Abstract
Objectives:
To evaluate levels of anti-carbamylated protein (anti-CarP) antibodies in rheumatoid arthritis (RA) patients and to determine their association with serological parameters and disease activity.
Methods:
A cross-sectional study involving 105 multiethnic RA patients (48 rheumatoid factor [RF]-positive and 57 RF-negative patients) was conducted at Hospital Universiti Sains Malaysia, Kelantan, Malaysia, from January 2015 to February 2016. Fifty healthy controls (HCs) were included. C-reactive protein (CRP), RF, anti-cyclic citrullinated peptide (anti-CCP) and anti-CarP antibodies were measured. A health assessment questionnaire (HAQ) was administered to the study participants and 28-joint Disease Activity Score (DAS28) were obtained.
Results:
The level of anti-CarP antibodies was significantly increased in the RA patients compared with HCs (p=0.042). The presence of anti-CarP antibodies was significantly associated with RF (p=0.019) and the HAQ (p=0.010). A significant association between the presence of anti-CarP antibodies and the DAS28 was not found (p=0.632).
Conclusion:
Our study provides further evidence that the level of anti-CarP antibodies is significantly elevated in RA patients.
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterised by progressive joint destruction and affects 0.5-1.0% of adult populations annually.1 The exact cause of RA is not yet known, although both genetic and environmental factors have been implicated as having a role in disease development.2 Of 291 conditions, RA was ranked as the 42nd highest contributor to global disability in the Global Burden of Disease 2010 study.3 Rheumatoid arthritis can be classified using the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) RA classification criteria.4 The scores of the classification system are calculated from the number and site of involved joints, abnormal serology, acute-phase reactants and symptom duration. Determination of the presence of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs) is commonly used to diagnose RA.4 Different combinations of markers are employed to improve RA diagnostic ability.5 The RF and anti-cyclic citrullinated peptide (anti-CCP) can be detected in the serum of healthy individuals years before they develop RA.6,7 RF positivity has been associated with aggressive and poorer outcomes.8,9 Likewise, ACPAs have been associated with disease severity, disability and radiological progression of the disease.10,11Anti-carbamylated protein (anti-CarP) antibodies have been extensively described in RA patients12 and their presence is associated with radiological damage.13
Antibodies to carbamylated proteins (anti-CarP antibodies) have been detected in the serum of 36-45% of RA patients.14,15 However, risk factors that have been proposed to influence the production of anti-CarP antibodies remain unsubstantiated.16 Carbamylation is a post-translational modification as a result of the conversion of amino acid lysine into homocitrulline, which the presence of cyanate is required.17,18 It has been shown that homocitrulline is present in the joints.19 In addition, elevated carbamylation have been reported in other conditions, including renal failure and chronic inflammation.17,20 Anti-CarP antibodies have been shown to be associated with the development of RA in patients with arthralgia21 and more severe radiographical progression in the total and ACPA-negative RA population.14,22 Furthermore, anti-CarP antibodies are also present in inflammatory arthritis, indicating that they potentially contribute to the development of chronic disease.23 The objectives of this study were to determine the levels of anti-CarP antibodies in RA patients and to establish whether or not there was an association with disease activity in relation to RF status.
Methods
Patient population
A cross-sectional study was performed on a cohort of 105 patients (48 RF-positive and 57 RF-negative patients) attending the rheumatology clinic at Hospital Universiti Sains Malaysia (HUSM), Kubang Kerian, Malaysia, between January 2015 and February 2016 and 50 healthy controls (comprising staff and students at USM). All patients met the 2010 ACR/EULAR classification criteria for RA.4 The RA patients and healthy controls who fulfilled the inclusion and exclusion criteria were enrolled in the study. Subjects who had been diagnosed with infectious mononucleosis, sarcoidosis, systemic lupus erythematosus and SjÖgren’s syndrome were excluded. The study protocol and written consent were approved by the ethics committee of USM according to the Declaration of Helsinki.
Laboratory tests
The presence of C-reactive protein (CRP) and RF were determined by latex agglutination using commercial latex test kits (CRP® Direct Latex and RF® Direct Latex, Veda Lab, Cerisé, France). The tests were considered positive when agglutination was observed. The level of anti-CCP antibodies in the serum of RA patients and healthy controls was quantified by enzyme-linked immunosorbent assay (ELISA) (Aeskulisa CCP®, Aesku Diagnostics, Wendelsheim, Germany). The cut-off value for a positive reaction was established as>18 U/ml, as suggested by the manufacturer.
The levels of anti-CarP antibodies in the serum of RA patients and healthy controls were determined using the OxiSelect™ Protein Carbamylation Sandwich ELISA kit (Cell Biolabs, Inc. San Diego, USA). Undiluted serum samples from the patients, together with the carbamyl-lysine (CBL)-bovine serum albumin (BSA) as the standard (using two-fold dilution), were coated on the anti-CBL antibody plate overnight (100 µl/well). After being washed, the wells were incubated with 100 µl anti-CBL antibody (1:1000 dilution), washed again, and then incubated with 100 µl horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000 dilution). The wells were then incubated with substrate solution to catalyse the enzymatic activity of the HRP (100 µl/well). The enzymatic reaction was terminated by adding a stop solution (H2SO4, 0.5N) to each well. The absorbance of both the anti-CCP and anti-CarP antibodies was measured as 450 nm using an ELISA microplate reader (Tecan Sunrise, Salzburg, Austria) and Magellan™ Data Analysis software (version 7.2).
Outcome measures
Disease activity was evaluated using the Disease Activity Score 28 (DAS28).24 Patients rated their pain from 0 (indicating no pain) to 100 (indicative of the worst pain). The counts for tender and swollen joints and the erythrocyte sedimentation rates were recorded. According to the DAS28 scores, disease activity in the RA patients was categorised as low to moderate, with a cut-off value of 3.2.25 A self-assessment questionnaire (HAQ) was administered to the 105 RA patients, who were asked about their ability to perform daily living activities. The disability index scores were determined through responses to questions within the eight categories that covered dressing and grooming, getting up, eating, walking, hygiene, reaching, grip strength and outside activity.26 According to the HAQ results, the RA patients in the study were categorised as having mild to moderate disability, with the cut-off value of >1.
Statistical analysis
The Youden index was used to establish the cut-off point for anti-CarP positivity in RA patients and the healthy controls. It was calculated using receiver operating characteristics (ROC) as described in a previous study.27 Youden index represents a biomarker’s maximum potential effectiveness when equal weight is given to both sensitivity and specificity derived from ROC curve.28 A c2 analysis was performed to determine any association between the serological and clinical parameters for the RA patients. A comparison was conducted on the anti-CarP and anti-CCP antibody levels in the RA patients and healthy controls, assessed using the Mann-Whitney U test. Data entry and statistical analysis were performed using The Statistical Package for the Social Sciences (IBM Corp., Armonk, NY, USA) version 22.
Results
Demographic data
Serum samples were collected from 105 patients with RA (89 women and 16 men; a mean age of 55.0±14.0 years and a median age of 57.0 years) and 50 healthy controls (30 women and 20 men; a mean age of 37.0±12.2 years and a median age of 32.5 years). Most of the RA patients (87.6%) were Malays (n=92/105), 9.5% were Chinese (n=10/105), 1.9% were Indians (n=2/105) and the remainder (1%) was of another race (n=1/105). The RA patients were classified as either RF positive or negative, as shown in Table 1.
Table 1.
Demographic data of rheumatoid arthritis patients and healthy controls.

The association between anti-carbamylated protein antibody level and rheumatoid arthritis patients. Increased levels of anti-CCP antibodies (p<0.001), in addition to significantly higher levels of circulating anti-CarP antibodies (p=0.042), were observed in the RA patients in comparison with those in the healthy controls following a Mann-Whitney U test (Figure 1).
Figure 1.

The levels (OD 450nm) of anti-CCP antibodies A) and anti-CarP antibodies B) in RA patients and healthy controls, RA - rheumatoid arthritis.
The correlation between anti-CarP and anti-CCP antibodies in rheumatoid arthritis patients
The levels of anti-CCP antibodies were not significantly correlated with levels of anti-CarP antibodies in our cohort of 105 RA patients (r=0.065; p=0.509) (Figure 2).
Figure 2.
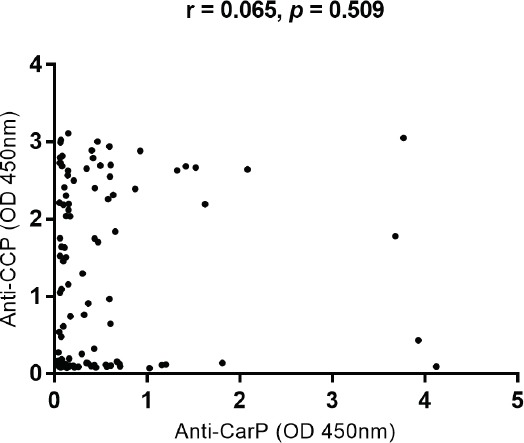
The levels of anti-CarP and anti-CCP antibodies in 105 RA patients, (r=0.065; p=0.509), nti-Carp - Antibodies to carbamylated proteins, Anti-CCp - anti-cyclic citrullinated peptide
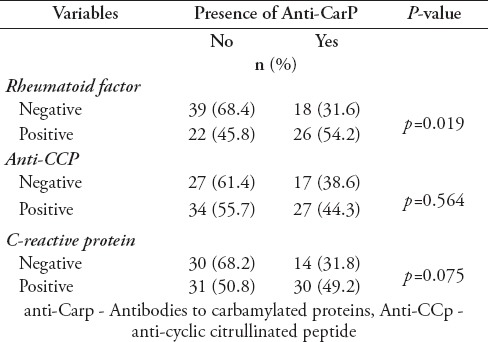
In order to compare the proportion of RA patients who were positive for anti-CarP autoantibodies with that of the healthy controls (namely, the categorical variables for both were compared), a ROC curve was generated to calculate the Youden index to establish the optimal cut-off value for anti-CarP antibody positivity. The group was classified according to positivity or negativity for anti-CarP antibodies following the determination of the highest Youden index value [the optical density (OD) cut-off value reflective of the most optimal or balanced sensitivity and specificity]. The optimal cut-off value for anti-CarP antibodies was set at OD450: 0.3380; with sensitivity of 42.0% and specificity of 78.0% when the Youden index was at its highest (Table 2). Based on the cut-off value, a higher number of RA patients in our cohort were positive for anti-CarP antibodies than that in the healthy control group (p=0.016). Anti-CarP antibodies were associated with RF (p=0.019). A significant association was not found between anti-CarP and anti-CCP antibodies. A pattern of significance was observed in relation to anti-CarP positivity and a positive CRP status (p=0.075) (Table 3).
Table 2.
Youden Index for anti-CarP antibodies.

Table 3.
Association of anti-CarP with rheumatoid factor, anti-CCP and C-reactive protein.
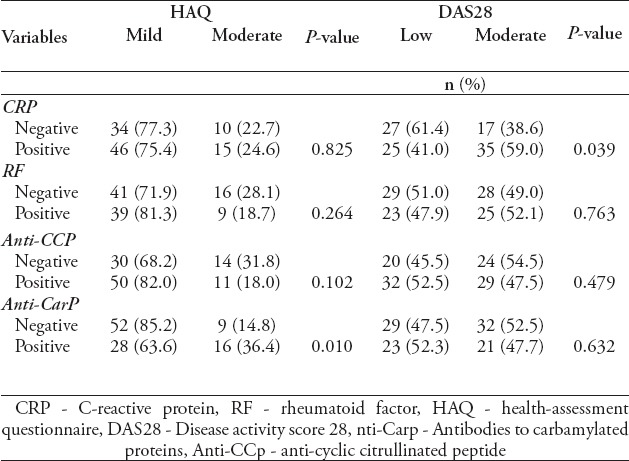
A significant association was not observed between the HAQ results and CRP (p=0.825), RF (p=0.264) and anti-CCP p=0.102. However, the presence of anti-CarP was significantly associated with the HAQ results (p=0.010). A significant association was not found between a low (< 2.6 to 3.2) or moderate (>3.2 to >5.1) DAS28 and antibodies of RF (p=0.763), anti-CCP (p=0.479) and anti-CarP (p=0.632) (Table 4). However, such an association was observed between the DAS28 and CRP status (p=0.039).
Table 4.
Association of HAQ and DAS28 with serological parameters.
Discussion
Anti-CarP antibodies were demonstrated to be present in RA patients in our study, consistent with the findings of a previous study14 in which it was reported that anti-CarP immunoglobulin (Ig)G (45%) and IgA antibodies (43%) were present in the serum of RA patients.” Elsewhere, the percentage of individuals who were positive for anti-CarP antibodies was reported to be 44.9% for RA patients and 2.3% for the controls.16 Elevated anti-CarP antibodies levels were observed (46.1%) in a recent study29 on seropositive patients with established RA (n=167), consistent with the findings of yet another study,30 in which 40-55% anti-CarP antibody positivity was seen in RA patients.
In other study, 36 of 120 RA patients (30%) were positive for anti-CarP antibodies.31 Similarly, of the 105 RA patients in our study, only 44 (41.9%) RA patients were positive for anti-CarP antibodies, with a corresponding figure of 11 (22%) for the control group. It has been reported that anti-CarP antibodies can be present in healthy individuals several years before the development of clinical RA symptoms.32,33 A significant increased incidence of anti-CarP antibodies positivity in RA patients was found in our study, when compared with that for the healthy controls (p=0.016). This is consistent with the findings of a previous study,16 in which significantly elevated levels of anti-CarP antibodies in RA patients were reported, in comparison with those for the control group (p<0.001). Increased levels of anti-CarP antibodies were demonstrated in RA patients, when compared with the healthy controls, in our study. This finding is similar to that in the study performed by Verheul et al34 in which significantly varied anti-CarP levels were observed in a Japanese RA cohort (p<0.001). A significant difference in anti-CarP antibodies between the healthy controls and RA patients was also reported elsewhere (p<0.001).14,35
In our study, we demonstrated that patients with anti-CarP-positive were more disabled than those with anti-CarP-negative. This is at least partially explained by the significant positive association between anti-CarP antibodies and the HAQ results. This is similar to the findings of another study,36 in which it was demonstrated that anti-CarP antibodies were associated with increased disability and higher disease activity in patients with inflammatory polyarthritis (n=1995). This indicates that anti-CarP antibodies might be predictive of a more severe disease course and could contribute to the pathogenesis of RA.37 The presence of anti-CarP antibodies was not significantly associated with the DAS28 results. This supports the findings of the study by Yee et al31 who reported that no individual marker (anti-CCP2, anti-CCP3, ACPA1-3, RF and anti-CarP) correlated with disease activity (as measured by the DAS28) but a significant correlation was found when ACPA1 and anti-CarP antibodies positivity were present (p=0.026). In addition, it was demonstrated in the study by Humphreys et al36 that the DAS28 score at baseline differed between anti-CarP-positive and negative patients with inflammatory polyarthritis (p<0.001).
Elsewhere, an association was not observed between the presence of anti-CarP antibodies and disease activity.38 Although RF is considered to be the main serum marker to use to diagnose RA, it was shown in one study that it did not correlate with disease activity.39 Anti-CarP status was not independently associated with remission40 and the lack of association between DAS remission and ACPA status has been previously reported.41 More studies are needed to determine the utility of anti-CarP antibodies with regard to any correlation with disease activity.
CRP has been used for ≥80 years as measure of inflammation in diseases such as RA during clinical disease activity evaluations.42 A significant association was not observed between CRP and disease activity, based on the DAS28 scores (p=0.084), in our cohort of RA patients. However, Yildirim et al43 demonstrated a significant association between CRP and the DAS28 score (p<0.001) in RA patients (n=47). The authors also showed that of the various acute phase reactant tests investigated, CRP had the closest relationship with disease activity in their cohort of RA patients.43 In contrast, a significant association was not observed between the presence of CRP and the HAQ results (p=0.825) in our cohort of RA patients, suggesting that different geographical populations of RA patients might respond differently to a HAQ evaluation or underlying genetic differences that influence CRP levels.
The participants in the control group in our study were much younger in age compared with the RA patients. This owes to the baseline screening of the health condition of the controls in order to exclude individuals with chronic illnesses such as diabetes mellitus, hypertension, acute viral infection and inflammatory conditions.
Of the 3 main ethnic groups (Malays, Chinese and Indians), RA patients in our study were mainly from Kelantan, Malaysia (located in the northeast region of Peninsular Malaysia) and were predominantly Malays (87.6%). A small proportion of other ethnic groups participated including Chinese (9.5%) and Indian RA patients (1.9%). It was demonstrated in a study by Gomez et al44 that positivity to any of the RF isotypes and anti-CCP2 in a series of 147 RA patients was almost similar across the 3 ethnic groups in Malaysia. However, IgG and IgM RF contributed to severe RA in Chinese patients, compared with that in Indian and Malay patients.45
Smoking was found to be associated with multiple autoantibody positivity (RF, anti-CCP2, and anti-CarP antibodies) in a previous multicentre cohort study (Netherlands, n=678; United Kingdom, n=761 and Sweden, n=795).46 In addition, Too et al47 found that smoking was associated with an increased risk of developing ACPA-positive RA in a multiethnic population in Malaysia.47 In contrast, African-Americans with early-onset RA did not report an association between smoking and ACPA-positive RA.48
It has been reported that anti-CarP antibodies were associated with more severe radiological damage, measured using the Sharp-van der Heijde method, when X-rays of the hands and feet of RA patients were taken.14 The authors also showed that the presence of anti-CarP antibodies was associated with more severe joint damage in the anti-CCP2-negative subgroup. The presence of anti-CarP antibodies correlated with joint erosion scores (p=0.033), whereas a correlation was not observed between the ACPA and the joint erosion scores in the RA patients (n=40).31
In conclusion, anti-CarP antibodies were found to be significantly elevated in RA patients and significantly associated with the HAQ results in our study, which provides further evidence that anti-CarP antibodies could be a potential biomarker for the diagnosis of RA in patients. However, additional studies are required using a larger, multi-institutional cohort or meta-analysis to definitively measure differences between anti-CarP levels in RA patients and those in the healthy controls, as well as any correlation with disease activity.
Footnotes
Copyright.
Whenever a manuscript contains material (tables, figures, etc.) which is protected by copyright (previously published), it is the obligation of the author to obtain written permission from the holder of the copyright (usually the publisher) to reproduce the material in Saudi Medical Journal. This also applies if the material is the authors own work. Please submit copies of the material from the source in which it was first published.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Verheul M, Fearon U, Trouw L, Veale D. Biomarkers for rheumatoid and psoriatic arthritis. Clin Immunol. 2015;161:2–10. doi: 10.1016/j.clim.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 4.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 5.Jaskowski TD, Hill HR, Russo KL, Lakos G, Szekanecz Z, Teodorescu M. Relationship between rheumatoid factor isotypes and IgG anti-cyclic citrullinated peptide antibodies. J Rheumatol. 2010;37:1582–1588. doi: 10.3899/jrheum.091236. [DOI] [PubMed] [Google Scholar]
- 6.Majka DS, Deane KD, Parrish LA, Lazar AA, Barón AE, Walker CW, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67:801–807. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielen MM, van Schaardenburg D, Reesink HW, Van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 8.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 9.Ning X, Jian Z, Wang W. Low Serum Levels of Interleukin 35 in Patients with Rheumatoid Arthritis. Tohoku J Exp Med. 2015;237:77–82. doi: 10.1620/tjem.237.77. [DOI] [PubMed] [Google Scholar]
- 10.van der Helm-van AH, Mil KNV, Breedveld FC, Toes R, Huizinga T. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R949–R58. doi: 10.1186/ar1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forslind K, Ahlmén M, Eberhardt K, Hafström I, Svensson B. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP) Ann Rheum Dis. 2004;63:1090–1095. doi: 10.1136/ard.2003.014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conigliaro P, Chimenti M, Triggianese P, Sunzini F, Novelli L, Perricone C, et al. Autoantibodies in inflammatory arthritis. Autoimmun Rev. 2016;15:673–683. doi: 10.1016/j.autrev.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Montes A, Regueiro C, Perez-Pampin E, Boveda MD, Gomez-Reino JJ, Gonzalez A. Anti-Carbamylated Protein Antibodies as a Reproducible Independent Type of Rheumatoid Arthritis Autoantibodies. PLoS One. 2016;11:e0161141. doi: 10.1371/journal.pone.0161141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GM, van Veelen PA, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci USA. 2011;108:17372–17377. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Trouw LA, van Wesemael TJ, Shi J, Bengtsson C, Källberg H, et al. Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann Rheum Dis. 2014;73:1761–1768. doi: 10.1136/annrheumdis-2013-205109. [DOI] [PubMed] [Google Scholar]
- 16.Verheul M, van Erp S, van der Woude D, Levarht E, Mallat M, Verspaget H, et al. Anti-carbamylated protein antibodies: a specific hallmark for rheumatoid arthritis. Comparison to conditions known for enhanced carbamylation;renal failure, smoking and chronic inflammation. Ann Rheum Dis. 2016;0:1–2. doi: 10.1136/annrheumdis-2016-209248. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, van Veelen PA, Mahler M, Janssen GM, Drijfhout JW, Huizinga TW, et al. Carbamylation and antibodies against carbamylated proteins in autoimmunity and other pathologies. Autoimmun Rev. 2014;13:225–230. doi: 10.1016/j.autrev.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Burska AN, Hunt L, Boissinot M, Strollo R, Ryan BJ, Vital E, et al. Autoantibodies to posttranslational modifications in rheumatoid arthritis. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/492873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mydel P, Wang Z, Brisslert M, Hellvard A, Dahlberg LE, Hazen SL, et al. Carbamylation-dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol. 2010;184:6882–6890. doi: 10.4049/jimmunol.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalim S, Karumanchi SA, Thadhani RI, Berg AH. Protein carbamylation in kidney disease: pathogenesis and clinical implications. Am J Kidney Dis. 2014;64:793–803. doi: 10.1053/j.ajkd.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J, van de Stadt LA, Levarht E, Huizinga TW, Toes RE, Trouw LA, et al. Anti–Carbamylated Protein Antibodies Are Present in Arthralgia Patients and Predict the Development of Rheumatoid Arthritis. Arthritis Rheum. 2013;65:911–915. doi: 10.1002/art.37830. [DOI] [PubMed] [Google Scholar]
- 22.Ajeganova S, Humphreys J, Verheul M, van Steenbergen H, van Nies J, Hafström I, et al. Anticitrullinated protein antibodies and rheumatoid factor are associated with increased mortality but with different causes of death in patients with rheumatoid arthritis: a longitudinal study in three European cohorts. Ann Rheum Dis. 2016;75:1924–1932. doi: 10.1136/annrheumdis-2015-208579. [DOI] [PubMed] [Google Scholar]
- 23.Chimenti MS, Triggianese P, Nuccetelli M, Terracciano C, Crisanti A, Guarino MD, et al. Auto-reactions, autoimmunity and psoriatic arthritis. Autoimmun Rev. 2015;14:1142–1146. doi: 10.1016/j.autrev.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Fransen J, Stucki G, van Riel PL. Rheumatoid arthritis measures: Disease Activity Score (DAS), Disease Activity Score-28 (DAS28), Rapid Assessment of Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis Disease Activity Index (RADAI) Arthritis Care Res. 2003;49:S214–S224. [Google Scholar]
- 25.Sahin O, Kaptanoglu E, Bakici MZ, Sezer H, Elden H, Hizmetli S. Diagnostic value of autoantibodies against citrullinated peptide antigens in rheumatoid arthritis: comparison of different commercial kits/romatoid artritte sitrulinli peptit antikorlarinin tanisal degeri: farkli ticari kitlerin karsilastirilmasi. Turk J Rheumatol. 2011;26:13–19. [Google Scholar]
- 26.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 27.Wong KK, Ch’ng ES, Loo SK, Husin A, Muruzabal MA, Møller MB, et al. Low HIP1R mRNA and protein expression are associated with worse survival in diffuse large B-cell lymphoma patients treated with R-CHOP. Exp Mol Pathol. 2015;99:537–545. doi: 10.1016/j.yexmp.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and Optimal Cut-Point Estimated from Observations Affected by a Lower Limit of Detection. Biom J. 2008;50:419–430. doi: 10.1002/bimj.200710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Challener GJ, Jones JD, Pelzek AJ, Hamilton BJ, Boire G, de Brum-Fernandes AJ, et al. Anti-carbamylated Protein Antibody Levels Correlate with Anti-Sa (Citrullinated Vimentin) Antibody Levels in Rheumatoid Arthritis. J Rheumatol. 2016;43:273–281. doi: 10.3899/jrheum.150179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scinocca M, Bell DA, Racapé M, Joseph R, Shaw G, McCormick JK, et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J Rheumatol. 2014;41:270–279. doi: 10.3899/jrheum.130742. [DOI] [PubMed] [Google Scholar]
- 31.Yee A, Webb T, Seaman A, Infantino M, Meacci F, Manfredi M, et al. Anti-CarP antibodies as promising marker to measure joint damage and disease activity in patients with rheumatoid arthritis. Immunol Res. 2015;61:24–30. doi: 10.1007/s12026-014-8560-x. [DOI] [PubMed] [Google Scholar]
- 32.Shi J, van de Stadt LA, Levarht EN, Huizinga TW, Hamann D, van Schaardenburg D, et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis. 2014;73:780–783. doi: 10.1136/annrheumdis-2013-204154. [DOI] [PubMed] [Google Scholar]
- 33.Brink M, Verheul MK, Rönnelid J, Berglin E, Holmdahl R, Toes RE, et al. Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res Ther. 2015;17:25. doi: 10.1186/s13075-015-0536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verheul MK, Shiozawa K, Levarht EN, Huizinga TW, Toes RE, Trouw LA, et al. Anti-carbamylated protein antibodies in rheumatoid arthritis patients of Asian descent. Rheumatology (Oxford) 2015;54:1930–1932. doi: 10.1093/rheumatology/kev250. [DOI] [PubMed] [Google Scholar]
- 35.Alessandri C, Bartosiewicz I, Pendolino M, Mancini R, Colasanti T, Pecani A, et al. Anti-carbamylated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients: lack of correlation with anti-cyclic citrullinated protein antibodies and rheumatoid factor. Clin Exp Rheumatol. 2015;33:824–830. [PubMed] [Google Scholar]
- 36.Humphreys JH, Verheul MK, Barton A, MacGregor AJ, Lunt M, Toes RE, et al. Anticarbamylated protein antibodies are associated with long-term disability and increased disease activity in patients with early inflammatory arthritis: results from the Norfolk Arthritis Register. Ann Rheum Dis. 2015;0:1–6. doi: 10.1136/annrheumdis-2015-207326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez G, Gómez JA, Bang H, Martínez-Gamboa L, Roggenbuck D, Burmester G-R, et al. Carbamylated vimentin represents a relevant autoantigen in Latin American (Cuban) rheumatoid arthritis patients. Rheumatol Int. 2016;36:781–791. doi: 10.1007/s00296-016-3472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albers H, Brinkman D, Kamphuis S, van Suijlekom-Smit L, van Rossum M, Hoppenreijs E, et al. Clinical course and prognostic value of disease activity in the first two years in different subtypes of juvenile idiopathic arthritis. Arthritis Care Res. 2010;62:204–212. doi: 10.1002/acr.20069. [DOI] [PubMed] [Google Scholar]
- 39.Šenolt L, Grassi W, Szodoray P. Laboratory biomarkers or imaging in the diagnostics of rheumatoid arthritis? BMC Med. 2014;12:49. doi: 10.1186/1741-7015-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barra L, Pope JE, Orav JE, Boire G, Haraoui B, Hitchon C, et al. Prognosis of seronegative patients in a large prospective cohort of patients with early inflammatory arthritis. J Rheumatol. 2014;41:2361–2369. doi: 10.3899/jrheum.140082. [DOI] [PubMed] [Google Scholar]
- 41.Nell V, Machold KP, Stamm TA, Eberl G, Heinzl H, Uffmann M, et al. Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis. 2005;64:1731–1736. doi: 10.1136/ard.2005.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowson CS, Rahman MU, Matteson EL. Which measure of inflammation to use? A comparison of erythrocyte sedimentation rate and C-reactive protein measurements from randomized clinical trials of golimumab in rheumatoid arthritis. J Rheumatol. 2009;36:1606–1610. doi: 10.3899/jrheum.081188. [DOI] [PubMed] [Google Scholar]
- 43.Yildirim K, Karatay S, Melikoglu MA, Gureser G, Ugur M, Senel K. Associations between acute phase reactant levels and disease activity score (DAS28) in patients with rheumatoid arthritis. Ann Clin Lab Sci. 2004;34:423–426. [PubMed] [Google Scholar]
- 44.Gomez EL, Gun SC, Somnath SD, D’souza B, Lim AL, Chinna K, et al. The prevalence of rheumatoid factor isotypes and anti-cyclic citrullinated peptides in Malaysian rheumatoid arthritis patients. Int J Rheum Dis. 2011;14:12–17. doi: 10.1111/j.1756-185X.2010.01573.x. [DOI] [PubMed] [Google Scholar]
- 45.Gomez EL, Gun SC, Somanath SD, Chinna K, Radhakrishnan AK. Ethnic differences in the prognostic utility of rheumatoid factor isotypes and anticyclic citrullinated peptides in rheumatoid arthritis patients: a cross-sectional study. Mod Rheumatol. 2013;23:716–721. doi: 10.1007/s10165-012-0718-6. [DOI] [PubMed] [Google Scholar]
- 46.van Wesemael TJ, Ajeganova S, Humphreys J, Terao C, Muhammad A, Symmons DP, et al. Smoking is associated with the concurrent presence of multiple autoantibodies in rheumatoid arthritis rather than with anti-citrullinated protein antibodies per se: a multicenter cohort study. Arthritis Res Ther. 2016;18:285. doi: 10.1186/s13075-016-1177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Too CL, Yahya A, Murad S, Dhaliwal JS, Larsson PT, Muhamad NA, et al. Smoking interacts with HLA-DRB1 shared epitope in the development of anti-citrullinated protein antibody-positive rheumatoid arthritis: results from the Malaysian Epidemiological Investigation of Rheumatoid Arthritis (MyEIRA) Arthritis Res Ther. 2012;14:R89. doi: 10.1186/ar3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikuls TR, Hughes LB, Westfall AO, Holers VM, Parrish L, van der Heijde D, et al. Cigarette smoking, disease severity and autoantibody expression in African Americans with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2008;67:1529–1534. doi: 10.1136/ard.2007.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]


