events (AEs). In this study, we systematically collected package insert-reported AEs associated with CVDs used in China, and developed and analyzed an Ontology of Cardiovascular Drug AEs (OCVDAE). Extending the Ontology of AEs (OAE) and NDF-RT, OCVDAE includes 194 CVDs, CVD ingredients, mechanisms of actions (MoAs), and CVD-associated 736 AEs. An AE-specific drug class effect is defined to exist when all the drugs (drug chemical ingredients or drug products) in a drug class are associated with an AE, which is formulated as a new proportional class level ratio (“PCR”) = 1. Our PCR-based heatmap analysis identified many class level drug effects on different AE classes such as behavioral and neurological AE and digestive system AE. Additional drug-AE correlation tests (i.e., class-level PRR, Chi-squared, and minimal case reports) were also modified and applied to further detect statistically significant drug class effects. Two drug ingredient classes and three CVD MoA classes were found to have statistically significant class effects on 13 AEs. For example, the CVD Active Transporter Interactions class (including reserpine, indapamide, digoxin, and deslanoside) has statistically significant class effect on anorexia and diarrhea AEs.
Introduction
Worldwide, the incidence of cardiovascular diseases has been increasing in recent decades1. Coronary heart disease and stroke remain the two leading causes of death in the poorer regions of the world, including China, which will probably remain unchanged in 20202, though the mortality from cardiovascular disease has declined in the US3 and most Western European countries4–6. In 1998, cardiovascular diseases claimed approximately 2.6 million lives, accounting for 40% of total deaths in China7. In 2014, cardiovascular diseases ranked the first major reason for death in China, accounting for 44.60% in rural areas, and 42.51% in cities8. The prevalence of cardiovascular diseases has been very high in the past decades. Consequently, the usage of cardiovascular drugs (CVDs), which are used to treat cardiovascular diseases, increased fast. A recent study revealed that Chinese hospital utilization of cardiovascular and cerebrovascular drugs increased 3.3-fold between 2006 and 20129.
CVDs may lead to various adverse events (AEs) including serious adverse events (SAEs). In Dutch, 34% of all hospital admissions due to adverse drug events (ADEs) were associated with CVDs10. The rate of AEs induced by CVDs in Iran was 24.2%1. In 2013, 81.3% of suspected drugs responsible for ADEs in China were chemical compounds, of which 10% were drugs used for diseases of the cardiovascular system11. Using two-year clinical data from a tertiary care hospital in India, Palaniappan et al. identified 463 AEs from 397 patients, and 18.1% of the total AEs are related to CVDs12. In USA, FDA AE reporting system (FAERS) is the official drug-associated AE surveillance system13. FAERS has been used to mine AEs associated with CVDs such as statin14. However, no systematic study has been reported in analyzing AEs associated with large-scale CVDs using the FAERS database.
Biomedical ontologies with logical classification hierarchies have emerged and played important roles in knowledge management and data integration compared to vocabulary resources15. Specifically, a biomedical ontology is a human- and computer-interpretable set of terms and relations that represent entities in a specific biomedical domain and how these terms relate to each other. A biomedical ontology is computer-interpretable since the ontology is generated using a standard computer-understandable language such as the Web Ontology Language (OWL; https://www.w3.org/OWL/). A signature usage of ontology is the wide usage of the Gene Ontology (GO)16 to support gene expression data analyses. Since its first publication in 200016, the original GO ontology has been cited by 18,000 publications. The Ontology for Biomedical Investigations (OBI)17, co-developed by over 20 biomedical communities, provides integrative representations of data in various areas of life-science and clinical investigations18–21. Other example usages of ontology include knowledge base construction22, data exchange23, natural language processing24–29, and metadata generation20,30,31.
The Ontology of Adverse Events (OAE) is a biomedical ontology designed from bottom to up to logically represent various AEs observed after medical interventions including drug administration32. Compared to controlled vocabulary terminologies such as the Medical Dictionary for Regulatory Activities (MedDRA)33 and the World Health Organization (WHO)’s Adverse Reaction Terminology (WHO-ART)34, OAE has many advantages such as the inclusion of textual definitions and references, logical axioms, and well-formed hierarchical structure32. Instead of definining an adverse event as an AE as shown in MedDRA and WHO-ART, OAE defines an AE as a bodily pathological process that occurs after a medical intervention and has an unintended outcome of a symptom, a sign, or a pathological process (e.g., infection)32. Such OAE definition logically links the AE with the medical intervention (e.g., drug administration), patient records, adverse health outcome, and temporal relation between the medical intervention and health outcome32,35. Empirical evidences36–39 show that OAE provides a more robust hierarchical structure defintions than MedDRA in terms of AE classification.
Developed by the Veterans Health Administration, the National Drug File - Reference Terminology (NDF-RT) uses a description logic-based, formal reference model that links drugs with active ingredients, groups ingredients into a hierarchy, and categorizes drugs and ingredients into classes of Mechanism of Actions (MoA)40. The combinatorial usage of OAE, and NDF-RT would allow the mapping and study of the relations among AEs, drugs, chemical elements, and drug mechanisms of action.
In pharmacovigilance, the term drug “class effect” was first used to describe the efficacy of beta-blockers in reducing total mortality for myocardial infarction41, and the term is now taken to mean the same or similar therapeutic or adverse effects of a “class” of drugs, i.e., drugs in the same group of chemical structures, mechanisms of action, or pharmacological effects42. To identify drug class effects, the levels of evidence from clinical experiments used to compare the efficacy and safety of drugs within the same class have been proposed43. Biomedical studies have found various beneficial class effects of drugs in terms of treatments. For example, beta-blockers exert a possible class effect in treating acute myocardial infarction44, and angiotensin converting enzyme-inhibitors (ACEIs) in treating heart failure patients45. It is also known that angiotensin receptor blockers have beneficial effects on glucose and lipid metabolism46. Adverse class effects of drugs also exist. A 2015 study uses visual and computational methods to explore the contribution of individual drugs to the class signal using MEDLINE literature data47. In this article, aligned with the previous definitions43–45,47–49, a class effect of drugs on a specific AE is also defined as an effect where all drugs in a defined class (or called category or group) are associated with the same AE. Identification of class effects of drugs on specific ADEs is important to drug development and patient treatment.
In this study, using drug package inserts and ontology-based technologies, we systematically studied the patterns of AEs associated with all available CVDs in China with a focus on drug class effect analysis.
Methods
CVD-specific AE data extraction
The names of active ingredients of all CVDs were first extracted from the Chinese textbook of New Pharmacology (17th version)49. Using these ingredient names, the database from the China Food and Drug Administration (CFDA) (http://app1.sfda.gov.cn/datasearch/face3/dir.html) was searched to identify the product names of the drugs that are manufactured domestically in China or imported abroad to China. The drug package insert documents were retrieved from the CFDA drug administration website (http://www.sda.gov.cn/WS01/CL1038/), or from drug manufacturers’ websites.
The CVDs, associated AEs, and other related information were initially collected using Microsoft Excel files. The section of “Adverse Reactions” of drug package insert documents contains text description of known AEs associated with the CVDs. These AEs were collected and inserted into an Excel file (see Supplemental File F1) with a pre-defined Excel template format. The Excel file also includes other information such as Chinese drug name, Chinese AE term, and English drug name.
English translation of Chinese drug and AE names
English drug names of those CVDs are usually provided by Chinese drug package inserts. Manual English translation was conducted only for those drugs of which English names are unavailable in the package inserts. All Chinese AE names were also manually translated into English. To ensure correctness, all authors manually reviewed the Chinese and English translations until agreements were achieved.
Ontology mapping and new OAE term addition
Translated English AE names were manually mapped to OAE terms. If there was no mapping, the new term was annotated and added into OAE by following the standard OAE term editing procedure32.
English drug names were mapped to NDF-RT (version 01/19/2012). Such mapping enables the query of drug products based on their active ingredient(s), ingredient classification, and the mechanisms of action (MoAs) for different drugs. Most drug names in NDF-RT contain dosage information. However, some cardiovascular drug package inserts do not contain drug dosage information. In this case, we mapped the drug dosage to the lowest dosage of the drug from NDF-RT.
Development and query of OCVDAE
The Ontology of Cardiovascular Drug Adverse Events (OCVDAE) was developed using the format of W3C standard Web Ontology Language (OWL2) (http://www.w3.org/TR/owl-guide/). To develop OCVDAE, we first used OntoFox (http://ontofox.hegroup.org/) to extract two ontology subsets: (1) an OAE ontology subset that includes all the CVD-associated AE terms, and their related terms in OAE; and (2) a NDF-RT ontology subset that includes all the CVDs and associated ingredients and the mechanisms of actions (MoAs). Like OAE, OCVDAE is aligned with the upper level Basic Formal Ontology (BFO)50. For ontology consistency, the extracted NDF-RT terms were also aligned with the BFO structure. Specifically, the drug products and drug ingredients are aligned under BFO:material entity branch, and the MoAs are aligned under the BFO:role branch. After the two ontology subsets are aligned under OCVDAE, we used Ontorat (http://ontorat.hegroup.org/)51 to automatically generate annotations (e.g., Chinese drug names) and axioms that link drugs with AEs in OCVDAE. The input files of the Ontorat operation were the Excel files described above. The Ontorat output OWL files were then directly imported into OCVDAE. The overall ontology results were visualized and manually edited using a Protégé OWL editor.
The OCVDAE source code and related documents have been released to the OCVDAE GitHub website: https://github.com/OCVDAE. The ontology was deposited to the Ontobee website: http://www.ontobee.org/ontology/OCVDAE as well as BioPortal: http://purl.bioontology.org/ontology/OCVDAE.
The OCVDAE information is queriable using SPARQL52. The SPARQL queries can be performed under the Protégé OWL editor, or conducted using the Ontobee SPARQL web page after its deposition in the Ontobee RDF triple store53.
Calculation of drug proportional class level ratio (PCR) for an AE
To calculate the class effect of a drug class on an AE, we designed and calculated a proportional class level ratio (PCR) of drugs in a drug class for a specific AE. Such a PCR score represents the ratio between the number of drugs in a class that are associated with an AE and the total number of drugs in the class. For a specific drug class and a specific AE, the PCR score is defined in Equation (1):
| 1 |
where the numerator is the total number of drugs associated with the AE in the drug class, and the denominator is the total number of drugs in the class. To count if a drug belongs to a drug class or not, mechanisms of action (MoA) hierarchy or related drug ingredient hierarchy as defined in NDF-RT can be utilized. All drugs under a specific ontological hierarchy of a class are considered as a drug under this class. Note that the specific AE can be a bottom level AE or intermediate or top level AE.
Our development of the PCR score is designed to mathematically calculate the drug “class effect”. Specifically, a drug “class effect” on AE is defined as a condition when all drugs in a class have the same AE47. Correspondingly, with the background of the total number of drugs from the same class, PCR calculates the percentage of drugs in the drug class associated with specific AE. Therefore, the drug class effect can be defined as the condition when PCR = 1. It is clear that PCR is critical to the analysis of drug class effect to drug AE. Furthermore, we used the PCR scores for heatmap analysis for better exploration of drug class effects on different AEs.
A drug can mean a drug chemical ingredient or a drug product. Drug chemical ingredient and drug product are different. A drug ingredient is typically matched to one or more drug products. In this study, the drug-AE class effect associations are defined at the ingredient level instead of drug product level. It means that an identified AE associated with an ingredient class occurs in at least one drug product containing the ingredient. For example, different dosage forms (e.g., tablet or lipid solution) of a drug may be associated with different AEs. As long as one dosage form (e.g., tablet) having the drug ingredient is associated with an AE, the ingredient of the drug is counted as a positive association with the AE. However, it is likely that the other dosage form (e.g., lipid solution) for the same drug is not associated with this AE. The study of the dosage form effect on the AE outcome is not within the scope of this study.
PCR-based heatmap exploration of drug class effects
Heatmap analysis was performed using R 3.1.3 to explore the correlation between drug classes and various AE classes from OAE. The hierarchical drug and AE classes were identified using NDF-RT and OAE, respectively. The heatmap was created using n × m count matrix, the value of each cell in the matrix is the PCR corresponding to the specific drug class and AE class. In this way, PCR scores were used to cluster the drug classes and AE classes.
Detection of statistically significant drug class effects on AEs
While a PCR of 1 meets the definition of class effect, such a class effect may not be statistically significant within background of all drugs and all AEs considered. To identify statistically significant drug class effects on AEs, we adopted and modified the traditional methods (e.g., PRR, χ2, and minimal case filtering) of defining specific drug-AE associations.
The proportional reporting ratio (PRR) is a major statistical method for detecting the associations between individual drugs and AEs54. In this study, we extended the PRR algorithm to detect the class effect between drug classes and AEs. Specifically, the drug class level PRR (C-PRR) for a specific AE was computed to measure if a class (or group) of drugs is more associated with the specific AE. A 2-by-2 contingency table was constructed for the C-PRR calculation (Table 1). The number of drugs with an AE and a drug class is defined as a. The number of drugs that belong to all other drug groups and are associated with the AE is assigned as b. The number of drugs belonging to the drug class, but having no association with the AE, is defined as c. The number of drugs unrelated to the AE or the drug class is defined as d. The C-PRR is then defined in equation (2):
| 2 |
Table 1.
A two-by-two contingency table for C-PRR calculation.
| All drugs in a drug class | All drugs in all other drug groups | |
|---|---|---|
| an AE | a (No. of drugs) | b (No. of drugs) |
| Not the AE | c (No. of drugs) | d (No. of drugs) |
| Total | a + c | b + d |
A large C-PRR score of the AE indicates that the drug class-AE association is richly reported compared with other drug class-AE associations available in the database. In our study, all CVDs and all their associated AEs are our database for the calculation. In pharmacovigilance field, PRR usually uses a cutoff of 254. The same cutoff can be used for C-PRR calculation.
Based on the same 2-by-2 contingency table, the Chi-squared score can be calculated and used as a criterion for AE significance test. The cutoff of Chi-squared test is usually >4, which is approximately of P-value of <0.0554. A similar method can be easily used to derive the drug class Chi-squared (C-χ2) score.
An additional filtering method commonly used in drug AE analysis is based on the minimal case reporting number54. Similarly, our class effect analysis includes a cutoff of at least 3 drug ingredients under a specific drug class category for a specific AE.
Results
Overall, this project aimed to systematically collect, ontologically represent, and analyze chemical drugs used to treat cardiovascular diseases (i.e., CVDs) and their associated AEs recorded in package insert documents, and we have focused our analysis on drug class effects on AEs (Fig. 1).
Figure 1.
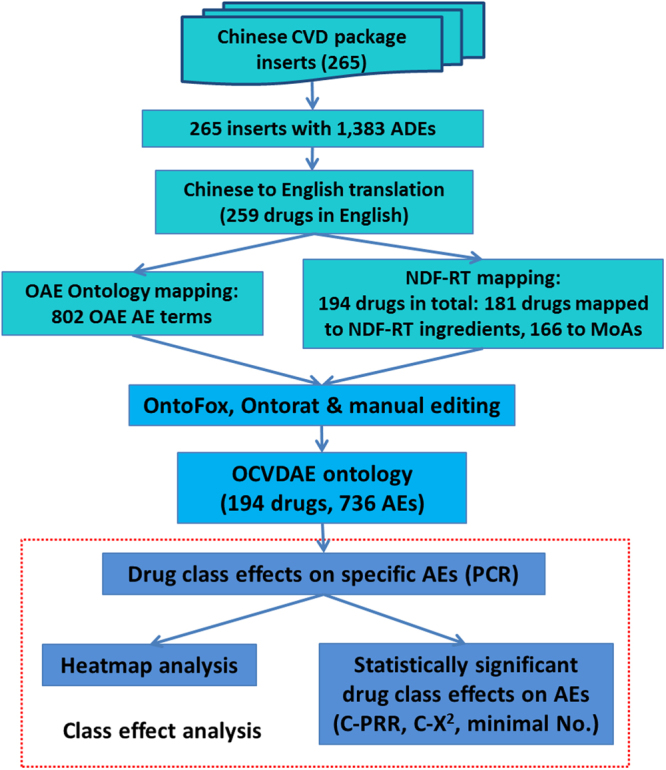
Workflow of our ontology-based cardiovascular drug class level effects on different adverse events (AEs). The results of collected CVDs, associated AEs, and ontology mappings are also labeled in this figure.
Collection and annotation of CVDs and their associated AEs
Our focus on the CVDs used in China market is because the health issue of cardiovascular diseases is very severe in China2,7,8, and the usage of various CVDs to treat cardiovascular diseases has dramatically increased8,9. The development of OCVDAE allowed us to seamlessly integrate different types of data, including CVDs, CVD ingredient classes and MoAs, and AEs, facilitating systematic queries and class effect analysis.
In total, 259 drugs used in China were identified to treat cardiovascular diseases. These CVDs were associated with 1,383 unique AEs in Chinese, corresponding to 802 unique English AE terms (see Supplemental File F1). One unique English AE term may include multiple Chinese translation names. Therefore, the number of the English terms is less than the number of Chinese terms. Among the 802 unique AE terms, 391 AEs already existed in OAE, and 411 AEs were added into OAE as new terms. In total 194 drugs can be mapped to NDF-RT, of which 181 drugs can be mapped to ingredients and associated classes, and 166 drugs can be mapped to MOA and associated classes. Supplemental File F1 is a master data Excel file that contains all the collected information of CVDs, NDF-RT terms and IDs of these CVDs, CVD-associated AEs, and OAE terms and IDs of these AEs.
To better understand the top AEs associated with licensed CVDs, the hierarchical structure of the top 10 AEs was extracted from OAE and visualized using the Protégé OWL editor (Fig. 2). Half of the top 10 most frequently reported AEs belong to behavioral and neurological AEs, followed by 3 digestive system AEs (i.e., diarrhea, nausea, and vomiting AE), one skin AE (e.g., rash), and one cardiovascular AE (i.e., hypotension). Note that hypotension raises alert for clinical use of CVDs in patients with the same condition.
Figure 2.
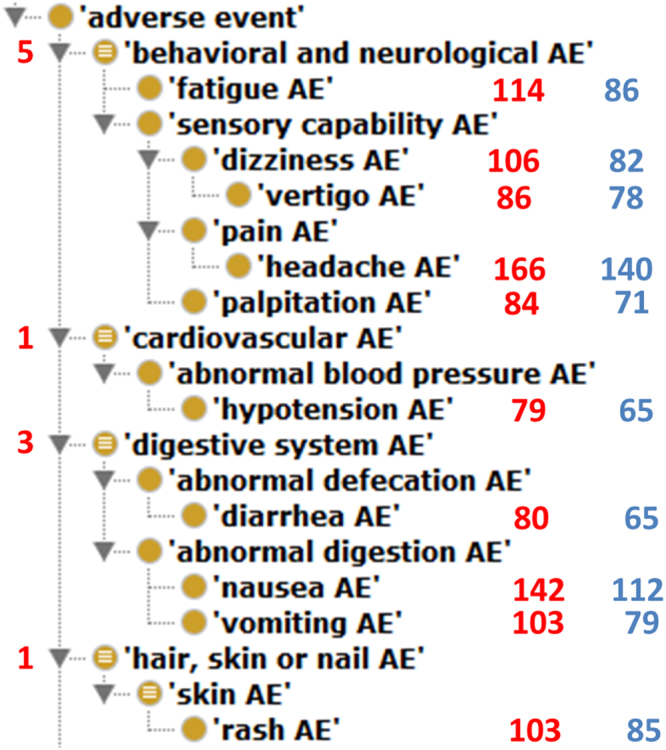
Classification of top 10 AEs associated with CVDs. These OAE terms are visualized using Protégé OWL editor. The left-side digits represent the numbers of specific AEs directly associated with CVDs. The right-side numbers represent the numbers of drugs associated with corresponding AEs. The red color-highlighted right-side numbers come from the total of 259 CVD drugs identified in our study. The blue color -highlighted numbers come from the total of 194 CVD drugs that were mapped to NDF-RT and included in the OCVDAE ontology.
The OCVDAE: Systematic representation of CVD-specific AE information
The purpose of generating OCVDAE is to ontologically represent and seamlessly link all the information of CVDs, CVD ingredients, CVD mechanisms of action, and CVD-specific AEs at different hierarchical levels. The machine-readable OCVDAE can be reused and serve as a platform for further CVD-specific AE research.
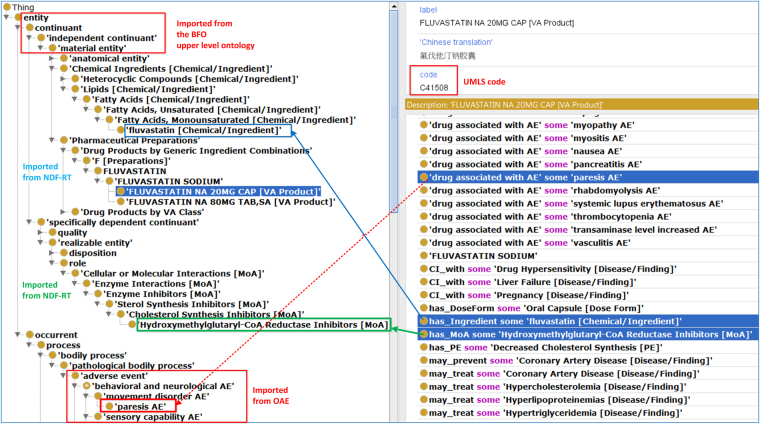
Formatted using the Web Ontology Language (OWL)55 format, our developed OCVDAE ontology includes 194 CVD classes, 2,948 classes and 126 annotation properties. It is noted that since some CVD classes mapped to more than one Chinese name, these 194 CVD drug classes were matched to 198 CVDs defined in Chinese. For these 194 CVD classes, OCVDAE includes corresponding CVD ingredients, MoAs, and associated 736 AEs (Fig. 1). To illustrate the hierarchical structure of OCVDAE, Fig. 3 was generated to show a subset of the OCVDAE that contains two fluvastatin drugs and their related terms, axioms, and hierarchies in OCVDAE. As seen in the figure, OCVDAE imports related terms from OAE32, and NDF-RT40. Like OAE, OCVDAE uses the Basic Formal Ontology (BFO)50 as the upper level ontology.
Figure 3.
Integrative OCVDAE ontology representation of drugs, AEs, drug ingredients, and MoAs. This is a screenshot of Protégé OWL editor of a subset of OCVDAE after OntoFox extraction of two Fluvastatin drugs and their associated terms from OCVDAE. As shown in this figure, OCVDAE imports many terms from NDF-RT and OAE and uses the BFO as the upper level ontology. Each drug (e.g., Fluvastatin NA 20 MG CAP) is associated with its ingredient, MoA, and specific AE terms (e.g., paresis AE). These terms and their parent upper level terms are organized in a well-defined hierarchy in OCVDAE.
OCVDAE knowledge queries and analysis
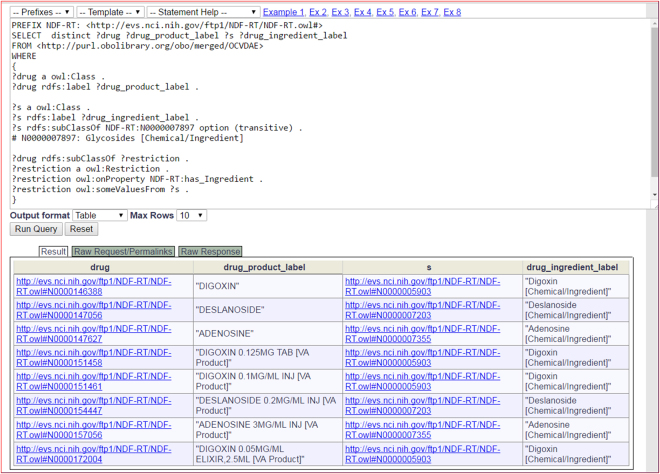
The contents of the OCVDAE OWL file were expressed with Resource Description Framework (RDF) triples and stored in the Ontobee RDF triple store53. The RDF data model makes statements about resources in the form of subject-predicate-object expressions (i.e., triples). The SPARQL RDF query language52 is used to retrieve data stored in a RDF triple store. Figure 4 provides an example of querying the OCVDAE ontology using the Ontobee SPARQL query web page (http://www.ontobee.org/sparql)53. In this example, the SPARQL script was developed to query the chemical ingredients under the Glycosides class and their associated drug products (Fig. 4). Similar scripts can be developed to query other information in OCVDAE.
Figure 4.
OCVDAE SPARQL script query example. The Ontobee SPARQL server53 was used to perform this query that identified drug-ingredient pairs with the condition of ingredients under the branch of NDF-RT “Glycosides [Chemical/Ingredient]” (N0000007897). This query returned 8 results, all related to digoxin, deslanoside, and adenosine.
Analysis of CVD drug class effects on AEs based on drug ingredient classification
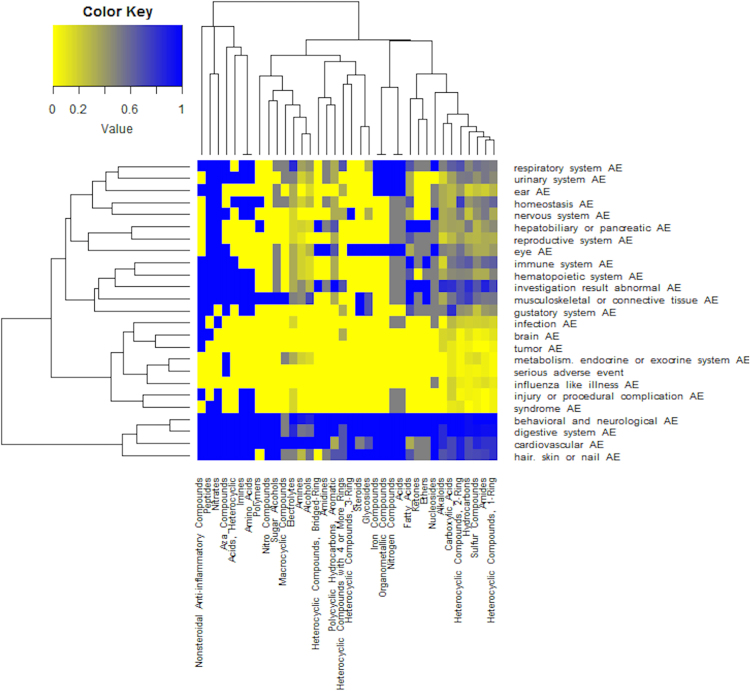
To illustrate our drug class effect analysis using drug ingredient classification, we generated the Fig. 5 heatmap that shows how 36 top level NDF-RT ingredient classes are related to AE classes based on the PCR scores. This level of 36 ingredient classes (e.g., “Fatty Acids”) is located at the third layer starting from “Chemical Ingredients” in NDF-RT (Fig. 3). Each of these 36 ingredient classes, e.g., Fatty Acids, includes its own hierarchy of chemical ingredients at different levels. A drug’s active ingredient (e.g., fluvastatin) usually is located at the bottom (or leaf) level of such a hierarchy (Fig. 3). Figure 3 also illustrates how our ontology represents the relation between a drug and its chemical ingredient component (or other pharmacological classes like MoAs).
Figure 5.
Heatmap analysis between 36 ingredient classes and AE classes based on PCR. Yellow, purple and blue are ordered from low to high number.
These ingredient classes include 181 drugs used to treat cardiovascular diseases. The use of PCR scores in the heatmap allowed us to clearly visualize which drug class-AE associations represent the class effects. Specifically, since PCR = 1 indicates that the drug class effect, those cells with PCR = 1 represent class effects of drug classes on corresponding AEs or AE classes.
According to Fig. 5, 32 out of 36 ingredient classes have a class effect on behavioral and neurological AE, and 26 of 36 ingredient classes on digestive system AE. Many class effects on AEs are found from different ingredient classes, e.g., “Ethers” ingredient class and Electrolytes class (Fig. 5). Glycosides and other 12 ingredient class such as Iron Compounds and Peptides were found to have vision blurred AE.
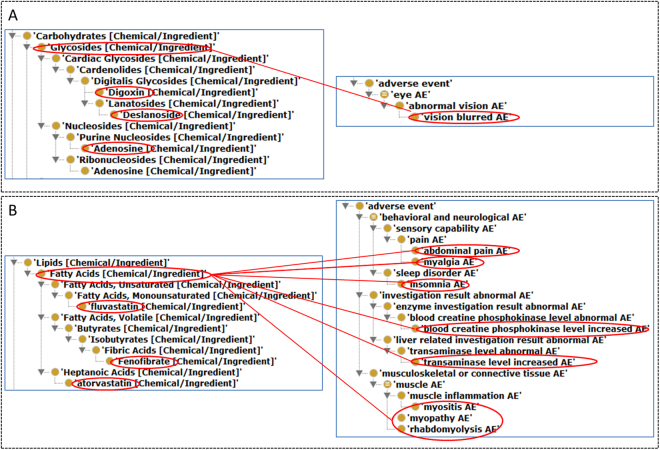
When the three additional criteria (i.e., C-PRR, Chi-squared, and drug number filtering) were used, only 2 of these 36 ingredient classes, i.e., Glycosides class (Fig. 6A) and Fatty Acids (Fig. 6B), were statistically significantly (Supplemental Table S1). As shown in Fig. 6A, Glycosides class (which has 3 ingredients: digoxin, deslanoside, and adenosine) has a class effect on vision blurred AE, which indicates that each of these 3 drug ingredients has an association with vision blurred AE. Furthermore, such a class effect is found to be statistically significant based on the three other factors: C-PRR, χ 2, and minimal drug number filtering.
Figure 6.
Statistically significant CVD class effects on different AEs based on 36 CVD ingredient classification. (A) Statistically significant class effect of glycosides on vision blurred AE, meaning that each of all three drugs under this class (i.e., adenosine, deslanoside, and digoxin) is statistically significantly associated with the AE. (B) Statistically significant class effects of fatty acids on 8 AEs. Only three drug ingredients (i.e., atorvastatin, fenofibrate, and fluvastatin) are placed under the fatty acids class in NDF-RT. Each of these ingredients is statistically significantly associated with each of these 8 AEs. See the text for more detail.
Similarly, as shown in Fig. 6B and Supplemental Table S1, the fatty acids class has a class effect on 8 AE classes such as insomnia, abdominal pain, myopathy, myalgia, and myositis AEs. According to the NDF-RT, the fatty acid drug class of all the collected CVDs includes three ingredients: atorvastatin, fluvastatin, and fenofibrate (Fig. 6B). These three ingredients have been used for lowering lipids in patients.
Analysis of CVD drug class effects on AEs based on drug MoA classification
Insights were gained by examining the association of NDF-RT mechanisms of action (MoA) with drugs. Specifically, our OCVDAE analysis identified a total of 26 NDF-RT MoA classes that are related to 166 CVDs (Supplemental Table S2). These 26 NDF-RT MoA classes are organized under the MoA class of ‘Cellular or Molecular Interactions’, which includes four direct subclasses: Biological Macromolecular Activity, Enzyme Interactions, Physiochemical Activity, and Receptors Interactions. Our study found that 97 CVDs are associated with Receptor Interactions, which further includes 8 drugs for active transporter interactions, 32 drugs for G-protein-linked receptor interactions, and 57 drugs for ion channel interactions. Among 33 CVDs for the Enzyme Interactions MoA class, 31 belong to the Enzyme inhibitors class, which includes 4 drugs for nucleic acid synthesis inhibitors, 1 for phosphodiesterase inhibitors, 11 for protease inhibitors, and 11 for sterol synthesis inhibitors (Supplemental Table S2).
Our MoA-based class effect analysis identified that 3 specific CVD MoA classes, each MoA class associated with different drug ingredients, have statistically significant class effects on 5 AEs (Table 2). Specifically, the Active Transporter Interactions MoA class has a class effect on anorexia and diarrhea AEs. The Active Transporter Interactions class includes 4 ingredients, i.e., reserpine, indapamide, digoxin, and deslanoside (Table 2). The Angiotensin-converting Enzyme Inhibitors class, which also includes 4 drug ingredients (e.g., enalapril) (Table 2), has a class effect on both cough increased and angioedema AEs (Table 2). In addition, the Cholesterol Synthesis Inhibitors class, which has 5 statin-related ingredients (Table 2), has a class effect on myalgia AE. It should be noted that the difference exists in that the class level effect is on specific class levels. For example, at the chemical structure level the CVD Lipids class includes three ingredients: atorvastatin, aluvastatin, and fenofibrate. While at the mechanism level, 5 statin-related ingredients (i.e., simvastatin, rosuvastatin, pravastatin, fluvastatin, and atorvastatin) are classified to have the same mechanism as Cholesterol Synthesis Inhibitors. NDF-RT classifies different drugs under these two categories. Different classifications may result in different results.
Table 2.
CVD class effect based on CVD MOA classification.
| MOA class and ingredients | AE | Ingredient No. | C-PRR | χ2 | PCR |
|---|---|---|---|---|---|
| Active Transporter Interactions (reserpine, indapamide, digoxin, and deslanoside) | anorexia AE | 4 | 3.05 | 7.28 | 1 |
| diarrhea AE | 4 | 2.10 | 4.13 | 1 | |
| Cholesterol Synthesis Inhibitors (simvastatin, rosuvastatin, pravastatin, fluvastatin, and atorvastatin) | myalgia AE | 5 | 4.00 | 12.19 | 1 |
| Angiotensin-converting enzyme inhibitors (enalapril, captopril, benazepril, and perindopril) | cough increased AE | 4 | 5.55 | 14.21 | 1 |
| angioedema AE | 4 | 5.55 | 14.21 | 1 |
Discussion
The contributions of this study are multiple. First, we for the first time systematically collected CVDs and annotated their corresponding AEs. Second, we developed an ontology OCVDAE to ontologically represent the CVD-AE information. Third, we developed and applied a combinatorial strategy to calculate statistically significant drug class-AE associations. Fourth, such a combinatorial strategy was applied to identify many scientific insights in terms of the AE patterns associated with CVDs at the drug class level.
To our best knowledge, our work represents the first study on collecting CVD-associated AEs from drug package insert information and ontologically representing and studying these AEs. Most studies on AEs of CVDs belong to retrospective studies without randomized and well-controlled clinical experiments, which primarily used clinical records of patients56–58, or belong to prospective case-series study that was based on information collected by interviewing patients, reviewing patients’ charts, laboratory test monitoring, and confirmation of physicians59. Existing studies mostly focused on AEs associated with specific classes of CVDs, i.e., cutaneous adverse reactions or suicide risk of calcium channel blockers56,60, or overall AEs of a specific class of CVDs, i.e., the retrospective evaluation of adverse drug reactions induced by antihypertensive treatment58. The systematic study of adverse reactions induced by CVDs at one hospital in Iran used prospective case-series method59. Different from these retrospective and prospective studies, our package insert data collection came from well-controlled randomized clinical trials, therefore providing accurate knowledge on drug-associated AEs. Meanwhile, exploratory studies are necessary in order to detect unknown drug AEs. Therefore, the evidences extracted from package insert documents and identified from spontaneously reported case reports or new clinical studies are complementary to each other.
OCVDAE is the first ontology targeted to represent AEs associated with drugs used to treat a category of diseases, i.e., cardiovascular diseases. Previously we have developed an Ontology of Drug Neuropathy Adverse Events (ODNAE), which ontologically represents all drugs leading to a specific category of AEs, i.e., neuropathy AEs61. Therefore, OCVDAE and ODNAE study drug AEs from two different aspects. It is noted that both OCVDAE and ODNAE extend OAE. To describe and organize liver injury induced by drugs, a drug-induced liver injury (DILI) ontology (DILIo)62. DILIo uses the Unified Medical Language System (UMLS) tool and the standardized terminology of the Systematized Nomenclature of Medicine-Clinical Terms (SNOMED CT). However, unlike OCVDAE or ODNAE, DILIo does not provide one-to-one associations between drugs and DILI histophological terms62. ADEpedia is a standardized knowledge base of AE using semantic web technology (38), which integrates general aspects of AEs and associated drugs. However, ADEpedia is not organized as an integrated ontology, which differs from our OCVDAE strategy.
The naturally hierarchical OCVDAE structure and knowledge provide an ideal framework for systematically analyzing the class effects of drugs on AEs. First of all, OCVDAE provides different hierarchical levels of class categorization of drugs, ingredients, and MoAs, and AEs. These hierarchical classes allow us to analyze class effects at different levels and with different class categorization. OCVDAE directly links drugs to AEs, allowing advanced queries and analyses. Second, we developed the mathematic PCR score to calculate class effects of drug classes on different AEs based on different drug hierarchies (e.g., ingredients, and MoAs). The PCR score is aligned with the general class effect definition, i.e., an effect of all drugs in a defined class having on the same AE. The PCR score provides a simple but powerful method to study class effects. For example, our PCR-based heatmap analysis allowed us to clearly visualize different class effects (Fig. 5).
In addition to the PCR-based class effect calculation and heatmap analysis, we further modified classical AE-drug association methods (i.e., C-PRR, C-χ2, and minimal number of drugs in a drug class) to identify statistically significant class effects. For the statistical drug class effect analysis, the background of C-PRR and C-χ2 is the total number of CVDs and all possible AEs associated with these CVDs with different drug classes. The C-PRR/C-χ2 methods test whether the specific AE is significantly associated with the specific drug class compared to all other AEs and all other drug classes in the database. We also use a minimal number of drugs in a drug class as a cutoff, which reflects the traditional method of using minimal case reporting number filtering for defining specific drug-AE association54. Such a combinatorial method provides a feasible strategy for detecting statistically significant drug class effect for specific AEs.
Our study differs in many ways from another study of drug class effect analysis using MEDLINE literature data and heatmap data analysis47. In that study, Winnenburg et al. extracted drug-AE pairs from MEDLINE by aggregating drugs into the Anatomical Therapeutic Chemical (ATC) classes and drug AEs into high-level MeSH terms. The association between drugs and AEs at the drug class level was explored using heatmaps and k-means clustering based on the PRR values that specified the significance of AE-drug associations47. In comparison, our study differs in data source, standard terminology, and method to identify class effect. Instead of using the MEDLINE literature, we used drug package insert data. Our semantic system used OAE for AE terms/hierarchy and NDF-RT for drugs, drug ingredients, and MoA classes. For the heatmap and clustering analysis, the biggest difference between these two methods is that our method uses the PCR while their method uses PRR. Since PRR does not include the information of drug classification, the PRR-based heatmap and k-means clustering analysis could only approximately and indirectly explore class effects (which rely on drug classification). Instead of using PRR values, we directly used the PCR scores that provide a simple and accurate method for directly calculating drug class effects on AEs based on drug ingredient or MoA classifications. Furthermore, we calculated and applied class level PRR (i.e., C-PRR), Chi-square (i.e., C-χ2) and minimal number filtering to generate statistically significant drug class effects on different AEs and AE classes.
In addition to the PRR and Chi-square methods used in this study for drug-AE association analysis, there are also other reported statistical methods, including the Bayesian Confidence Propagation Neural Network (BCPNN)63,64, Gamma Poisson Shrinkage (GPS)65, Reporting Odds Ratio (ROR)66, Reporting Fisher’s Exact Test (RFET)67. An R package PhViD68 is also developed to include these methods. All these methods are developed for general drug-AE association studies. In comparison, our PCR method is designed specificially for drug class effect analysis. The PCR value of 1 indicates a class effect result. Since PCR does not test statistical significance, the combined useage of PCR and statistical methods (e.g., PRR and Chi-square in this study) will allow the detection of statistical significant drug class effects.
Our findings showed that CVDs are associated with cardiac disorder AE significantly. Hypotension AE and palpitation AE are among the top 10 most frequently reported AEs (Fig. 2). Previous studies also revealed the potential of having hypotension AE following treatment with cardiovascular drugs, e.g., verapamil (a drug to treat hypertension)69,70. Nifedipine, a calcium antagonistic drug for treating high blood pressure, was also associated with short lasting palpitation event without arrhythmias71. Our findings confirmed the alert of potential risks of cardiac disorder AEs after the administration of CVDs.
Our OCVDAE-based drug class effect analysis identified many insightful drug class effects on different levels of AEs based on the drug ingredient or MoA classifications. For example, our statistically significant class effect analysis found that out of 36 CVD ingredient classes, the Glycosides class and Fatty Acids class was found to have class effects on one and eight AEs, respectively (Fig. 6). Three Glycosides class ingredients (i.e., digoxin, deslanoside, and adenosine) are all associated with blurred eye AE. The association between deslanoside (or adenosine) and blurred eye AE has not been reported in the literature; however, digoxin has been reported to be associated with blurred eye AE in peer-reviewed publications72,73. In such a class level analysis, drug classes were based on NDF-RT hierarchical definition, and AE or AE classes were derived from the OAE ontology.
Our statistical analysis found three MoA drug classes having class effects on different AEs (Table 2). Our MoA-based study found that the 4 drug ingredients under the MoA class of angiotensin-converting enzyme inhibitors have a class effect on increased cough and angioedema AEs (Table 2). Interestingly, a 2013 report has clearly indicated that angioedema and cough are the two most important adverse effects of angiotensin-converting enzyme inhibitors74. In addition, our study found that 5 statin-related ingredients (i.e., simvastatin, rosuvastatin, pravastatin, fluvastatin, and atorvastatin), which have the mechanism as Cholesterol Synthesis Inhibitors, all have the class effect on myalgia AE. Our result is consistent with many literature reports. For example, it has been reported that statin-associated muscle symptoms, which most often consist of myalgia, may occur in more than 10% of patients using statins75,76. Muscle toxicity caused by the statin has been focused, such as myalgia, myopathy and rhabdomyolysis77–79. Furthermore, myalgia is found to be the most common adverse event of statin use according to the review of MEDLINE database English articles80. While the mechanism by which statins produce muscle injuries is still unclear, reduction of the cholesterol content of skeletal muscle membrane is considered as a possible mechanism81,82. The other possible mechanisms include mitochondrial mechanisms83 and inhibition of farnesyl pyrophosphate (an intermediate for the production of ubiquinone (Coenzyme Q10))81. All these mechanisms are likely interconnected. For example, coenzyme Q is a member of the electron transport system of the inner mitochondrial membrane where it converts the energy in carbohydrates and fatty acids into ATP to drive cellular machinery and synthesis84, and treatment with Coenzyme Q10 significantly affects cholesterol production85. A recent report indicates that Coenzyme Q10 protects against statin-induced myotoxicity in zebrafish larvae86. How these individual mechanisms are interconnected to cause statin-induced myotoxicity deserves further investigations.
Our study also found that the Active Transporter Interactions drug class (with 4 ingredients) have a class effect on anorexia and diarrhea AEs (Table 2). The Active Transporter Interactions drug class includes 4 drug chemical ingredients: reserpine, indapamide, deslanoside, and digoxin. The finding of such a class effect on anorexia and diarrhea AEs means that each of the 4 chemical ingredients in the drug ingredient class has been found to be associated with these two AEs according to the package insert data we have collected. Such a finding suggests that some active transporter interaction(s), triggered by these drug ingredients, might participate in the formation of anorexia and diarrhea AEs. For example, reserpine is able to bind to the catecholamine transport system of synaptic vesicles and deplete catecholamines from peripheral sympathetic nerve endings87. Catecholamines regulate appetite control which is closely related to anorexia88. Deslanoside inhibits the Na-K-ATPase membrane pump, resulting in an increase in intracellular sodium and calcium concentrations89. The imbalance of electrolytes (including sodium and calcium) may result in anorexia. It has been found that anorectic patients have altered erythrocyte Na-K-ATPase pump90. Metabolic turnover of Na-K-ATPase may be regulated by catecholamines and other hormones91. Indapamide is an inhibitor of the the Na-Cl co-transporter (NCCT). The chloride depletion can cause electrolyte perturbations, hypokalemic alkalosis, and anorexia92. While digoxin-induced anorexia mechanism is unclear, digoxin-induced toxicity (including anorexia) is often elevated when digoxin is combined with other drugs (e.g., quinidine)89. Digoxin is a substrate of P-glycoprotein, an efflux transporter found throughout the epithelial cells of the intestine. It was found that quinidine inhibits the P-glycoprotein in the intestine and at sites of digoxin elimination (e.g., kidney)89. Therefore, digoxin-induced anorexia is often due to a drug-drug interaction that affects digoxin elimination and absorption through the P-glycoprotein efflux transporter system. Therefore, these four chemical ingredients (i.e., reserpine, indapamide, deslanoside, and digoxin) all interact with some transporters, which might lead to anorexia AE. By integrating the possible anorexia formation mechanisms induced by different drugs as described above, we hypothesize that these drugs induce anorexia by interacting with some components of an integrative transporter-mediated pathway, which likely includes a chain of transporter-mediated interactions that regulate the metabolic levels and activities of catecholamine and electrolytes. However, one drug may interact with an off-target receptor or transporter, and there might be other mechanisms of these drugs that contribute to the anorexia AE. Therefore, more experimental investigations are required to test this hypothesis and the role of the drug-transporter interactions in anorexia AE formation.
Our innovative technique revealed drug class effects based on the structural level as shown in the Fig. 5 heatmap and Mechanisms of actions level (Table 2). In our studies, it appears that the classification based on the Mechanisms of actions is more useful to generate plausible hypotheses, for example, our hypothesis about the Active Transporter Interactions drugs that have a class effect on anorexia and diarrhea. Meanwhile, our structure-based classification was only focused on the top two level structure classes. It is possible that class effects based on more specific and bioactive structural classification may become more useful, which can be further explored in the future.
Our study represents the first ontology-based methodology that identifies drug class effects on adverse events. Most existing drug class effect studies focus on the drug therapeutic effects. Since no systematical reference textbook or electronic resource is available to introduce drug class effects on certain adverse events, our study also provides the first source of summarized drug class effects on adverse events.
In our future work, we may examine the effects of different variables (e.g., age, gender, and drug dose form) on the class effect outcomes93. These variables may change the health outcomes and the classification of drug class effects on AEs. For example, a Korean study, analyzed the patterns of ADEs in different age groups and showed that CVDs, among other drugs, was reported more frequently as causative drugs for ADEs in the elderly94. Clear sex differences between women and men (54% vs. 46%, respectively) were seen in a Dutch study in hospital admissions for ADEs due to CVDs10. In addition, different drug forms (e.g., capsule vs tablet) may induce different AEs. A detailed examination of these variables to drug class effects will be very important for precision medicine.
In the future, we will also use our OCVDAE ontology as a platform to further add updated information to explain the basic mechanisms of CVD-associated AEs. For example, a new publication by Imbrici-2017 et al.95 discloses ClC-K channels as a novel target of the AT1 receptor blockers valsartan and olmesartan. The ion Channel Interactions [MoA] in OCVDAE does not mention the ClC-K chloride channels. To explain the AEs of valsartan and olmesartan by this mechanism of action, we can improve OCVDAE and include the reported reference.
Our current study focused on the PCR score of 1, which represents a class effect, i.e., all drugs in the drug class being associated with an AE. In the future, we may also examine the scenario with a PCR score less than 1, which indicates that only a portion of the drugs in a drug class are associated with a corresponding AE. The portion of the drugs may have its special meaning under different conditions; for example, this portion of drugs may be classified into a more specific drug subclass. The higher the PCR score, the more likely the drugs in a drug class are associated with a corresponding AE or AE class.
In this study, we focused our analysis of package insert documents of the CVDs used in China market. Our future work may also focus on the systematic analysis of class effects of different CVDs or other drug classes used in USA and European markets using package insert documents or other types of drug AE related data. The knowledge obtained will be added to OCVDAE. We will also periodically update OCVDAE when new information of drug AEs is obtained.
While package inserts contain the AE information for the patients, the Summaries of product characteristics is the information for the doctors, which is available in the European Union)96. Since the Summaries of product characteristics is not available in China, we did not use such information. In the future, we will consider the collection of the Summaries of product characteristics in Europe and use them for our class level effect drug adverse event studies.
FAERS contains publically available data of over 2 million drug AE case reports97. Compared to package insert information, the FARES data were spontaneously reported by customers, distributor or health professionals, etc., and can be registered by arbitrary names, including trade names, abbreviations, and even typographical errors. Therefore, the FAERS data can be quite noisy and difficult to analyze. For Such FAERS data analysis, we envision that the conventional drug-AE correlation tests (i.e., PRR, Chi-squared, and minimal case reports) can be performed first, followed by a PCR analysis.
Conclusion
In this study, we systematically collected cardiovascular drugs (CVDs) used in China and CVD-associated AEs from package insert documents, generated the OCVDAE ontology by reusing terms from the OAE and NDF-RT and adding CVD-AE associations, and systematically analyzed drug class effects on AEs based on OCVDAE knowledge. For the class effect analysis, we for the first time derived a PCR score based on the class effect definition, and calculated and applied PCR scores for heatmap visualization and identification of drug class effects. We also modified classical AE tests to generate the methods of class level PRR (C-PRR), Chi-square (C-χ2), minimal number of drugs in a drug class for a specific AE (or AE class). These methods were used to identify statistically significant class effects at the drug ingredient class level and mechanisms of action class level. The usage of our new methods also identified many scientific insights, facilitating knowledge integration and exploration.
Electronic supplementary material
Acknowledgements
The study was supported by National Natural Science Foundation of China (NSFC #81601574) to LW, Graduate Innovation Fund of Jilin University (#2016110) to ML, and National Institute of Allergy and Infectious Diseases (NIH-NIAID 1R01AI081062) to YH. We also appreciate the anonymous reviewers’ insightful comments and suggestions.
Author Contributions
All co-authors are justifiably credited with authorship, according to the authorship criteria. Final approval is given by each co-author. In detail: L.W. – design, development, data collection, analysis of data, interpretation of results, and drafting of the manuscript; M.L. – data collection, analysis of data, interpretation of results; J.X. – data collection; Y.C. – analysis of data; H.L.- critical revision of manuscript; Y.H. – conception, design, development, analysis of data, interpretation of results, and critical revision of manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12580-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liwei Wang, Email: wang.liwei@mayo.edu.
Yongqun He, Email: yongqunh@umich.edu.
References
- 1.Mohebbi N, Shalviri G, Salarifar M, Salamzadeh J, Gholami K. Adverse drug reactions induced by cardiovascular drugs in cardiovascular care unit patients. Pharmacoepidemiology and drug safety. 2010;19:889–894. doi: 10.1002/pds.1916. [DOI] [PubMed] [Google Scholar]
- 2.Beaglehole R. Global cardiovascular disease prevention: time to get serious. The Lancet. 2001;358:661–663. doi: 10.1016/S0140-6736(01)05784-1. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, et al. Explaining the decrease in US deaths from coronary disease, 1980–2000. New England Journal of Medicine. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 4.Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–1107. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- 5.Laatikainen T, et al. Explaining the decline in coronary heart disease mortality in Finland between 1982 and 1997. American journal of epidemiology. 2005;162:764–773. doi: 10.1093/aje/kwi274. [DOI] [PubMed] [Google Scholar]
- 6.Björck L, Rosengren A, Bennett K, Lappas G, Capewell S. Modelling the decreasing coronary heart disease mortality in Sweden between 1986 and 2002. European heart journal. 2009;30:1046–1056. doi: 10.1093/eurheartj/ehn554. [DOI] [PubMed] [Google Scholar]
- 7.Zhao SP, Hu MJ. JP. The analysis of population mortality rate and causes of death in urban and rural areas of China. Chin J Health Stat. 1999;16:276–280. [Google Scholar]
- 8.Chen Weiwei GR, et al. Hu Shengshou. Report on Cardiovascular Disease in China. Chinese Circulation Journal. 2016;31:521–528. [Google Scholar]
- 9.Zeng W, et al. Analysis of the influence of recent reforms in China: cardiovascular and cerebrovascular medicines as a case history to provide future direction. Journal of comparative effectiveness research. 2014;3:371–386. doi: 10.2217/cer.14.28. [DOI] [PubMed] [Google Scholar]
- 10.Rodenburg EM, Stricker BH, Visser LE. Sex differences in cardiovascular drug‐induced adverse reactions causing hospital admissions. British journal of clinical pharmacology. 2012;74:1045–1052. doi: 10.1111/j.1365-2125.2012.04310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CFDA Annual report on adverse drug event surveillance in China (2013) Chinese Journal of Drug Evaluation. 2014;31:254–256. [Google Scholar]
- 12.Palaniappan, M. et al. Pattern of Adverse Drug Reactions Reported with Cardiovascular Drugs in a Tertiary Care Teaching Hospital. Journal of clinical and diagnostic research: JCDR9, FC01 (2015). [DOI] [PMC free article] [PubMed]
- 13.Harpaz, R., Haerian, K., Chase, H. S. & Friedman, C. In AMIA Annu Symp Proc. 281–285. [PMC free article] [PubMed]
- 14.Sakaeda, T., Kadoyama, K. & Okuno, Y. Statin-Associated Muscular and Renal Adverse Events: Data Mining of the Public Version of the FDA Adverse Event Reporting System. Plos One6, doi:ARTN e2812410.1371/journal.pone.0028124 (2011). [DOI] [PMC free article] [PubMed]
- 15.Bodenreider, O. Biomedical ontologies in action: role in knowledge management, data integration and decision support. Yearbook of medical informatics 67 (2008). [PMC free article] [PubMed]
- 16.Ashburner M, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkman RR, et al. Modeling biomedical experimental processes with OBI. Journal of biomedical semantics. 2010;1(Suppl 1):S7. doi: 10.1186/2041-1480-1-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng J, Manduchi E, Stoeckert CJ., Jr. Development of an Application Ontology for Beta Cell Genomics based On the Ontology for Biomedical Investigations. The 4th International Conference on Biomedical Ontology (ICBO-2013) 2013;1060:62–67. [Google Scholar]
- 19.Brinkman RR, et al. Modeling biomedical experimental processes with OBI. Journal of Biomedical Semantics, June 22. 2010;21(Suppl 21):S27. doi: 10.1186/2041-1480-1-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dugan VG, et al. Standardized metadata for human pathogen/vector genomic sequences. PloS one. 2014;9:e99979. doi: 10.1371/journal.pone.0099979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarntivijai S, et al. CLO: The Cell Line Ontology. Journal of biomedical semantics. 2014;5:37. doi: 10.1186/2041-1480-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcos E, Zhao B, He Y. The Ontology of Vaccine Adverse Events (OVAE) and its usage in representing and analyzing adverse events associated with US-licensed human vaccines. Journal of biomedical semantics. 2013;4:40. doi: 10.1186/2041-1480-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demir E, et al. The BioPAX community standard for pathway data sharing. Nature biotechnology. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettembourg C, Diot C, Burgun A, Dameron O. GO2PUB: Querying PubMed with semantic expansion of gene ontology terms. Journal of biomedical semantics. 2012;3:7. doi: 10.1186/2041-1480-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doms, A. & Schroeder, M. GoPubMed: exploring PubMed with the Gene Ontology. Nucleic acids research33, W783–786, doi:33/suppl_2/W783 [pii]10.1093/nar/gki470 (2005). [DOI] [PMC free article] [PubMed]
- 26.Plake C, Royer L, Winnenburg R, Hakenberg J, Schroeder M. GoGene: gene annotation in the fast lane. Nucleic acids research. 2009;37:W300–304. doi: 10.1093/nar/gkp429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hur J, Ozgur A, Xiang Z, He Y. Identification of fever and vaccine-associated gene interaction networks using ontology-based literature mining. Journal of biomedical semantics. 2012;3:18. doi: 10.1186/2041-1480-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hur J, Xiang Z, Feldman EL, He Y. Ontology-based Brucella vaccine literature indexing and systematic analysis of gene-vaccine association network. BMC immunology. 2011;12:49. doi: 10.1186/1471-2172-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozgur A, Xiang Z, Radev DR, He Y. Mining of vaccine-associated IFN-gamma gene interaction networks using the Vaccine Ontology. Journal of biomedical semantics. 2011;2(Suppl 2):S8. doi: 10.1186/2041-1480-2-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Beltran A, Maguire E, Sansone SA, Rocca-Serra P. linkedISA: semantic representation of ISA-Tab experimental metadata. BMC bioinformatics. 2014;15(Suppl 14):S4. doi: 10.1186/1471-2105-15-S14-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malladi, V. S. et al. Ontology application and use at the ENCODE DCC. Database: the journal of biological databases and curation2015, doi:10.1093/database/bav010 (2015). [DOI] [PMC free article] [PubMed]
- 32.He Y, et al. OAE: The Ontology of Adverse Events. Journal of biomedical semantics. 2014;5:29. doi: 10.1186/2041-1480-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannangelo, K. Healthcare code sets, clinical terminologies, and classification systems. (AHIMA, American Health Information Management Association, 2006).
- 34.Littell, R. C., Milliken, G. A., Stroup, W. W. & Wolfinger, R. D. SAS system for mixed models. (SAS Institute Inc., 1996).
- 35.Sarntivijai, S. et al. Linking MedDRA-Coded Clinical Phenotypes to Biological Mechanisms by the Ontology of Adverse Events: A Pilot Study on Tyrosine Kinase Inhibitors. Drug safety, doi:10.1007/s40264-016-0414-0 (2016). [DOI] [PMC free article] [PubMed]
- 36.Sarntivijai, S. et al. Ontology-based combinatorial comparative analysis of adverse events associated with killed and live influenza vaccines. PloS one7, e49941, doi:10.1371/journal.pone.0049941 PONE-D-12-19530 [pii] (2012). [DOI] [PMC free article] [PubMed]
- 37.Xie J, Codd C, Mo K, He Y. Differential adverse event profiles associated with BCG as a preventive tuberculosis vaccine or therapeutic bladder cancer vaccine identified by comparative ontology-based VAERS and literature meta-analysis. PloS one. 2016;11:e0164792. doi: 10.1371/journal.pone.0164792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie J, Zhao L, Zhou S, He Y. Statistical and ontological analysis of adverse events associated with monovalent and combination vaccines against hepatitis A and B diseases. Scientific reports. 2016;6:34318. doi: 10.1038/srep34318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y. Ontology-based vaccine and drug adverse event representation and theory-guided systematic causal network analysis toward integrative pharmacovigilance research. Curr Pharmacol Rep. 2016;2:113–128. doi: 10.1007/s40495-016-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Q, Jiang G, Chute CG. Profiling structured product labeling with NDF-RT and RxNorm. J. Biomedical Semantics. 2012;3:16. doi: 10.1186/2041-1480-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turi ZG, Braunwald E. The use of β-blockers after myocardial infarction. Jama. 1983;249:2512–2516. doi: 10.1001/jama.1983.03330420058038. [DOI] [PubMed] [Google Scholar]
- 42.Soares I, Carneiro AV. Drug class effects: definitions and practical applications. Revista portuguesa de cardiologia: orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology: an official journal of the Portuguese Society of Cardiology. 2002;21:1031–1042. [PubMed] [Google Scholar]
- 43.McAlister FA, Laupacis A, Wells GA, Sackett DL, Grp E-BMW. Users’ Guides to the Medical Literature - XIX. Applying clinical trial results - B. Guidelines for determining whether a drug is exerting (more than) a class effect. Jama-Journal of the American Medical Association. 1999;282:1371–1377. doi: 10.1001/jama.282.14.1371. [DOI] [PubMed] [Google Scholar]
- 44.Lin, T.-T., Chan, K. A., Chen, H.-M., Lai, C.-L. & Lai, M.-S. Class effect of beta-blockers in survivors of ST-elevation myocardial infarction: A nationwide cohort study using an insurance claims database. Scientific reports5 (2015). [DOI] [PMC free article] [PubMed]
- 45.Yancy CW, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Ernsberger P, Koletsky RJ. Metabolic actions of angiotensin receptor antagonists: PPAR-gamma agonist actions or a class effect? Current opinion in pharmacology. 2007;7:140–145. doi: 10.1016/j.coph.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winnenburg, R., Sorbello, A. & Bodenreider, O. Exploring adverse drug events at the class level. Journal of Biomedical Semantics 6, doi:ARTN1810.1186/s13326-015-0017-1 (2015). [DOI] [PMC free article] [PubMed]
- 48.Annigeri RA, Mani RM. Acute interstitial nephritis due to statin and its class effect. Indian Journal of Nephrology. 2015;25:54. doi: 10.4103/0971-4065.136883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen, X. et al. New pharmacology. 17th version edn, 34–156 (Beijing: People’s Health Publishing House, 2011).
- 50.Arp, R., Smith, B. & Spear, A. D. Building Ontologies Using Basic Formal Ontology. (Cambridge, MA, USA, 2015).
- 51.Xiang Z, Zheng J, Lin Y, He Y. Ontorat: automatic generation of new ontology terms, annotations, and axioms based on ontology design patterns. Journal of biomedical semantics. 2015;6:1. doi: 10.1186/2041-1480-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris, S. & Seaborne, A. SPARQL 1.1 Query Language, W3C Recommendation 21 March 2013. http://www.w3.org/TR/sparql11-query/, accessed on December 26, 2016 (2013).
- 53.Ong E, et al. Ontobee: A linked ontology data server to support ontology term dereferencing, linkage, query and integration. Nucleic acids research. 2017;45:D347–D352. doi: 10.1093/nar/gkw918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans S, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiology and drug safety. 2001;10:483–486. doi: 10.1002/pds.677. [DOI] [PubMed] [Google Scholar]
- 55.W3C. OWL 2 Web Ontology Language Quick Reference Guide (Second Edition), W3C Recommendation 11 December 2012, http://www.w3.org/TR/owl2-quick-reference/. Accessed on December 10, 2016 (2012).
- 56.Tuchinda P, Kulthanan K, Khankham S, Jongjarearnprasert K, Dhana N. Cutaneous adverse reactions to calcium channel blockers. Asian Pacific journal of allergy and immunology / launched by the Allergy and Immunology Society of Thailand. 2014;32:246–250. doi: 10.12932/AP0380.32.3.2014. [DOI] [PubMed] [Google Scholar]
- 57.Britt J, Moffett BS, Bronicki RA, Checchia PA. Incidence of adverse events requiring intervention after initiation of oral beta-blocker in pediatric cardiac intensive care patients. Pediatr Cardiol. 2014;35:1062–1066. doi: 10.1007/s00246-014-0899-1. [DOI] [PubMed] [Google Scholar]
- 58.Rende P. Retrospective evaluation of adverse drug reactions induced by antihypertensive treatment. Journal of pharmacology & pharmacotherapeutics. 2013;4:S47–50. doi: 10.4103/0976-500X.120954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niayesh M, Gloria S, Mojtaba S, Jamshid S, Kheirollah G. Adverse drug reactions induced by cardiovascular drugs in cardiovascular care unit patients. Pharmacoepidemiology & Drug Safety. 2010;19:889–894. doi: 10.1002/pds.1916. [DOI] [PubMed] [Google Scholar]
- 60.Lindberg G, Bingefors K, Ranstam J, Rastam L, Melander A. Use of calcium channel blockers and risk of suicide: ecological findings confirmed in population based cohort study. Brit Med J. 1998;316:741–745. doi: 10.1136/bmj.316.7133.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo A, et al. Ontology-based collection, representation and analysis of drug-associated neuropathy adverse events. Journal of biomedical semantics. 2016;7:1. doi: 10.1186/s13326-016-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang YP, et al. A Unifying Ontology to Integrate Histological and Clinical Observations for Drug-Induced Liver Injury. American Journal of Pathology. 2013;182:1180–1187. doi: 10.1016/j.ajpath.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 63.Bate A, Lindquist M, Orre R, Edwards IR, Meyboom RH. Data-mining analyses of pharmacovigilance signals in relation to relevant comparison drugs. European journal of clinical pharmacology. 2002;58:483–490. doi: 10.1007/s00228-002-0484-z. [DOI] [PubMed] [Google Scholar]
- 64.Bate A, et al. A Bayesian neural network method for adverse drug reaction signal generation. European journal of clinical pharmacology. 1998;54:315–321. doi: 10.1007/s002280050466. [DOI] [PubMed] [Google Scholar]
- 65.DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. The American Statistician. 1999;53:177–190. [Google Scholar]
- 66.van Puijenbroek EP, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiology and drug safety. 2002;11:3–10. doi: 10.1002/pds.668. [DOI] [PubMed] [Google Scholar]
- 67.Ahmed I, et al. False discovery rate estimation for frequentist pharmacovigilance signal detection methods. Biometrics. 2010;66:301–309. doi: 10.1111/j.1541-0420.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed, I. & Poncet, A. PhViD: an R package for pharmacovigilance signal detection. R package version 1.6 (2013).
- 69.Holdgate, A. & Foo, A. Adenosine versus intravenous calcium channel antagonists for the treatment of supraventricular tachycardia in adults. Cochrane Database Syst Rev4 (2006). [DOI] [PubMed]
- 70.Holdgate, A. & Foo, A. Adenosine versus intravenous calcium channel antagonists for the treatment of supraventricular tachycardia in adults. The Cochrane Library (2012). [DOI] [PubMed]
- 71.Olivari MT, et al. Treatment of hypertension with nifedipine, a calcium antagonistic agent. Circulation. 1979;59:1056–1062. doi: 10.1161/01.CIR.59.5.1056. [DOI] [PubMed] [Google Scholar]
- 72.Nadeem A, Allegretti P. Blurred vision and weakness in a 60-year-old woman. The American journal of emergency medicine. 2010;28:536 e531–532. doi: 10.1016/j.ajem.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 73.Closson RG. Visual hallucinations as the earliest symptom of digoxin intoxication. Archives of neurology. 1983;40:386. doi: 10.1001/archneur.1983.04050060086017. [DOI] [PubMed] [Google Scholar]
- 74.Mahmoudpour SH, et al. Pharmacogenetics of ACE inhibitor-induced angioedema and cough: a systematic review and meta-analysis. Pharmacogenomics. 2013;14:249–260. doi: 10.2217/pgs.12.206. [DOI] [PubMed] [Google Scholar]
- 75.Bitzur R, Cohen H, Kamari Y, Harats D. Intolerance to statins: mechanisms and management. Diabetes care. 2013;36:S325–S330. doi: 10.2337/dcS13-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovascular Drugs and Therapy. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 77.Armitage J. The safety of statins in clinical practice. The Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 78.Beltowski J, Wojcicka G, Jamroz-Wisniewska A. Adverse effects of statins-mechanisms and consequences. Current drug safety. 2009;4:209–228. doi: 10.2174/157488609789006949. [DOI] [PubMed] [Google Scholar]
- 79.Brown WV. Safety of statins. Current opinion in lipidology. 2008;19:558–562. doi: 10.1097/MOL.0b013e328319baba. [DOI] [PubMed] [Google Scholar]
- 80.Ramkumar S, Raghunath A, Raghunath S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiologica Sinica. 2016;32:631. doi: 10.6515/ACS20160611A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. Jama. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 82.Choi HK, Won EK, Choung SY. Effect of Coenzyme Q10 Supplementation in Statin-Treated Obese Rats. Biomolecules & therapeutics. 2016;24:171–177. doi: 10.4062/biomolther.2015.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Golomb BA, Evans MA. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. American journal of cardiovascular drugs: drugs, devices, and other interventions. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manoukian AA, et al. Rhabdomyolysis secondary to lovastatin therapy. Clinical chemistry. 1990;36:2145–2147. [PubMed] [Google Scholar]
- 85.Modi K, Santani DD, Goyal RK, Bhatt PA. Effect of coenzyme Q10 on catalase activity and other antioxidant parameters in streptozotocin-induced diabetic rats. Biological trace element research. 2006;109:25–34. doi: 10.1385/BTER:109:1:025. [DOI] [PubMed] [Google Scholar]
- 86.Pasha R, Moon TW. Coenzyme Q10 protects against statin-induced myotoxicity in zebrafish larvae (Danio rerio) Environmental toxicology and pharmacology. 2017;52:150–160. doi: 10.1016/j.etap.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 87.Lajtha A. Book Review: Basic Neurochemistry: Molecular, Cellular, and Medical Aspects Sixth Edition. Neurochemical Research. 1999;24:973–974. doi: 10.1023/A:1020966216216. [DOI] [Google Scholar]
- 88.Lehnert H, Schrezenmeir J, Beyer J. Central nervous appetite regulation: mechanisms and significance for the development of obesity. Zeitschrift fur Ernahrungswissenschaft. 1990;29:2–12. doi: 10.1007/BF02019529. [DOI] [PubMed] [Google Scholar]
- 89.That, S. D. Drug interactions with digoxin: the role of P-glycoprotein. Pharmacy times45 (2004).
- 90.Pasquali R, et al. Altered erythrocyte Na-K pump in anorectic patients. Metabolism: clinical and experimental. 1985;34:670–674. doi: 10.1016/0026-0495(85)90096-4. [DOI] [PubMed] [Google Scholar]
- 91.Sweadner KJ, Goldin SM. Active transport of sodium and potassium ions: mechanism, function, and regulation. The New England journal of medicine. 1980;302:777–783. doi: 10.1056/NEJM198004033021404. [DOI] [PubMed] [Google Scholar]
- 92.Mathot M, et al. Pseudo-Bartter syndrome in a pregnant mother and her fetus. Pediatric nephrology. 2006;21:1037–1040. doi: 10.1007/s00467-006-0123-5. [DOI] [PubMed] [Google Scholar]
- 93.Harpaz R, et al. Performance of Pharmacovigilance Signal‐Detection Algorithms for the FDA Adverse Event Reporting System. Clinical Pharmacology & Therapeutics. 2013;93:539–546. doi: 10.1038/clpt.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu YM, et al. Patterns of Adverse Drug Reactions in Different Age Groups: Analysis of Spontaneous Reports by Community Pharmacists. PloS one. 2015;10:e0132916. doi: 10.1371/journal.pone.0132916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imbrici P, et al. Pharmacovigilance database search discloses ClC-K channels as a novel target of the AT1 receptor blockers valsartan and olmesartan. British journal of pharmacology. 2017;174:1972–1983. doi: 10.1111/bph.13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arguello B, Salgado TM, Fernandez‐Llimos F. Assessing the information in the Summaries of Product Characteristics for the use of medicines in pregnancy and lactation. British journal of clinical pharmacology. 2015;79:537–544. doi: 10.1111/bcp.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L, Jiang G, Li D, Liu H. Standardizing adverse drug event reporting data. Journal of biomedical semantics. 2014;5:36. doi: 10.1186/2041-1480-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.