Atrial fibrillation prevalence is increasing with age, reaching up to 5% of patients older than 65 years, and is associated with 20%–30% of stroke episodes in that population.[1],[2]
Oral anticoagulation is still currently the reference treatment of thromboembolic risk prevention in patients with atrial fibrillation (AF).[3],[4] In patients with non-valvular AF, percutaneous left atrial appendage closure (LAAC) has emerged as an alternative approach to reduce the risk of stroke.[5]–[9] However, one should worry about the potential hemodynamics consequences of left atrial appendage (LAA) exclusion. The role of the LAA in cardiac hemodynamics has been previously investigated in both animal and human studies.[10]–[15] While percutaneous LAAC and its specific indications are rapidly expanding, we aimed to non-invasively assess the impact of percutaneous LAA occlusion on atrial anatomy and cardiac hemodynamics.
In this prospective study, we included all consecutive patients with non-valvular AF who underwent LAAC in our institution using either the amplatzer cardiac plug (ACP) device (St. Jude Medical, Minneapolis, Minnesota) or the watchman device (Boston Scientific, Natick, Massachusetts). Our local protocol included a complete screening at baseline before the procedure and after three months of follow-up, including clinical evaluation, electrocardiogram transthoracic echocardiography (TTE) and contrast-enhanced cardiac computed tomography (CT). Patients with changes in heart rhythm [from Sinus rhythm (SR) to AF or the other way around] between baseline and post implantation follow up were excluded.
LAAC procedural techniques and TTE assessment of the left ventricular (LV) diastolic function and filling pressures have been previously described.[6],[7],[16] ECG-gated cardiac CT was performed pre-procedurally – for LAA anatomical assessment and to rule out the presence of LAA thrombi – and 3 months post-procedurally – to evaluate device position, device-related thrombus and the presence of residual peri device leaks on a 64–slice CT scanner (Somatom Definition Dual Source®, Siemens Medical Solutions, Erlangen, Germany). LA volume assessment was performed by two experienced independent readers using the OsiriX® software package (OsiriX MD, Pixmeo Inc, Geneva, Switzerland), with a threshold-based region-growing 3D segmentation of the left atrial cavity, as previously described.[17],[18]
Sixty-three patients underwent successful LAAC during the study period. Table 1 summarizes patients' demographic, TTE, and CT data at baseline and after three months of follow-up. After 3 months of follow-up, no recurrent stroke nor other thromboembolic complications were observed. No significant difference was seen regarding patients' functional status (New-York Heart Association I: 33% vs. 34%, P = 0.82; NYHA II: 60.3% vs. 55.7%, P = 0.34; NYHA III: 6.3% vs. 9.8%; P = 0,74), BNP rates (155.6 ± 107 pg/mL baseline vs. 155 ± 150.6 pg/m at three months; P = 0.85), or the daily dose of loop diuretics (69.4 ± 58.1 pg/ml vs 67 ± 50.4 pg/mL; P = 0.16).
Table 1. Patients demographic, echographic and computed demography data at baseline and after three months of follow-up.
| Variable | Baseline | Three months | P |
| Age, yrs | 73.7 ± 8.9 | - | |
| Male Gender | 38 (60.3%) | - | |
| CHA2DS2-VASc score | 4.3 ± 1.3 | - | |
| HASBLED Score | 3.4 ± 0.9 | - | |
| Sinus rhythm/AF | 27/36 | 24/39 | 0.42 |
| Hear rate, beats/min | 70 ± 17 | 72 ± 17 | 0.88 |
| BNP levels, pg/mL | 155 ± 107 | 155 ± 150 | 0.85 |
| Loop diuretic treatment, Yes/No | 9/54 | 12/51 | 0.33 |
| Loop diuretic dose, mg | 69 ± 58 | 67 ± 50 | 0.16 |
| TTE measurments | |||
| LVEF, % | 58 ± 7 | 60 ± 5 | 0.78 |
| Peak mitral E-wave, m/s | 84.2 ± 22.7 | 86.7 ± 26 | 0.62 |
| Peak mitral A-wave, m/s | 65.4 ± 18.4 | 68.5 ± 22.2 | 0.66 |
| Peak lateral E' wave, cm/s | 11.1 ± 2.8 | 10 ± 2.9 | 0.15 |
| E/A ratio | 1.1 ± 0.4 | 1.1 ± 0.6 | 0.96 |
| E/E' ratio | 7.9 ± 2.1 | 9.1 ± 3.6 | 0.03 |
| CT left atrial volume, mL | |||
| Overall population | 145.5 ± 55.4 | 144.3 ± 50.5 | 0.30 |
| Sinus rhythm patients | 99.7 ± 19.1 | 103,.8 ± 21 | 0.32 |
| AF patients | 173.2 ± 54.2 | 171.7 ± 48.6 | 0.59 |
Data was presented as Mean ± SD or n (%). AF: atrial fibrillation. BNP: brain natriuretic peptide; CHA2DS2-VASc: cardiac failure, hypertension, age, diabetes, stroke, vascular disease; HASBLED: hypertension, zbnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol; LVEF: left ventricular ejection fraction. TTE: transthoracic echocardiography.
Five patients who could not have contrast-enhanced cardiac CT due to renal failure were excluded from subsequent analysis. The mean heart rate during CT acquisition did not differ between baseline and follow-up studies neither in the overall population (70 ± 17 vs. 72 ± 17 bpm, P = 0.88), nor in the SR (P = 0.16) and the AF subgroups (P = 0.99). Intra-and inter-observer variability of LA volume measurement was excellent, with an intraclass correlation coefficient of 0.999 (95% CI: 0.996; 1.000) and 0.975 (95% CI: 0.937; 0.991), respectively.
Overall, mean LA volume was 145 ± 55 mL and 144 ± 50 mL at baseline and during follow-up, respectively, showing no statistical difference (P = 0.30) (Figure 1). No difference was seen between baseline and follow-up in SR or AF patient's subgroups (99.7 ± 19 vs. 103.8 ± 21 mL, P = 0.32; 173.2 ± 54 baseline vs. 171.7 ± 48.6 mL, P = 0.59, respectively) (Table 1). No thrombus was seen on the atrial side of the device. Peri-device leaks (defined as presence of dye in LAA beyond the device) were observed in 23 patients (39%) but were trivial or mild.
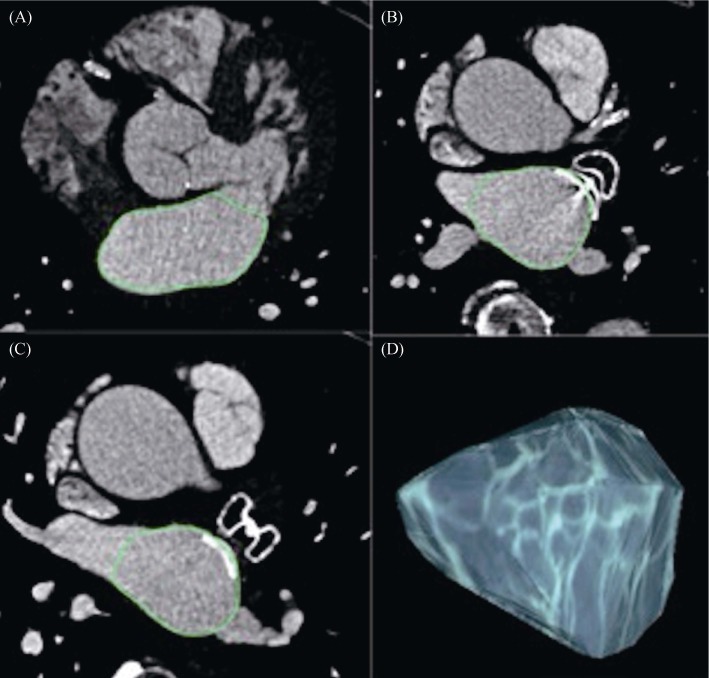
Figure 1. Measurement of left atrial volume using 3D volume threshold-based method of cardiac multidetector CT.
Volumetric assessment was performed on sets of axial images. The outline of the LA was traced manually on each slice, excluding the mitral valve (A) pulmonary veins (B, C) and the LAA (B, C). After selecting all the regions of interest within one series, OsiriX® automatically calculated the volume by multiplying surface and slice thickness and then adding up individual slice volumes. OsiriX® also provided 3D images using the “ROI volume” tool (D). CT: computed tomography; LAA: left atrial appendage; ROI: region of interest.
Regarding TTE assessment of diastolic function, no significant difference was observed in the overall population between baseline and follow-up, except for E/E' ratio which increased after LAA closure (7.9 ± 2.1 vs. 9.1 ± 3.6; P = 0,038). No difference was observed in SR and AF subgroups (Table 1).
The LAA is an embryologic remnant of the left atrium where 90% of thrombi are located in patients with non-valvular AF. It seems to play, among others, a significant haemodynamic role.[10]–[15] Davis, et al.[10] evaluated the compliance of the left atrium with and without the LAA in six isolated canine left atria. They found that the slope of the pressure in the left atrium without the appendage was significantly greater than with the appendage intact. In another animal study, Hoit, et al.[11] similarly showed that the left atrial reservoir volume significantly decreased after appendectomy at matched left atrial pressures. These experimental data suggest that the LAA may play a critical role in maintaining hemodynamic function when filling pressures are elevated. This hypothesis was further confirmed by Tabata and colleagues, who showed that LAA temporary exclusion during cardiac surgery in 15 patients led to an increase in LA pressure.[14] The LAA reservoir function in the presence of LA pressure and/or volume overload was therefore established. Other that its haemodynamic function, the LAA also has an endocrine role, as it contains stretch- sensitive receptors that may influence natriuretic peptide secretion in response to variations of left atrial pressures.[15]
On the other hand, the LAA may have deleterious effects as it is known to be the most common source of embolic stroke in non-valvular AF (up to 90% of cases).[5] Therefore, LAAC has emerged as a valuable therapeutic option for thromboembolism prevention in AF patients with a high bleeding risk. However, the haemodynamic impact of LAA transcatheter closure has not been clearly assessed and raises questions, as percutaneous LAAC is expanding in elderly people (most of them having LV diastolic dysfunction), and may involve younger patients in the future. Indeed, since the study of Hanna and colleagues in 2004 which did not demonstrate a significant impact of the plaato device on the mitral valve and pulmonary veins six months after LAAC, there is no recent study evaluating the possible hemodynamic effect of LAAC.[19] In a series of 11 patients implanted with a plaato device and evaluated using TEE, the authors showed no significant change on LA size, mitral regurgitation severity, and mitral valve peak E-wave velocities between baseline and 6 months after LAAC.
In this preliminary study, we aimed to non-invasively evaluate the impact of LAAC on left sided haemodynamic based on basic imaging parameters including TTE conventional Doppler measurements and CT-derived LA volume. After three months, in patients with stable clinical conditions, we found no significant LA remodelling in the overall population, nor in SR and AF patients' subgroups. However, we demonstrated a significant increase of E/E' ratio, suggesting a possible elevation of LV filling pressures.
By losing the reservoir function of the LAA after closure, we might have expected a more significant post-procedural LA remodelling. Several factors may explain our results: (1) our evaluation was relatively early (three months) regarding the potentially slow remodelling of cardiac chambers and should be repeated after a longer follow-up, (2) the persistence of temporary small atrial septal defect following trans septal puncture is not rare, with an incidence of 20% after six months, and may allow a left-to-right shunt that may impact volume/pressure atrial patterns, which is supported by the observed elevation of TTE E/E' ratio, and (3) the presence of residual peri-device leak, although small and probably without haemodynamic consequences, must be taken into account.[20]
The left ventricular diastolic function depends on many parameters: relaxation and LV compliance, ventricular systolic function, preload and afterload conditions, and LA function.[21],[22] In healthy subjects, LA function contributes 20% to 30% to the LV filling. Echocardiography is the cornerstone for the non-invasive evaluation and quantitation of diastolic function.[23] Peak mitral early filling velocity E (early and passive LV filling) in conjunction with early mitral annular velocity (E') obtained with the use of tissue Doppler imaging (LV myocardial relaxation) and peak mitral A wave (end-diastole atrial contraction) are used to estimate diastolic function and left filling pressures using E/A and E/E' ratios.[23]
In our study, SR patients presented at baseline with a pseudo normal pattern of E/A ratio of 1.1 traducing a decrease in LV myocardial relaxation, which is usually observed in elderly patients. E/A ratio was not modified at three months in the SR group. Conversely, in the overall population, we found a significant increase in E/E' ratio between baseline and three months after LAAC. This trend toward a moderate elevation of LV filling pressures was observed in patients who remained clinically stable, with no modification of diuretics dose nor increase of BNP values. However, one must be cautious with the conclusions drawn from this result for several reasons: first, the mean absolute value of E/E' remained < 12 which is the cut-off indicating elevated LV filling pressures, secondly a recent meta-analysis showed that there was insufficient evidence to support that E/E' ratio can reliably estimate LV filling pressures in patients with preserved ejection fraction, which was the case of our study population.[23],[24]
This preliminary study shows intermediary results but sheds some light on the potential impact of LAAC. Indeed, as this therapy is expanding to a wide population, the potential mid- to long term drawbacks of the technique should be anticipated. This paper also has the merit of opening the door to other works, including for instance acute haemodynamic studies during LAAC. Our results also encourage us to be very cautious when performing LAAC in patients with patent heart failure, whether with preserved or altered LV ejection fraction, and to monitor the possible occurrence of cardiac failure.
This is a non-randomized, hypothesis-generating observational study reporting a prospective single-centre experience. Regarding TTE analysis, other diastolic function parameters such as deceleration time or isovolumetric relaxation time to better refine our results. The LA mechanical function could also have been assessed using speckle tracking. Invasive haemodynamic data could also have been useful to determine the impact of LAAC but it was not ethically possible to perform such a study in this very fragile population, which is why we chose to perform a non-invasive assessment. Finally, the follow-up duration is relatively short and LA remodelling might be longer. The evaluation should therefore be repeated after a longer follow-up period.
In summary, this preliminary study is the first to non-invasively evaluate the left atrial haemodynamic impact of percutaneous LAA closure. We showed no early significant LA remodelling but a trend toward an increase in LV filling pressure. Our results also encourage us to be very cautious when performing LAAC in patients with patent heart failure. This paper also has the merit of opening the door to other works, including for instance acute haemodynamic studies during LAAC and assessment of LA remodelling after a longer follow-up period.
Acknowledgments
This study received financial support from the French Government as part of the ‘Investments of the future’ program managed by the National Research Agency (ANR), Grant reference ANR–10–IAHU–04. Thambo JB and Iriart X act as proctors for St. Jude Medical and Boston Scientific. The other authors have no conflicts of interest to declare.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke. 2014;45:520–526. doi: 10.1161/STROKEAHA.113.003433. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Lai HC, Chien WC, Chung CH, et al. Atrial fibrillation, liver disease, antithrombotics and risk of cerebrovascular events: a population-based cohort study. Int J Cardiol. 2016;223:829–837. doi: 10.1016/j.ijcard.2016.08.297. [DOI] [PubMed] [Google Scholar]
- 5.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 6.Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs. warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988–1998. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 7.Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER cardiac plug. EuroIntervention. 2016;11:1170–1179. doi: 10.4244/EIJY15M01_06. [DOI] [PubMed] [Google Scholar]
- 8.Meier B, Blaauw Y, Khattab AA, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace. 2014;16:1397–1416. doi: 10.1093/europace/euu174. [DOI] [PubMed] [Google Scholar]
- 9.Freixa X, Abualsaud A, Chan J, et al. Left atrial appendage occlusion: initial experience with the Amplatzer™ Amulet™. Int J Cardiol. 2014;174:492–496. doi: 10.1016/j.ijcard.2014.03.154. [DOI] [PubMed] [Google Scholar]
- 10.Davis CA, 3rd, Rembert JC, Greenfield JC, Jr, et al. Compliance of left atrium with and without left atrium appendage. Am J Physiol. 1990;259:1006–1008. doi: 10.1152/ajpheart.1990.259.4.H1006. [DOI] [PubMed] [Google Scholar]
- 11.Hoit BD, Shao Y, Tsai LM, et al. Altered left atrial compliance after atrial appendectomy. Influence on left atrial and ventricular filling. Circ Res. 1993;72:167–175. doi: 10.1161/01.res.72.1.167. [DOI] [PubMed] [Google Scholar]
- 12.Massoudy P, Beblo S, Raschke P, et al. Influence of intact left atrial appendage on hemodynamic parameters of isolated guinea pig heart. Eur J Med Res. 1998;3:470–474. [PubMed] [Google Scholar]
- 13.Hondo T, Okamoto M, Yamane T, et al. The role of the left atrial appendage. A volume loading study in open-chest dogs. Jpn Heart J. 1995;36:225–234. doi: 10.1536/ihj.36.225. [DOI] [PubMed] [Google Scholar]
- 14.Tabata T, Oki T, Yamada H, et al. Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am J Cardiol. 1998;81:327–332. doi: 10.1016/s0002-9149(97)00903-x. [DOI] [PubMed] [Google Scholar]
- 15.Regazzoli D, Ancona F, Trevisi N, et al. Left Atrial Appendage: Physiology, Pathology, and Role as a Therapeutic Target. Biomed Res Int. 2015;2015:205013. doi: 10.1155/2015/205013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Christiaens L, Varroud-Vial N, Ardilouze P, et al. Real three-dimensional assessment of left atrial and left atrial appendage volumes by 64-slice spiral computed tomography in individuals with or without cardiovascular disease. Int J Cardiol. 2010;140:189–196. doi: 10.1016/j.ijcard.2008.11.055. [DOI] [PubMed] [Google Scholar]
- 18.Scolozzi P, Jaques B. Computer-aided volume measurement of posttraumatic orbits reconstructed with AO titanium mesh plates: accuracy and reliability. Ophthal Plast Reconstr Surg. 2008;24:383–389. doi: 10.1097/IOP.0b013e318185a72c. [DOI] [PubMed] [Google Scholar]
- 19.Hanna IR, Kolm P, Martin R, et al. Left atrial structure and function after percutaneous left atrial appendage transcatheter occlusion (PLAATO): six-month echocardiographic follow-up. J Am Coll Cardiol. 2004;43:1868–1872. doi: 10.1016/j.jacc.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 20.Alkhouli M, Sarraf M, Zack CJ, et al. Iatrogenic atrial septal defect following transseptal cardiac interventions. Int J Cardiol. 2016;209:142–148. doi: 10.1016/j.ijcard.2016.02.068. [DOI] [PubMed] [Google Scholar]
- 21.Appleton CP, Galloway JM, Gonzalez MS, et al. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22:1972–1982. doi: 10.1016/0735-1097(93)90787-2. [DOI] [PubMed] [Google Scholar]
- 22.Maragiannis D, Nagueh SF. Echocardiographic evaluation of left ventricular diastolic function: an update. Curr Cardiol Rep. 2015;17:3. doi: 10.1007/s11886-014-0561-9. [DOI] [PubMed] [Google Scholar]
- 23.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 24.Sharifov OF, Schiros CG, Aban I, et al. Diagnostic accuracy of tissue Doppler index E/è for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002530. [DOI] [PMC free article] [PubMed] [Google Scholar]