Abstract
Detection of motion is a feature essential to any living animal. In vertebrates, mechanosensory hair cells organized into the lateral line and vestibular systems are used to detect external water or head/body motion, respectively. While the neuronal components to detect these physical attributes are similar between the two sensory systems, the organizational pattern of the receptors in the periphery and the distribution of hindbrain afferent and efferent projections are adapted to the specific functions of the respective system. Here we provide a concise review comparing the functional organization of the vestibular and lateral line systems from the development of the organs to the wiring from the periphery and the first processing stages. The goal of this review is to highlight the similarities and differences to demonstrate how evolution caused a common neuronal substrate to adapt to different functions, one for the detection of external water stimuli and the generation of sensory maps and the other for the detection of self-motion and the generation of motor commands for immediate behavioral reactions.
Keywords: lateral line, vestibular system, hair cells, placodes, evolution, efferent system
Introduction
Maintaining body position within the earth’s gravitational field as well as stabilizing gaze and posture during self-generated motion or externally induced perturbations is essential for survival in all freely moving vertebrates. Although specialized somatosensory cells in the skin and joints can detect postural changes and visual circuits in the eyes and the brain contribute to the detection of body motion in space, hair cell mechanoreceptive organs are the main sensors that inform the CNS about body position in space and various aspects of externally induced and self-generated motion [Fritzsch and Straka 2014]. Ciliated mechanotransducer cells appeared early in animal evolution, indeed among protists, but the detection of different aspects of motion by mechanosensory hair cell systems in vertebrates depends on later evolved secondary structural elements that determine the specific modality and the dynamic range of the sensory organs [Fritzsch and Straka 2014]. Thus, non-visual motion detection is based on a relatively ancient invention [Duncan and Fritzsch 2013]. However, once incorporated into appropriate secondary structures, such as semicircular canals and otolith organs early in the vertebrate lineage, few further modifications have occurred [Straka and Baker 2013]. Although all vertebrates possess a vestibular apparatus in the inner ear, mechanosensory motion detection is mediated to a substantial degree by the lateral line system in amphibians and fish (Fig. 1A). In other vertebrate classes it is mediated solely by the vestibular system [Fritzsch and Straka 2014].
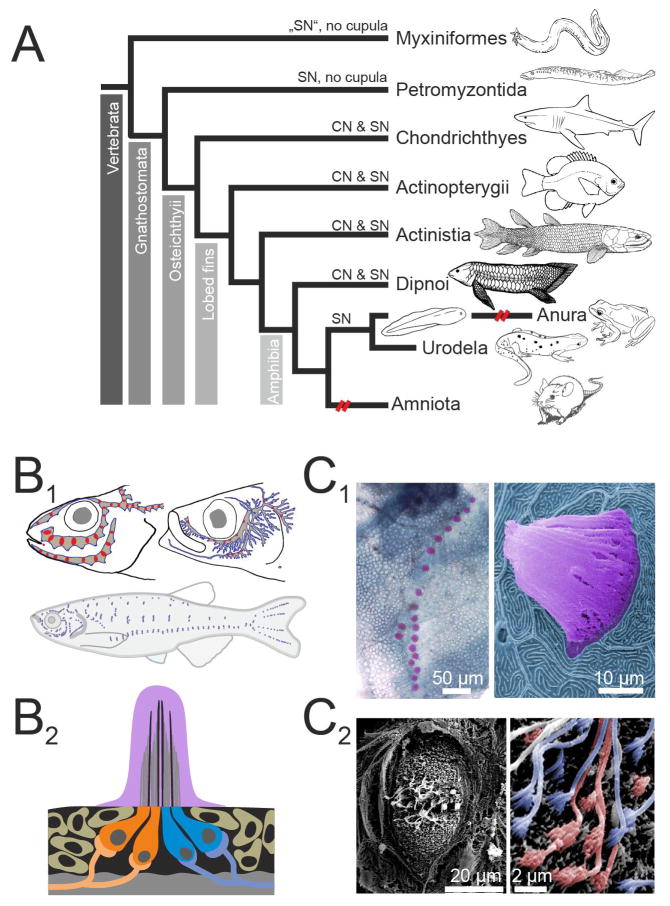
Figure 1.
Distribution and organization of the lateral line system A. Schematic cladogram of the distribution of lateral line mechanoreceptors across taxa. Secondary loss of the ancestral pattern is indicated by broken (red) lines. B. Schematics illustrating the varying distributions of superficial neuromasts (SNs; blue) and canal neuromasts (CNs; red) in three different species (Percarina demidoffi, Sprattus and Danio rerio; data modified after [Webb 1989b] (B1). Schematic illustration of a cross section through a superficial neuromast, coloration of cupula and hair cell somata corresponds to coloration used in photomicrographs (B2). Note that oppositely oriented hair cells are innervated separately. C. Photomicrographs show methylene green stained SNs of a scale on the trunk of an adult zebrafish (C1 left), and a colored SEM picture of a single SN including the cupula digitally colored in pink from an adult goldfish (C1 right). Photomicrograph of a goldfish SN following removal of the cupula (C2 left), with details of the hair cell polarization is shown in the magnified part (C2 right). Here hair cells of opposing orientation as determined by the position of their kinocilium and stereovilli are digitally colored in orange and blue.
Vertebrate hair cells in the inner ear organs and the lateral line neuromasts are specialized asymmetric bipolar cells that possess a set of stereocilia and a single kinocilium on the apical side and a synaptic zone for neurotransmitter release on the basal side [Hudspeth 2005]. Based on structural similarities, these cells are evolutionarily linked to ciliated choanoflagellates [Fritzsch and Straka 2014]. The staircase arrangement of tip-linked stereocilia assigns a functional polarity to the ciliary bundle since the direction and degree of bundle deflection determines the magnitude of the electrical response [Hudspeth 2005]. Shearing of the bundle towards and away from the kinocilium causes a depolarization and hyperpolarization of the hair cell, respectively, while bundle deflections perpendicular to this axis yield no change in the membrane potential. Thus, mechanosensory hair cells transform shear forces with vectors parallel to their apical surfaces into electric signals with directional specificity.
Hair cells in the inner ear and the lateral line neuromasts share many morphological and physiological properties, but their topographical arrangement within peripheral endorgans differs, as does peripheral endorgan anatomy (Fig. 1B). Thus, the neuronal response to a stimulus that is finally relayed to the CNS, and in particular the spatio-temporal motion sensitivity, depends on the particular anatomical structure and orientation of the organ in which the hair cells are inserted. Lateral line neuromasts are distributed across the entire surface of the body (Fig. 1B1) as well as within tubular structures (canals) mainly along the lateral surface of the trunk [Montgomery et al. 2014]. The cilia of lateral line hair cells are deflected by water motion and as such provide information about biotically (e.g. by prey or predators) and non-biotically induced (e.g. by waves or gravity) motion [Bleckmann et al. 2014]. Vestibular endorgans are organized into sensory epithelia with specific orientations relative to the head and with associated structures that anchor the hair cell cilia and couple head movement and position to cilial deflection. Hair cells in both the vestibular endorgans and the lateral line neuromasts are contacted by afferent fibers that project centrally to alar (dorsolateral) regions of the hindbrain where they transmit the encoded sensory signals onto second-order neurons.
In line with their particular anatomical features, the two mechanosensory systems are also characterized by different but molecularly overlapping developmental processes, whereby particular sets of developmental patterning genes interact and collaborate to generate sensory epithelial domains with the appropriate distribution and orientation of hair cells. Major differences, however, exist between the two systems in how spatial information in the sensory stimulus is encoded centrally. For instance, lateral line afferent fibers project in two segregated streams either to the Mauthner cell system where these projections can initiate quick motor behavior, or to an ascending pathway where an ordered topographical representation of the sensory surface is formed in the CNS that mirrors the body surface [Alexandre and Ghysen 1999; Pujol-Martí and López-Schier 2014; Pujol-Martí et al. 2012]. By contrast, vestibular afferent terminations from the various inner ear endorgans overlap extensively, reflecting convergence and synaptic interactions that relate directly to secondary neuron populations that mediate motor outputs [Straka and Dieringer 2004]. Thus, the two systems differ considerably in their developmental programs, their anatomical organization and their underlying principles of sensory processing. This review describes the genetic specification and developmental construction of these two systems and outlines the major morphological and physiological differences that relate to their specific roles in encoding body motion. We will present the thesis that functional relevance to the animal is the likely parameter that determines which type of central organization is used to process sensory signals: a topographical or a motor target-specific representation of the sensory surface.
Placodes and dedicated hindbrain nuclei: pivotal vertebrate inventions necessary for the creation of vestibular and lateral line mechanosensory systems
Central to the evolution of hair cell mechanoreception and the representation and processing of hair cell-derived signals in the brain are two vertebrate evolutionary innovations: the common placodal origin of the sensory periphery and the presence of compartmentalized hindbrain regions containing the secondary sensorimotor neurons. The first of these inventions is the set of epidermal placodes that are present only in craniate chordates [Northcutt and Gans 1983]. In addition to placodes associated with gustatory [Iskusnykh et al. 2016; Qian et al. 2001], paratympanic and spiracular organs [O’Neill et al. 2012], regionally localized placodes give rise to lateral line, electroreceptive and vestibular/auditory neurons, which project to specific nuclear areas in the dorsolateral part of the hindbrain [Dabdoub et al. 2016; Straka et al. 2014]. The evolutionary origin of these placodes is not clear. Discussion on this question revolves around two alternative arguments. 1) Placodes as well as their derivative neurons and sensory cells evolved de novo without any homologies in invertebrates. 2) Placodes and their derivatives evolved as a developmental program through co-option of preexisting building blocks required for neurosensory development in differently organized invertebrate sensory systems [Fritzsch et al. 2015; Gasparini et al. 2013].
Molecular evidence currently favors the latter argument. In particular, vertebrates and invertebrates exhibit molecular similarities in neurosensory development through cell fate decision-making transcription factors (TFs), and some of these have been successfully interchanged between vertebrates and invertebrates [Fritzsch et al. 2015; Fritzsch and Straka 2014]. Not all components of the gene regulatory networks for placode development in vertebrates and for neurosensory development in invertebrates are yet clear [Chen and Streit 2013; Peter and Davidson 2015; Roellig and Bronner 2016], but certain predictions of a homology seem to hold. For example, inner ear hair cells and their associated sensory afferent neurons likely arose after gene duplication and diversification [Fritzsch et al. 2000; Fritzsch et al. 2010]. This lineage relation would allow cell fate switching between afferent neurons and hair cells, given their common precursor. Indeed, loss of hair cells [Ma et al. 2000; Matei et al. 2005], cross-regulation of Atoh1 and Neurog1 [Raft and Groves 2015], development of intra- ganglionic hair cells when Neurod1 is removed [Jahan et al. 2015c] and the ability of Neurog1 to govern some aspects of hair cell development [Jahan et al. 2015b] support the notion of a molecular overlap in inner ear hair cell and sensory neuron lineages [Fritzsch et al. 2010]. What remains still unclear is at which stage of placode evolution these lineages diverged.
It is of interest to note with respect to evolution of functional cell types that hair cells closely resemble the unicellular ancestor of metazoans, the choanoflagellates, which possess a single flagellum surrounded by a collar of microvilli [Fritzsch and Straka 2014]. Mechanosensory hair cells of the inner ear and of the lateral line neuromast exhibit a similar arrangement in which a single kinocilium is asymmetrically placed relative to an organ pipe-like assembly of actin-rich elongated stereocilia (which bear some similarities to microvilli). This arrangement provides a directionality to the hair cell’s mechanoreceptive ability [Hudspeth 2005]. The later evolving electroreceptors lack these mechanosensory capabilities but have a variable assembly of kinocilia and/or microvilli resembling developmentally arrested mechanosensory hair cells.
The second evolutionary invention is the partitioning of the hindbrain into a matrix of subdivisions characterized by different patterns of neuronal differentiation. Developmental analyses spanning the last 100 years have revealed an organization of the spinal cord and brain into parallel longitudinal columns, containing specific categories of neuron types, which in the hindbrain are intersected by transverse neuromeres, called rhombomeres. More recently, both the longitudinal columns and the rhombomeres have been characterized according to their unique profiles of gene expression, particularly with respect to the expression of transcription factors (TFs) involved in cell specification and differentiation programs [Nieuwenhuys and Puelles 2015]. For example, each rhombomere is characterized by a unique code of Hox gene expression [Krumlauf 2016], and each longitudinal column expresses a specific set of dorsoventrally disposed TFs. Some of the longitudinal columns in the hindbrain are continuous with those in the spinal cord, including columns in the alar plate that express the TFs Atoh1 [Bermingham et al. 2001], Neurog1/2 [Fritzsch et al. 2006], Ascl1 [Qian et al. 2001] and Ptf1a [Bermingham et al. 2001; Fritzsch et al. 2006; Iskusnykh et al. 2016; Ma et al. 1997]. Others, however, are not [Nieuwenhuys and Puelles 2015]. These latter, hindbrain-specific columns contain parts of the vestibular, lateral line, electroreceptive, auditory and gustatory nuclei. Thus, the developmental partitioning of gene expression within the hindbrain defines in each rhombomere domains that are molecularly partially continuous with the spinal cord but develop neural progenitors with a unique TF profile [Nieuwenhuys and Puelles 2015]. These then give rise to distinct populations of central neurons, organized in clusters that to varying degrees are associated with specific grid domains [Di Bonito et al. 2013; Glover 2001]. The rhombomeric scaffold terminates rostrally at the sharp midbrain-hindbrain boundary, which evolved only in craniate chordates [Fritzsch and Glover 2007; Fritzsch et al. 2015], and this also delimits rostrally the extent of the first order vestibular and lateral line nuclei. While the rostro-caudal Hox code expression is shared with invertebrates, the dorso-ventral patterning mechanism through diffusible factors (Shh, BMP, Wnt) gradients and the formation of longitudinal bands of bHLH transcription factors driven by these dorso ventral gradients of diffusible factors is unique to vertebrates [Fritzsch et al. 2006].
Evolution and development of peripheral sensory structures and target hindbrain neuron populations
Peripheral sensory structures
The placodes, which give rise to sensory neurons and in most instances also associated sensory cells, initially form as a pre-placodal field around the invaginating future brain part of the neuroectoderm [Patthey et al. 2014; Schlosser et al. 2014]. This is an essential step in the developmental formation of the craniate vertebrate head [Maier et al. 2014]. The pre-placodal field then resolves into distinct placodes, with the otic placode forming lateral to the middle region of the hindbrain, and, in aquatic anamniotes, an anterior and a posterior lateral line placode forming respectively rostral and caudal to the otic placode. The morphological development of the vestibular peripheral organs has been described in detail in several species from fish to human [Fritzsch et al. 2006; Lim and Brichta 2016; Rinkwitz et al. 2001]. Briefly summarized, the otic placode invaginates to form the otocyst, from which the various vestibular endorgans and the cochlea derive through protrusive expansion (and, in the case of the semicircular canals, fusion and subsequent loss of intermediate regions) of specific patches of the nascent sensory epithelium. A number of genes are involved in specifying the distinct functional compartments of the sensory epithelium giving rise to the otolith organs and semicircular canals [Bober et al. 2003; Fritzsch et al. 2006]. The different endorgans acquire their anatomical structure through ensuing morphogenetic changes, which are generally conserved throughout the vertebrate radiation, although the number of semicircular canals increased from 2 to 3 and the number of otolith organs increased from one to three (two) during evolution from agnathans to fish (mammals) [Fritzsch et al. 2006]. As endorgan morphogenesis transpires, hair cell differentiation takes place.
The morphological development of the lateral line sensory organ may be less familiar to many readers since this organ is only found in aquatic anamniotes (Fig. 1A). Lateral line sensory organ formation involves two morphogenetic mechanisms exerted on the lateral line placodes: elongation and migration [Piotrowski and Baker 2014]. The first leads to the formation of sensory ridges that fragment and generate neuromasts and surrounding ampullary organs. The second leads to the formation of a sensory primordium that migrates over the trunk and head thereby depositing neuroblasts. This is the dominant feature in the formation of neuromasts in both fish and amphibians [Pichon and Ghysen 2004]. In fish, additional neuromasts are added during the transition from larval to adult, either through further deposition of neuroblasts between proliferating primary neuromasts, or in some species by a late developing second placode. The latter process, classically referred to as stitching, generates neuromasts that occur close to each other forming lines of several neighboring neuromasts along vertical lines on the trunk and tail fin (Fig. 1B1 and C1) [Ledent 2002]. The variability of neuromast arrangement in teleosts [Gibbs 2004; Janssen 2004] can thus arise through post-embryonic migration and stitching [Bricaud et al. 2001] or splitting of placodes prior to migration [Ghysen et al. 2007]. The secondary form of post-embryonic modification essentially causes the formation of two functional sub-modalities, i.e. superficial and canal neuromasts. The two classes become distinct during the larval period when future canal neuromasts change their shape and become distinct from superficial neuromasts [McCormick 1989; Tarby and Webb 2003]. Canal formation commences in the vicinity of presumptive canal neuromasts as bony canal walls ossify within epithelial ridges, forming an open groove containing the canal neuromast. These epithelial ridges then fuse over the individual canal neuromast and the bony walls fuse, thereby forming the bony canal roof of a single canal segment [Tarby and Webb 2003].
Despite the distinctiveness of placodes as developmental Anlagen, the gene regulatory networks that initiate and guide their further neurosensory development overlap with those involved in the development of the nearby neural crest and of central neurons. For example, in addition to being essential for the development of hair cells in the inner ear and lateral line, Atoh1 also regulates the development of the dorsal part of the rhombic lip, the most dorsomedial part of the spinal cord and most mammalian cerebellar granule cells as well as Paneth cells in the intestine and Merkel cells in the skin [Bermingham et al. 2001; Fritzsch et al. 2015; Jahan et al. 2015a]. On the other hand, all proximal ganglion neurons of the brainstem require Neurog1 for their development whereas distal placode-derived neurons require Neurog2 [Ma et al. 2000; Ma et al. 1998]. This underscores the context-dependent actions of nearly any TF thus far associated with placode development. In fact, a challenging question regarding the evolution of the peripheral nervous system is the molecular and anatomical interrelationships between placode and neural crest derivatives. For example, in the trigeminal system, placode- and neural crest-derived neurons intermingle. Similarly, the geniculate ganglion contains epibranchial placode-derived neurons as well as a small component of neural crest-derived neurons [Fritzsch et al. 1997]. A similar intermingling of placode and neural crest-derived neurons has been suggested for the lateral line and the inner ear [Freyer et al. 2011], with a report of variable numbers of neurons from the spiral ganglion and vestibular neurons as well as hair cells being derived from Pax3-expressing neural crest precursors [Freyer et al. 2011]. However, in a different transgenic mouse line expressing Pax3-cre, transgene expression labeled only Schwann cell precursors that tightly interacted with the developing vestibular and cochlear ganglion neurons [Sandell et al. 2014]. Thus, the neural crest contribution to the inner ear and lateral line systems does not appear to include the neuronal lineage [Mao et al. 2014].
Central target neurons in the hindbrain
The target neurons of the vestibular and lateral line organs reside within the hindbrain and are specified through the patterning that establishes the rhombomeres and the longitudinal TF expression columns. Rhombomeres are established through the action of anteroposterior signaling gradients, most notably of retinoids and Fgf8 [Glover et al. 2006; Marín and Charnay 2000; Parker et al. 2016] whereas the longitudinal columns are established through the action of dorso-ventral signaling gradients, most notably of Shh, BMPs and Wnts [Wilson and Maden 2005]. Within this developmental patterning framework, central vestibular and lateral line target neurons are organized into coherent clusters with specific locations within the hindbrain grid, and with specific identities related to their central axon trajectories and synaptic targets. This is particularly evident in the vestibular system, where central vestibular projection neuron clusters identified as vestibulo-spinal, vestibulo-ocular, combined vestibulo-spinal/vestibulo-ocular and vestibulo-cerebellar groups are segregated into well-defined domains in a pattern that has been called the vestibular hodological mosaic [Díaz et al. 1998; Glover 2000]. Several elements of this pattern are highly conserved from fish to mammals while others exhibit phylogenetic variants that are likely to be related to specific functional adaptations [Díaz et al. 1998; pasqualetti et al. 2007]. Recent work has identified some of the key TF genes involved in specifying this pattern, and investigated the effects of manipulating these genes. For example, the TF Hoxb1 is crucial for the specification of the neuron group that gives rise to the lateral vestibulo-spinal tract (LVST), such that this group is essentially absent in Hoxb1 null mutant mice [Di Bonito et al. 2015].
Although the developmental patterning of the central lateral line projection neuron populations has not been characterized to the same extent as in the vestibular system, the stereotyped locations of the Mauthner cell and the other specific cell groups (medial octavolateral nucleus, cerebellar field) indicates a similarly conserved link between patterning and second-order neuron function [Fame et al. 2006; pujol-Martí and López-Schi er 2014; Puzdrowski 1989]. This indicates that the identities of second-order neurons within the hindbrain are specified early and provide molecularly defined targets for different subsets of afferents. Moreover, these second-order hair cell mechanoreceptive neuron groups relay sensory signals through distinct pathways to higher centers for sensory modality-specific information processing, and in the case of vestibular signals and some lateral line signals, directly to premotor and motor command centers for activating respectively vestibulo-motor reflexes and escape reflexes.
Directed growth of sensory afferent axons and selective central synapse formation
Shortly after their formation, inner ear and neuromast sensory afferent neurons extend axons into the hindbrain. Given their location at about the same anteroposterior level as the central target neurons, this brings them into close proximity to their synaptic targets. Despite the potential for anatomical overlap, the sensory afferent axons segregate among central target neuron groups according to their peripheral targets and function. Importantly, from their initial ingrowth, sensory afferents of a variety of modalities not only selectively enter specific rhombomeres but also specific longitudinal columns (Fig. 2), thereby faithfully segregating trigeminal, inner ear, mechanosensory and electrosensory lateral line afferent projections [Fritzsch et al. 2005]. This indicates that lateral line and otic placode-derived sensory neurons are differentially attracted by as yet incompletely understood molecular signals to innervate selectively both their appropriate targets in the periphery, and within the hindbrain the appropriate lateral line and vestibular neuron groups. Thus, it is likely that the specification of uniquely placode-derived hair cell and afferent neurons bestows them with distinct pathfinding properties that restrict their axons to the appropriate hindbrain targets. This is supported by experimental manipulations that provide the sensory afferents with the opportunity to innervate inappropriate targets. In particular, ears and placodes can be heterotopically transplanted, so that the mechano- and electrosensory hair cells and organs differentiate independently of their new locations [Elliott et al. 2015b; Modrell et al. 2011; Northcutt et al. 1995]. Notably, transplanted ears can form connections with the spinal cord and the midbrain, albeit without uniform termination patterns [Elliott and Fritzsch 2011]. This implies that synapse formation per se is not limited to the appropriate central targets, and that it is the initial pathfinding of inner ear afferents that generates this restriction. Whether lateral line afferents behave similarly after heterotopic transplantation remains to be determined.
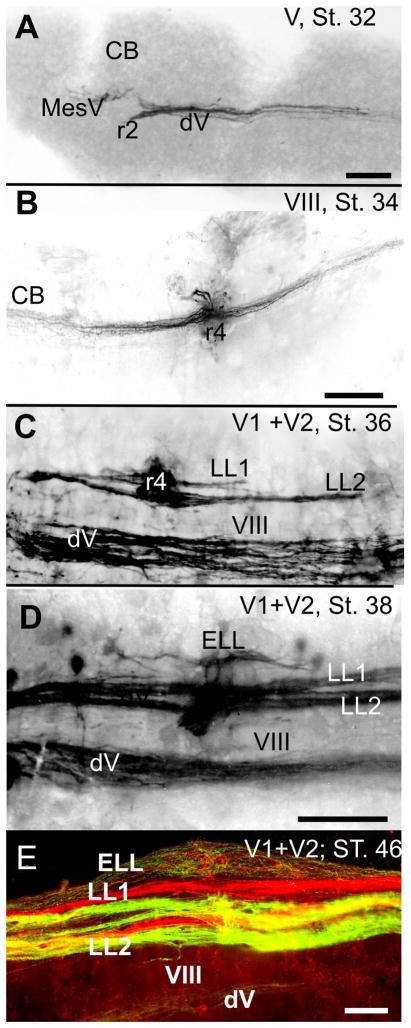
Figure 2.
Central projections of trigeminal, lateral line and inner ear afferents develop at different time. The earliest projection is trigeminal (A) followed by inner ear (B) and mechanosensory lateral line (LL,C). the ampullary electroreceptor projections (ELL) develop after the mechanosensory lateral line (D) and are completely adult only several stages later (E). Note the gap of labeled afferent fibers (in C,D,E) between the lateral line projections and the descending tract of the trigeminal projection (dV) that contains the unlabeled VIIIth nerve projection (VIII). Note also that the mechanosensory lateral line has two mostly discrete bundle per labeled trigeminal nerve (V1, supraorbital nerve; V2 infraorbital nerve) whereas the ampullary organ afferents are intermixed. Bars indicate 100μm, Modified after [Fritzsch et al. 2005].
Such manipulations offer the opportunity to explore how novel sensory systems can be added to a pre-existing system. Making a new central sensory projection requires the formation of new neurons that selectively receive a novel input. How afferent fibers terminate centrally in a regionally segregated pattern has been experimentally explored in the context of ocular dominance column formation in the visual system [Constantine-Paton and Law 1978; Hubel et al. 1977] and a similar phenomenon was recently shown for the vestibular afferent projection in three-eared frogs [Elliott et al. 2015b]. The timing of lateral line and inner ear afferent segregation in salamanders [Fritzsch et al. 2005] and zebrafish [Zecca et al. 2015] suggests that the capacity for selective pathfinding by placode-derived neurons is present prior to the development of the central targets (Fig. 2). This is particularly obvious in mammals, where cochlear and vestibular afferents grow to separate central regions while cochlear nucleus neurons are still proliferating. Indeed, the central projections of cochlear afferents can form a topographical map in Atoh1 null mice in which neither hair cells nor an organ of Corti form, and in which the equally Atoh1-dependent cochlear nuclei are also absent [Fritzsch et al. 2006]. While only a few sensory neurons survive in these Atoh1 null mice due to lack of neurotrophic support, the simple fact that these remaining neurons develop a cochleotopic projection rules out several currently discussed hypotheses about how cochleotopic projections form. It is thus likely that afferents generate topographically roughly correct projections (Fig. 2), independent of Atoh1, which, however, are refined by the expression of the latter.
Whereas the afferents of placode-derived sensory neurons lacking both a peripheral and a central target can form connections with other available targets, central neurons undergo a critical phase during which they are highly sensitive to the absence of afferent input. For example, the size of the cochlear nuclei are reduced through cell death after cochlear and spiral ganglion neuron removal in postnatal mice and chickens [Rubel and Fritzsch 2002]. Likewise, some, but not all, central vestibular neurons undergo a critical phase and may be lost after very early removal of sensory input [Elliott et al. 2015a]. The weaker dependence of central vestibular neurons on afferent inputs might be related to their multi-modality, since they also receive proprioceptive and indirectly also visual inputs, in contrast auditory neurons are strictly unimodal [Rubel and Fritzsch 2002]. From an evolutionary perspective it is important to note that separate ‘auditory’ projections appear even in animals with a clearly convergent evolution of a sound pressure auditory system, such as catfish [Bleckmann et al. 1991; Fritzsch et al. 1990], implying that the developmental segregation of afferents from distinct endorgans with different functions is possible under such circumstances Thus far only very few molecules, such as GATA3 [Duncan and Fritzsch 2013; Goodrich 2016] and Neurod1 [Jahan et al. 2010], have been identified that play a role in this process. A first step in disentangling the puzzle is to understand how the apparent stimulus-dependent segregation of placodal afferents [Elliott et al. 2015b] combines with afferent-specific molecular signals to provide the very early and clear segregation of ingrowing afferents to specific nuclei prior to second-order neuron differentiation [Fritzsch et al. 2005; Zecca et al. 2015]. This would then allow the assessment of how the concurrent loss of a specific set of afferents and the appearance of a novel set of afferents can be combined to form new hindbrain nuclei dedicated to the detection of sound and electrical stimuli [Fritzsch 1991]. One possible evolutionary outcome, namely a replacement of the mechanosensory lateral line system by the auditory system in tetrapods, clearly does not hold across the board, as lateral line and auditory nuclei co-exist in some aquatic frogs [Fritzsch et al. 1988].
Hair cell mechanoreceptive organs - peripheral organization and stimulus detection
All hair cell mechanoreceptive organs, with the exception of the derived electroreceptive system, transduce mechanical stimuli into electrical responses [Fritzsch and Straka 2014]. Whereas vestibular and lateral line organs are each sensitive to a specific modality (body and water motion, respectively), the two organs also share a common responsiveness to less specific mechanosensory stimuli such as high frequency substrate or water vibrations [see discussion in Straka et al. 2003]. The distinct transduction features of vestibular and lateral line organs engender them with the capability to respond to different aspects of self-motion, passive motion and surrounding water motion. For aquatic organisms, this provides two sources of information that permit orientation in the water column: body orientation in the gravitational field within an egocentric frame of reference and sensation of water motion over the body surface.
Detection of vestibular stimuli
Motion-sensitive vestibular organs in the inner ear of all vertebrates are subdivided into two major classes that detect specific vectorial components of actively and passively induced head/body movements (Fig. 3A) [Straka and Dieringer 2004]. The spatial arrangement of the different endorgans as well as the overall appearance of the vestibular apparatus is remarkably conserved among vertebrates, independent of life style or eco-physiological niche. All vertebrates have three otolith organs (utricle, saccule, lagena), except for therian mammals [de Burlet 1929; Lewis et al. 1985], which lack the lagena [Fritzsch et al. 2013]. Depending on the spatial arrangement within the inner ear, the otolith organs detect linear translational acceleration in the horizontal (utricle) or vertical plane (lagena/saccule) as well as head position relative to the Earth’s gravitational field. The capacity to detect these vector components of acceleration depends on the inertia of a heavy mass (provided by the otoconia, small crystals of calcium carbonate), which in fishes usually forms a single solid otolith. These otoconia/otoliths are positioned on the epithelial sensor, thereby creating a directionally specific shear force on the hair cell cilia. A systematic shift in the alignment of the hair cells within each otolith organ is such that the ciliary bundle axes in toto cover 360°, permitting the detection and encoding of motion in all directions (Fig. 3B1) [Fritzsch and Straka 2014]. Accordingly, otolith organs detect three-dimensional linear acceleration components during translational or tilting movements of the head [Fritzsch and Straka 2014]. Depending on the intrinsic electrical properties of the hair cell and associated sensory afferent, the discharge pattern that is transmitted to the CNS upon stimulation may be transient and related to the onset/offset of the motion or tonic and directly proportional to the magnitude, rate and duration of the head movement.
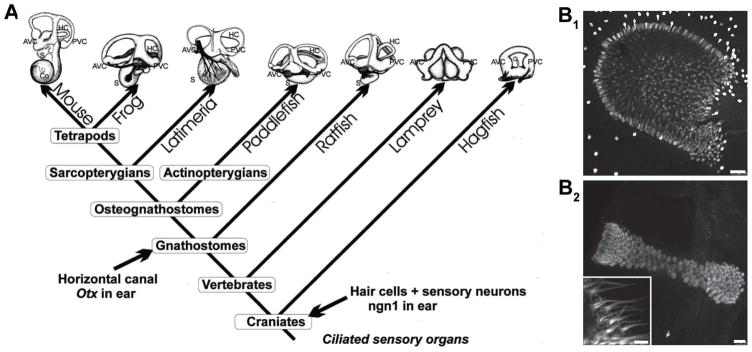
Figure 3.
Evolution of the inner ear and morpho-physiological organization of the vestibular system. A. Increase of morphological complexity of the inner ear. Note that the formation of a horizontal canal in jawed vertebrates correlates with expression of several genes only in this lineage. B. Calretinin-positive hair cells in saccular crista of Xenopus laevis larvae have a disc-shaped distribution with short kinocilia and stereocilia, while those in the anterior semicircular canal form a continuous band with long kinocilia and stereocilia. Scale bars represent 50 μm (B1) and 25 μm (inset 5 μm) (B2). Images were kindly provided by Céline Gravot. A is adapted with permission from [Fritzsch and Beisel 2001].
The second type of vestibular endorgan is an interconnected duct system of semicircular canals in all vertebrates [Fritzsch and Straka 2014]. All jawed vertebrates have three semicircular canals with associated ampullae containing sensory cristae. In contrast, jawless vertebrates have only two canal cristae but have only a single torus (hagfish) or vertical canals/horizontal canals, possibly presenting a convergent evolution of a structurally different horizontal canal system [Maklad et al. 2010]. The evolutionary appearance of the third, horizontal semicircular canal is directly related to the expression of the otx gene in the respective inner ear substrate [Fritzsch et al. 2001; Mazan et al. 2000]. The perpendicular arrangement of the three semicircular canals in the latter vertebrate groups [Fritzsch and Straka 2014] is approximately aligned with the pulling direction of the eye muscles (Fig. 4A). An enlargement of each canal, called the ampulla, contains the sensory epithelium (crista) where hair cell cilia protrude into a gelatinous matrix (cupula) that spans across the ampullary space (Fig. 3B2) [Fritzsch and Straka 2014]. At variance with otolith sensory epithelia, all hair cells in a given semicircular canal crista have the same ciliary bundle orientation and thus a single directional sensitivity, aligned with the plane of the canal [Goldberg 2000]. The anatomically more complex semicircular canals appeared later phylogenetically than the simpler otolith organs [Fritzsch and Straka 2014; Straka and Baker 2013]. The semicircular canal cristae detect angular head acceleration and given their mutually orthogonal arrangement decompose any such movement into three separate vector components [Rohregger and Dieringer 2002]. The mechanistic principle for detection of this stimulus relies on the inertia of the endolymph within the semicircular canals and the anchoring of the cilia in the cupula [Fritzsch and Straka 2014]. With any acceleration or deceleration, the endolymph lags the head movement thereby creating a deflection of the cupula and the hair cell ciliary bundle. However, due to the dynamics of the endolymph and the mechanics of the cupula displacement, the parameter encoded by the afferent firing rate is in fact the velocity (first derivative of acceleration) of the head movement during the acceleration or deceleration [Highstein et al. 2005]. The wide dynamic range of possible motion stimuli is not compatible with a relay of impulses to the CNS by a single prototype afferent neuron, but rather requires the presence of frequency-tuned information channels from the sensory periphery to the central vestibular nuclei [Straka et al. 2009]. This is indicated by the presence of a range of differently tuned vestibular afferent fibers, each with distinct morpho-physiological properties and differential innervation patterns of dynamically comparable hair cells across the respective sensory epithelia [Straka and Dieringer 2004]. Thus, three-dimensional head motion signals are not only spatially decomposed by the different spatial orientations of the endorgans but also dynamically decomposed into position, velocity and acceleration components by afferents with different computational capabilities [Goldberg 2000; Straka et al. 2005]. Accordingly, any head motion is encoded in distinct spatio-temporal components that are transmitted by the vestibular afferents to the central vestibular nuclei in the hindbrain [Straka and Dieringer 2004].
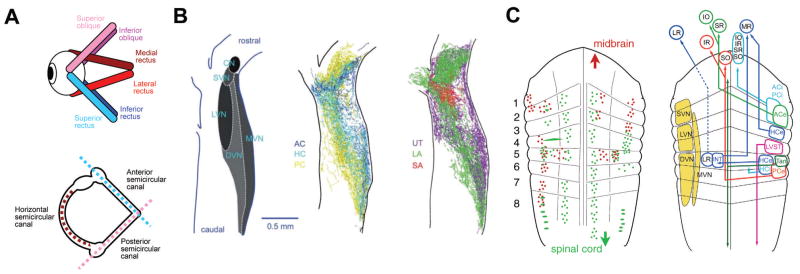
Figure 4.
Organization of the vestibular system. A. Schematics depicting the spatial arrangement of the six eye muscles (top) and the three semicircular canals of the ipsilateral labyrinth (bottom). Color-coded antagonistic pairs of eye muscles are spatially aligned with individual semicircular canals. B. Spatial arrangement of the vestibular nuclei on a horizontal section through the dorsal hindbrain (left) and color-coded overlay of afferent projections from the three semicircular canals (middle) and the three otolith organs (right). C. Schematic depicting the rhombomeric arrangement of spinal projecting (green) and midbrain oculomotor nucleus projecting (red) neurons (left) in the amphibian. Right: Allocation of classical vestibular nuclei nomenclature (yellow) onto the amphibian rhombomeric scaffold and tentative segmental location of vestibular subgroups that mediate sensory signals from specific semicircular canals onto spatially matching extraocular and spinal motoneuronal populations. ACe/ACi/PCe/PCi, excitatory/inhibitory neurons of anterior/posterior semicircular canal; ATD, ascending tract of Deiters; DVN/LVN/MVN/SVN, descending/lateral/medial/superior vestibular nucleus; HCe/HCi, horizontal semicircular canal excitatory/inhibitory neurons; INT, abducens internuclear neurons; IO/IR, inferior oblique/rectus motoneurons; MR/LR, medial/lateral rectus motoneurons; LVST, lateral vestibulo-spinal tract; r1–r8, rhombomeres 1–8; SO/SR, superior oblique/rectus motoneurons; Tan, tangential nucleus; VN, vestibular nuclei. B and C are adapted with permission from [Straka et al. 2014; Straka et al. 2002], respectively.
Detection of lateral line stimuli
With the exception of some aquatic caecilians, anurans and terrestrial vertebrates (Fig. 1A), all aquatic anamniotes possess a mechanosensory lateral line system that is composed of functional sensory units, the so-called neuromasts, that are distributed over the skin (Fig. 1B1). Each neuromast is typically covered by a gelatinous cupula and contains hair cells with opposite polarity, which, as in the inner ear, act as mechano-electrical transducers [Flock and Wersäll 1962]. The neuromasts are distributed over the head and trunk in clusters or rows each of which is innervated by separate placode-derived lateral line nerve branches [Webb 2014]. Neuromasts are largely conserved in their morphology and vary mainly in the size and shape of the cupula, the number of hair cells and the stiffness of the hair cell cilia. Substantial morphological diversity arises, however, on account of accessory structures. Through this diversity, neuromasts may be located either superficially on the body surface, recessed to various degrees within structures ranging from shallow dermal pits to cartilaginous grooves (so-called pit organs) or completely surrounded by bony or cartilaginous canals that open to the surrounding water through pores [Webb 1989a]. Except in amphibians, which lack a duct system, seven distinct canal lines are found: supraorbital, infraorbital, mandibular, otic, temporal and supra-temporal and trunk (Fig. 1B1). The structural diversity of the lateral line has been attributed to the varying life-style and/or habitat as well as differences in behavior [Dijkgraaf 1963]. Accordingly, wide canals as in notothenioid fishes enhance the sensitivity of canal neuromasts and as such are an adaptation to low-noise aquatic habitats [Denton and Gray 1983; Denton and Gray 1988; Janssen 2004; Schwalbe et al. 2012]. This correlation also highlights the possibility that much of the morphological adaptation in the lateral line periphery does not change the frequency tuning of canal or superficial neuromasts [Coombs and Montgomery 1994], but rather serves to maintain the tuning independent of hydrodynamic habitat or life-style. Likewise the density of canal and superficial neuromasts potentially correlates with the hydrodynamics of a given habitat and/or lifestyle with limnophilic species tending to have more superficial neuromasts with wide but often reduced canals compared to rheophilic species [Janssen 2004]. However, despite correlations between morphological differences of superficial and canal neuromasts, no unifying pattern has yet been found to link hydrodynamic conditions and/or lifestyle with a particular morphology [Beckmann et al. 2010; Schmitz et al. 2008].
Compatible with the idea that lateral line morphology correlates with the habitat, populations of the three-spine stickleback in different environments show significant variations in the density of superficial neuromasts [Wark and Peichel 2010]. A causal relation between the hydrodynamics of the aquatic environment and spatial arrangement of the canal system is supported by findings in rainbow trout where the arrangement of canals forms a hydrodynamic “fovea”, with canals being densest at the head [Ristroph et al. 2015]. This location experiences the strongest spatio-temporal variations of pressure during swimming or exposure to bulk water flow, regardless of the yaw angle of the animal with respect to the flow [Ristroph et al. 2015]. The comparison of bilateral pressure fluctuations by superficial neuromasts appears to make this system uniquely responsible for flow-orientation within a current [Montgomery et al. 1997]. However, a contribution of canal neuromasts to this rheotactic behavior is likely [van Trump and McHenry 2013] since a bilateral comparison is effective in both neuromast systems.
For the detection of water flow the cupula and accessory structures serve as biomechanical interface between water- and hair cell movements. Flow over any surface is affected by the fluids’ viscosity, causing the fluid to resist shearing and to adhere to the surface. This creates a spatial gradient of flow velocity known as the boundary layer, schematically illustrated in Fig. 5A. The spatial extent of the boundary layer is proportional to the velocity of the water outside of the boundary layer. The cupula thus acts like a transducer that extends into this boundary layer. The force acting on the cupula and thus the shear stress that is acting on the neuromast [Prandtl 1904; Schlichting and Gersten 1979] depends on the water velocity as higher water velocities result in a thinner boundary layer. This renders the movement of the cupula, and hence the responsiveness of the superficial neuromasts, sensitive to the velocity of local water motion [Chagnaud et al. 2008a; Chagnaud et al. 2008b; Engelmann et al. 2002; Goulet et al. 2008; Goulet et al. 2012; Kroese and Schellart 1992; Kroese et al. 1978; Voigt et al. 2000]. The sensitivity of canal neuromasts depends on both, the filter properties of the cupula (see above) and the morphology of the canal. While the cupula of canal neuromasts is as sensitive as that of superficial neuromasts to the flow velocity within the canal, the generation of this flow inside of the canal requires a pressure difference between pores at the outside water [Denton and Gray 1989]. This renders canal neuromasts sensitive to the pressure gradient (i.e. the spatial velocity gradient = acceleration) over the sensory surface [Denton and Gray 1983; Denton and Gray 1988, 1989; Kroese and Schellart 1992; van Netten 2006]. The biomechanical/physiological threshold of canal neuromasts is ~0.1 mm/s2 [van Netten and McHenry 2014], a value that is in agreement with the behaviorally determined threshold for a presumably canal neuromast-mediated orienting response [Coombs and Janssen 1990]. In contrast, superficial neuromasts are generally much less sensitive due to reduced numbers of hair cells per organ and smaller cupulae [van Netten and McHenry 2014].
Figure 5.
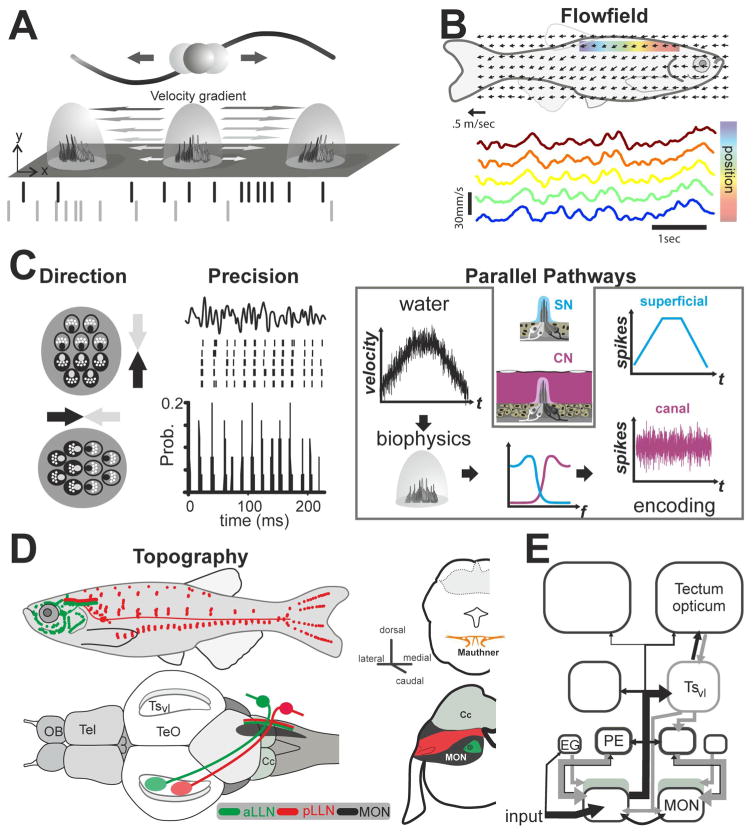
Stimulus encoding and processing in the lateral line system. A. Schematic representation of the boundary layer effect between an oscillating sphere and superficial neuromasts (SN) on the skin of a fish. Sphere displacement is shown by the sine wave. Note that the cupula movement results in hair cell deflection. Depending on the hair cell polarity (grey and black hair cells), the corresponding afferents respond 180° out of phase (raster plot in grey and black). B. Schematic of the flow field over a fish’s body. Arrows represent the mean local flow direction and magnitude obtained in small interrogation areas. As shown for five of these areas located along the dorsal trunk of a fish being held stationary in flow, these local fluctuations of the velocity are strongly correlated over the sensory surface of the fish. Data redrawn and modified after [Chagnaud et al. 2008b]. C. Overview of the features of hydrodynamic stimuli encoded in the periphery: The hair-cells and their primary afferents respond in a directionally sensitive manner such that the response of a given afferent changes in a cosine-like fashion with stimulus angle. Neuromasts are typically oriented with their long axis either parallel to the anterior-posterior or the dorso-ventral axis of the animals, leading to a neuronal representation of local flow direction. Given that the precision at which the afferents respond, this separation in two groups of neuromasts with their hair cells being sensitive in a mirror-symmetric fashion leads to a precise encoding of the minute spatiotemporal differences characteristic for global flow-fields. The separation in a superficial and a canal system results in two complementary channels of information with different filter characteristics (see blue and pink input-output functions). The highly sensitive SNs convey low-pass filtered information of the flow properties whereas the less sensitive canal neuromasts (CNs) convey high-pass filtered information. As the flow inside the canals will depend on the placement of the pores, CNs typically sample more local aspects of the otherwise highly correlated global flow field. This suggests that SNs encode the global flow, whereas CNs encode local fluctuations. D. Afferents of the anterior and posterior lateral line nerve project to the medial octavolateral nucleus in the medulla in a topographical manner. Here afferents bifurcate and make synaptic contacts along the length of the MON. The projection of afferents of the anterior nerve course more medially and more ventrally within the MON than afferents of the posterior lateral line nerve. A parallel path of fibers contacts the Mauthner cell; it is presently unknown if this projection is topographically ordered. E. Schematic overview of the major ascending and descending pathways of the mechanosensory lateral line. Abbreviations: Cc: Crista cerebellaris; EG: eminentia granularis; OB olfactory bulb; MON: medial octavolateral nucleus; PE: preeminential nucleus; Tel: Telencephalon; TeO Tectum opticum; TSvl: ventrolateral nucleus of the torus semicircularis.
Neighboring superficial and canal neuromasts are innervated by the same cranial nerve branches, however, each population of neuromasts is connected by a separate set of afferent fibers. Hair cells of opposing polarity are contacted by different afferents (orange and blue fibers in Fig. 1B2) [Faucherre et al. 2009; Fritzsch and López-Schier 2014]. In the superficial neuromast system, a given afferent fiber typically innervates more than one neuromast in a given stitch, whereas multiple neuromast innervation is rare for canal neuromasts. The separate innervation of hair cells of opposing polarity suggests that afferents from both neuromast systems provide parallel and complementary aspects of sensory information to the central nervous system: 1) a low sensitivity pathway transmitting signals about water velocity and 2) a high sensitivity pathway tuned to the acceleration of the external flow field (Fig. 5B). In both pathways information on the direction of stimuli is maintained such that both pathways supply amplitude and direction information of water velocity and water acceleration, respectively. In both pathways this information is encoded through the temporal modulation of the discharge rate of the corresponding afferents. For the superficial neuromast system the precision of this encoding strategy is remarkable (Fig. 5C) [Goulet et al. 2012], suggesting that the low sensitivity pathway is well suited to encode the minute spatio-temporal differences characteristic for global flow fields, i.e., hydrodynamic patterns spanning an animals sensory surface (Fig. 5B). The ability to analyze the flow pattern in detail could serve as a means to decipher the hydrodynamic patterns that swimming fish or prey produce. Such hydrodynamic trails have been found to be temporarily stable hydrodynamic patterns that have been implicated in prey detection and trail following behavior of fish [Pohlmann et al. 2001].
Central termination patterns of vestibular and lateral line afferent fibers
Afferent fibers of all hair cell mechanosensory sub-systems terminate in independent non-overlapping columns of the hindbrain (Fig. 2). These are the ventral (vestibular), intermediate (lateral line) and dorsal longitudinal columns (electrosensory), respectively (Fig. 2) [Wullimann and Grothe 2014]. Depending on the number of placodes (see above), two (Sarcopterygians), three (Myxoids and Petromyzontids) or four (Chondrichthyes and ray-finned fish) separate nerves innervate the lateral line neuromasts, of which only the posterior lateral line nerve innervates neuromasts on the trunk. Where present, electroreceptor afferents share the same nerves, however terminate in a separate column. In contrast, afferents from all vestibular endorgans form a single nerve that enters the hindbrain. Despite the morphologically similar connectivity between the sensory periphery and the CNS, vestibular and lateral line projections differ considerably in their topographical arrangement of afferent termination patterns. Thus, the question arises as to whether this difference is mirrored by equally differential projection patterns and topography in these two sensory systems.
Transmission of vestibular afferent signals and organizational arrangement of central projections
Vestibular afferents that supply all inner ear endorgans join into a single nerve [Straka et al. 2014] that splits after entering the hindbrain into an ascending and descending fiber bundle [Birinyi et al. 2001]. While afferent fibers from different mechanosensory modalities terminate in non-overlapping central areas, afferent projections from different vestibular sensory endorgans overlap extensively within their dedicated hindbrain recipient nuclei (Fig. 4B) [Straka and Dieringer 2004]. An exception are the lagena and saccule in fish and the saccule in amphibians, which have a dual function with variable contributions to the encoding of auditory and vestibular signals [Straka and Dieringer 2004]. In particular, striolar hair cells in the latter otolith organs encode high-frequency accelerations (vibration), while hair cells in extra-striolar areas are sensitive to changes of the position relative to the earth’s gravitational vector (low-frequency; [see discussion in Fritzsch et al. 2001; Straka and Dieringer 2004]). Given such a dual function of otolith organs, afferent fibers innervating either of the two different sensory areas project separately to auditory or vestibular central nuclei in fish and amphibians [Birinyi et al. 2001; McCormick and Wallace 2012; Straka et al. 2003; Will and Fritzsch 1988]. In contrast, semicircular canal and utricular/lagenar afferents occupy the same dorso-lateral hindbrain area without any evidence for an endorgan-related topographical segregation (Fig. 4B) [Straka et al. 2014]. In addition, vestibular afferents project into the cerebellum and in an endorgan-specific manner (horizontal semicircular canal and utricular afferents) into the r5/r6 reticular formation [Birinyi et al. 2001; Will et al. 1985]. In the vestibular nuclei, afferent nerve projections from different semicircular canals and otolith organs as well as afferent fibers with different diameters and thus with different response dynamics overlap to a considerable extent within the termination area [Straka and Dieringer 2004; Straka et al. 2014]. The absence of endorgan-specific regionally restricted terminal zones, comparable to the retinotopic projections of the visual system, suggests that vestibular signals are not sorted in the CNS based on a sensory topography. Moreover, the extensive overlap of afferent hindbrain projections along with the large dendritic tree of most second-order vestibular neurons that spreads across multiple hindbrain segments and a considerable medio-lateral extent theoretically allows a convergence of vestibular sensory signals from all vestibular endorgans.
However, despite the dense overlap of vestibular afferent projections, second-order vestibular neurons select their inputs such that individual neurons receive monosynaptic inputs from only one semicircular canal or otolith organ [Straka and Dieringer 2004]. With respect to combining semicircular canal and otolith signals in second-order vestibular neurons, the convergence of afferent inputs is also not ubiquitous but spatially specific with respect to the endorgan origin. In any given second-order vestibular neuron, afferent inputs from a semicircular canal converge with afferent inputs from a spatially matching otolith epithelial sector that is co-activated (i.e. originates from hair cells with the same direction tuning as the canal hair cells) during natural head/body motion [Straka et al. 2002]. Thus, horizontal semicircular canal signals predominantly merge with utricular signals and vertical semicircular canal signals with signals from a vertically oriented otolith organ [Straka et al. 2002]. Accordingly, second-order vestibular neurons process spatially specific motion signals that are related to motion vectors rather than endorgan-specific origin. The generation of afferent signal convergence is likely consolidated and directionally tuned during a developmental period when semicircular canal and otolith organs become fully functional and co-activated during naturally occurring head/body motion [Branoner and Straka 2015; Straka 2010].
Instead of processing vestibular signals within a sensory topographical map, sensory-motor transformations of vestibular inputs occurs within a structural framework that is defined by premotor or motor targets (Fig. 4C) [Straka and Dieringer 2004]. In fact, vestibulo-ocular neurons that form the central element of the three-neuronal reflex pathway and reciprocally excite/inhibit specific populations of extraocular motoneurons are arranged in segment-specific hindbrain locations (right scheme in Fig. 4C) [Straka et al. 2001]. The distribution of vestibular subgroups with projections to spatially specific sets of extraocular motoneurons in separate rhombomeric compartments complies with a processing of head/body motion signals within a motor map that likely also includes vestibulo-spinal neurons for posture and locomotor control (left scheme in Fig. 4C) [Straka and Baker 2013; Straka et al. 2014]. As will be shown in the next section, this is at variance with the lateral line system, where signals related to water motion are processed within a sensory map.
Transmission of lateral line afferent signals and organizational arrangement of central projections
In most fish and all amphibian species, lateral line sensory signals arrive at the respective hindbrain nucleus through the anterior (aLLN) and the posterior lateral line nerve (pLLN). The two nerves enter the dorso-lateral hindbrain at the level r4 and r6, respectively where the axons bifurcate into an ascending and descending tract [Alexandre and Ghysen 1999] along the length of the medial octavolateral nucleus (MON, Fig. 5D,E). Lateral line afferents terminate in a topographic manner suggesting that the position of afferent terminals along the dorso-ventral axis of the MON mirrors the spatial distribution of the neuromasts along the rostro-caudal axis of the periphery (Fig. 5D). This sensory topography appears to be defined by neurogenic timing [Pujol-Martí et al. 2012] compatible with findings in cartilaginous fish [Koester 1983; Puzdrowski and Leonard 1993]. In contrast axonal projections to the Mauthner cell are non-topographic, compatible with the low-threshold sensitivity for lateral line mediated escape behavior [Mirjany et al. 2011; Stewart et al. 2013]. A third target of the lateral line afferent projections is the cerebellum [Fame et al. 2006; Puzdrowski 1989], where the termination fields of aLLN and pLLN segregate along an antero-ventral and postero-dorsal axis and partly overlap with those of vestibular afferents [Wullimann and Grothe 2014].
The typical gnathostome MON is covered by the cerebellar crest (Fig. 5E) with peripheral sensory inputs reaching ventral dendrites and central feedback via parallel fibers reaching apical dendrites of the same principal neurons [Mugnaini and Maler 1987]. This cerebellar-like network in the MON acts as an adaptive filter that assists the cancellation of reafferent hydrodynamic noise such as during breathing [Montgomery et al. 1996], a mechanism shared with a similar network in the electrosensory DON of elasmobranch fish [Montgomery and Bodznick 1999]. The axons of Purkinje-like cells in the MON give rise to second-order lateral line and commissural projections. This secondary projection reaches the ventro-lateral nucleus of the midbrain torus semicircularis through the ascending lemniscus, with a contralateral dominance (Fig. 5E) [Wullimann and Grothe 2014]. As a general principle water motions are represented in a somatotopic manner [Engelmann and Bleckmann 2004; Plachta et al. 2003]. This sensory representation appears to re-align the map for the afferent terminations to the MON from a dorso-ventral in a rostro-caudal representation of the periphery in the torus. In addition, a second weaker projection from the MON reaches the midbrain optic tectum. Whether a somatotopic lateral line map in the optic tectum is present in teleost fish as in anurans [Bartels et al. 1990; Behrend et al. 2006; Zittlau et al. 1985] remains to be determined. Nonetheless, the presence of a sensory topography at multiple hierarchical brain levels for lateral line signals is a marked difference with the motor organizational pattern of vestibular sensory signals.
Efferent control of hair cell mechanoreception
A major commonality of vestibular and lateral line organization is an efferent neural innervation of hair cells and/or afferent fibers [Hellmann and Fritzsch 1996]. A high sensitivity enables hair cells to detect very small shearing angles of the cilial bundle, but impairs the ability to encode larger and/or more rapid cilial deflections. By changing the sensitivity of the hair cells, via dynamic manipulation of the membrane potential and stiffness of the stereovilli, the working range of cilial deflections can be considerably increased [Sienknecht et al. 2014]. A central control of the mechanoreceptive sensitivity in both systems of aquatic anamniotes is mediated by a set of hindbrain efferent neurons. Shared efferent neurons, with an origin in r4 project to all inner ear endorgans as well as to neuromasts supplied by the aLLN [Chagnaud et al. 2015; Hellmann and Fritzsch 1996]. In contrast, a second set of efferent neurons, which derives from r6 project to those neuromasts along the body that are innervated by the pLLN [Chagnaud et al. 2015; Gilland et al. 2014]. Despite the common origin, the functional consequence of efferent activation differs between the vestibular and lateral line system. Axonal projections of efferent neurons to the inner ear form synapses on both hair cells and afferent fibers, whereas efferent axon collaterals in the lateral line system contact only hair cells [Boyle and Highstein 1990; Highstein and Baker 1985; Rabbitt et al. 2004]. While lateral line afferents respond with a decrease in afferent fiber activity upon efferent activation, e.g. during locomotor activity, vestibular afferents show either an increased or decreased activity [e.g., Chagnaud et al. 2015]. This difference in response pattern is likely related to the different requirements related to the mechanistic principles for the encoding of head/body versus water motion [Chagnaud et al. 2015]. The processing of vestibular signals usually relies on sensory inputs from both sides and a modulation of a bilaterally symmetric resting rate. This can only be achieved if efferent mediated changes in the efficacy of hair cell sensitivity are compensated by a corresponding alteration of the activity within the overall population of afferent fibers [Chagnaud et al. 2015]. In contrast, effective encoding of water motion requires only single patches of neuromasts with hair cells of opposite polarity. The innervation of the latter by separate afferents allows unilateral encoding of bidirectional water motion without the necessity to maintain a balanced resting rate of the lateral line nerves on both sides. The different functional control of afferent activity is likely achieved by an efferent synaptic innervation of both hair cells and afferent fibers in the vestibular system and of hair cells alone in the lateral line system.
Conclusion
Vestibular and lateral line mechanoreception share a number of genetic and morphological traits, such as development from adjacent placodal regions, identical sensory cell types with similar mechano-electrical transduction mechanisms and projections to adjacent hindbrain recipient areas. While the general functionality of the sensory cells is comparable and designed to transduce mechanical stimuli into a neuronal response, the specific adequate stimulus for vestibular and lateral line sense organs depends on accessory structural elements that define the effective responsiveness of the two sensory organs and the dynamic range for their motion sensitivity. Despite the many shared organizational features of these two closely related sensory systems, there are fundamental differences in the central representation of the encoded sensory signals. Also, differences in computational mechanisms for higher order processing are reflected in distinct central projections to defined second order nuclei. While lateral line signals are topographically represented at the first and subsequent levels within a sensory map that increases in precision (i.e., receptive fields become sharper from MON to torus), vestibular signals are processed in neuronal elements that are organized into distinct channels according to the motor output. This difference relates to the respective roles of the two sensory systems in the detection of body motion and the functional significance of this motion detection for the animal in its environment: The vestibular system senses body position and motion within the earth’s gravitational field and as such uses an egocentric reference frame with an immediate control of motor output that continuously ensures gaze and postural stability. In contrast, the lateral line detects water motion over the body’s surface that requires a topographic mapping of the impinging sensory stimuli. Despite the same sensory cell types, neuronal coding and adjacent hindbrain recipient areas, the two systems take advantage of different representation strategies to map sensory signals onto central circuits.
Acknowledgments
This review originated from the symposium “hair cell sensory systems from wiring to firing” held at the 8th European Conference on Comparative Neurobiology. We thank the program committee and in particular Dr. Mario Wullimann for organizing the meeting. Funding was provided by the German Science Foundation (DFG, CRC870 to BPC and HS; EN 825/4-1 and EXC 277 to JE), the NIH (BF, P30 DC 010362) and by the Research Council of Norway (NFR 251282 to JCG).
References
- Alexandre D, Ghysen A. Somatotopy of the lateral line projection in larval zebrafish. Proc Natl Acad Sci USA. 1999;96:7558–7562. doi: 10.1073/pnas.96.13.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, Münz H, Claas B. Representation of lateral line and electrosensory systems in the midbrain of the axolotl, Ambystoma mexicanum. J Comp Physiol A. 1990;167:347–356. [Google Scholar]
- Beckmann M, Erős T, Schmitz A, Bleckmann H. Number and distribution of superficial neuromasts in twelve common european cypriniform fishes and their relationship to habitat occurrence. Int Rev Hydrobiol. 2010;95:273–284. [Google Scholar]
- Behrend O, Branoner F, Zhivkov Z, Ziehm U. Neural responses to water surface waves in the midbrain of the aquatic predator Xenopus laevis. Eur J Neurosci. 2006;23:729–744. doi: 10.1111/j.1460-9568.2006.04577.x. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- Birinyi A, Straka H, Matesz C, Dieringer N. Location of dye-coupled second order and of efferent vestibular neurons labeled from individual semicircular canal or otolith organs in the frog. Brain Res. 2001;921:44–59. doi: 10.1016/s0006-8993(01)03075-x. [DOI] [PubMed] [Google Scholar]
- Bleckmann H, Niemann U, Fritzsch B. Peripheral and central aspects of the acoustic and lateral line system of a bottom dwelling catfish, Ancistrus sp. J Comp Neurol. 1991;314:452–466. doi: 10.1002/cne.903140304. [DOI] [PubMed] [Google Scholar]
- Bleckmann H, Mogdans J, Coombs SL. Flow Sensing in Air and Water. New York: Springer; 2014. [Google Scholar]
- Bober E, Rinkwitz S, Herbrand H. Molecular basis of otic commitment and morphogenesis: a role for homeodomain-containing transcription factors and signaling molecules. Curr Top Dev Biol. 2003;57:151–175. doi: 10.1016/s0070-2153(03)57005-3. [DOI] [PubMed] [Google Scholar]
- Boyle R, Highstein S. Efferent vestibular system in the toadfish: action upon horizontal semicircular canal afferents. J Neurosci. 1990;10:1570–1582. doi: 10.1523/JNEUROSCI.10-05-01570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branoner F, Straka H. Semicircular canal-dependent developmental tuning of translational vestibulo-ocular reflexes in Xenopus laevis. Dev Neurobiol. 2015;75:1051–1067. doi: 10.1002/dneu.22234. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Chaar V, Dambly-Chaudière C, Ghysen A. Early efferent innervation of the zebrafish lateral line. J Comp Neurol. 2001;434:253–261. doi: 10.1002/cne.1175. [DOI] [PubMed] [Google Scholar]
- Chagnaud BP, Bleckmann H, Hofmann MH. Lateral line nerve fibers do not code bulk water flow direction in turbulent flow. Zoology (Jena) 2008a;111:204–217. doi: 10.1016/j.zool.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Chagnaud BP, Brücker C, Hofmann MH, Bleckmann H. Measuring flow velocity and flow direction by spatial and temporal analysis of flow fluctuations. J Neurosci. 2008b;28:4479–4487. doi: 10.1523/JNEUROSCI.4959-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnaud BP, Banchi R, Simmers J, Straka H. Spinal corollary discharge modulates motion sensing during vertebrate locomotion. Nat Commun. 2015;6:7982. doi: 10.1038/ncomms8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Streit A. Induction of the inner ear: stepwise specification of otic fate from multipotent progenitors. Hear Res. 2013;297:3–12. doi: 10.1016/j.heares.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Law MI. Eye-specific termination bands in tecta of three-eyed frogs. Science. 1978;202:639–641. doi: 10.1126/science.309179. [DOI] [PubMed] [Google Scholar]
- Coombs S, Janssen J. Behavioral and neurophysiological assessment of lateral line sensitivity in the mottled sculpin, Cottus bairdi. J Comp Physiol A. 1990;167:557–567. doi: 10.1007/BF00190827. [DOI] [PubMed] [Google Scholar]
- Coombs S, Montgomery J. Function and evolution of superficial neuromasts in an Antarctic notothenioid fish. Brain Behav Evol. 1994;44:287–298. doi: 10.1159/000113590. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Fritzsch B, Popper AN, Fay RR. The Primary Auditory Neurons of the Mammalian Cochlea. New York: Springer; 2016. [Google Scholar]
- de Burlet HM. Zur Vergleichenden Anatomie der Labyrinthinnervation. J Comp Neurol. 1929;47:155–169. [Google Scholar]
- Denton EJ, Gray J. Mechanical factors in the excitation of clupeid lateral lines. Proc R Soc B. 1983;218:1–26. doi: 10.1098/rspb.1983.0023. [DOI] [PubMed] [Google Scholar]
- Denton EJ, Gray JAB. Mechanical factors in the excitation of the lateral lines of fishes. In: Atema J, Fay RR, Popper AN, Tavolga WN, editors. Sensory Biology of Aquatic Animals. New York: Springer-Verlag; 1988. pp. 595–617. [Google Scholar]
- Denton EJ, Gray JAB. Some observations on the forces acting on neuromasts in fish lateral line canals. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line Neurobiology and Evolution. New York: Springer Verlag; 1989. pp. 229–246. [Google Scholar]
- Di Bonito M, Narita Y, Avallone B, Sequino L, Mancuso M, Andolfi G, Franzè AM, Puelles L, Rijli FM, Studer M. Assembly of the auditory circuitry by a Hox genetic network in the mouse brainstem. PLoS Genet. 2013;9:e1003249. doi: 10.1371/journal.pgen.1003249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bonito M, Boulland J-L, Krezel W, Setti E, Studer M, Glover JC. Loss of projections, functional compensation, and residual deficits in the mammalian vestibulospinal system of Hoxb1-deficient mice. eneuro. 2015:2. doi: 10.1523/ENEURO.0096-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz C, Puelles L, Marín F, Glover JC. The relationship between rhombomeres and vestibular neuron populations as assessed in quail–chicken chimeras. Dev Biol. 1998;202:14–28. doi: 10.1006/dbio.1998.8986. [DOI] [PubMed] [Google Scholar]
- Dijkgraaf S. The functioning and significance of the lateral-line organs. Biol Rev. 1963;38:51–105. doi: 10.1111/j.1469-185x.1963.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Fritzsch B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS One. 2013;8:e62046. doi: 10.1371/journal.pone.0062046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott KL, Fritzsch B. Transplantation of Xenopus laevis ears reveals the ability to form afferent and efferent connections with the spinal cord. Int J Dev Biol. 2011;54:1443–1451. doi: 10.1387/ijdb.103061ke. [DOI] [PubMed] [Google Scholar]
- Elliott KL, Houston DW, DeCook R, Fritzsch B. Ear manipulations reveal a critical period for survival and dendritic development at the single-cell level in Mauthner neurons. Dev Neurobiol. 2015a;75:1339–1351. doi: 10.1002/dneu.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott KL, Houston DW, Fritzsch B. Sensory afferent segregation in three-eared frogs resemble the dominance columns observed in three-eyed frogs. Sci Rep. 2015b;5:8338. doi: 10.1038/srep08338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann J, Hanke W, Bleckmann H. Lateral line reception in still- and running water. J Comp Physiol A. 2002;188:513–526. doi: 10.1007/s00359-002-0326-6. [DOI] [PubMed] [Google Scholar]
- Engelmann J, Bleckmann H. Coding of lateral line stimuli in the goldfish midbrain in still and running water. Zoology. 2004;107:135–151. doi: 10.1016/j.zool.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Fame RM, Brajon C, Ghysen A. Second-order projection from the posterior lateral line in the early zebrafish brain. Neural Dev. 2006;1:1–28. doi: 10.1186/1749-8104-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucherre A, Pujol-Martí J, Kawakami K, López-Schier H. Afferent neurons of the zebrafish lateral line are strict selectors of hair-cell orientation. PLoS One. 2009;4:e4477. doi: 10.1371/journal.pone.0004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock Å, Wersäll J. A study of the orientation of the sensory hairs of the receptor cells in the lateral line organ of fish, with special reference to the function of the receptors. J Cell Biol. 1962;15:19–27. doi: 10.1083/jcb.15.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer L, Aggarwal V, Morrow BE. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development. 2011;138:5403–5414. doi: 10.1242/dev.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Wahnschaffe U, Bartsch U. The Evolution of the Amphibian Auditory System. Wiley; New York: 1988. Metamorphic changes in the octavolateralis system of amphibians; pp. 359–376. [Google Scholar]
- Fritzsch B, Niemann U, Bleckmann H. A discrete projection of the sacculus and lagena to a distinct brainstem nucleus in a catfish. Neurosci Lett. 1990;111:7–11. doi: 10.1016/0304-3940(90)90335-7. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The Neocortex. New York: Springer; 1991. On the coincidence of loss of electroreception and reorganization of brain stem nuclei in vertebrates; pp. 103–109. [Google Scholar]
- Fritzsch B, Sarai P, Barbacid M, Silos-Santiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Dev Neurosci. 1997;15:563–576. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Bermingham NA. Developmental evolutionary biology of the vertebrate ear: conserving mechanoelectric transduction and developmental pathways in diverging morphologies. Neuroreport. 2000;11:R35–R44. doi: 10.1097/00001756-200011270-00013. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Signore M, Simeone A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol. 2001;211:388–396. doi: 10.1007/s004270100166. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel K. Evolution and development of the vertebrate ear. Brain Res Bull. 2001;55:711–721. doi: 10.1016/s0361-9230(01)00558-5. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Gregory D, Rosa-Molinar E. The development of the hindbrain afferent projections in the axolotl: evidence for timing as a specific mechanism of afferent fiber sorting. Zoology. 2005;108:297–306. doi: 10.1016/j.zool.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Feng F, Matei V, Nichols D. The molecular and developmental basis of the evolution of the vertebrate auditory system. Int J Comp Psychol. 2006;19:1–25. [Google Scholar]
- Fritzsch B, Glover J. Evolution of the deuterostome central nervous system: an intercalation of developmental patterning processes with cellular specification processes. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 2. Elsevier; 2007. pp. 1–24. [Google Scholar]
- Fritzsch B, Eberl DF, Beisel KW. The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell Mol Life Sci. 2010;67:3089–3099. doi: 10.1007/s00018-010-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pan N, Jahan I, Duncan JS, Kopecky BJ, Elliott KL, Kersigo J, Yang T. Evolution and development of the tetrapod auditory system: an organ of Corti-centric perspective. Evol Dev. 2013;15:63–79. doi: 10.1111/ede.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Straka H. Evolution of vertebrate mechanosensory hair cells and inner ears: toward identifying stimuli that select mutation driven altered morphologies. J Comp Physiol A. 2014;200:5–18. doi: 10.1007/s00359-013-0865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, López-Schier H. Evolution of polarized hair cells in aquatic vertebrates and their connection to directionally sensitive neurons. In: Bleckmann H, Mogdans J, Coombs SL, editors. Flow Sensing in Air and Water. New York: Springer; 2014. pp. 271–294. [Google Scholar]
- Fritzsch B, Jahan I, Pan N, Elliott KL. Evolving gene regulatory networks into cellular networks guiding adaptive behavior: an outline how single cells could have evolved into a centralized neurosensory system. Cell Tissue Res. 2015;359:295–313. doi: 10.1007/s00441-014-2043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Degasperi V, Shimeld SM, Burighel P, Manni L. Evolutionary conservation of the placodal transcriptional network during sexual and asexual development in chordates. Dev Dynam. 2013;242:752–766. doi: 10.1002/dvdy.23957. [DOI] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudière C, Raible D. Making sense of zebrafish neural development in the Minervois. Neural Dev. 2007;2:15. doi: 10.1186/1749-8104-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs MA. Lateral line receptors: where do they come from developmentally and where is our research going? Brain Behav Evol. 2004;64:163–181. doi: 10.1159/000079745. [DOI] [PubMed] [Google Scholar]
- Gilland E, Straka H, Wong TW, Baker R, Zottoli SJ. A hindbrain segmental scaffold specifying neuronal location in the adult goldfish, Carassius auratus. J Comp Neurol. 2014;522:2446–2464. doi: 10.1002/cne.23544. [DOI] [PubMed] [Google Scholar]
- Glover JC. Neuroepithelial ‘compartments’ and the specification of vestibular projections. Prog Brain Res. 2000;124:3–21. doi: 10.1016/S0079-6123(00)24004-1. [DOI] [PubMed] [Google Scholar]
- Glover JC. Correlated patterns of neuron differentiation and Hox gene expression in the hindbrain: a comparative analysis. Brain Res Bull. 2001;55:683–693. doi: 10.1016/s0361-9230(01)00562-7. [DOI] [PubMed] [Google Scholar]
- Glover JC, Renaud JS, Rijli FM. Retinoic acid and hindbrain patterning. J Neurobiol. 2006;66:705–725. doi: 10.1002/neu.20272. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res. 2000;130:277–297. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV. Early development of the spiral ganglion. In: Dabdoub A, Fritzsch B, Popper AN, Fay RR, editors. The Primary Auditory Neurons of the Mammalian Cochlea. New York: Springer; 2016. pp. 11–48. [Google Scholar]
- Goulet J, Engelmann J, Chagnaud B, Franosch J-M, Suttner M, van Hemmen J. Object localization through the lateral line system of fish: theory and experiment. J Comp Physiol A. 2008;194:1–17. doi: 10.1007/s00359-007-0275-1. [DOI] [PubMed] [Google Scholar]
- Goulet J, van Hemmen JL, Jung SN, Chagnaud BP, Scholze B, Engelmann J. Temporal precision and reliability in the velocity regime of a hair-cell sensory system: the mechanosensory lateral line of goldfish, Carassius auratus. J Neurophysiol. 2012;107:2581–2593. doi: 10.1152/jn.01073.2011. [DOI] [PubMed] [Google Scholar]
- Hellmann B, Fritzsch B. Neuroanatomical and histochemical evidence for the presence of common lateral line and inner ear efferents and of efferents to the basilar papilla in a frog, Xenopus laevis. Brain Behav Evol. 1996;47:185–194. doi: 10.1159/000113238. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Baker R. Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. J Neurophysiol. 1985;54:370–384. doi: 10.1152/jn.1985.54.2.370. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Rabbitt RD, Holstein GR, Boyle RD. Determinants of spatial and temporal coding by semicircular canal afferents. J Neurophysiol. 2005;93:2359–2370. doi: 10.1152/jn.00533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond Biol. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear’s works work: mechanoelectrical transduction and amplification by hair cells. C R Biol. 2005;328:155–162. doi: 10.1016/j.crvi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Iskusnykh IY, Steshina EY, Chizhikov VV. Loss of Ptf1a leads to a widespread cell-fate misspecification in the brainstem, affecting the development of somatosensory and viscerosensory nuclei. J Neurosci. 2016;36:2691–2710. doi: 10.1523/JNEUROSCI.2526-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Kersigo J, Pan N, Fritzsch B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010;341:95–110. doi: 10.1007/s00441-010-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Fritzsch B. Opportunities and limits of the one gene approach: the ability of Atoh1 to differentiate and maintain hair cells depends on the molecular context. Front Cell Neurosci. 2015a;9:26. doi: 10.3389/fncel.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurog1 can partially substitute for Atoh1 function in hair cell differentiation and maintenance during organ of Corti development. Development. 2015b;142:2810–2821. doi: 10.1242/dev.123091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Elliott KL, Fritzsch B. The quest for restoring hearing: understanding ear development more completely. Bioessays. 2015c;37:1016–1027. doi: 10.1002/bies.201500044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen J. Adaptations for the reception of natural stimuli. In: von der Emde G, Mogdans J, Kapoor BG, editors. The Senses of Fish. New Delhi: Narosa Publishing House; 2004. pp. 231–264. [Google Scholar]
- Koester DM. Central projections of the octavolateralis nerves of the clearnose skate, Raja eglanteria. J Comp Neurol. 1983;215:199–215. doi: 10.1002/cne.902210208. [DOI] [PubMed] [Google Scholar]
- Kroese AB, Van der Zalm JM, Van den Bercken J. Frequency response of the lateral-line organ of Xenopus laevis. Pflugers Arch. 1978;375:167–175. doi: 10.1007/BF00584240. [DOI] [PubMed] [Google Scholar]
- Kroese AB, Schellart NA. Velocity- and acceleration-sensitive units in the trunk lateral line of the trout. J Neurophysiol. 1992;68:2212–2221. doi: 10.1152/jn.1992.68.6.2212. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Chapter thirty-four-hox genes and the hindbrain: a study in segments. Curr Top Dev Biol. 2016;116:581–596. doi: 10.1016/bs.ctdb.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Ledent V. Postembryonic development of the posterior lateral line in zebrafish. Development. 2002;129:597. doi: 10.1242/dev.129.3.597. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The Vertebrate Inner Ear. Boca Raton: CRC Press; 1985. [Google Scholar]
- Lim R, Brichta AM. Anatomical and physiological development of the human inner ear. Hear Res. 2016 doi: 10.1016/j.heares.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Ma Q, Sommer L, Cserjesi P, Anderson DJ. Mash1 and neurogenin1 expression patterns define complementary domains of neuroepithelium in the developing CNS and are correlated with regions expressing notch ligands. J Neurosci. 1997;17:3644–3652. doi: 10.1523/JNEUROSCI.17-10-03644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EC, Saxena A, Alsina B, Bronner ME, Whitfield TT. Sensational placodes: neurogenesis in the otic and olfactory systems. Dev Biol. 2014;389:50–67. doi: 10.1016/j.ydbio.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Kamel S, Wong E, Fritzsch B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010;340:303–321. doi: 10.1007/s00441-010-0944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Reiprich S, Wegner M, Fritzsch B. Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PLoS One. 2014;9:e94580. doi: 10.1371/journal.pone.0094580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín F, Charnay P. Hindbrain patterning: FGFs regulate Krox20 and mafB/kr expression in the otic/preotic region. Development. 2000;127:4925–4935. doi: 10.1242/dev.127.22.4925. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel K, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev Dynam. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazan S, Jaillard D, Baratte B, Janvier P. Otx1 gene-controlled morphogenesis of the horizontal semicircular canal and the origin of the gnathostome characteristics. Evol Dev. 2000;2:186–193. doi: 10.1046/j.1525-142x.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- McCormick CA. Central lateral line mechanosensory pathways in bony fish. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line Neurobiology and Evolution. New York: Springer; 1989. pp. 79–97. [Google Scholar]
- McCormick CA, Wallace AC. Otolith end organ projections to auditory neurons in the descending octaval nucleus of the goldfish, Carassius auratus: a confocal analysis. Brain Behav Evol. 2012;80:41–63. doi: 10.1159/000339746. [DOI] [PubMed] [Google Scholar]
- Mirjany M, Preuss T, Faber DS. Role of the lateral line mechanosensory system in directionality of goldfish auditory evoked escape response. J Exp Biol. 2011;214:3358. doi: 10.1242/jeb.052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrell MS, Bemis WE, Northcutt RG, Davis MC, Baker CV. Electrosensory ampullary organs are derived from lateral line placodes in bony fishes. Nat Commun. 2011;2:496. doi: 10.1038/ncomms1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Bodznick D, Halstead M. Hindbrain signal processing in the lateral line system of the dwarf scorpionfish Scopeana papillosus. J Exp Biol. 1996;199:893–899. doi: 10.1242/jeb.199.4.893. [DOI] [PubMed] [Google Scholar]
- Montgomery J, Baker CF, Carton AG. The lateral line can mediate rheotaxis in fish. Nature. 1997;389:960–963. [Google Scholar]
- Montgomery J, Bleckmann H, Coombs S. The Lateral Line System. New York: Springer; 2014. [Google Scholar]
- Montgomery JC, Bodznick D. Signals and noise in the elasmobranch electrosensory system. J Exp Biol. 1999;202:1349–1355. doi: 10.1242/jeb.202.10.1349. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Maler L. Cytology and immunocytochemistry of the nucleus of the lateral line lobe in the electric fish Gnathonemus petersii (mormyridae): evidence suggesting that GABAergic synapses mediate an inhibitory corollary discharge. Synapse. 1987;1:32–56. doi: 10.1002/syn.890010107. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Puelles L. Towards a New Neuromorphology. New York: Springer; 2015. [Google Scholar]
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol. 1983;58:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Brandle K, Fritzsch B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev Biol. 1995;168:358–373. doi: 10.1006/dbio.1995.1086. [DOI] [PubMed] [Google Scholar]
- O’Neill P, Mak S-S, Fritzsch B, Ladher RK, Baker CV. The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nat Commun. 2012;3:1041. doi: 10.1038/ncomms2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H, Bronner M, Krumlauf R. The vertebrate Hox gene regulatory network for hindbrain segmentation: Evolution and diversification: Coupling of a Hox gene regulatory network to hindbrain segmentation is an ancient trait originating at the base of vertebrates. Bioessays. 2016;38:526–538. doi: 10.1002/bies.201600010. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Díaz C, Renaud J-S, Rijli FM, Glover JC. Fate-mapping the mammalian hindbrain: segmental origins of vestibular projection neurons assessed using rhombomere-specific Hoxa2 enhancer elements in the mouse embryo. J Neurosci. 2007;27:9670–9681. doi: 10.1523/JNEUROSCI.2189-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthey C, Schlosser G, Shimeld SM. The evolutionary history of vertebrate cranial placodes–I: cell type evolution. Dev Biol. 2014;389:82–97. doi: 10.1016/j.ydbio.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Peter I, Davidson EH. Genomic Control Process: Development and Evolution. Academic Press; 2015. [Google Scholar]
- Pichon F, Ghysen A. Evolution of posterior lateral line development in fish and amphibians. Evol Dev. 2004;6:187–193. doi: 10.1111/j.1525-142X.2004.04024.x. [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Baker CVH. The development of lateral line placodes: Taking a broader view. Dev Biol. 2014;389:68–81. doi: 10.1016/j.ydbio.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Plachta DT, Hanke W, Bleckmann H. A hydrodynamic topographic map in the midbrain of goldfish Carassius auratus. J Exp Biol. 2003;206:3479–3486. doi: 10.1242/jeb.00582. [DOI] [PubMed] [Google Scholar]
- Pohlmann K, Grasso FW, Breithaupt T. Tracking wakes: the nocturnal predatory strategy of piscivorous catfish. Proc Natl Acad Sci USA. 2001;98:7371–7374. doi: 10.1073/pnas.121026298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prandtl L. Über Flüssigkeitsbewegung bei sehr kleiner Reibung. Verhandlungen des dritten Int Math Heidelberg. 1904:484–491. [Google Scholar]
- Pujol-Martí J, Zecca A, Baudoin J-P, Faucherre A, Asakawa K, Kawakami K, López-Schier H. Neuronal birth order identifies a dimorphic sensorineural map. J Neurosci. 2012;32:2976–2987. doi: 10.1523/JNEUROSCI.5157-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol-Martí J, López-Schier H. Developmental and architectural principles of the lateral-line neural map. Front Neural Circ. 2014;7:274–281. doi: 10.3389/fncir.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzdrowski RL. Peripheral distribution and central projections of the lateral-line nerves in goldfish, Carassius auratus. Brain Behav Evol. 1989;34:121–131. doi: 10.1159/000116496. [DOI] [PubMed] [Google Scholar]
- Puzdrowski RL, Leonard RB. The octavolateral systems in the stingray, Dasyatis sabina. I. Primary projections of the octaval and lateral line nerves. J Comp Neurol. 1993;332:21–37. doi: 10.1002/cne.903320103. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen C-L, Choi Y, Ma Q. Formation of brainstem (nor) adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt RD, Boyle R, Holstein GR, Highstein SM. Hair-cell versus afferent adaptation in the semicircular canals. J Neurophysiol. 2004;93:424–436. doi: 10.1152/jn.00426.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Groves AK. Segregating neural and mechanosensory fates in the developing ear: patterning, signaling, and transcriptional control. Cell Tissue Res. 2015;359:315–332. doi: 10.1007/s00441-014-1917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkwitz S, Bober E, Baker R. Development of the vertebrate inner ear. Ann N Y Acad Sci. 2001;942:1–14. doi: 10.1111/j.1749-6632.2001.tb03730.x. [DOI] [PubMed] [Google Scholar]
- Ristroph L, Liao JC, Zhang J. Lateral line layout correlates with the differential hydrodynamic pressure on swimming fish. Phys Rev Lett. 2015;114:018102. doi: 10.1103/PhysRevLett.114.018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roellig D, Bronner ME. The epigenetic modifier DNMT3A is necessary for proper otic placode formation. Dev Biol. 2016;411:294–300. doi: 10.1016/j.ydbio.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohregger M, Dieringer N. Principles of linear and angular vestibuloocular reflex organization in the frog. J Neurophysiol. 2002;87:385. doi: 10.1152/jn.00404.2001. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Sandell LL, Tjaden NEB, Barlow AJ, Trainor PA. Cochleovestibular nerve development is integrated with migratory neural crest cells. Dev Biol. 2014;385:200–210. doi: 10.1016/j.ydbio.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting DH, Gersten K. Boundary-layer theory. Eur J Mech - B/Fluids. 1979;20:817. [Google Scholar]
- Schlosser G, Patthey C, Shimeld SM. The evolutionary history of vertebrate cranial placodes II. Evolution of ectodermal patterning. Dev Biol. 2014;389:98–119. doi: 10.1016/j.ydbio.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Bleckmann H, Mogdans J. Organization of the superficial neuromast system in goldfish, Carassius auratus. J Morphol. 2008;269:751–761. doi: 10.1002/jmor.10621. [DOI] [PubMed] [Google Scholar]
- Schwalbe MAB, Bassett DK, Webb JF. Feeding in the dark: lateral-line-mediated prey detection in the peacock cichlid Aulonocara stuartgranti. J Exp Biol. 2012;215:2060. doi: 10.1242/jeb.065920. [DOI] [PubMed] [Google Scholar]
- Sienknecht UJ, Köppl C, Fritzsch B. Evolution and development of hair cell polarity and efferent function in the inner ear. Brain Behav Evol. 2014;83:150–161. doi: 10.1159/000357752. [DOI] [PubMed] [Google Scholar]
- Stewart WJ, Cardenas GS, McHenry MJ. Zebrafish larvae evade predators by sensing water flow. J Exp Biol. 2013;216:388. doi: 10.1242/jeb.072751. [DOI] [PubMed] [Google Scholar]
- Straka H, Baker R, Gilland E. Rhombomeric organization of vestibular pathways in larval frogs. J Comp Neurol. 2001;437:42–55. doi: 10.1002/cne.1268. [DOI] [PubMed] [Google Scholar]
- Straka H, Holler S, Goto F. Patterns of canal and otolith afferent input convergence in frog second-order vestibular neurons. J Neurophysiol. 2002;88:2287–2301. doi: 10.1152/jn.00370.2002. [DOI] [PubMed] [Google Scholar]
- Straka H, Holler S, Goto F, Kolb FP, Gilland E. Differential spatial organization of otolith signals in frog vestibular nuclei. J Neurophysiol. 2003;90:3501–3512. doi: 10.1152/jn.00372.2003. [DOI] [PubMed] [Google Scholar]
- Straka H, Dieringer N. Basic organization principles of the VOR: lessons from frogs. Prog Neurobiol. 2004;73:259–309. doi: 10.1016/j.pneurobio.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol. 2005;76:349–392. doi: 10.1016/j.pneurobio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Straka H, Lambert FM, Pfanzelt S, Beraneck M. Vestibulo-ocular signal transformation in frequency- tuned channels. Ann N Y Acad Sci. 2009;1164:37–44. doi: 10.1111/j.1749-6632.2008.03740.x. [DOI] [PubMed] [Google Scholar]
- Straka H. Ontogenetic rules and constraints of vestibulo-ocular reflex development. Curr Opin Neurobiol. 2010;20:689–695. doi: 10.1016/j.conb.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Straka H, Baker R. Vestibular blueprint in early vertebrates. Front Neural Circuits. 2013;7:182. doi: 10.3389/fncir.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka H, Fritzsch B, Glover JC. Connecting ears to eye muscles: evolution of a ‘simple’ reflex arc. Brain Behav Evol. 2014;83:162–175. doi: 10.1159/000357833. [DOI] [PubMed] [Google Scholar]
- Tarby ML, Webb JF. Development of the supraorbital and mandibular lateral line canals in the cichlid, Archocentrus nigrofasciatus. J Morphol. 2003;255:44–57. doi: 10.1002/jmor.10045. [DOI] [PubMed] [Google Scholar]
- van Netten S. Hydrodynamic detection by cupulae in a lateral line canal: functional relations between physics and physiology. Biol Cyber. 2006;94:67–85. doi: 10.1007/s00422-005-0032-x. [DOI] [PubMed] [Google Scholar]
- van Netten SM, McHenry MJ. The biophysics of the fish lateral line. In: Coombs S, Bleckmann H, Fay RR, Popper NA, editors. The Lateral Line System. New York, NY: Springer New York; 2014. pp. 99–119. [Google Scholar]
- van Trump WJ, McHenry MJ. The lateral line system is not necessary for rheotaxis in the Mexican blind cavefish (Astyanax fasciatus) Integr Comp Biol. 2013;53:799–809. doi: 10.1093/icb/ict064. [DOI] [PubMed] [Google Scholar]
- Voigt R, Carton AG, Montgomery JC. Responses of anterior lateral line afferent neurones to water flow. J Exp Biol. 2000;203:2495–2502. doi: 10.1242/jeb.203.16.2495. [DOI] [PubMed] [Google Scholar]
- Wark AR, Peichel CL. Lateral line diversity among ecologically divergent threespine stickleback populations. J Exp Biol. 2010;213:108–117. doi: 10.1242/jeb.031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JF. Developmental constraints and evolution of the lateral line system in teleost fishes. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line Neurobiology and Evolution. New York: Springer; 1989a. pp. 79–97. [Google Scholar]
- Webb JF. Gross morphology and evolution of the mechanoreceptive lateral-line system in teleost fishes. Brain Behav Evol. 1989b;33:34–53. doi: 10.1159/000115896. [DOI] [PubMed] [Google Scholar]
- Webb JF. Morphological diversity, development, and evolution of the mechanosensory lateral line system. In: Coombs S, Bleckmann H, Fay RR, Popper NA, editors. The Lateral Line System. New York: Springer New York; 2014. pp. 17–72. [Google Scholar]
- Will U, Luhede G, Görner P. The area octavo-lateralis in Xenopus laevis. Cell Tissue Res. 1985;239:163–175. [Google Scholar]
- Will U, Fritzsch B. The eighth nerve of amphibians: peripheral and central distribution. In: Fritzsch B, editor. The Evolution of the Amphibian Auditory System. New York: Wiley; 1988. pp. 159–183. [Google Scholar]
- Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev Biol. 2005;282:1–13. doi: 10.1016/j.ydbio.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Grothe B. The central nervous organization of the lateral line system. In: Coombs S, Bleckmann H, Fay RR, Popper AN, editors. The Lateral Line System. Vol. 48. Springer; New York: 2014. pp. 195–251. [Google Scholar]
- Zecca A, Dyballa S, Voltes A, Bradley R, Pujades C. The order and place of neuronal differentiation establish the topography of sensory projections and the entry points within the hindbrain. J Neurosci. 2015;35:7475–7486. doi: 10.1523/JNEUROSCI.3743-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittlau KE, Claas B, Münz H, Görner P. Multisensory interaction in the torus semicircularis of the clawed toad Xenopus laevis. Neurosci Lett. 1985;60:77–81. doi: 10.1016/0304-3940(85)90384-2. [DOI] [PubMed] [Google Scholar]