Abstract
Despite new treatment options in lung cancer, there is still a need for better biomarkers to assist in therapy decisions. Angiogenesis has been associated with tumour growth and dissemination, and the vascular proliferation index (VPI) is a valuable prognostic marker in other tumours. Nestin, a marker of immature endothelium, was previously applied in combination with Ki67 for proliferating endothelium as a novel marker (Nestin‐Ki67) of ongoing angiogenesis. Here, the prevalence and prognostic impact of vascular proliferation on lung cancer‐specific survival (LCSS) in lung adenocarcinomas was studied. Selected tumour slides from a cohort of 210 patients treated surgically for adenocarcinoma at Haukeland University Hospital (Norway) from 1993 to 2010 were stained for Nestin‐Ki67. VPI, the ratio between the density of proliferating vessels and the overall microvessel density were used, and the cut‐off value was set at 4.4% (upper quartile). High VPI was associated with the presence of blood vessel invasion (p = 0.007) and tumour necrosis (p = 0.007). Further, high VPI was significantly associated with reduced LCSS (p = 0.020). By multivariate analysis, VPI remained an independent prognostic factor for reduced LCSS (HR 1.7; 95% CI 1.04–2.68; p = 0.033) when adjusted for other prognostic clinico‐pathological features. In conclusion, microvessel proliferation assessed using the VPI was associated with aggressive tumour features such as blood vessel invasion and tumour necrosis and, independently, decreased LCSS. This marker should be further explored in separate cohorts, and in trials of anti‐angiogenesis therapy.
Keywords: microvessel proliferation, lung adenocarcinoma, survival, neoangiogenesis
Introduction
The incidence of non‐small cell lung carcinoma (NSCLC) is still rising and, despite new and targeted treatment strategies, the mortality rate is still high (5 year relative survival rate 14% for men and 21% for women in Norway) 1. Adenocarcinomas (AC) comprise around 40% of all new cases of lung cancer 2 and, for this patient group, several new treatment options such as EGFR mutations and EML‐ALK translocations have emerged during the past two decades based on molecular changes in tumour cells 3. Lately, changes in the tumour microenvironment have received attention, especially with the emergence of immunotherapy 4.
Angiogenesis, i.e. the sprouting of new vessels from existing vasculature, has long been considered a hallmark of cancer 5, 6, and a prerequisite for tumours to grow beyond 1–2 mm in diameter and spread 7. For NSCLC, there are now FDA approved anti‐angiogenic drugs targeting the vascular endothelial growth factor receptor system, with effects superior to non‐angiogenic inhibitors according to a recent review by Hong et al 8, and there are several trials ongoing. There are, however, no predictive biomarkers for selecting patients who might benefit from such therapy 9.
Evaluation of tumour angiogenesis has been the subject of several studies, with microvessel density (MVD) regarded as an appropriate marker 10. In 1991, MVD was first described to have an influence on patient prognosis in breast cancer 11. In NSCLC, the first report was presented the following year, describing higher MVD to indicate increased metastatic rate 12. Since then, reports regarding the prognostic benefits of counting vessels in NSCLC have yielded somewhat conflicting results 13, 14, 15, 16. The predictive value of MVD for anti‐angiogenic treatment effect has also been debated 17, 18, and there have been discussions on the quantification methods as well as use of antibodies to detect microvessels 19, 20, 21.
Traditionally, MVD has been detected using factor VIII, CD31, or CD34 22, 23. Previous studies have indicated that co‐expression of factor VIII or CD34 with the proliferation marker Ki67 in endothelial cells is a better marker for prognosis in breast, endometrial, pancreatic, and prostate cancer 24, 25, 26, 27, 28, 29. For NSCLC, there have been limited data supporting this suggestion 30. Notably, combining MVD with proliferation markers into a vascular proliferation index (VPI) provided better information on associations with aggressive tumour features and patient prognosis in several tumours 25, 27, 29. Such information has not been examined and presented for NSCLC.
Since factor VIII, CD31, or CD34 also stain pre‐existing vessels in solid organs, there have been attempts to find markers for newly formed vessels, such as vasohibin‐1 30, 31. Similarly, the intermediate filament protein Nestin has been promoted as a marker of immature endothelium 32. Nestin was initially thought to be expressed in neuroepithelial stem cells alone, but has since been found in several other undifferentiated cells, including blood vessel endothelium 33. Using Nestin‐Ki67 in dual immunohistochemical staining of activated angiogenesis, we previously reported that this novel angiogenesis marker was more sensitive for prognostic impact than standard MVD 24. In lung cancer, Nestin expression has been studied before, but mainly as a tumour cell marker 34, 35, 36, 37, 38. In addition, one single study indicated that Nestin positive vessels correlated with a higher tumour stage in NSCLC 39, although no survival data were presented, and the frequency of proliferating vessels was not assessed.
Here, we examined the presence and density of microvascular proliferation using the novel angiogenesis marker Nestin‐Ki67, in relation to patient prognosis and clinico‐pathological features in lung AC.
Materials and methods
Patients
Selected tissue blocks from 213 patients surgically treated for lung AC from 1993 to 2010 at Haukeland University Hospital, Bergen, Norway, were retrieved from the archives of the Department of Pathology, Haukeland University Hospital. Twenty‐two of these cases had matched surgical biopsies from metastatic lesions, and these were also retrieved from the archives. After immunohistochemical staining (details below), 210 primary tumours and 19 matched metastatic tumours had sufficient tissue to be included in the study. There were 109 men (52%) and 101 women (48%) in the cohort, with a median age of 67 years (range 32–84). Of these AC, the most frequent growth patterns were solid and acinar predominant (38% each), followed by papillary and lepidic predominant (9% each), and five cases of mucinous adenocarcinoma (2%); there were three cases of micropapillary predominant pattern, and two cases of minimally invasive adenocarcinoma (1% each). Finally, 90% of patients were current or former smokers.
Clinical data were obtained by reviewing hospital records, and complete follow‐up data for all 210 patients were collected by 16 May 2015 (median follow‐up of survivors was 81 months, range 1–222). At latest follow‐up, 93 patients (44%) were dead because of lung cancer, 51 (24%) were dead from other causes, 7 (3%) were alive with disease recurrence, and 59 patients (28%) were fully recovered.
The study was approved by a regional ethics committee (#2013–529 REK‐Nord) prior to tissue collection and retrieval of patient data. The requirement for informed consent was waived.
Tissue handling and immunohistochemistry
From the surgical specimens, initially submerged in 10% formalin for an average of 5.8 days, sections from tumour tissue was paraffin embedded (an average of four blocks per tumour, range 1–16). Selected sections from primary tumours and metastatic lesions were stained by immunohistochemistry for Nestin‐Ki67. Briefly, 4 μm sections where deparaffinised in xylene and rehydrated in alcohol and distilled water. Target retrieval was obtained by boiling in buffered solution at pH6 (Dako S1699) using a microwave oven for 20 min. After cooling, distilled water was added to reduce the fluid to room temperature. Slides were then transferred to a humidifying chamber (Magnetic Immuno Staining Tray, Cell Path, UK). Dual Endogenous Enzyme Block (Dako 2003) was added for 8 min to block endogenous peroxidase. Buffered saline solution (Dako S3006) was used in between steps. Incubation with primary antibody was performed at room temperature for 30 min (Nestin mouse IgG SC‐23927, Santa Cruz Biotech 10c2, dilution 1:50 and Ki67 rabbit IgG Clone SP6, Thermo Sciences, dilution 1:100, both in diluent with background reducing components, Dako S0809). A HRP‐labelled polyclonal anti‐mouse polymer (Dako 4007 for Nestin) and goat anti‐rabbit IgG (H + L) alcalic‐phosphatase (Southern Biotech, Cat. No. 4050–04, for Ki67) in the ratio Gar‐AP:EnV‐M 1:100 as secondary antibody were added for 30 min at room temperature. For visualization, the Ferangie Blue TM Chromogen System (Biocare Medical) and AEC + Substrate Chromogen (Dako K3469) were added sequentially for 10 and 20 min, respectively, rinsing with distilled water between steps. Slides were mounted using Faramount mounting media (Dako S3025).
At evaluation, there were two cases with insufficient tumour tissue left for evaluation of intra‐tumoural vessels, and in one case the tissue was destroyed during the staining procedure. Thus, 210 primary tumours and 19 matched metastatic lesions were eligible for evaluation of microvessels.
Variables recorded
Clinico‐pathological data
Histological type and grade were determined according to World Health Organization criteria 40, and cases with well to moderate differentiation were merged into a low‐grade group, whereas poorly differentiated and undifferentiated tumours were merged into a high‐grade category 41, 42. The predominant growth pattern in these AC was recorded according to the International Association for the Study of Lung Cancer 43. Blood vessel invasion (BVI), lymphatic vessel involvement, pleural invasion, necrosis, and tumour inflammation were recorded from routine haematoxylin and eosin stained sections without the use of additional staining as previously described 44. Briefly, necrosis was regarded as present when areas of dead tumour cells were found (lower threshold: clusters of at least 10 necrotic tumour cells). Tumour stage was determined using the TNM Classification of Malignant Tumours (7th edition) 45. Data on age at surgery, fixation time (in days), tumour size (defined as largest diameter measured macroscopically), time of disease recurrence, time of death, cause of death, type of recurrence (local, regional, distant), and sites of metastasis (liver, adrenals, brain, bone, skin, and others such as intra‐thoracic sites, distant lymph nodes, and kidneys) were included from the clinical records.
Microvessel density/proliferating microvessel density
The MVD was recorded in accordance with the method described by Weidner, with minor modifications 24, 46. Sections stained with Nestin‐Ki67 were first scanned at low magnification (×40 and ×100) to identify areas of the tumour with the highest number of Nestin‐positive vessels (red stain). This ‘hot‐spot’‐area was indicated, and 10 non‐overlapping high power fields (HPF, ×400, field size 0.239 mm2) were examined sequentially for the total number of positive vessels. Areas of necrosis and fibrosis formation were avoided, and there had to be at least 50% viable tumour cells in the HPF for it to be examined. All stained vessels were counted, including single endothelial cells. Lumen formation was not mandatory for counting a vessel, but cells needed to morphologically resemble endothelial cells or cell clusters 11. Elements with non‐specific staining, nerves, fibroblasts, and positive tumour cells were excluded from these counts. The number of stained vessels per square‐millimetre was recorded as the MVD.
The number of proliferating vessels proliferating microvessel density (pMVD) was obtained from the same ten HPF as MVD. A vessel positive for Nestin that contained at least one Ki67‐positive nucleus (blue) within the endothelial cells, counted as a proliferative vessel (Figure 1). The intensity of Ki67 in endothelial nuclei was compared to the intensity in tumour cell nuclei in each case (as an internal positive control). The number of proliferating vessels per square‐millimetre was recorded as the pMVD in each case.
Figure 1.
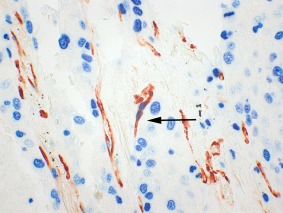
Nestin‐Ki67 dual immunohistochemical staining (×400). A nestin‐positive (red) immature endothelial cell co‐expressing Ki67 (blue) in a proliferating vessel is indicated by an arrow.
Vascular proliferation index
The VPI was obtained by dividing the number of proliferating vessels by the microvessel density (pMVD/MVD), and is presented as a percentage.
Intra‐ and inter‐observer agreement
All cases were scored twice by one experienced pathologist (MR), blinded to clinical data. For MVD, the Spearman correlation coefficient (rho) was 0.75 (p < 0.001), and the measure of intra‐observer agreement (Kappa) for cut‐off values median and upper quartiles were 0.58 and 0.54, respectively. Rho for pMVD was 0.75 (p < 0.001), with Kappa 0.54 and 0.71 for median and upper quartile cut‐off values. The Spearman rho for VPI was 0.66 (p < 0.001), and the Kappa values were 0.50 and 0.55, respectively.
We then performed an inter‐observer variability test in a subset of 25 randomly selected cases, and these were counted independently by another researcher (SA) with experience in counting microvessels by this method. We found significant correlations between counts, with Spearman's correlation coefficient (rho) being 0.61 for MVD (p = 0.001), 0.56 for pMVD (p = 0.003), and 0.58 for VPI (p = 0.002). Using the upper quartile as cut‐off gave Kappa values of 0.26 for MVD, 0.56 for pMVD, and 0.75 for VPI.
Cut‐off values
For the vascular markers, cut‐off values for dichotomization were determined prior to statistical analyses by exploring the frequency distribution. By default, we examined median and quartile values. Number of events across quartile subgroups was considered in order to secure sufficient statistical power in survival analyses. This is also in accordance with our previous studies 27, 29, 47. In the final analyses, associations and survival studies were performed using the upper quartile as cut‐off value. We also performed survival analyses using the median as cut‐off value.
Statistical analyses
For associations between categorical data, Pearson chi‐square test was performed, and odds ratios were computed by the Mantel–Haenzel method. Associations between continuous and categorical data were analysed using the independent samples Mann–Whitney U‐test. Correlations between primary tumours and metastatic lesions were analysed by the Wilcoxon signed rank test, and frequency distributions were analysed by the McNemar test for related samples. The Spearman correlation test was used to analyse intra‐ and inter‐observer agreement of continuous variables, and Cohen's kappa for dichotomized variables. Lung cancer‐specific survival (LCSS), recorded as time from surgery to the time of death from lung cancer was regarded as the primary end‐point for survival analyses. Patients alive at last follow‐up or dead from other causes were censored. For survival analysis, Kaplan‐Meier curves were computed and differences between groups were analysed using the log‐rank test. Univariate survival analyses were performed by the Cox' proportional hazards method. Assumptions of proportionality were checked using log‐log plots. For multivariate analyses by the Cox' proportional hazards method, variables with P values ≤ 0.10 were included in the final model (likelihood‐ratio test for differences). Interactions were tested by applying the product‐term method. Significance was set at P values less than 0.05. The statistical analyses were performed using SPSS (IBM, versions 22–24).
Results
Median MVD/mm2 was 39.5 (mean 43.8, range 4.4–149.8), median pMVD/mm2 was 0.9 (mean 1.2, range 0–7.9), and median VPI was 1.9% (mean 2.9%, range 0–21%). The interquartile range for MVD was 25.2–59.5%, for pMVD 0–1.7%, and for VPI 0–4.4%. These continuous variables were dichotomized using the upper quartile limit (59.5, 1.7, and 4.4%, respectively) as the cut‐off value (see above).
Fixation time did not affect these results. When correlating VPI with fixation time as a continuous variable, no significance was found (p = 0.73 by Pearson correlation; Spearman's rho −0.05, p = 0.47). Also, no significance was found comparing pMVD and MVD with fixation time (data not shown). No difference in mean VPI, pMVD, or MVD was found when comparing cases above or below the mean fixation time (data not shown).
Associations
Microvessel density
High MVD was associated with lower tumour stage (OR 0.5; 95% CI 0.27–0.98; p = 0.043), but not with any of the other clinico‐pathological factors.
Proliferating microvessel density
The number of proliferating vessels showed a significant association with high tumour grade (corresponding to poorly differentiated or undifferentiated tumours versus well to moderately differentiated tumours) (OR 2.0; 95% CI 1.11–3.76; p = 0.022) and presence of necrosis (OR 2.7; 95% CI 1.38–5.11; p = 0.003). No associations with other clinico‐pathological factors were found, including adenocarcinoma subtypes.
Vascular proliferation index
The VPI was significantly associated with invasion into blood vessels (OR 2.4; 95% CI 1.27–4.63; p = 0.007) and presence of tumour necrosis (OR 2.7; 95% CI 1.35–5.33; p = 0.004), but not with other clinico‐pathological factors (Table 1). High VPI showed a trend towards being associated with distant metastasis as first site of recurrence (OR 2.4; 95% CI 0.90–5.59; p = 0.089), compared to recurrence at a local or regional site.
Table 1.
Demographic and clinico‐pathological data with associations [by Pearson chi‐square test and odds ratios (OR)] with VPI (cut‐off by upper quartile) (n = 210)
| VPI low | VPI high | |||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | OR (95% CI) | P | |
| Age (median) | ||||||
| <67 | 75 | (75.0) | 25 | (25.0) | 1 | ns |
| ≥67 | 82 | (74.5) | 28 | (25.5) | 1.0 (0.55–1.91) | |
| Sex | ||||||
| Male | 77 | (70.6) | 32 | (29.4) | 1 | ns |
| Female | 80 | (79.2) | 21 | (20.8) | 0.6 (0.34–1.19) | |
| Histological grade | ||||||
| Low | 89 | (79.5) | 23 | (20.5) | 1 | 0.094 |
| High | 68 | (69.4) | 30 | (30.6) | 1.7 (0.91–3.20) | |
| BVI | ||||||
| Absent | 117 | (80.1) | 29 | (19.9) | 1 | 0.007 |
| Present | 40 | (62.5) | 24 | (37.5) | 2.4 (1.27–4.63) | |
| LVI | ||||||
| Absent | 116 | (74.4) | 40 | (25.6) | 1 | ns |
| Present | 41 | (75.9) | 13 | (24.1) | 0.9 (0.45–1.89) | |
| Tumour necrosis | ||||||
| Absent | 77 | (84.6) | 14 | (15.4) | 1 | 0.004 |
| Present | 80 | (67.2) | 39 | (32.8) | 2.7 (1.35–5.33) | |
| Pleural invasion | ||||||
| Absent | 107 | (73.3) | 39 | (26.7) | 1 | ns |
| Present | 50 | (78.1) | 14 | (21.9) | 0.8 (0.38–1.54) | |
| Tumour stage | ||||||
| I | 67 | (76.1) | 21 | (23.9) | 1 | ns |
| II‐IV | 88 | (74.6) | 30 | (25.4) | 1.1 (0.57–2.07) | |
BVI, blood vessel invasion; LVI, lymphatic vessel involvement; VPI, vascular proliferation index (dichotomized by upper quartile); OR, odds ratio; CI, confidence interval; P values from Pearson chi‐square test; ns, non‐significant (P value > 0.05).
Survival analysis
A high VPI (by upper quartile) was significantly associated with reduced LCSS (log‐rank test, p = 0.020), with estimated median survival of 42 months compared with low VPI of 135 months (Figure 2A). VPI above the upper quartile level was also significantly associated with shorter time to recurrence (log‐rank test, p = 0.012) (Figure 2B). Neither MVD nor pMVD above the upper quartile level were significantly associated with survival. In addition, a high VPI and pMVD (with median as cut‐off value) were significantly associated with reduced LCSS (p = 0.020 and p = 0.005 by log‐rank test), whereas MVD was not.
Figure 2.
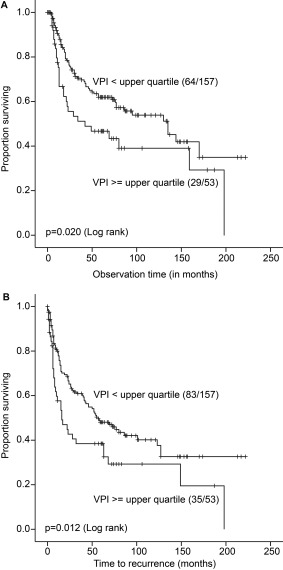
(A) Comparison of LCSS between adenocarcinoma cases according to low or high VPI (by upper quartile; 4.4%; Kaplan‐Meier method). Numbers in brackets indicate events and total number of cases in each subgroup. (B) Comparison of time to recurrence between adenocarcinoma cases according to low or high VPI (by upper quartile; 4.4%; Kaplan‐Meier method). Numbers in brackets indicate events and total number of cases in each subgroup.
By univariate analysis, tumour grade, blood and lymphatic vessel invasion, tumour necrosis, and tumour stage were significantly associated with LCSS (Table 2), whereas patient age and smoking history were not significant. In a multivariate model where clinico‐pathological variables significant in univariate analysis were included, VPI above the upper quartile level remained an independent prognostic factor for LCSS (HR 1.7; 95% CI 1.04–2.68; p = 0.033), along with lymph vessel invasion and tumour stage (Table 2). The proportional hazards assumption was tested and met for all variables entered into the model.
Table 2.
Univariate and multivariate analysis (by Cox' proportional hazards method) of lung cancer specific survival for VPI (cut‐off by upper quartile) and other known prognostic factors in lung AC (n = 210)
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | ||
| Histological grade | Absent | 1.0 | 1.0 | ||||
| Present | 1.6 | (1.10–2.46) | 0.017 | 1.2 | (0.76–1.84) | ns | |
| BVI | Absent | 1.0 | 1.0 | ||||
| Present | 2.2 | (1.42–3.27) | <0.001 | 1.3 | (0.84–2.05) | ns | |
| LVI | Absent | 1.0 | 1.0 | ||||
| Present | 2.7 | (1.79–4.15) | <0.001 | 1.8 | (1.10–2.77) | 0.017 | |
| Tumour necrosis | Absent | 1.0 | 1.0 | ||||
| Present | 2.2 | (1.39–3.32) | 0.001 | 1.5 | (0.92–2.40) | ns | |
| VPIa | Low | 1.0 | 1.0 | ||||
| High | 1.7 | (1.08–2.60) | 0.022 | 1.7 | (1.04–2.68) | 0.033 | |
| Tumour stage | I | 1.0 | 1.0 | ||||
| II–IV | 4.3 | (2.63–7.18) | <0.001 | 3.1 | (1.78–5.38) | <0.001 | |
HR, hazard ratio; CI, confidence interval; VPI, vascular proliferation index; BVI, blood vessel invasion; LVI, lymph vessel invasion; ns, non‐significant (P value > 0.05).
By upper quartile, 4.4%.
Vascular proliferation in metastatic lesions
For the metastatic lesions, 19 cases with sufficient tissue were left for analysis. In these, the median MVD/mm2 was 16.6, median pMVD/mm2 was 0.9, and median VPI 5.0%. The upper quartile values for MVD/mm2, pMVD/mm2, and VPI were 29.3, 1.7, and 9.5%, respectively.
The median MVD/mm2 was significantly higher in primary tumours versus metastatic lesions (39.5 versus 17.1; p = 0.001 by Wilcoxon signed rank test). No significant differences were found for pMVD/mm2 or VPI, comparing metastases with paired primary tumours. No association with sites of metastasis was found (data not shown).
Nestin expression in tumour cells
Nestin positivity in tumour cells was found in 45 cases (21%) and was associated with high tumour grade (OR 1.9; 95% CI 1.10–3.89; p = 0.043), and high tumour stage (OR 2.5; 95% CI 1.19–5.10; p = 0.014). Further, the solid predominant subtype of AC had significantly higher frequency of Nestin positive tumour cells compared to acinar or other growth patterns (p = 0.005). Nestin was also associated with lymph vessel invasion (OR 2.1; 95% CI 1.04–4.25; p = 0.037), whereas tumour cell expression of Nestin was not related to LCSS.
Discussion
We present here a novel marker for evaluation of active angiogenesis in lung adenocarcinoma by the use of Nestin‐Ki67 co‐expression given as a VPI. We found that high VPI was significantly associated with aggressive tumour features such as BVI and presence of tumour necrosis, and reduced cancer specific survival in lung AC.
Vascular proliferation has been proposed as a marker of neoangiogenesis for other solid tumours such as endometrial carcinoma 29, prostatic adenocarcinoma 24, breast carcinoma 26, 27, and pancreatic adenocarcinoma 25. To the best of our knowledge, this marker has not been presented for lung cancer.
Previous studies of MVD as a potential predictive marker have given conflicting results 17, 18, 48. A recent review on anti‐angiogenic therapies by Bugyik et al proposes that the focus should be on patients where primary tumours have endothelial proliferation 49. We found that proliferating vessels, expressed as VPI, have a stronger impact on prognosis than vessel density itself, and we propose that further studies on the predictive impact of VPI are needed.
Comparing the frequencies of MVD to previous studies on lung cancer is in part difficult due to the range of methodologies for vessel counts 12, 13, 14, 23, 30, 50, 51, 52. Our mean MVD/mm2 of 39.5 is somewhat low, and this could be due to our use of Nestin as it is assumed to stain newly formed vessels only. Previously, factor VIII, CD31, or CD34 have been used to identify vessels 23, 50, 53, 54, but as these antibodies also stain existing vessels, they may not reflect only the ongoing tumour‐induced angiogenesis. The lung is a highly vascularized organ, especially around alveoli, and the use of pan‐endothelial markers is likely to give higher numbers of microvessel density. By using Nestin and counting the number of proliferating vessels, presented as VPI, we hypothesize that this will provide a better estimate of the neovascularization in lung tumours.
We found that the level of MVD was higher in primary tumours compared with paired metastatic lesions. Differences in vascularity between primary tumours and metastatic lesions have been previously reported for NSCLC 39, as well as for other tumours 55, 56, 57, 58, with the main result being that metastatic lesions had lower MVD than primary tumours. The reasons for reduced MVD in metastatic lesions could be that aggressive tumour cells are less dependent on angiogenesis to form lesions at a distant site. There are reports of metastatic tumour cells exploiting existing vessels for nutrient supply, by a mechanism called vascular co‐option 59. This could in part explain the lower microvascular density and also the low vascular proliferation that we observed in the metastatic lesions.
We are aware of limitations to our study in that we used a retrospective cohort. However, our series consists of tumour specimens from one institution where the treatment of AC did not change much during the study period. Of note, no patients received anti‐angiogenic treatment or tyrosine kinase inhibitors. We have included all patients surgically treated for lung AC in our county and in the time period, and our cohort is considered to be representative for the population. As to the possibility of sampling bias, we acknowledge that the metastatic tissues used in this study are biopsies and not surgical specimens, and this may potentially account for some of the discrepancies between vascular density in primary and metastatic lesions.
As this is an early biomarker study, we have not assessed vascular proliferation by the use of an image analysis system. We are aware of the potential benefits of such a method with increased standardization. Still, a subjective evaluation by trained and experienced personnel would be necessary to determine the areas of interest, and to exclude parts of the tumour that should not be included for assessment, such as areas close to necrotic tissue and within tumour scars. Also, most markers for endothelial cells (factor VIII, CD 31, Nestin) stain other cells (fibroblasts, nerves, tumour cells), and careful separation of these cells would be needed. An automated counting system should thus be thoroughly calibrated regarding these measures, and could then possibly improve inter‐observer variability and also reduce the labour‐intensive task of counting MVD manually.
We also acknowledge that since this is the first study showing a potential prognostic value of VPI in lung cancer, this marker should be validated in a similar and independent cohort of surgically resected NSCLC.
The potential clinical implications of our findings could be to use this angiogenesis marker in patient stratification for closer follow‐up after surgery. Also, there is a possibility for using VPI as a tool for prediction of response to anti‐angiogenic treatment, although this has not been the focus of our present study. Currently, there is no validated predictor for anti‐angiogenic therapy, and this has been raised as one of the problems concerning lack of improvement in overall survival for the majority of patients 60. The other problem is the toxicity linked to angiogenesis inhibitors, which could possibly be reduced if drugs are given to patients most likely to benefit from the treatment 61.
In this cohort of lung AC, we find that the novel angiogenesis marker Nestin‐Ki67, given as a VPI, was associated with aggressive tumour features, such as BVI and tumour necrosis and, independently, with decreased LCSS. This marker should be explored further in separate cohorts, including those that include patients who have received anti‐angiogenesis therapy.
Author contributions statement
MR: performed the research, analysed the data and wrote the paper; CA: collected data and revised the paper; SA: performed the inter‐observer test and revised the paper; LH: revised the paper; LAA: designed the study, participated in data analyses, and revised the paper.
Acknowledgements
The authors would like to thank Gerd Lillian Hallseth, Randi Hope Lavik, and Bendik Nordanger for excellent technical assistance. The authors would also like to thank the Department of Pathology, Haukeland University Hospital, Bergen, Norway for giving access to the archival material. This work was partly supported by the Research Council of Norway through its Centre of Excellence funding scheme, project number 223250. The study was also supported by the University of Bergen and the Norwegian Cancer Society.
No conflicts of interest were declared.
References
- 1. Norway CRO, Cancer Registry of Norway . Cancer in Norway 2015 ‐ Cancer Incidence, Mortality, Survival and Prevalence in Norway. Cancer Registry of Norway: Oslo, Norway: 2016. [Google Scholar]
- 2. Norway CRO . Cancer Registry of Norway. Nasjonalt Kvalitetsregister for Lungekreft. Årsrapport 2013‐2014, 2015.
- 3. Helsedirektoratet. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av lungekreft, mesoteliom og thymom . In. Health Mo, Ministry of Health, Norway: Norway, 2017.
- 4. Brahmer JR, Pardoll DM. Immune checkpoint inhibitors: making immunotherapy a reality for the treatment of lung cancer. Cancer Immunol Res 2013; 1: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 7. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990; 82: 4–6. [DOI] [PubMed] [Google Scholar]
- 8. Hong S, Tan M, Wang S, et al Efficacy and safety of angiogenesis inhibitors in advanced non‐small cell lung cancer: a systematic review and meta‐analysis. J Cancer Res Clin Oncol 2015; 141: 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis PM. Anti‐angiogenesis in personalised therapy of lung cancer. Adv Exp Med Biol 2016; 893: 91–126. [DOI] [PubMed] [Google Scholar]
- 10. Hasan J, Byers R, Jayson GC. Intra‐tumoural microvessel density in human solid tumours. Br J Cancer 2002; 86: 1566–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weidner N, Semple JP, Welch WR, et al Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 12. Macchiarini P, Fontanini G, Hardin MJ, et al Relation of neovascularisation to metastasis of non‐small‐cell lung cancer. Lancet 1992; 340: 145–146. [DOI] [PubMed] [Google Scholar]
- 13. Chandrachud LM, Pendleton N, Chisholm DM, et al Relationship between vascularity, age and survival in non‐small‐cell lung cancer. Br J Cancer 1997; 76: 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fontanini G, Lucchi M, Vignati S, et al Angiogenesis as a prognostic indicator of survival in non‐small‐cell lung carcinoma: a prospective study. J Natl Cancer Inst 1997; 89: 881–886. [DOI] [PubMed] [Google Scholar]
- 15. Mattern J, Koomagi R, Volm M. Vascular endothelial growth‐factor expression and angiogenesis in nonsmall cell lung carcinomas. Int J Oncol 1995; 6: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 16. Yuan A, Yang PC, Yu CJ, et al Tumor angiogenesis correlates with histologic type and metastasis in non‐small‐cell lung cancer. Am J Respir Crit Care Med 1995; 152: 2157–2162. [DOI] [PubMed] [Google Scholar]
- 17. Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst 2002; 94: 883–893. [DOI] [PubMed] [Google Scholar]
- 18. Zhao YY, Xue C, Jiang W, et al Predictive value of intratumoral microvascular density in patients with advanced non‐small cell lung cancer receiving chemotherapy plus bevacizumab. J Thorac Oncol 2012; 7: 71–75. [DOI] [PubMed] [Google Scholar]
- 19. Bremnes RM, Camps C, Sirera R. Angiogenesis in non‐small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer 2006; 51: 143–158. [DOI] [PubMed] [Google Scholar]
- 20. Meert AP, Paesmans M, Martin B, et al The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta‐analysis. Br J Cancer 2002; 87: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vermeulen PB, Gasparini G, Fox SB, et al Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer 2002; 38: 1564–1579. [DOI] [PubMed] [Google Scholar]
- 22. Bing Z, Jian‐Ru Y, Yao‐Quan J, et al Evaluation of angiogenesis in non‐small cell lung carcinoma by CD34 immunohistochemistry. Cell Biochem Biophys 2014; 70: 327–331. [DOI] [PubMed] [Google Scholar]
- 23. Giatromanolaki A, Koukourakis MI, Theodossiou D, et al Comparative evaluation of angiogenesis assessment with anti‐factor‐VIII and anti‐CD31 immunostaining in non‐small cell lung cancer. Clin Cancer Res 1997; 3: 2485–2492. [PubMed] [Google Scholar]
- 24. Gravdal K, Halvorsen OJ, Haukaas SA, et al Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res 2009; 69: 4708–4715. [DOI] [PubMed] [Google Scholar]
- 25. Hoem D, Straume O, Immervoll H, et al Vascular proliferation is associated with survival in pancreatic ductal adenocarcinoma. APMIS 2013; 121: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 26. Kraby MR, Kruger K, Opdahl S, et al Microvascular proliferation in luminal A and basal‐like breast cancer subtypes. J Clin Pathol 2015; 68: 891–897. [DOI] [PubMed] [Google Scholar]
- 27. Kruger K, Stefansson IM, Collett K, et al Microvessel proliferation by co‐expression of endothelial nestin and Ki‐67 is associated with a basal‐like phenotype and aggressive features in breast cancer. Breast 2013; 22: 282–288. [DOI] [PubMed] [Google Scholar]
- 28. Nalwoga H, Arnes JB, Stefansson IM, et al Vascular proliferation is increased in basal‐like breast cancer. Breast Cancer Res Treat 2011; 130: 1063–1071. [DOI] [PubMed] [Google Scholar]
- 29. Stefansson IM, Salvesen HB, Akslen LA. Vascular proliferation is important for clinical progress of endometrial cancer. Cancer Res 2006; 66: 3303–3309. [DOI] [PubMed] [Google Scholar]
- 30. Yazdani S, Miki Y, Tamaki K, et al Proliferation and maturation of intratumoral blood vessels in non‐small cell lung cancer. Hum Pathol 2013; 44: 1586–1596. [DOI] [PubMed] [Google Scholar]
- 31. Tamaki K, Moriya T, Sato Y, et al Vasohibin‐1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci 2009; 100: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sugawara K, Kurihara H, Negishi M, et al Nestin as a marker for proliferative endothelium in gliomas. Lab Invest 2002; 82: 345–351. [DOI] [PubMed] [Google Scholar]
- 33. Mokry J, Cizkova D, Filip S, et al Nestin expression by newly formed human blood vessels. Stem Cells Dev 2004; 13: 658–664. [DOI] [PubMed] [Google Scholar]
- 34. Ahmed MB, Nabih ES, Louka ML, et al Evaluation of nestin in lung adenocarcinoma: relation to VEGF and Bcl‐2. Biomarkers 2014; 19: 29–33. [DOI] [PubMed] [Google Scholar]
- 35. Chen Z, Wang J, Cai L, et al Role of the stem cell‐associated intermediate filament nestin in malignant proliferation of non‐small cell lung cancer. PLoS One 2014; 9: e85584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Narita K, Matsuda Y, Seike M, et al Nestin regulates proliferation, migration, invasion and stemness of lung adenocarcinoma. Int J Oncol 2014; 44: 1118–1130. [DOI] [PubMed] [Google Scholar]
- 37. Ryuge S, Sato Y, Jiang SX, et al Prognostic impact of nestin expression in resected large cell neuroendocrine carcinoma of the lung. Lung Cancer 2012; 77: 415–420. [DOI] [PubMed] [Google Scholar]
- 38. Ryuge S, Sato Y, Wang GQ, et al Prognostic significance of nestin expression in resected non‐small cell lung cancer. Chest 2011; 139: 862–869. [DOI] [PubMed] [Google Scholar]
- 39. Skarda J, Kolar Z, Janikova M, et al Analysis of the prognostic impact of nestin expression in non‐small cell lung cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2012; 156: 135–142. [DOI] [PubMed] [Google Scholar]
- 40. Travis WD, Brambilla E, Muller‐Hermelink HK, Harris CC. (Eds.). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC Press: Lyon, 2004. [Google Scholar]
- 41. Park SY, Lee HS, Jang HJ, et al Tumor necrosis as a prognostic factor for stage IA non‐small cell lung cancer. Ann Thorac Surg 2011; 91: 1668–1673. [DOI] [PubMed] [Google Scholar]
- 42. Shimada Y, Ishii G, Hishida T, et al Extratumoral vascular invasion is a significant prognostic indicator and a predicting factor of distant metastasis in non‐small cell lung cancer. J Thorac Oncol 2010; 5: 970–975. [DOI] [PubMed] [Google Scholar]
- 43. Travis WD, Brambilla E, Noguchi M, et al International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramnefjell M, Aamelfot C, Helgeland L, et al Vascular invasion is an adverse prognostic factor in resected non‐small‐cell lung cancer. APMIS 2017; 125: 197–206. [DOI] [PubMed] [Google Scholar]
- 45. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (7th edn). International Union Against Cancer, Wiley‐Blackwell Oxford 2009.
- 46. Weidner N, Folkman J, Pozza F, et al Tumor angiogenesis: a new significant and independent prognostic indicator in early‐stage breast carcinoma. J Natl Cancer Inst 1992; 84: 1875–1887. [DOI] [PubMed] [Google Scholar]
- 47. Arnes JB, Stefansson IM, Straume O, et al Vascular proliferation is a prognostic factor in breast cancer. Breast Cancer Res Treat 2012; 133: 501–510. [DOI] [PubMed] [Google Scholar]
- 48. Jubb AM, Hurwitz HI, Bai W, et al Impact of vascular endothelial growth factor‐A expression, thrombospondin‐2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 2006; 24: 217–227. [DOI] [PubMed] [Google Scholar]
- 49. Bugyik E, Renyi‐Vamos F, Szabo V, et al Mechanisms of vascularization in murine models of primary and metastatic tumor growth. Chin J Cancer 2016; 35: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Angeletti CA, Lucchi M, Fontanini G, et al Prognostic significance of tumoral angiogenesis in completely resected late stage lung carcinoma (stage IIIA‐N2). Impact of adjuvant therapies in a subset of patients at high risk of recurrence. Cancer 1996; 78: 409–415. [DOI] [PubMed] [Google Scholar]
- 51. Yamazaki K, Abe S, Takekawa H, et al Tumor angiogenesis in human lung adenocarcinoma. Cancer 1994; 74: 2245–2250. [DOI] [PubMed] [Google Scholar]
- 52. Maeda R, Ishii G, Ito M, et al Number of circulating endothelial progenitor cells and intratumoral microvessel density in non‐small cell lung cancer patients: differences in angiogenic status between adenocarcinoma histologic subtypes. J Thorac Oncol 2012; 7: 503–511. [DOI] [PubMed] [Google Scholar]
- 53. Matsuyama K, Chiba Y, Sasaki M, et al Tumor angiogenesis as a prognostic marker in operable non‐small cell lung cancer. Ann Thorac Surg 1998; 65: 1405–1409. [DOI] [PubMed] [Google Scholar]
- 54. Ohta Y, Tomita Y, Oda M, et al Tumor angiogenesis and recurrence in stage I non‐small cell lung cancer. Ann Thorac Surg 1999; 68: 1034–1038. [DOI] [PubMed] [Google Scholar]
- 55. Guidi AJ, Berry DA, Broadwater G, et al Association of angiogenesis in lymph node metastases with outcome of breast cancer. J Natl Cancer Inst 2000; 92: 486–492. [DOI] [PubMed] [Google Scholar]
- 56. Hillen HF, Hak LE, Joosten‐Achjanie SR, et al Microvessel density in unknown primary tumors. Int J Cancer 1997; 74: 81–85. [DOI] [PubMed] [Google Scholar]
- 57. Miliaras D, Kamas A, Kalekou H. Angiogenesis in invasive breast carcinoma: is it associated with parameters of prognostic significance? Histopathology 1995; 26: 165–169. [DOI] [PubMed] [Google Scholar]
- 58. Mooteri S, Rubin D, Leurgans S, et al Tumor angiogenesis in primary and metastatic colorectal cancers. Dis Colon Rectum 1996; 39: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 59. Donnem T, Hu J, Ferguson M, et al Vessel co‐option in primary human tumors and metastases: an obstacle to effective anti‐angiogenic treatment? Cancer Med. 2013; 2: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giuliano S, Pages G. Mechanisms of resistance to anti‐angiogenesis therapies. Biochimie 2013; 95: 1110–1119. [DOI] [PubMed] [Google Scholar]
- 61. Li BT, Barnes TA, Chan DL, et al The addition of anti‐angiogenic tyrosine kinase inhibitors to chemotherapy for patients with advanced non‐small‐cell lung cancers: a meta‐analysis of randomized trials. Lung Cancer 2016; 102: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


