Abstract
Objectives:
To assess mammography utilization and knowledge, and to determine barriers associated with mammography utilization among Saudi women.
Methods:
We conducted a cross-sectional survey in 5 main geographic regions of Saudi Arabia from February 2015 to May 2015. The sample comprised women aged ≥40 years. Associations between socio-demographic factors and mammography use were tested using chi-square test. Predictors of mammography use were assessed by logistic regression.
Results:
A total of 3,245 women were surveyed, with 40% reporting ever having a mammogram. As indicated by the univariable analyses, older age (≥60 years), being single or divorced, having <2 children, not completing high school, and having a family history (hx) of breast cancer were significantly associated with never having a mammogram. Participants of older age (odds ratio [OR] 51-60 versus 41-50 = 0.6, 95% CI: 0.5-0.7 and OR >60 versus 41-50 = 0.5, 95% CI: 0.3-0.8), and divorced (OR divorced versus married = 0.6, 95% CI: 0.5-0.8] were less likely to have had a mammogram, while participants with no family hx of breast cancer (OR no family hx versus family hx = 1.5, 95% CI: 1.3-1.8)were more likely to have had a mammogram.
Conclusion:
Mammography utilization and knowledge are low in Saudi Arabia. Increasing the awareness of breast cancer screening through educational programs could help women overcome existing barriers and misconceptions.
Breast cancer is a serious public health issue worldwide. Approximately 1.67 million new cases of breast cancer, representing 25% of all cancers, were diagnosed among women in 2012.1 Its incidence is the highest in developed countries, with rates as high as 92 per 100,000 people in North America compared with 27 per 100,000 people in Middle Africa and Eastern Asia.1 It is the leading cause of death among women in developing countries and the fifth cause of mortality among all cancers.1 In Saudi Arabia, a total of 1,473 cases of breast cancer were diagnosed among women in 2010, representing 27.4% of all newly diagnosed cancers.2 It ranked first among all cancers diagnosed in women in the same year, with patients aged 48 years on average at the time of diagnosis.
Mammography screening has been associated with a decreased risk of death from breast cancer.3 The National Comprehensive Cancer Network recommends annual mammograms for women ≥ 40 years.4 Approximately 6.5% of breast cancers occur in women aged 30-40 years,5 and a large proportion of these cancers occur in women without a family history of breast cancer.6 In these cases, the disease tends to be aggressive and is often associated with a poorer prognosis.7 Consequently, early identification, irrespective of family history of breast cancer, is beneficial.
In Saudi Arabia, a few studies investigated women’s knowledge, attitudes, and practices about breast cancer. In a study conducted on adult women attending primary health care centers in Al-Khobar, it was found that approximately 85% of the 400 surveyed women were knowledgeable about mammography.8 Unfortunately, the authors surveyed women who fell in the age bracket of 18 to 29 years, for whom mammography is not typically recommended. In another survey conducted in the eastern province,9 only 6.5% of the women reported ever having a mammogram. A multistage survey of individuals aged 15 years and older found that 92% of women aged 50-74 years old reported never having had a mammogram.10 In addition, women were more likely to have had a mammogram in the past 2 years if they were educated, had received a routine medical exam within the last 2 years and were diagnosed with hypertension.
Most studies that explored women’s knowledge and practices of breast cancer screening in Saudi Arabia were conducted in specific regions or cities. In addition, in many studies, mammography use was assessed among women with a wide age range, including ages not intended for mammography. This study is novel as it attempts to assess in a comprehensive manner the breast cancer screening practices of women more than 40 years old in 5 geographic regions of Saudi Arabia. The aims of our study were: (i) to assess mammography use and the factors associated with it, (ii) to investigate mammography knowledge and the factors associated with it, and (iii) to determine the barriers associated with mammography utilization.
Methods
Research design and data collection
This cross-sectional survey is part of a larger study designed to assess men and women’s knowledge of breast cancer and mammography use. We selected a convenience sample of women residing in the 5 main geographic regions of Saudi Arabia (Northern, Southern, Western, Eastern, and Central). The inclusion criteria were; (i) to be fluent in English or Arabic, (ii) to have no mental disabilities, (iii) to be residents of Saudi Arabia. Participants who did not meet these criteria were not eligible to participate. After reviewing the published literature, a structured questionnaire was developed based on questions used in a previous study that investigated breast cancer knowledge and mammography use.11 The questionnaire was reviewed by 4 consultants in different fields that dealt with breast cancer—i.e., medical oncologist, radiation oncologist, imaging radiologist and breast surgeon. The questionnaire was structured into the following sections: (i) socio-demographic characteristics of the participants, (ii) knowledge of breast cancer symptoms and risk factors, (iii) mammography use and knowledge of mammography practice, (iv) barriers to the utilization of mammography screening and (v) perceptions about breast cancer awareness and campaigns in Saudi Arabia. Questionnaire sections (i), (iii) and (iv) were the focus of this analysis.
In section (i), information about age, the number of children, marital status, family income, education, the region of residence and the family history of breast cancer were collected. Section (iii) included questions about mammography use and knowledge. First, mammography use was measured by asking women the following question: “Have you ever had a mammogram?” Second, information about mammography practice knowledge was obtained by asking women 3 questions: “Is the mammogram the standard method to identify breast cancer?”, “At what age should a woman obtain a mammogram?”, and “How often should a woman obtain a mammogram?” Third, women were asked to self-rate their knowledge about mammograms. In section (iv), the women were asked to identify the barriers to mammography use, including reasons for not getting a mammogram and feelings before a mammogram appointment.
Electronic and paper-based questionnaires were distributed and promoted through social media. The electronic questionnaires were distributed through Twitter, Facebook and WhatsApp. To ensure broad distribution of the questionnaires, especially to individuals with limited access to social media, hard copies of the questionnaire were distributed at schools and malls in each geographic region. The data were collected from the beginning of February 2015 to the end of May 2015. The questionnaire and the study proposal were approved by the Biomedical Ethics Research Committee of King Abdulaziz University, Jeddah, Saudi Arabia.
Statistical Analysis
Because the study focused on mammography use, the analyses were restricted to women >40 years. The main outcome variables were mammography use (never/ever had a mammogram) and the mammography knowledge score. The knowledge of mammography practice was evaluated by assessing perceived knowledge and actual knowledge. Perceived knowledge was measured by asking the women to rate their own knowledge with one of 4 responses: “Excellent”, “Very good”, “Fair” and “Poor”. Actual knowledge was measured by asking women three general questions about mammography: whether mammograms were the best methods for breast cancer diagnosis, the age at which women should start having mammograms, and the frequency of mammograms for women of applicable age. The response to each knowledge question was assigned a score of 1 for a correct answer and 0 for “don’t know” or an incorrect answer. For each woman, a total knowledge score was calculated by summing the scores of the responses, with a final score ranging from 0 to 3. The main predictors in this study were age, the number of children, marital status, education, family income, region and family history of breast cancer.
Frequencies and percentages are reported for categorical data, while means and standard deviations are reported for continuous data. Associations between socio-demographic predictors and mammography use and between actual knowledge and perceived knowledge were tested using the chi-square test. To identify the significant predictors of mammography use, a logistic regression was performed using stepwise selection technique. The significance levels for removal and entry of variables from the model were 0.1 and 0.05, respectively. Associations between socio-demographic predictors and actual knowledge scores were tested using a one-way ANOVA, followed by Tukey’s post-hoc test if the results were significant. Associations between mammography knowledge and mammography use were tested using a chi-square test and t-test, as indicated. The significance level was set at 0.05. All data analyses were performed using Stata version 13.0 (StataCorp LP, College Station, TX, USA).
Results
A total of 3,245 women above 40 years old were included in this study. The majority of the participants were aged between 41 and 50 years (77%), married (78%), and had more than one child (85%). Thirteen percent of the participants did not have a high school degree, while 44% had a university degree (Table 1). Approximately 35% of the women resided in the western region, 31% in the central region, 21% in the eastern region, and 7% each in the northern and southern regions. Twenty-six percent of women reported a family history of breast cancer.
Table 1.
Associations between socio-demographic characteristics and mammography use.

Forty percent of the participants reported ever having a mammogram. Mammography use decreased with age; 44% of women aged 41-50 had ever had a mammogram versus women aged 51-60 (33%) and women aged >60 (24%). Mammography use was higher among women with more than one child (42%). Mammography use also varied by marital status; it was highest among married women (42%) and lowest among single women (26%). Compared with more educated women, women with an educational level below high school reported ever having a mammogram less often (33%). The highest prevalence of mammography use was reported by residents of the western and central regions (43%), followed by the southern (38%) and northern regions (36%), and the eastern region (34%). Women who had a family history of breast cancer reported lower mammography use (32%) than those without a family history of the disease (Table 1).
Approximately 36% of the women perceived their knowledge as poor; 24% perceived their knowledge as excellent, 22% perceived their knowledge as fair and 19% of the respondents perceived their knowledge as very good (Table 2). A large percentage of women correctly stated that mammography was the standard method to identify breast cancer (72%) and that a woman should have a mammogram every 1-2 years (60%). Only 44% of the respondents reported the correct age women should start having mammograms.
Table 2.
Association between self-perceived knowledge and actual knowledge about mammography practice.

Women’s self-rating of their mammography knowledge (perceived knowledge) was significantly associated with their corresponding answers to actual knowledge questions (Table 2). Ninety-six percent of the women who perceived that they had excellent knowledge responded that a mammogram was the standard method to detect breast cancer. The relative frequency of a correct response to the question regarding the age at which a woman should start having mammograms varied by perceived knowledge: excellent, 40%; very good, 52%; fair, 46%; and poor knowledge, 42%. Approximately 78% of the participants who perceived that they had excellent knowledge correctly determined how often women should obtain mammograms. The actual knowledge scores were significantly associated with perceived knowledge (p-value=0.01). Women who perceived that they had excellent knowledge had higher scores than participants who perceived that they had lower knowledge levels.
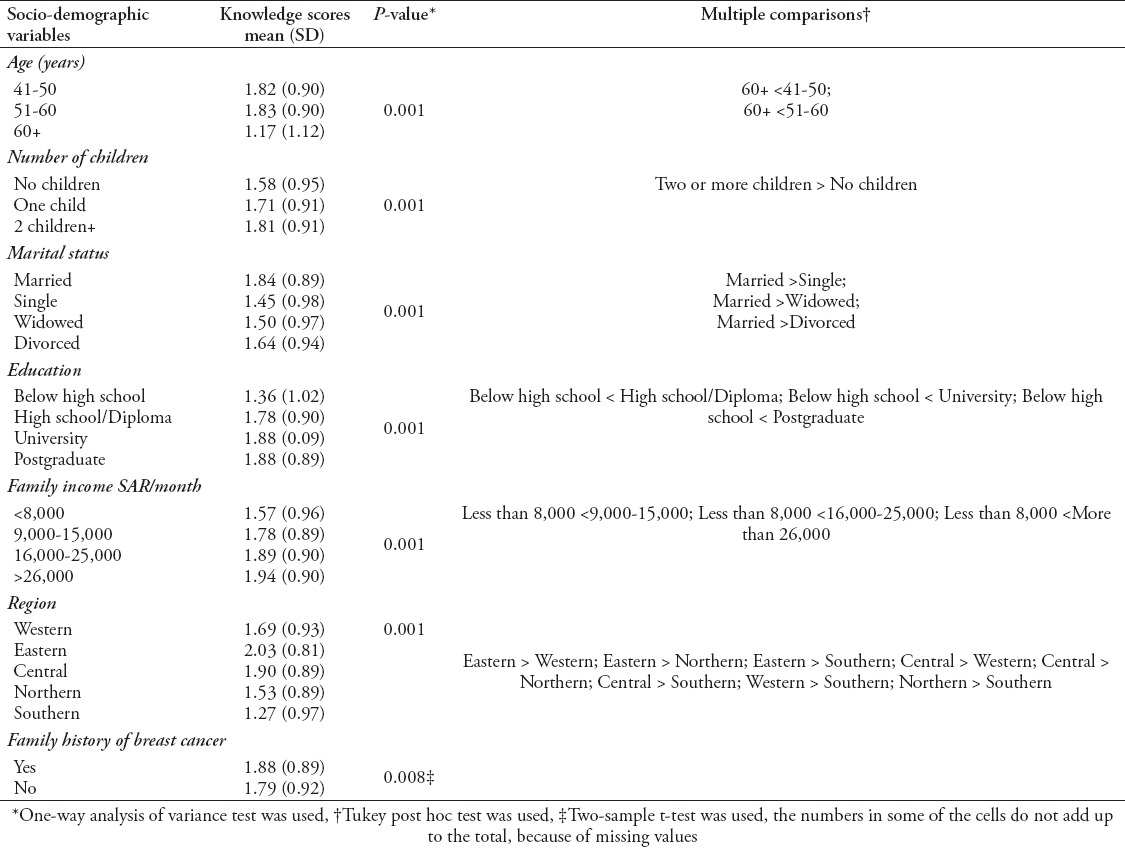
The associations between socio-demographic characteristics and mammography knowledge scores are illustrated in Table 3. Knowledge of mammography was significantly associated with age, number of children, marital status, education, income and area of residence (p-value=0.001) as well as family history of breast cancer (p-value=0.008). The mean knowledge scores were lowest among single and older women aged ≥60 years, participants with less than a high school degree, and those with a monthly family income <8,000 SAR. Conversely, the mean knowledge scores were highest among women with more than one child, residents of the eastern and central regions, and those with a family history of breast cancer.
Table 3.
Association between socio-demographic characteristics and mammography knowledge scores.
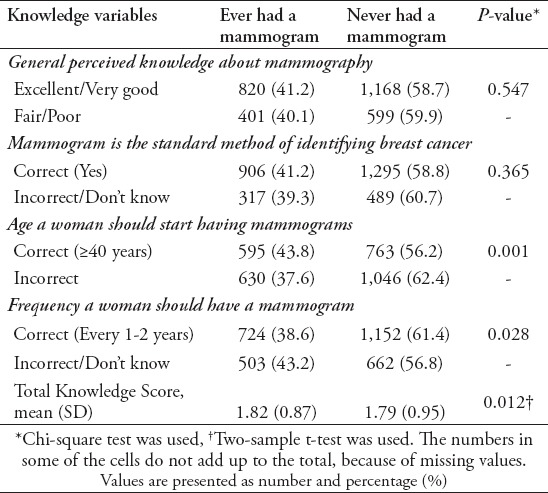
As shown in Table 4, women who correctly reported the recommended age to start getting mammograms were more likely to report ever having a mammogram compared with women who responded incorrectly (p-value=0.001). However, mammography use was significantly higher among women who responded incorrectly to the question concerning mammography frequency (p-value=0.028). Perceived knowledge about mammography and knowledge on whether mammograms were the best methods for breast cancer diagnosis were not significantly associated with mammography use. Subjects with higher knowledge scores were more likely to have had a mammogram (p-value=0.012).
Table 4.
Association between mammography knowledge and mammography use.
The main reasons why women failed to obtain a mammogram were the belief that the examination was not important (31%) and worries about the results (25%). Regarding how participants would feel before a mammography appointment, 45.5% reported they would be worried about the results, 15.7% reported they would be scared of pain, 11.1% reported they would not be able to sleep, 10.3% reported that they would be embarrassed, and 7.5% reported that they would not want to go (Table 5).
Table 5.
Barriers to mammography use among women.
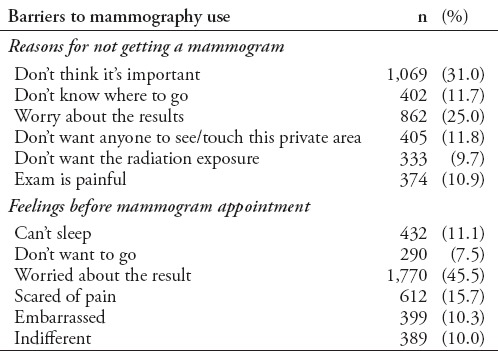
Table 6 presents the logistic regression univariable and multivariable associations of socio-demographic indicators and mammography knowledge with mammography use. In the univariable analyses, participants aged >51 years were less likely to have had mammography than younger participants. Respondents with the following characteristics were more likely to have had a mammogram, higher than high school education level vs. lower level, low, intermediate and high mammography knowledge scores vs. a score of zero. Single, and divorced participants compared with married participants, and participants from the eastern province compared with those from the western province, were less likely to have had a mammogram. Participants with no family history of breast cancer were 1.5 times more likely to have had a mammogram, compared with those with a family history. Age [OR 51-60 vs. 41-50= 0.6, 95% CI= 0.5-0.7 and OR >60 vs. 41-50=0.5, 95% CI= 0.3-0.8, respectively], marital status [OR divorced vs. married= 0.6, 95% CI= 0.5-0.8] and family history of breast cancer [OR no family hx vs. family hx=1.5, 95% CI=1.3-1.8] remained statistically significant in the multivariable model.
Table 6.
Logistic regression of sociodemographic characteristics and mammography knowledge score.

Discussion
We found that 40% of the respondents reported ever having a mammogram, which may reflect women’s limited awareness of this screening tool. Similar to our findings, a previous report indicated a low rate of mammography use among women in Saudi Arabia,10 although our reported prevalence is relatively higher. In their study, El Bcheraou et al.11 reported that 8% of women aged 50–74 years old had ever had a mammogram.
Low levels of mammography utilization have also been reported in various Middle Eastern population-based studies conducted in Iran,12 Egypt,13 the United Arab Emirates,14 and Lebanon.15 The situation is different from developed countries; mammography rates of 75% have been reported in Australia and 83% have been reported in Scotland.16 In the United States, the proportion of women aged 40 years and above who had a mammogram within the previous two years was 66.8% in 2014.17 While breast screening services are available at no cost to all women in the screening age group in Saudi Arabia, as is also the case in Scotland and Australia, it is not clear why mammography rates are lower in our setting. One possible reason to explain this observation is the difference in the screening service delivery between these countries and Saudi Arabia. In Australia and Scotland, healthcare authorities use mobile breast screening coupled with local advertising and invitation letters to promote and ensure that the rural population has access to screening mammography services.16 On the other hand, mobile screening units have only been used in the eastern region of Saudi Arabia. From 2009-2010, the program covered only four centers, and 14 centers were covered by October 2013.18
Similar to our findings, other authors reported an association between socio-demographic characteristics, such as age, marital status, income, and women’s likelihood of getting a mammogram.19 Among women aged <65 years and those aged ≥65 years, screening mammography adherence behaviors were reportedly higher among married women and respondents with higher incomes.19 Conversely, other researchers did not find an association between age and marital status and women’s likelihood of having a mammogram.9 However, the effect of demographic characteristics on mammography could be affected by other factors, such as access to screening services,20 which we did not measure.
Of note, our finding that women with a family history of breast cancer reported lower mammography use than their peers without a family history of the disease is contrary to that reported by other authors.21 Although the investigators thought women with a family history of breast cancer would have an increased perceived risk than their peers without a family history, they did not place much emphasis on the lack of family history of breast cancer and how it would influence women’s attitudes towards screening. Previous studies also suggested a moderate increase in mammography use among women with a family history of breast cancer.6,22 For example, an analysis of data from the 2005 California Health Interview Survey demonstrated that 83.5% of women with a family history of breast cancer in a first-degree relative had a screening mammogram in the past 2 years, compared to 76% of those at average risk.22 Our finding of a low level of mammography knowledge has been reported by other investigators in studies conducted in Saudi Arabia.23,24 In a study conducted in Abha,23 only 22% of the 1,092 women surveyed had ever heard about mammography. In another survey of 200 women aged ≥ 20 years who resided in Jeddah, it was observed that participants had poor knowledge of mammography use as a screening tool.24
We found that mean knowledge scores were lowest among women with primary and secondary school education. Education level was also found to be significantly associated with better mammography knowledge in previous studies conducted in Saudi Arabia.8,11, This finding was reported elsewhere26,27 and is expected, as education level reflects the individuals’ social environment and affects their understanding of disease and prevention.28 Our finding that underprivileged women had a lower knowledge about mammography is consistent with those of other authors.27,29 The relationship between income and knowledge score may show that women with a higher income had better access to medical and screening services. Thus, they were more likely to learn about breast health practices. Although an association was found in our study between marital status and mammography knowledge, no clear reason explains why married women appeared to be more knowledgeable about mammography.8 Moreover, other investigators30 did not find a significant association between marital status and mammography knowledge.
A previous study demonstrated that perceived self-efficacy to perform breast health practices was strongly related to perceived knowledge and not actual knowledge because perceived knowledge appears to be a crucial component of successful behavior change.31 Health campaigns aimed at promoting breast cancer screening should focus on this construct. On the other hand, perceived knowledge may not be easy to determine, especially for individuals who believe they have excellent knowledge to begin with. Indeed, prior research suggests that people are not usually accurate in differentiating between perceived and actual knowledge32 and tend to judge the accuracy of their knowledge too positively.33 However, this was not the case in our participants, whose self-rating of their mammography knowledge was significantly associated with their actual knowledge.
Women who were aware of the recommended age for women to start getting mammograms were more likely to have received a mammogram. Previous data suggested that the lack of knowledge and the awareness about breast screening procedures were factors associated with the underutilization of mammography.34 On the contrary, women who responded correctly to the question concerning mammography frequency were less likely to report ever having a mammogram. There is no clear explanation for this finding. Despite the consensus that mammography is a valuable screening tool for early breast cancer detection, some researchers35 have shown that many women remain unconvinced of its efficacy. Mammography has been available in all regions of Saudi Arabia since 2005,10 and recently, a nationwide breast cancer screening program was initiated in Riyadh.36 However, only 19% of men and 24% of women in our study were aware of these breast cancer-screening programs. Similarly, a recent study reported low rates of breast cancer screening in Saudi Arabia, despite the fact that mammography is free and widely available.10 Furthermore, while breast cancer awareness campaigns are widespread in Saudi Arabia (for example, the “Think Pink” campaign), it has been reported that they are not providing sufficient knowledge about the disease.11
In the current survey, one of the main reasons why women failed to obtain a mammogram was the belief that the examination was not important, which highlights the importance of educational programs to enhance women’s perceived self-efficacy associated with having a mammography. Another interesting finding was women’s fear of mammography results, which was the second most frequent barrier to having a mammogram. One of the possible consequences of underlying fear is that women may not show up for their mammography even after booking an appointment.35 While fear of breast cancer diagnosis has also been reported to preclude Saudi women from getting a mammogram,8 other authors suggest that fear or anxiety regarding getting cancer may, in general, facilitate screening, especially when the target population has access to and is aware of available screening procedures.9,37
This study has a few limitations; first, although the study covered five geographic regions in Saudi Arabia, random sampling was not used to select the regions and/or participants; thus, the results might not be generalizable to all women living in Saudi Arabia. Second, the data were self-reported so they might suffer from reporting bias. Third, the questionnaire was not validated; however, it was reviewed by several experts in the field. Fourth, there may be differences in breast cancer screening between rural and urban/cosmopolitan areas (e.g., Riyadh or Jeddah), but information was not collected to examine rural and urban differentials.
In conclusion, the utilization of mammography is low in Saudi Arabia, especially among older, married and less educated women as well as women with a family history of breast cancer. A considerable percentage of women did not know that mammography is the standard method to detect breast cancer and did not know the correct frequency and age to have a mammogram. Increasing the awareness of breast cancer screening through education and community programs could help women overcome existing barriers and misconceptions.
Footnotes
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon (France): International Agency for Research on Cancer; 2013. [[Accessed 2016 May]]. Available from: http://globocan.iarc.fr . [Google Scholar]
- 2.Council of Health Services. Cancer Incidence Report Saudi Arabia 2010. Riyadh, Saudi Arabia: Council of Health Services; 2010. [[Cited April 2014; Accessed May 2016] ]. http://www.chs.gov.sa/Ar/mediacenter/NewsLetter/2010%20Report%20(1).pdf . [Google Scholar]
- 3.Independent UK. Panel on Breast Cancer Screening The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–1786. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis. [[Accessed 2016 March; Cited 2017 March]]. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp .
- 5.Jardines L, Goyal S, Fisher P, Weitzel J, Royce M, Goldfarb SB. Breast Cancer Overview: Risk Factors, Screening, Genetic Testing, and Prevention. Cancer Management. [[Published June 2015; Accessed Mar 2017]]. Available from: http://www.cancernetwork.com/cancer-management/breast-cancer-overview-risk-factors-screening-genetic-testing-and-prevention .
- 6.Haber G, Ahmed NU, Pekovic V. Family history of cancer and its association with breast cancer risk perception and repeat mammography. Am J Public Health. 2012;102:2322–2329. doi: 10.2105/AJPH.2012.300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013;5:S2–S8. doi: 10.3978/j.issn.2072-1439.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dardas NMY, Taha AZ. Knowledge and Awareness About Breast Cancer Among Primary Care Attendees in Al-Khobar City, Eastern Saudi Arabia. Int J Med Health Sci. 2013;2:95–105. [Google Scholar]
- 9.Rehmani R, Elzubair AG, Al Maani M, Chaudary IY, Al Qarni A, Khasshogi T, et al. Population-based health survey in Eastern region of Saudi Arabia. Eastern Mediterr Health J. 2013;19:417–425. [PubMed] [Google Scholar]
- 10.El Bcheraoui C, Basulaiman M, Wilson S, Daoud F, Tuffaha M, AlMazroa MA, et al. Breast cancer screening in Saudi Arabia: free but almost no takers. PLoS One. 2015;10:e0119051. doi: 10.1371/journal.pone.0119051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagi SK, Khafaji MA. Do Women in Saudi Arabia “Think Pink”? American J Res Comm. 2013;1:43–58. [Google Scholar]
- 12.Ahmadian M, Samah AA, Redzuan M, Emby Z. Predictors of mammography screening among Iranian women attending outpatient clinics in Tehran, Iran. Asian Pac J Cancer Prev. 2012;13:969–974. doi: 10.7314/apjcp.2012.13.3.969. [DOI] [PubMed] [Google Scholar]
- 13.Uddin N, Fateem E, Hablas A, Seifeldin IA, Brown E, Merajver SD, et al. Public and professional educational needs for downstaging breast cancer in Egypt. J Cancer Educ. 2012;27:149–155. doi: 10.1007/s13187-011-0282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elobaid YE, Aw TC, Grivna M, Nagelkerke N. Breast cancer screening awareness, knowledge, and practice among arab women in the United Arab Emirates: a cross-sectional survey. PLoS One. 2014;9:e105783. doi: 10.1371/journal.pone.0105783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elias N, Bou-Orm IR, Adib SM. Patterns and determinants of mammography screening in Lebanese women. Prev Med Rep. 2016;5:187–193. doi: 10.1016/j.pmedr.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung J, Macleod C, McLaughlin D, Woods LM, Henderson R, Watson A, et al. Screening mammography uptake within Australia and Scotland in rural and urban populations. Prev Med Rep. 2015;2:559–562. doi: 10.1016/j.pmedr.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Health, United States 2014: With special Feature on Adults Aged 55-64. [[Accessed April 2016 12]]. Available from: http://www.cdc.gov/nchs/data/hus/hus14.pdf#076 .
- 18.Al Mulhim FA, Syed A, Bagatadah WA, Al Muhanna AF. Breast cancer screening programme: experience from Eastern province, Saudi Arabia. East Mediterr Health J. 2015;21:111–119. doi: 10.26719/2015.21.2.111. [DOI] [PubMed] [Google Scholar]
- 19.Madadi M, Zhang S, Yeary KH, Henderson LM. Analyzing factors associated with women’s attitudes and behaviors toward screening mammography using design-based logistic regression. Breast Cancer Res Treat. 2014;144:193–204. doi: 10.1007/s10549-014-2850-9. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadian M, Samah AA. A Literature Review of Factors Influencing Breast Cancer Screening in Asian Countries A Literature Review of Factors Influencing Breast Cancer Screening in Asian Countries. Life Science Journal. 2012;9:585–594. [Google Scholar]
- 21.Bird Y, Moraros J, Banegas MP, King S, Prapasiri S, Thompson B. Breast cancer knowledge and early detection among Hispanic women with a family history of breast cancer along the U.S.-Mexico border. J Health Care Poor Underserved. 2010;21:475–88. doi: 10.1353/hpu.0.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponce NA, Tsui J, Knight SJ, Afable-Munsuz A, Ladabaum U, Hiatt RA, et al. Disparities in cancer screening in individuals with a family history of breast or colorectal cancer. Cancer. 2012;118:1656–1663. doi: 10.1002/cncr.26480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahfouz AA, Hassanein MH, Nahar S, Farheen A, Gaballah II, Mohamed A, et al. Breast cancer knowledge and related behaviors among women in Abha City, southwestern Saudi Arabia. J Cancer Educ. 2013;28:516–520. doi: 10.1007/s13187-013-0495-8. [DOI] [PubMed] [Google Scholar]
- 24.Radi SM. Breast Cancer awareness among Saudi females in Jeddah. Asian Pac J Cancer Prev. 2013;14:4307–4312. doi: 10.7314/apjcp.2013.14.7.4307. [DOI] [PubMed] [Google Scholar]
- 25.Al-Zalabani AH, Alharbi KD, Fallatah NI, Alqabshawi RI, Al-Zalabani AA, Alghamdi SM. Breast Cancer Knowledge and Screening Practice and Barriers Among Women in Madinah, Saudi Arabia. J Cancer Educ. 2016 doi: 10.1007/s13187-016-1057-7. [DOI] [PubMed] [Google Scholar]
- 26.Banegas MP, Bird Y, Moraros J, King S, Prapsiri S, Thompson B. Breast cancer knowledge, attitudes, and early detection practices in United States-Mexico border Latinas. J Womens Health (Larchmt) 2012;21:101–107. doi: 10.1089/jwh.2010.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider IJ, Corseuil MW, Boing AF, d’Orsi E. Knowledge about mammography and associated factors: population surveys with female adults and elderly. Rev Bras Epidemiol. 2013;16:930–42. doi: 10.1590/s1415-790x2013000400013. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Lee SK, Lee J, Choi MY, Jung SP, Kim MK, et al. Breast Cancer Screening Knowledge and Perceived Health Beliefs among Immigrant Women in Korea. J Breast Cancer. 2014;17:279–286. doi: 10.4048/jbc.2014.17.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian P, Oranye NO, Masri AM, Taib NA, Ahmad N. Breast cancer knowledge and screening behaviour among women with a positive family history: a cross sectional study. Asian Pac J Cancer Prev. 2013;14:6783–6790. doi: 10.7314/apjcp.2013.14.11.6783. [DOI] [PubMed] [Google Scholar]
- 30.Abdallah AS, El-Gharabawy RM, AL-Suhaibany HO. Knowledge, Attitude and Practice about Breast Cancer among Women in Saudi Arabia. Int Arch Med. 2015;8:1–13. [Google Scholar]
- 31.Abolfotouh MA, BaniMustafa AA, Mahfouz AA, Al-Assiri MH, Al-Juhani AF, Alaskar AS. Using the health belief model to predict breast self examination among Saudi women. BMC Public Health. 2015;15:1163. doi: 10.1186/s12889-015-2510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sivakumar H, Hanoch Y, Barnes AJ, Federman AD. Cognition, Health Literacy, and Actual and Perceived Medicare Knowledge Among Inner-City Medicare Beneficiaries. J Health Commun. 2016;21:155–163. doi: 10.1080/10810730.2016.1193921. [DOI] [PubMed] [Google Scholar]
- 33.Atir S, Rosenzweig E, Dunning D. When Knowledge Knows No Bounds: Self-Perceived Expertise Predicts Claims of Impossible Knowledge. Psychol Sci. 2015;26:1295–1303. doi: 10.1177/0956797615588195. [DOI] [PubMed] [Google Scholar]
- 34.Mamdouh HM, El-Mansy H, Kharboush IF, Ismail HM, Tawfik MM, El-Baky MA, et al. Barriers to breast cancer screening among a sample of Egyptian females. J Family Community Med. 2014;21:119–124. doi: 10.4103/2230-8229.134771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolma EL, Batterton C, Hamm RM, Thompson D, Engelman KK. American Indian women and screening mammography: Findings from a qualitative study in Oklahoma. Am J Health Educ. 2012;43:18–30. [Google Scholar]
- 36.Alhazmi FG. Comparison of Breast and Colorectal Cancer Screening Programs in the Netherlands and the Kingdom of Saudi Arabia. Int J Acad Sci Res. 2016;4:157–165. [Google Scholar]
- 37.Rosenbaum L. Invisible risks, emotional choices--mammography and medical decision. N Engl J Med. 2014;371:1549–1552. doi: 10.1056/NEJMms1409003. [DOI] [PubMed] [Google Scholar]


