Abstract
Objective
Current therapies often have limited efficacy and untenable side effects when used to treat persistent incisional pain following cancer-related surgery. Lidocaine patches reduce neuropathic pain from herpes zoster but their benefits for persistent cancer-related postsurgical incisional pain remain unclear.
Study design
Multicenter, double-blind, randomized, two-period crossover trial.
Materials and methods
Twenty-eight cancer patients with postsurgical incisional pain were randomly assigned to receive either lidocaine patches followed by placebo patches or the reverse. Each study period lasted 4 weeks. Patches were applied daily upon waking and left in place for a maximum of 18 h. The primary outcome measure, an 11-point pain intensity rating scale, was administered weekly. Secondary outcomes were administered weekly (Brief Pain Inventory-Short Form(BPI-SF), Subject Global Impression of Change) and at the end of each study period (Short Form-Magill Pain Questionnaire, Linear Analogue Self Assessment Scale, Neuropathy Pain Scale, Pain Catastrophizing Scale, Profile of Mood States Short Form). Results Twenty-one patients completed the first period and 18 completed their crossover second phase. No significant intergroup differences were detected in pain intensity ratings. Few secondary end points were significantly different when subjects used the lidocaine versus placebo patches. BPI-SF interference scores were lower in patients using the lidocaine patch during the first study period, including several scores that achieved statistical significance, general activity (p=0.02), work (p=0.04), and relations with others (p=0.02).
Conclusion
Lidocaine patch use did not significantly reduce pain intensity ratings or the majority of related secondary end points in cancer patients with persistent incisional pain.
Keywords: Lidocaine patch, Postsurgical neuropathic pain, Cancer, Cancer-related pain
Introduction
Persistent postsurgical incisional pain is an important survivorship issue for patients with cancer. Persistent pain occurs most commonly following thoracotomy and mastectomy, affecting up to 80% and 56% of patients undergoing these procedures, respectively [18, 39]. Incisional pain may persist for as long as 5 years after surgery in some patients and significantly degrade their quality of life [7, 23].
Persistent postsurgical pain has been attributed to damage of afferent nerves leading to ectopy and aberrant somatosensory processing [19, 33, 42] with some authors invoking plastic changes in the central nervous system (“central sensitization” or “wind up”) in this phenomenon [6, 20, 43]. Central sensitization is initiated when cumulative nociceptive input increases intracellular calcium (Ca++) concentrations in second-order central nervous system (CNS) neurons thereby triggering a cascade that ultimately leads to sustained alterations in neuronal processing and chronic pain [6, 24]. Once established, central sensitization may be sustained through nociceptor- or mechanoreceptor-generated input [4].
The well-characterized benefits of local anesthetics in managing acute postoperative incisional pain suggest that they may also be beneficial in controlling persistent incisional pain [17, 29]. Indeed, lidocaine, when administered transdermally via a topical patch, reduces chronic pain associated with postherpetic neuralgia [11, 12, 34]. In addition, its mechanism of action, stabilization of neuronal membranes and blockade of pain signals, suggests it should reduce postoperative incisional pain through at least two mechanisms [13]: inhibition of afferent nociceptive impulses and attenuation of central sensitization by limiting peripheral nerve input to the CNS. The latter mechanism would theoretically afford persistent analgesia beyond the peripheral effects of the lidocaine patch.
Lidocaine patches offer the additional benefits of minimal systemic drug absorption and a safe and tolerable side effect profile [13]. Anticonvulsants, the current first-line therapy for neuropathic pain, may cause sedation, gait instability, and memory deficits [22]. Toxicities and side effects are most common in the elderly, the population with the highest prevalences of cancer-related neuropathic pain states. The comparable numbers needed to treat (NNT) and numbers needed to harm (NNH) estimates for commonly utilized anticonvulsants, e.g., gabapentin (NNT=4.3, NNH=3.7) [40] and carbamazepine (NNT=2.5, NNH= 3.7) [41], demonstrate the problematic toxicity relative to benefit that characterizes these agents. Opioid analgesics, while capable of alleviating neuropathic pain, share similar toxicities that constrain clinical use [31].
This two-period placebo-controlled, phase III, double-blind crossover study was conducted to determine whether lidocaine patches (Lidoderm®) could provide pain relief in patients with cancer and persistent postsurgical pain at their incision site(s).
Materials and methods
Subjects
Study patients from ten clinical centers participated in this double-blind randomized controlled trial, which was conducted from September 2004 to May 2006. The study protocol and informed consent were approved by the institutional review boards of all the participating centers. The study period consisted of an initial 4-week treatment phase followed by a second 4-week treatment phase during which subjects switched study arms. There was no intervening washout period. Subjects were observed at three scheduled visits: baseline, week 4, and week 8.
Eligible subjects had persistent pain (≥1 month), rated ≥4 out of 10, with neuropathic features (e.g., burning, paresthesias, or allodynia) involving an affected area that could be encompassed by less than three lidocaine patches. Subjects’ pain had to be associated with a surgical procedure as part of cancer treatment, as well as temporally and anatomically related to the surgical site. Additionally, eligible subjects were required to be ≥18 years of age, without recent histories of drug or alcohol abuse, expected to live >6 months, without clinically evident cognitive or psychiatric morbidity, and neither pregnant nor nursing. The presence of non-surgical pain etiologies such as malignancy, dermal pathology, or chemotherapy-induced neuropathy at the painful site rendered subjects ineligible. Additional contraindications to participation included concurrent radiation therapy to the painful area; skin problems such as breakdown, infection, or extreme thinning at the painful site; use of topical medications on the affected area; history of an allergic reaction or intolerance to amide local anesthetics; use of class I antiarrhythmic drugs (e.g., tocainide and mexiletine); and aspartate amino transferase >2× the upper limit of normal. Non-opioid, opioid, and adjuvant analgesics were permitted, provided that subjects had received a stable regimen for at least 10 days prior to study entry.
Subject randomization utilized a dynamic allocation that balanced the marginal distribution of the stratification factors between the two groups. Stratification factors included the etiology of pain (breast surgery vs. lung surgery vs. amputation vs. other), the duration of pain (1–3 vs. >3–6 vs. >6 months) and current analgesic regimens (opioids vs. antidepressants vs. anticonvulsants vs. combination vs. other vs. none) [32] between the groups. Subjects were randomized to apply either lidocaine-impregnated or placebo patches.
Each commercially available 5.5×4-in. lidocaine patch was composed of an adhesive material containing 5% lidocaine, applied to a non-woven felt backing and covered with a polyethylene terephthalate film release liner (Endo Pharmaceuticals, Chadds Ford, PA). Lidocaine and placebo were supplied in identical appearing patches in a blinded fashion. Based on the size of the affected area, subjects were instructed to apply a patch or patches (up to a maximum of three patches) directly to the painful area upon waking and to leave them in place for 18 h or until their usual bedtime.
Subjects continued their stable pre-entry oral analgesic regimens upon entry into the trial and were permitted to decrease or discontinue analgesic use at the discretion of their physician. Introduction of new analgesics or adjuvant drugs led to withdrawal from the study. Subjects were contacted by telephone at 1-week intervals throughout the study to document compliance and address problems.
Outcome measures consisted of self-report instruments assessing pain intensity, quality of life, and degrees of interference with life domains. Subjects’ psychological status and quality of life were similarly assessed by self-report. The psychometric properties of study instruments are listed below.
Pain intensity rating
The pain intensity rating is the standard instrument in chronic pain studies [10]. The pain intensity rating is widely used and has evidence of validity in the assessment of cancer neuropathic pain [15]. Pain is measured on an 11-point pain intensity numerical rating scale, where 0 = no pain and 10 = worst possible pain.
Brief Pain Inventory-Short Form
The Brief Pain Inventory (BPI) consists of 15 items that assess the location of pain, its severity, its interference with daily activities, and extent of pain relief from analgesics [23–26]. All items except those concerning pain location and medication consist of visual analog scales ranging from 0 to 10. Higher scores indicate worsening pain or limitation. The BPI has been widely used and validated for use in cancer populations [5].
Neuropathy Pain Scale
The NPS assesses specific neuropathic pain characteristics which include sharp, hot, dull, cold, sensitive, itchy. This scale has been observed to be discriminative in trials of topical analgesics for neuropathic pain [1, 16].
The Subject Global Impression of Change
The Subject Global Impression of Change is a seven-point item in which patients rate the change in their overall status since the beginning of the study (ranging from much improved, moderately improved, minimally improved, no change, minimally worse, and moderately worse to much worse). The scale has been found to effectively discriminate treatment effects in neuropathic pain trials [10].
Short-Form McGill Pain Questionnaire
The Short-Form McGill Pain Questionnaire (SF-MPQ) is a tool used to assess the sensory and affective dimensions of pain quality. Completion of the SF-MPQ involves counting the number of responses to obtain the Number of Words Chosen score and adding the intensity of each response to obtain the Present Pain Intensity [27]. This questionnaire has undergone extensive psychometric evaluation. Its subscales are discriminative in neuropathic pain trials [8, 28].
Pain Catastrophizing Scale
The PCS uses five-point ratings of a range of 14 questions potentially experienced by a patient with pain. It has been successfully applied in the assessment of neuropathic and cancer-associated pain states [30].
Profile of Mood States Short Form
The Profile of Mood States Short Form (POMS-SF) consists of 30 items to assess mood states. It is valid for use and is discriminative in the evaluation of cancer- and pain-associated distress [2].
NCCTG patient quality-of-life linear analog self-assessment scale
The linear analog self-assessment (LASA) scale is a single-item measurement of global quality-of-life rating on a numerical rating scale from 0 to 10 with well-established validity data in cancer populations [14].
At study entry, subjects were given a booklet containing the questionnaires and assessments. The procedures for completing and returning the booklet were carefully explained. All the assessments, including the pain intensity rating, Neuropathy Pain Scale, BPI-SF, SF-MPQ, PCS, POMS-SF, and LASA were completed at baseline. Subsequently, the Neuropathy Pain Scale, Subject Global Impression of Change, and BPI-SF were completed weekly. The pain intensity rating was chosen at study initiation as the primary efficacy measure. A health care professional administered the pain intensity rating weekly by phone. The SF-MPQ, PCS, POMS-SF, and LASA scale were completed at baseline and at the end of each study period (weeks 4 and 8). Completed questionnaires were returned by mail to the local institution responsible for enrolling each subject.
Subjects were informed of possible dermal reactions. The development of persistent marked erythema or rash at the application site or symptoms suggesting an allergic or anaphylactic reaction led to immediate patch removal and study discontinuation. This study utilized the Common Terminology Criteria for Adverse Events (CTCAE) v3.0 for toxicity and adverse event reporting. Toxicity data were collected by weekly subject questionnaires. A CTCAE grade was recorded for each reported toxicity.
Statistical analysis
The analytic procedures used in this two-arm, double-blinded, placebo-controlled, randomized, two-period crossover clinical trial were based on methods used successfully in previous studies of a similar nature by the North Central Cancer Treatment Group (NCCTG) Cancer Control Program [21]. All subjective patient response data were transformed into 0–100-point scales [38]. All statistical hypothesis testing was done using a two-tailed alternative hypothesis and a 5% type I error rate unless otherwise specified.
The primary efficacy measure was the pain intensity rating. The average pain score was calculated for each treatment period for input into a classical crossover “sums and differences” analysis [35]. The score for the first treatment period was the average of the pain scores in weeks 1 through 4 and for the second period the average of the pain scores in weeks 5 through 8. These two treatment period scores were used in the “sums and differences” analysis. A Bayesian hierarchical modeling approach involving Markov Chain Monte Carlo procedures and Gibbs sampling [25] was used to validate the results of the standard “sums and differences” crossover analysis [26]. The “sums and differences” approach was supplemented by modeling the difference in the period 2 minus period 1 average pain scores for each subject as a linear function of treatment arm adjusted for age, race, gender, stratification factors, and baseline pain scores. Linear hierarchical models were included in the analytical process using a validated computer algorithm built specifically for the analysis of crossover designs [37]. Analyses were also done looking at the intergroup differences between baseline and the last treatment week of period 1. Secondary end points including the SF-MPQ, BPI-SF, Neuropathy Pain Scale, Subject Global Impression of Change, PCS, POMS-SF, and the LASA Scale were analyzed in a manner identical to that of the primary end point.
Overall toxicity scores were calculated for each phase and treatment arm by adding up the maximum toxicity grade for each toxicity recorded for each patient. Toxicity scores were also analyzed with the “sums and differences” approach.
Other end points included the proportion of patients who reported a preference for lidocaine patch or placebo and the proportion of patients who terminated protocol therapy prematurely on each treatment. These data were analyzed with chi-square and Fisher’s exact tests. Where appropriate, Bonferroni adjustments were made for multiple testing. All treatment comparisons were made using two-sided testing and a 5% type I error rate.
Missing data were handled in a number of ways as a sensitivity analysis of the robustness of results relative to missing data [9]. First, only subjects completing 8 weeks of treatment were used in the analysis. A series of analyses using various imputation methods (average value carried forward, last value carried forward, minimum or maximum value carried forward) were carried out to assess the impact of missing data on the results.
The original planned accrual goal was 50 patients per arm which was calculated to provide 80% power to detect differences in average pain scores of 0.58 standard deviations. This was considered a moderate effect size that would be clinically meaningful [36].
Results
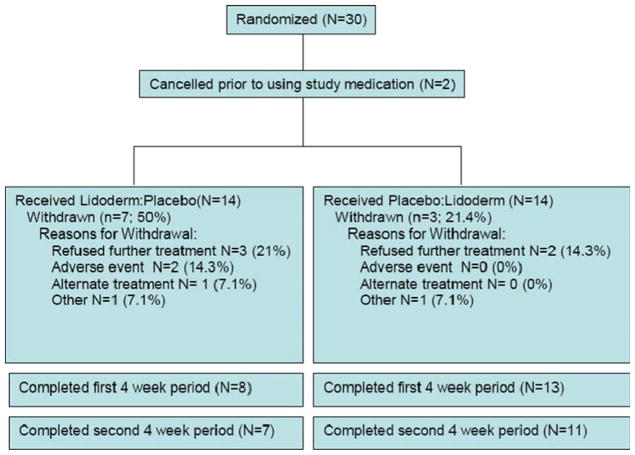
A consort diagram of the trial is presented in Fig. 1 [3]. Thirty subjects were enrolled and randomized between September 2004 and May 2006. The study was prematurely closed by the NCCTG Patient Safety Monitoring Committee due to a slow recruitment rate. Two subjects canceled after randomization and did not participate in the trail, resulting in 28 assessable subjects. Of the assessable subjects, 21 (75%) finished at least the first 4-week phase of the study and 18 (64%) finished both phases (i.e., 8 weeks). Ten of the 28 subjects did not finish the trial: two discontinued because of adverse events, seven discontinued for unknown reasons, and one switched to an alternate treatment. The two sequence groups were comparable with respect to the proportion of subjects who discontinued and the reasons for discontinuation (P=0.39). Notably, nine of the ten subjects who discontinued the study did so while using lidocaine (versus placebo) patches (P=0.02). Subjects who discontinued were more likely to have reported severe, ≥7/10, pain at baseline.
Fig. 1.
Study participant flow
Demographics and baseline clinical characteristics at randomization are presented in Table 1. Medications utilized by subjects during each study period are listed in Table 2.
Table 1.
Baseline demographics and pain characteristics of the study groups
| Lid:Plac (N=14) | Plac:Lid (N=14) | Total (N=28) | P value | |
|---|---|---|---|---|
| Age | ||||
| N | 14 | 14 | 28 | 0.05 |
| Mean (SD) | 65.0 (6.4) | 58.6 (11.3) | 61.8 (9.5) | |
| Gender | ||||
| Female | 8 (57%) | 11 (79%) | 19 (68%) | 0.22 |
| Male | 6 (42.9%) | 3 (21.4%) | 9 (32.1%) | |
| Race | ||||
| White | 10 (71%) | 9 (64%) | 19 (68%) | 0.79 |
| Black or African American | 3 (21%) | 3 (21%) | 6 (21%) | |
| American Indian or Alaska Native | 1 (7%) | 1 (7%) | 2 (7%) | |
| Unknown: patient unsure | 0 (0%) | 1 (7%) | 1 (4%) | |
| Current analgesic regimen | ||||
| Opioids (includes tramadol) | 2 (14%) | 3 (21%) | 5 (18%) | 0.63 |
| Antidepressants | 1 (7%) | 1 (7%) | 2 (7%) | |
| Anticonvulsants | 0 (0%) | 2 (14%) | 2 (7%) | |
| Combination | 4 (29%) | 4 (29%) | 8 (29%) | |
| Other | 2 (14%) | 2 (14%) | 4 (14%) | |
| None | 5 (36%) | 2 (14%) | 7 (25%) | |
| Duration of pain (months) | ||||
| 1–3 | 3 (21%) | 2 (14%) | 5 (18%) | 0.77 |
| >3–6 | 1 (7%) | 2 (14%) | 3 (11%) | |
| >6 | 10 (71%) | 10 (71%) | 20 (71%) | |
| Etiology of pain | ||||
| Breast surgery | 4 (29%) | 6 (43%) | 10 (36%) | 0.67 |
| Lung surgery | 8 (57%) | 7 (50%) | 15 (54%) | |
| Other | 2 (14%) | 1 (7%) | 3 (11%) | |
| Week 0: worst paina | ||||
| N | 13 | 14 | 27 | 0.27 |
| Mean (SD) | 7.5 (1.9) | 6.5 (2.4) | 7.0 (2.2) | |
| Week 0: least paina | ||||
| Mean (SD) | 2.9 (2.1) | 3.6 (1.6) | 3.3 (1.9) | 0.16 |
| Week 0: average paina | ||||
| Mean (SD) | 4.6 (1.8) | 5.1 (1.9) | 4.9 (1.8) | 0.40 |
| Week 0: pain nowa | ||||
| Mean (SD) | 5.0 (2.7) | 5.1 (2.1) | 5.1 (2.3) | 0.84 |
Values from Pain Intensity Rating Scale
Table 2.
Frequency of analgesic use by groups during study periods 1 and 2
| Medication | Lidocaine patch: placebo | Lidocaine patch: placebo | ||
|---|---|---|---|---|
|
|
|
|||
| Period 1 | Period 2 | Period 1 | Period 2 | |
| Naprosyn | 1 (7.1%) | Cont | 0 (0.0%) | |
| Aspirin | 1 (7.1%) | Cont | 0 (0.0%) | |
| Ibuprofen | 2 (14.3%) | 1 Cont, 1 D/C | 1 (7.1%) | Cont |
| Rofecoxib | 1 (7.1%) | D/C | 0 (0.0%) | |
| Acetaminophen | 0 (0.0%) | 1 (7.1%) | Cont | |
| Amitryptiline | 1 (7.1%) | Cont | 0 (0.0%) | |
| Propoxyphene–acetaminophen | 2 (14.3%) | 1 Cont, 1 D/C | 0 (0.0%) | |
| Oxycodone | 0 (0.0%) | 1 (7.1%) | D/C | |
| Hydrocodone–acetaminophen | 1 (7.1%) | D/C | 1 (7.1%) | Cont |
Cont continuous, D/C discontinued
Primary end point
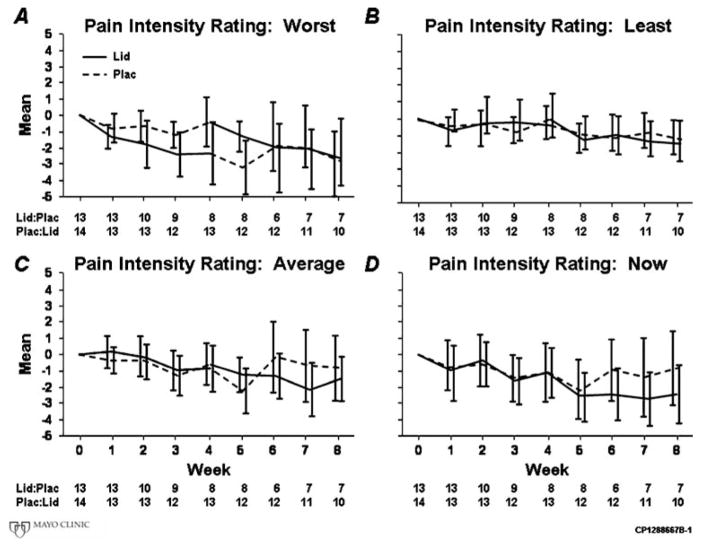
Average weekly pain intensity ratings were not significantly reduced while subjects used the lidocaine patch compared to placebo. This was true of the primary analysis (4.1 versus 3.8, P=0.36), the period 1 analysis (4.4 versus 4.8, P=0.92), and using an alternative approach, Senn’s test (0.75 versus 0.74, P=0.53), which accounts for the crossover design by analyzing within patient treatment differences during each study arm. Figure 2 graphically illustrates the general lack of a differential response between the lidocaine and placebo groups, providing data regarding the changes from baseline for average, least, worst, and current weekly pain scores. Table 3 illustrates P values for worst, least, and average pain intensity ratings, as well as “pain now” ratings by analysis strategy. Carryover effects of the randomized treatments were not observed for pain intensity ratings. Significant differences were similarly absent during period 1 between lidocaine and placebo groups with respect to the proportion of patients experiencing 10% relief (64% vs. 71%, respectively, P=0.69) or in week 4 minus baseline change (mean −0.9 vs. −0.6, respectively, P=0.91).
Fig. 2.
Mean pain intensity rating changes from baseline for: a average, b worst, c least, and d current weekly pain scores
Table 3.
Group means and P values using different statistical tests of pain intensity rating
| Variable | Complete data including cross over | First randomization period only | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Lid:Plac mean (SD) | Plac:Lid mean (SD) | Diff mean (SD) | P value | Lidocaine mean (SD) | Placebo mean (SD) | Diff mean SD) | P value | |
| Weekly pain intensity rating worst pain | 5.4 (2.57) | 5.4 (2.46) | −0.3 (1.45) | 0.96 | 5.8 (2.69) | 6.2 (1.90) | −0.4 (2.32) | 0.97 |
| Weekly pain intensity rating least pain | 2.3 (2.01) | 2.7 (2.17) | −0.0 (2.19) | 0.84 | 2.1 (2.05) | 3.7 (1.98) | −1.6 (2.02) | 0.19 |
| Weekly pain intensity rating average pain | 4.1 (2.13) | 4.1 (2.11) | −0.2 (1.83) | 0.36 | 4.4 (2.12) | 4.8 (1.71) | −0.4 (1.90) | 0.92 |
| Weekly pain intensity rating pain now | 4.4 (2.57) | 4.8 (2.63) | −0.3 (1.44) | 0.37 | 3.9 (2.66) | 4.6 (1.82) | −0.7 (2.28) | 0.56 |
The small sample size hampers the power for the original design specifications. Fourteen patients per sequence completing the study would provide 80% power to detect an effect size of 1.1 standard deviations in average pain scores. This is an exceptionally large effect size and statistical significance would only be achieved if the observed differences were very large. The observed effect size of 0.3 standard deviations, however, would not have been statistically significant even if the study reached its original accrual of 50 patients per treatment group.
Secondary end points
Without exception, all period 1 BPI interference scores improved to a greater degree in the lidocaine group. Significant improvements were detected in both physical (general activity, P=0.02; and work, P=0.04) and psychoemotional (mood, P=0.06; and relations with others, P=0.02) domains. Figure 3 suggests a potential intergroup difference in the degree of overall pain interference during period 1, although the confidence intervals do overlap. Few additional secondary end points were significantly different when subjects used the lidocaine versus placebo patches. Significant carryover effects were noted for most BPI interference scores. Table 4 lists P values for an overall and for each individual BPI interference score derived by analytic procedure.
Fig. 3.

Mean Brief Pain Inventory overall pain interference changes from baseline
Table 4.
P values of different statistical tests of BPI interference scales
| BPI interference scales | Carryover test | Period 1 test | Period 2 test | Senn’s test | Week 4 minus baseline | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| P value | P value | P value | P value | Mean lidocaine | Mean placebo | P value | |
| Overall interference | 0.04 | 0.05 | 0.95 | 0.95 | −1.8 | −0.1 | 0.02 |
| General Activity | 0.02 | 0.02 | 0.56 | 0.67 | −1.6 | −0.2 | 0.12 |
| Mood | 0.05 | 0.11 | 0.09 | 0.09 | −2.5 | −0.5 | 0.06 |
| Walking ability | 0.14 | 0.09 | 0.61 | 0.97 | −1.8 | −0.6 | 0.33 |
| Normal work (work outside the home and housework) | 0.07 | 0.32 | 0.18 | 0.18 | −2.3 | 0 | 0.04 |
| Relations with other people | 0.24 | 0.27 | 0.37 | 0.37 | −1.5 | 0.8 | 0.02 |
| Sleep | 0.05 | 0.14 | 0.84 | 0.56 | −0.9 | 0.3 | 0.2 |
| Enjoyment of life | 0.04 | 0.09 | 0.44 | 0.53 | −1.9 | −0.4 | 0.18 |
Despite the fact that more subjects discontinued while using the lidocaine patch, reported toxicities were not significantly different for either arm or time period (Table 5).
Table 5.
Reported toxicities during lidocaine and placebo use
| Toxicity | Lidocaine | Placebo | P value |
|---|---|---|---|
| Dizziness | |||
| None | 13 (93%) | 14 (100%) | 0.33 |
| Moderate | 1 (7%) | 0 (0%) | |
| Fatigue | |||
| None | 13 (93%) | 14 (100%) | 0.33 |
| Moderate | 1 (7%) | 0 (0%) | |
| Hypersensitivity | |||
| None | 14 (100%) | 12 (86%) | 0.15 |
| Moderate | 0 (0)% | 2 (14%) | |
| Neuromotor | |||
| None | 13 (93%) | 14 (100%) | 0.33 |
| Moderate | 1 (7%) | 0 (0%) | |
| Pain–chest | |||
| None | 14 (100%) | 13 (93%) | 0.33 |
| Moderate | 0 (0%) | 1 (7%) | |
| Rash–desquamation | |||
| None | 11 (79%) | 13 (93%) | 0.24 |
| Mild | 2 (14%) | 1 (7%) | |
| Moderate | 1 (7%) | 0 (0%) | |
| Worst toxicity | |||
| None | 10 (71%) | 10 (71%) | 0.79 |
| Mild | 2 (14%) | 3 (21%) | |
| Moderate | 2 (14%) | 1 (7%) | |
Discussion
This randomized, double-blind, placebo-controlled crossover study failed to detect significant intergroup differences in postsurgical pain intensity ratings between subjects using lidocaine versus placebo patches. This disappointing and perhaps surprising result contrasts with reports of significant analgesia associated with patch use in patients with postherpetic neuralgia [8, 11, 34].
Several possibilities may account for the absence of a treatment effect. The high dropout rate and low accrual constrain the study’s ability to detect the originally planned meaningful difference. The observed effect size (0.3 standard deviations), however, was remarkably small and it is doubtful that an increased sample size would have modified the overall conclusion. In fact, with an effect size of 0.3 standard deviations, 175 subjects per treatment arm would be required for 80% power. Alternatively, the study design may not have placed the lidocaine patch at its best clinical advantage. If lidocaine’s primary mechanism of action involves attenuation of central sensitization, peak responses may not be evident at 4 weeks. Misclassification bias toward the null arising from the inappropriate enrollment of patients with nociceptive rather than neuropathic pain could explain the lack of efficacy. However, the magnitude of misclassification would have to be significant and the study inclusion criteria were designed to minimize this possibility.
It is difficult to explain the high discontinuation rate among patients using the lidocaine patch, particularly given the absence of evidence of increased toxicity. The majority of subjects who discontinued reported severe levels of pain at baseline, ≥7/10. Little qualitative data are available to clarify subjects’ decisions to discontinue; therefore, possible explanations remain speculative.
A potentially significant treatment effect was noted among the BPI interference scores. During period 1, patients using the lidocaine patch reported lower BPI interference scores and their scores diminished during treatment. The size of the average difference was more than two points on the 0–10-point scale, which would tend to indicate a clinically meaningful effect [10]. In fact, all BPI interference scores were reduced to a greater degree in lidocaine patch patients when week 4 scores were compared to baseline. These results must be interpreted with caution due to the small sample size and multiple testing, as well as the notation that the patients who discontinued the study were more likely to be using the lidocaine patch and to have higher pain scores. Nonetheless, the magnitude and time course of the reduction in pain interference scores have important implications for future studies as well as lidocaine patch trials in individual patients. Our findings suggest that trials should extend beyond 4 weeks and that their success should be at least partially determined by the degree of pain interference.
Despite its disappointing accrual, early closure, and high discontinuation rate, this study represents the best data currently available regarding the efficacy of the lidocaine patch in managing chronic postoperative incisional pain among cancer patients. Our results can be considered respectable pilot data which suggest that future studies might benefit from evaluating the lidocaine patch for at least 6–8 weeks and assessing performance parameters such as physical function, given the reduced BPI interference scores noted with lidocaine patch use. Encouragingly, only 34 patients per arm would be needed to detect the observed effect size of 0.7 standard deviations in mean BPI interference score.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35103, CA-35119, CA-35415, and CA-35113.
Footnotes
Additional participating institutions include: Medcenter One Health Systems, Bismarck, ND 58501, USA (Edward J. Wos, M.D.); Illinois Oncology Research Assn. CCOP, Peoria, IL 61615-7828, USA (John W. Kugler, M.D.); Abbott Northwestern Hospital, Minneapolis, MN 55407, USA (Daniel M. Anderson, M.D.); Siouxland Hematology–Oncology Associates, Sioux City, IA 51105, USA (Donald B. Wender, M.D.)
Contributor Information
Andrea L. Cheville, Mayo Clinic and Mayo Foundation, Rochester, MN 55905, USA
Jeff A. Sloan, Mayo Clinic and Mayo Foundation, Rochester, MN 55905, USA
Donald W. Northfelt, Mayo Clinic Scottsdale, Scottsdale, AZ 85259-5404, USA
Anand P. Jillella, Mayo Clinic Scottsdale, Scottsdale, AZ 85259-5404, USA
Gilbert Y. Wong, Mayo Clinic and Mayo Foundation, Rochester, MN 55905, USA
James D. Bearden, III, Upstate Carolina CCOP, Spartanburg, SC 29303, USA.
Heshan Liu, Mayo Clinic and Mayo Foundation, Rochester, MN 55905, USA.
Paul L. Schaefer, Toledo Community Hospital Oncology Program CCOP, Toledo, OH 43623, USA
Benjamin T. Marchello, Montana Cancer Consortium, Billings, MT 59101, USA
Bradley J. Christensen, Mayo Clinic and Mayo Foundation, Rochester, MN 55905, USA
Charles L. Loprinzi, Mayo Clinic and Mayo Foundation, Rochester, MN 55905, USA. Mayo Clinic, 200 First Street, SW Rochester, MN 55905, USA
References
- 1.Argoff CE. Conclusions: chronic pain studies of lidocaine patch 5% using the Neuropathic Pain Scale. Curr Med Res Opin. 2004;20(Suppl 2):S29–S31. doi: 10.1185/030079904X12979. [DOI] [PubMed] [Google Scholar]
- 2.Baker F, Denniston M, Zabora J, Polland A, Dudley WN. A POMS short form for cancer patients: psychometric and structural evaluation. Psychooncology. 2002;11:273–281. doi: 10.1002/pon.564. [DOI] [PubMed] [Google Scholar]
- 3.Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 5.Cleeland C, et al. How to assess cancer pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. Guilford; New York: 1992. pp. 360–387. [Google Scholar]
- 6.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 7.Dajczman E, Gordon A, Kreisman H, Wolkove N. Long-term postthoracotomy pain. Chest. 1991;99:270–274. doi: 10.1378/chest.99.2.270. [DOI] [PubMed] [Google Scholar]
- 8.Dworkin RH, Corbin AE, Young JP, Jr, Sharma U, LaMoreaux L, Bockbrader H, Garofalo EA, Poole RM. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60:1274–1283. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- 9.Fairclough DPH, Cella D, Bonomi P. Comparison of several model-based methods for analyzing incomplete quality of life data in cancer trials. Stat Med. 1998;17:781–796. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<781::aid-sim821>3.0.co;2-o. doi:10.1002/(SICI) 1097-0258(19980315/15)17:5/7<781::AID-SIM821>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 11.Galer BS, Rowbotham MC, Perander J, Friedman E. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: results of an enriched enrollment study. Pain. 1999;80:533–538. doi: 10.1016/S0304-3959(98)00244-9. doi:10.1016/S0304-3959(98) 00244-9. [DOI] [PubMed] [Google Scholar]
- 12.Galer BS, Jensen MP, Ma T, Davies PS, Rowbotham MC. The lidocaine patch 5% effectively treats all neuropathic pain qualities: results of a randomized, double-blind, vehicle-controlled, 3-week efficacy study with use of the neuropathic pain scale. Clin J Pain. 2002;18:297–301. doi: 10.1097/00002508-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol. 2003;43:111–117. doi: 10.1177/0091270002239817. [DOI] [PubMed] [Google Scholar]
- 14.Hurny C, Bernhard J, Coates A, Peterson HF, Castiglione-Gertsch M, Gelber RD, Rudenstam CM, Collins J, Lindtner J, Goldhirsch A, Senn HJ. Responsiveness of a single-item indicator versus a multi-item scale: assessment of emotional well-being in an international adjuvant breast cancer trial. Med Care. 1996;34:234–248. doi: 10.1097/00005650-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 16.Jensen MP, Dworkin RH, Gammaitoni AR, Olaleye DO, Oleka N, Galer BS. Assessment of pain quality in chronic neuropathic and nociceptive pain clinical trials with the Neuropathic Pain Scale. J Pain. 2005;6:98–106. doi: 10.1016/j.jpain.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Johansson A, Kornfalt J, Nordin L, Svensson L, Ingvar C, Lundberg J. Wound infiltration with ropivacaine and fentanyl: effects on postoperative pain and PONV after breast surgery. J Clin Anesth. 2003;15:113–118. doi: 10.1016/S0952-8180(02)00511-1. [DOI] [PubMed] [Google Scholar]
- 18.Karmakar MK, Ho AM. Postthoracotomy pain syndrome. Thorac Surg Clin. 2004;14:345–352. doi: 10.1016/S1547-4127(04)00022-2. [DOI] [PubMed] [Google Scholar]
- 19.Katz J, McCartney CJ. Current status of pre-emptive analgesia. Curr Opin Anaesthesiol. 2002;15:435–441. doi: 10.1097/00001503-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 21.Loprinzi CL, Kugler JW, Sloan JA, Rooke TW, Quella SK, Novotny P, Mowat RB, Michalak JC, Stella PJ, Levitt R, Tschetter LK, Windschitl H. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. N Engl J Med. 1999;340:346–350. doi: 10.1056/NEJM199902043400503. [DOI] [PubMed] [Google Scholar]
- 22.Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist. 2004;9:571–591. doi: 10.1634/theoncologist.9-5-571. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer. 2005;92:225–230. doi: 10.1038/sj.bjc.6602304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malenka RC. The role of postsynaptic calcium in the induction of long-term potentiation. Mol Neurobiol. 1991;5:289–295. doi: 10.1007/BF02935552. [DOI] [PubMed] [Google Scholar]
- 25.Mandrekar JSD, Novotny PJ, Slaon JA. A general Gibbs sampling algorithm for analyzing linear models using the SAS system. Proceedings of the SUGI. 1999:1644–1649. [Google Scholar]
- 26.Mandrekar JSD, Novotny PJ, Slaon JA. A general Gibbs sampling algorithm for analyzing linear models using the SAS system. SUGI Proc. 1999;24:1644–1649. [Google Scholar]
- 27.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 28.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen A, Girard F, Boudreault D, Fugere F, Ruel M, Moumdjian R, Bouthilier A, Caron JL, Bojanowski MW, Girard DC. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg. 2001;93:1272–1276. doi: 10.1097/00000539-200111000-00048. [DOI] [PubMed] [Google Scholar]
- 30.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20:589–605. doi: 10.1023/A:1025570508954. [DOI] [PubMed] [Google Scholar]
- 31.Pergolizzi J, Boger RH, Budd K, Dahan A, Erdine S, Hans G, Kress HG, Langford R, Likar R, Raffa RB, Sacerdote P. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 32.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. doi: 10.2307/2529712. [DOI] [PubMed] [Google Scholar]
- 33.Reuben SS. Chronic pain after surgery: what can we do to prevent it. Curr Pain Headache Rep. 2007;11:5–13. doi: 10.1007/s11916-007-0015-9. [DOI] [PubMed] [Google Scholar]
- 34.Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996;65:39–44. doi: 10.1016/0304-3959(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 35.Senn S. Cross-over trials in clinical research. Wiley; New York: 1993. [Google Scholar]
- 36.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J Biopharm Stat. 2004;14:73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 37.Sloan JANP, Lorinzi CL, Nair S. Graphical and analytical tools for the analysis two-period crossover clinical trials. SUGI Proc. 1997;22:1312–1317. [Google Scholar]
- 38.Sloan JAOFJ, Suman VJ, Sargeant DJ. Incorporating quality of life measurement in oncology clinical trials. Proceedings of the American Statistical Association; 1998. pp. 281–287. [Google Scholar]
- 39.Wallace MS, Wallace AM, Lee J, Dobke MK. Pain after breast surgery: a survey of 282 women. Pain. 1996;66:195–205. doi: 10.1016/0304-3959(96)03064-3. [DOI] [PubMed] [Google Scholar]
- 40.Wiffen PJ, McQuay HJ, Edwards JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database Syst Rev. 2005:CD005452. doi: 10.1002/14651858.CD005452. [DOI] [PubMed] [Google Scholar]
- 41.Wiffen PJ, McQuay HJ, Moore RA. Carbamazepine for acute and chronic pain. Cochrane Database Syst Rev. 2005:CD005451. doi: 10.1002/14651858.CD005451. [DOI] [PubMed] [Google Scholar]
- 42.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 43.Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. J Pain. 2006;7:S13–S30. doi: 10.1016/j.jpain.2005.09.007. [DOI] [PubMed] [Google Scholar]