Summary
Programmed death axis 1 (PD-1) inhibitors have ushered in a new error of cancer immuno-therapeutics for advanced smoking associated non-small cell lung cancer. Their role in treating EGFR mutant and ALK rearranged lung cancer has yet to be determined.
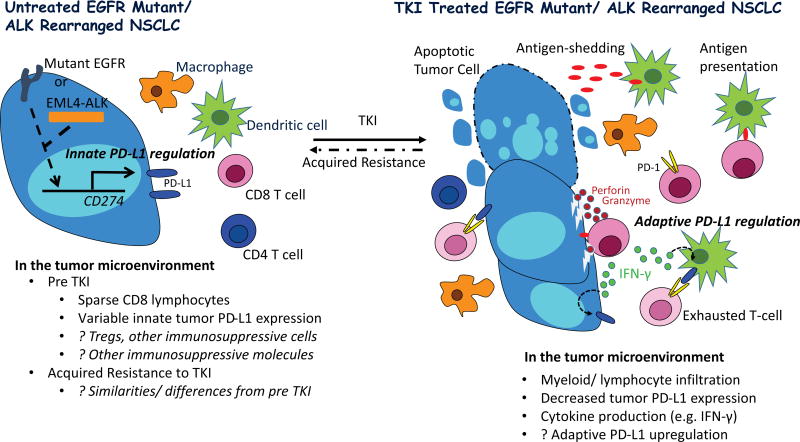
In this issue of Clinical Cancer Research, Dr. Gainor and colleagues report on effectiveness of programmed death 1 (PD-1) axis inhibitors in a small cohort of epidermal growth factor receptor (EGFR) mutant (n=22) and anaplastic lymphoma kinase (ALK) rearranged (n=6) non-small cell lung cancer (NSCLC). They further describe tumor programmed death ligand 1 (PD-L1) and CD8 staining patterns from a larger cohort of patients with EGFR and ALK driven NSCLC, prior to and at the time of acquired resistance to tyrosine kinase inhibitor (TKI) therapy (1). They propose that a lack of an inflamed tumor microenvironment in the majority of such patients, suggested by a dearth of tumor infiltrating CD8 positive lymphocytes, may explain the low response rate to PD-1 axis inhibitors observed among EGFR and ALK driven NSCLC. They also imply that PD-L1 tumor expression found in the minority of patients is primarily driven by intrinsic (i.e. constitutive oncogenic signaling) rather than adaptive processes (induction by local inflammatory signals), considering a lack of significant concomitant CD8 positive lymphocyte infiltrate (Figure 1).
Figure 1. Proposed Tumor immunity in TKI naïve and treated EGFR and ALK Driven Lung Cancer.
Oncogenic signaling through EGFR and ALK drives tumor PD-L1 expression in preclinical models (innate PD-L1 regulation), however, PD-L1 protein expression is variable in tumor specimens from patients with EGFR and ALK driven NSCLC (2–6). Predicted lower somatic mutational burden in such tumors, relative to typical smoking associated NSCLC, may result in less immunogenic tumors, potentially explaining reports describing scarcity of tumor infiltrating lymphocytes (TILs). Preclinical studies have additionally identified tumor infiltrating T regulatory cells in EGFR mutant models, which may further promote tumor immune evasion (2).
On treatment with a highly active tyrosine kinase inhibitor (TKI), apoptosis of EGFR and ALK driven NSCLC leads to tumor influx of immune cells and cytokine production, and tumor antigen processing by dendritic cells with presentation to antigen specific T cells which can then engage tumor cells, releasing cytotoxic enzymes (perforin and granzyme) and pro-inflammatory cytokines. The latter, in particular IFN-γ, can induce PD-L1 on myeloid and tumor cells (adaptive PD-L1 regulation), resulting in T cell exhaustion with blunting of the anti-tumor immune response. Of note, preclinical studies have demonstrated PD-1 inhibitors can relieve suppression of effector T cells in EGFR and ALK driven models, resulting in tumor cell death.
At the time of acquired resistance to TKI therapy, it is unclear if the tumor immune microenvironment regains the dominant immunosuppressive features of the pre TKI state, or if additional/ different mechanisms of immunosuppression emerge.
Initial interest in using PD-1 axis inhibitors in EGFR and ALK driven NSCLC was sparked after preclinical studies reported that aberrant oncogenic EGFR and ALK signaling drives PD-L1 expression, and that in-vitro treatment with PD-1 axis inhibitors in EGFR mutant and ALK rearranged tumor co-culture systems with immune cells compromised tumor cell viability (2–6). Furthermore, therapy with a PD-1 axis inhibitor in EGFR mutant mouse models resulted in improved survival (2). Of note, treatment with respective TKI therapy alone in cell line models of EGFR and ALK driven lung cancer led to PD-L1 down regulation, questioning the utility of combining a TKI with a PD-1 axis inhibitor. Indeed, such combination therapy did not lead to synergistic tumor killing in EGFR or ALK driven co-culture systems (3, 4).
In the clinic, response rates to PD-1 axis inhibitors across trials have been lower (approximately 10%) in patients with NSCLC whose tumors harbored EGFR mutations (less is known about responsiveness in ALK driven NSCLC), with lack of a survival benefit over salvage chemotherapy in two Phase III trials (7, 8). These disappointing results, along with the observation that NSCLC in never smokers is associated with lower response rates to PD-1 axis inhibitors, have led to pessimism about using such therapy in EGFR or ALK driven NSCLC (which primarily occur in patients with minimal to no smoking history). One proposed explanation for inferior activity here has been that NSCLC in patients with EGFR or ALK driven tumors and/or no smoking history generally have a lower somatic mutational burden, with less tumor immunogenicity. So, even if PD-L1 were overexpressed in EGFR mutant or ALK rearranged NSCLC, lack of immune recognition and tumor infiltrating lymphocytes (TILs) would limit the effectiveness of PD-1 axis inhibitor therapy. Notably, however, Dr. Gainor and colleagues found low tumor PD-L1 expression in such tumors, regardless of exposure to respective TKIs. This is contrary to other reports demonstrating an association between high PD-L1 expression and the presence of EGFR mutations in NSCLC tumor specimens (9, 10).
As interest in PD-1 axis inhibitors for TKI treated EGFR mutant and ALK rearranged NSCLC waned, focus shifted to patients with TKI naïve EGFR and ALK driven NSCLC. Multiple ongoing studies were initiated evaluating combination therapy with respective TKIs combined with a PD-1 axis inhibitor (NCT 02039674, 02013219, 02511184). This strategy is largely based on a presumption that a highly active therapy such as an EGFR TKI in EGFR mutant NSCLC will lead to tumor apoptosis and enhanced immune priming, with resultant tumor lymphocytic infiltration and induced up-regulation of PD-L1 (Figure 1). At least one of these trials requires serial tumor biopsies, including one just before and shortly after initiating TKI monotherapy, before PD-1 axis inhibitor therapy is added, and may help corroborate this hypothesis. Of note, data presented by Gainor and colleagues from a limited number of patients with paired tumor specimens collected before and at the time of acquired resistance to TKI did not find clear changes in TILs or PD-L1 expression; however, specimens from the time of response to TKI were not available for analysis. Based on the same hypothesis supporting evaluation in TKI naïve NSCLC, additional trials were launched paring next generation ALK TKIs and T790M EGFR mutant selective TKIs with PD-1 axis inhibitor therapy in both TKI naïve and treated NSCLC, including a phase III trial of osimertinib plus/ minus the anti-PD-L1 antibody durvalumab (NCT 02393625, 02584634, 02323126, 02143466, 02454933). Unfortunately, the latter trial was halted prematurely due to an apparent increased incidence of pneumonitis in this and a former trial evaluating concomitant treatment with osimertinib and durvalumab, with no plans to further pursue this combination.
We applaud Dr. Gainor and colleagues for presenting their experience with PD-1 axis inhibitor therapy in EGFR and ALK driven NSCLC, and conducting needed translational studies in an attempt to characterize the tumor immune microenvironment in such tumors, and understand limited activity with PD-1 axis inhibitors. Although their results are sobering, there is still much to learn about PD-1 axis inhibitors in EGFR and ALK driven NSCLC. Clearly, some patients benefit from such therapy, as demonstrated in the Checkmate 012 trial (NCT 01454102). One arm of this trial evaluated the combination of erlotinib and the anti-PD-1 antibody, nivolumab in 20 patients with EGFR mutant chemotherapy naive NSCLC and acquired resistance to erlotinib (11). Four patients had durable tumor regression; one ongoing at 18 and ½ months, one ongoing at 24 and ¾ months after removal of a solitary growing lesion at 15 and ¾ months, one ongoing at 24 months after initial pseudo-progression (initial growth of non-target lesions at 2 months followed by regression), and one lasting 18 and ½ months. Tumor biopsies obtained in these 4 patients prior to trial therapy, after the development of resistance to erlotinib, yielded the secondary T790M EGFR resistance mutation in two (one with an exon 19 deletion EGFR mutation, the other an L858R EGFR mutation) and c-MET amplification in a third (with L858R mutation). One EGFR TKI naïve patient was additionally treated with this combination, and achieved complete response which was ongoing at last data lock 24 months after initiating therapy. Other arms of this trial additionally evaluated combination therapy with nivolumab and ipilimumab, a cytotoxic T-lymphocyte associated protein 4 antagonist antibody, as first line therapy in unselected patients with advanced NSCLC. Preliminary results of these arms were recently presented, with 4 of 8 patients with EGFR mutant NSCLC treated with the standard dose of nivolumab (3 mg/kg) and ipilimumab achieving partial response (12).
Ongoing preclinical and translational efforts at several academic centers promise to elucidate unique mechanisms of tumor immune suppression in EGFR and ALK driven NSCLC. Considering the complexity of the immune response, and molecular heterogeneity of such tumors, this is no easy task. However, much has already been accomplished in understanding the biology of EGFR and ALK driven NSCLC, and molecular mechanisms of resistance have already been identified and exploited. This background, and the relatively less complex genotype of such tumors (compared to smoking related lung cancer), with routine biopsies collected at the time of acquired resistance to TKI therapy, provides a unique opportunity to dissect mediators of immune recognition, activation and suppression in EGFR and ALK driven NSCLC. As this understanding grows, we should increasingly be able to determine which patients will benefit from PD-1 axis inhibitor therapy, other immunotherapies, or combinations of such therapy.
Acknowledgments
Katerina Politi
Consulting: Novartis Pharmaceuticals, NCCN for Afatinib Request for Proposals Adviser
Research Support: AstraZeneca, Roche, Kolltan Pharmaceuticals
Footnotes
Conflicts of Interest: Scott Gettinger, Consultant, Bristol Myers Squibb and Ariad Pharmaceuticals
Katerina Politi, IP: Molecular MD/MSKCC for licensing a patent for T790M testing
Contributor Information
Scott Gettinger, Yale University School of Medicine, Yale Cancer Center
Katerina Politi, Yale University School of Medicine, Yale Cancer Center
References
- 1.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer (NSCLC): A Retrospective Analysis. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-3101. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–63. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol. 2015;10:910–23. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 4.Hong S, Chen N, Fang W, Zhan J, Liu Q, Kang S, et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: Implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology. 2015;5:e1094598. doi: 10.1080/2162402X.2015.1094598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21:4014–21. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 6.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci. 2008;105:20852–7. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 9.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–40. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 10.D'Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gettinger S, Chow LQ, Borghaei H, Shen Y, Harbison C, Nathan F, et al. Safety and Response With Nivolumab (Anti-PD-1; BMS-936558, ONO-4538) Plus Erlotinib in Patients (pts) With Epidermal Growth Factor Receptor Mutant (EGFR MT) Advanced Non-Small Cell Lung Cancer (NSCLC). Poster/ Abstract; Chicago Multidisciplinary Symposium in Thoracic Oncology (CMSTO) Meeting; 2014. [Google Scholar]
- 12.Hellmann MD, Gettinger SN, Goldman JW, Brahmer JR, Borghaei H, Chow LQ, et al. CheckMate 012: Safety and efficacy of first-line (1L) nivolumab (nivo; N) and ipilimumab (ipi; I) in advanced (adv) NSCLC. Oral presentation. 2016 American Society of Clinical Oncology Annual Meeting, J Clin Oncol (Meeting Abstracts) 2016;34(suppl 3001) [Google Scholar]