Abstract
Background:
Radiation therapy is among the most conventional cancer therapeutic modalities with effective local tumor control. However, due to the development of radio-resistance, tumor recurrence and metastasis often occur following radiation therapy. In recent years, combination of radiotherapy and gene therapy has been suggested to overcome this problem. The aim of the current study was to explore the potential synergistic effects of N-Myc Downstream-Regulated Gene 2 (NDRG2) overexpression, a newly identified candidate tumor suppressor gene, with radiotherapy against proliferation of prostate LNCaP cell line.
Materials and Methods:
In this study, LNCaP cells were exposed to X-ray radiation in the presence or absence of NDRG2 overexpression using plasmid PSES- pAdenoVator-PSA-NDRG2-IRES-GFP. The effects of NDRG2 overexpression, X-ray radiation or combination of both on the cell proliferation and apoptosis of LNCaP cells were then analyzed using MTT assay and flow cytometery, respectively.
Results:
Results of MTT assay showed that NDRG2 overexpression and X-ray radiation had a synergistic effect against proliferation of LNCaP cells. Moreover, NDRG2 overexpression increased apoptotic effect of X-ray radiation in LNCaP cells synergistically.
Conclusion:
Our findings suggested that NDRG2 overexpression in combination with radiotherapy may be an effective therapeutic option against prostate cancer.
Keywords: Gene Therapy , Radiation Therapy , Prostate Cancer , N-Myc Downstream-Regulated Gene 2
Introduction
Prostate cancer (PCa) is the second mostly frequent cancer in men worldwide [1]. It is estimated that PCa will overtake lung cancer as the most common cancer in men in the near future [2].
In our country, prostate cancer is also considered as an underlying health problem in male population, ranking as the second most common cancer after gastric cancer [3,4]. Despite recent advances in early detection and treatment, the emergence of recurrent hormone-refractory metastatic prostate cancer is still a major therapeutic challenge. Therefore, development of novel therapeutic strategies is desperately needed [5].
Radiotherapy is a keystone therapeutic strategy in cancer treatment including prostate carcinoma [6]. Radiation dose is a key parameter in tumor control so important that dose escalation has been shown to improve local tumor control. In prostate carcinoma; however, administration of high dose radiation is limited by safety concern including the proximal critical normal structure toxicity such as bladder and rectum [7-9]. On the other hand, development of radioresistance in cancer cells due to cell cycle deregulation, DNA damage repair mechanisms inhibition and apoptosis induction lead to local recurrence and distant metastasis after radiotherapy [10]. Over the last decade, extensive efforts have been made in radiotherapy and gene therapy combinatorial approaches. Many studies have documented that such strategies would appear to be a more logical way to overcome this problem.
N-myc downstream-regulated gene 2 (NDRG2) belongs to NDRG family which is composed of four members. So far, NDRG2 has been reported to contribute to multiple biological processes such as proliferation, differentiation, cell stress and cell cycle, neurodegeneration and embryonic development [11]. Under pathological conditions, NDRG2 functions as a tumor suppressor gene and its downregulation has been broadly observed in various human cancers including glioblastoma, breast cancer, colorectal cancer, lung cancer, liver cancer, cancer, PCa and bladder cancer [12-22]. Furthermore, accumulating lines of evidence show that the overexpression of NDRG2 is significantly correlated with favorable prognosis in several malignancies [23]. These findings suggest that NDRG2 could play a crucial role in suppressing carcinogenesis. Altogether, considering the fact that synergistic effects of specific overexpression of NDRG2 combined with radiotherapy has not still been studied in androgen-dependent prostate cancer cell lines, the aim of the current study is to explore the potential benefits of this combinatorial approach in the proliferation and apoptosis of LNCaP cell line.
Material and Methods
Cell Culture and Reagents
Human androgen-dependent LNCaP cell line was obtained from the cell bank of Pasteur Institute of Iran. Cells were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 100 unit/ml penicillin-streptomycin. Cells were cultured in tissue culture flasks at 37°C, 5% CO2 and 95% humidity atmosphere.
Cell Transfection
We previously constructed PSES-pAdenoVator-PSA-NDRG2-IRES-GFP plasmid and confirmed that it can specifically express in LNCaP cell line (Unpublished data). One LNCaP cell group was transfected with this plasmid at 75-90% confluency using Lipofectamine 3000 reagent according to the manufacturer’s instructions (Invitrogen). The second group was placed in a Plexiglas phantom providing full-scatter conditions and irradiated with a dose 8 Gy using 6 MV X-rays of an Elekta Compact linear accelerator (Elekta, UK) at a dose rate of approximately 300 cGy/min. The dosimetric stability of the linear accelerator had previously been established [24]. The relevant quality assurance tests were repeated just before cell irradiation. The third group was first transfected with PSES- pAdenoVator-PSA-NDRG2-IRES-GFP plasmid at 75-90% confluency and then subjected to radiation 24 h after transfection to investigate the synergistic effects of specific overexpression of NDRG2 combined with radiotherapy. The other group was irradiated with 8 Gy of X-ray at a dose rate of 300 cGy/min using linear accelerator. The third group was first transfected with PSES- pAdenoVator-PSA-NDRG2-IRES-GFP plasmid at 75-90% confluency and then subjected to radiation 24h after transfection to investigate the synergistic effects of specific overexpression of NDRG2 combined with radiotherapy.
MTT Assay
Cell proliferation was measured using MTT assay. In brief, LNCaP cells were seeded in an initial density of 5000 cell/well in 96-well plates in triplicate. Five days after treatments, 20 μL/well (5 mg/mL) of MTT solution [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added into each well and incubated at 37°C for 4 h. Supernatant was removed and 150 μL/ well DMSO was added to each well to stop the reaction followed by shaking for 10 min. The absorbance was detected at 490nm wavelength.
Apoptosis Analysis
LNCaP cells were seeded in 6 well plates and treated with PSES- pAdenoVator-PSA-NDRG2-IRES-GFP and/or 8 Gy X ray radiation. One cell group was transfected with PSES- pAdenoVator-PSA -IRES-GFP as mock group. Transfected LNCaP cells and mock group were trypsinized 72 h after transfection, washed with cold PBS and resuspended in PBS. AnnexinV and 7 AAD (BD Biosciences, San Jose, CA, USA) were added to the mixture containing 100 µl of cell suspension and binding buffer (BD Biosciences). The cells were then incubated for 15 min at room temperature in the dark, followed by adding 400 µl of binding buffer for flow cytometric analysis. In each experiment, unstained cells, fixed cells with formaldehyde and stained with annexin V and 7 AAD or both were used for the compensation of flow cytometer.
Statistical Analysis
All statistical analyses were carried out using SPSS 16.0 statistical software package (SPSS, Chicago, IL, USA). All represented data were expressed as mean ± S.D of at least three independent experiments. MTT results were analyzed using one-way ANOVA followed by LSD post-hoc. The results of flow cytometery for apoptosis measurements were analyzed using t-test. P values < 0.05 were considered statistically significant.
Results
Cell Transfection
For specific overexpression of GFP-tagged NDRG2 in LNcap cells, the cells were transfected with PSES- pAdenoVator-PSA-NDRG2-IRES-GFP. Green fluorescent (GFP) production was confirmed by fluorescent microscopy as shown in Figure 1.
Figure1.
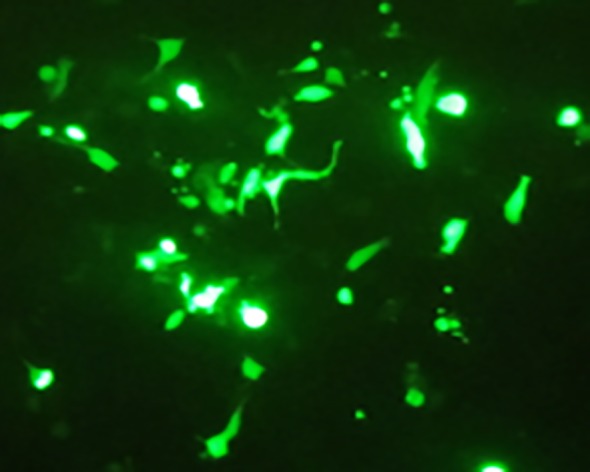
Specific overexpression of NDRG2 in LNCaP cell line.GFP expression show successful transfection and specific overexpression of NDRG2
The synergistic effect of specific overexpression of NDRG2 combined with radiotherapy against viability of LNCaP cells
The effects of specific overexpression of NDRG2 and radiotherapy, as well as, combination of both treatments on LNCaP cells viability were evaluated using MTT assay 5 days after transfection of LNCaP cells with PSES- pAdenoVator-PSA-NDRG2-IRES-GFP and/or X ray radiation as compared with transfected cells with PSES- pAdenoVator-PSA-IRES-GFP as mock group (control group). As represented in Figure 2, overexpression of NDRG2 (33.96± 4.15%) or X ray radiation (21.26± 1.43%) resulted in significant (P<0.001) inhibition of LNCaP cells’ viability as compared with mock group (95.6±1.4%). Statistical analysis using ANOVA method revealed that NDRG2 overexpression of LNCaP cells followed by X ray radiation had more inhibitory effect on cell viability (12.25±2.33%) as compared with mock (P<0.001), NDRG2 overexpression (P<0.01) or radiation alone (P<0.01) suggesting a synergistic effect of combination of NDRG2 overexpression and radiotherapy against cell viability.
Figure2.
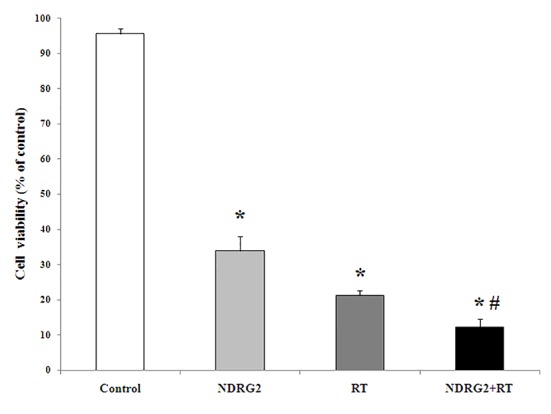
The effect of NDRG2 overexpression, radiotherapy (RT) and combination of NDRG2 overexpression and RT (NDRG2+RT group) on LNCaP cells viability. LNCaP cells were transfected with PSES-pAdenoVator-PSA-NDRG2-IRES-GFP plasmid (NDRG2 group) or plasmid without NDRG2 (Control group) in the presence or absence of X-ray radiation (8 Gy) and cell viability was evaluated using MTT assay. *P<0.001 compared to control group, # P<0.01 compared to NDRG2 group and RT group (one-way ANOVA followed with LSD post hoc test).
Synergistic apoptotic effect of NDRG2 overexpression combined with radiotherapy in LNCaP cells
AnnexinV and 7 AAD flow cytometric analyses were used to investigate the role of apoptosis in cell death inducing effects of NDRG2 overexpression, X-ray radiation or combination of both in LNCaP cells. Representative flow cytometry charts illustrating the effect of NDRG2 overexpression (Chart B), X-ray radiation (Chart C) or combination of both (Chart D) are shown in Figure 3. As can be seen in Figures 3 and Figure 4, both NDRG2 overexpression and X-ray radiation increased percentage of total cell apoptosis (early apoptotic cells + late apoptotic cells) and necrosis compared with control group. Combination of NDRG2 overexpression and X-ray radiation increased percent total cell apoptosis as well as cell necrosis compared to NDRG2 and X-ray-treated groups indicating synergistic effect of NDRG2 overexpression and radiotherapy on apoptosis of LNCaP cells.
Figure3.
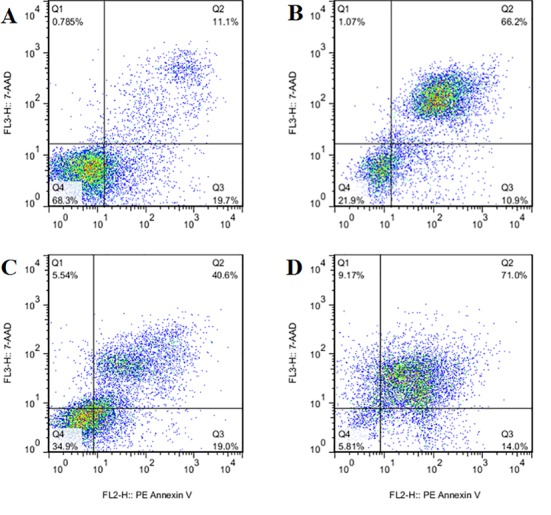
Representative flow cytometry charts illustrating percentage of live cells (Q4), early apoptotic cells (Q3), late apoptotic cells (Q2) and necrotic cells (Q1) in control cells (Chart A), cells overexpressing NDRG2 (Chart B), X-ray treated cells (Chart C) or NDRG2 overexpressing cells which treated with X-ray radiation (Chart D). As can be seen in chart D combination of NDRG2 overexpression and X-ray radiation increased percent total cell apoptosis (early apoptotic cells+ late apoptotic cells) as well as cell necrosis in a synergistic manner.
Figure4.
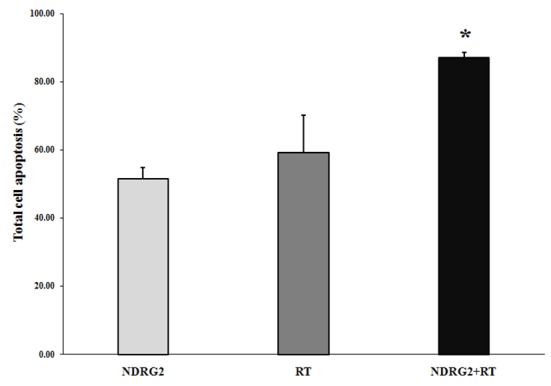
Comparison of percentage of total apoptosis between cells treated with PSES-pAdenoVator-PSA-NDRG2-IRES-GFP (NDRG2 group), X-ray radiation (RT group) or combination of both (NDRG2+RT). As results indicated combination of X-ray radiation and NDRG2 overexpression have the potential to induce apoptosis about 30% more than each of the treatment alone represented a positive synergistic effect. *P<0.05 compared to NDRG2 and RT groups (Student t-test).
Discussion
Despite recent advances in radiotherapy methods, radioresistance of cancer cells still remains a major impediment to successful cancer therapy. In the case of the prostate cancer, radiation is capable of eradicating localized prostate tumors; however, about 40% of patients with clinically localized prostate cancer relapse within 5 years after receiving curative doses of standard radiation [24]. In recent years, combined modality approaches are frequently considered in order to improve the clinical outcome and quality of life for patients with prostate cancer. Gene therapy is one of the most promising therapies which can be combined with radiation in cancer treatment. There are known mechanisms responsible for the potential benefits in such combinatorial approaches to achieve enhanced antitumor effects. Radiotherapy can improve the effect of gene therapy by increasing the efficiency of gene transfer and transgene integration [25]. Gene therapy and radiation therapy target different parts of cell cycle, so that gene therapy requires the “S” phase of cell cycle while “M” and “G2” phases are target of radiotherapy [26]. Another mechanism probably contributing to the synergistic effect of this approach is that phosphorylated prodrugs are incorporated into newly synthesized DNA leading to termination of DNA synthesis and, thus, cell death. This may increase DNA susceptibility to radiation damage. On the other hand, by incorporation into the DNA, phosphorylated prodrugs may interfere with repair of radiation-induced DNA damage. Radiation may enhance the “bystander effect” of gene therapy. This may be due to the products released from the radiation-damaged cells followed by presentation of tumor antigens by immune effector cells attracting immunocytes and mediating an antitumor response [9,28].
Numerous preclinical studies of combined radio-gene therapy have revealed synergistic effects of the combination both in vitro and in vivo [6,29,30]. The cytolytic effect of the prostate specific CV 706 virus is also synergistically enhanced when it is combined with radiotherapy in prostate cancer LNCaP cell line as compared with radiotherapy alone [31].
A line of evidence releasing from different studies confirmed reduced expression of NDRG2 as a tumor suppressor gene in a variety of tumors including prostate cancer. Furtheremore, a significant association between the overexpression of NDRG2 and inhibition of proliferation and invasiveness potential of cancer cells has been shown in several studies including our two recent studies in lung and colon tumor cell lines [16-18]. In the current study, we desined a plasmid constract that facilitated specific expression of NDRG2 in LNCaP cells. Our MTT results showed that overexpression of NDRG2 reduced the viabilty of LNCaP cells. Our results also revealed that NDRG2 overexpression could enhance cytotoxic effect of radioterapy, synergistically.
To explore the mechanism of this synergistic effect, we then detected the effects of NDRG2 overexpression and radiotherapy on cell apoptosis. The results suggest that combination of both treatment has the potential to induce apoptosis synergistically in LNCaP cells as compared to monotherapy. However, the molecular mechanisms for the induced apoptosis by combined NDRG2 and radiation remain unknown. So, further studies are encouraged to elucidate the interaction between NDRG2 and other apopototic and anti-apoptotic molecules. Furthermore, more studies will be desirable to investigate the relationship between different doses of radiation and such effects. In addition, more detailed assessment is neeeded to evaluate both in vitro and in vivo to verify such effects. Future directions include the evaluation of radio-gene therapy effects on other prostate cancer characteristics such as migration and invasion, as well as, the influence of this combination therapy in the pattern of gene expression involved in prostate cancer progression and metastasis are needed as we are planning to explore them in our future studies.
Altogether, the findings highlight the potential therapeutic benefits of combination of specific overexpression of NDRG2 and radiotherapy in LNCaP cell line as compared with monotherapy which could be a prospect for prostate cancer adenovirus gene therapy in the future.
Footnotes
Conflict of interests: None.
References
- 1.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Jain S, Saxena S, Kumar A. Epidemiology of prostate cancer in India. Meta Gene. 2014;2:596–605. doi: 10.1016/j.mgene.2014.07.007. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosseini M, SeyedAlinaghi S, Mahmoudi M, McFarland W. A case-control study of risk factors for prostate cancer in Iran. Acta Med Iran. 2010;48:61–6. [PubMed] [Google Scholar]
- 4.Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20:556–63. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 5.Akbari ME, Hosseini SJ, Rezaee A, Hosseini MM, Rezaee I, Sheikhvatan M. Incidence of genitourinary cancers in the Islamic Republic of Iran: a survey in 2005. Asian Pac J Cancer Prev. 2008;9:549–52. [PubMed] [Google Scholar]
- 6.Fujita T, Satoh T, Timme TL, Hirayama T, Zhu JX, Kusaka N, et al. Combined therapeutic effects of adenoviral vector-mediated GLIPR1 gene therapy and radiotherapy in prostate and bladder cancer models. Urologic Oncology: Seminars and Original Investigations: Elsevier; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollack A, Zagars GK. External beam radiotherapy dose response of prostate cancer. International Journal of Radiation Oncology Biology Physics. 1997;39:1011–8. doi: 10.1016/S0360-3016(97)00508-7. [DOI] [PubMed] [Google Scholar]
- 8.Zelefsky MJ, Cowen D, Fuks Z, Shike M, Burman C, Jackson A, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer. 1999;85:2460–8. doi: 10.1002/(SICI)1097-0142(19990601)85:11<2460::AID-CNCR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Teh BS, Aguilar-Cordova E, Vlachaki MT, Aguilar L, Mai WY, Caillouet J, et al. Combining radiotherapy with gene therapy (from the bench to the bedside): a novel treatment strategy for prostate cancer. Oncologist. 2002;7:458–66. doi: 10.1634/theoncologist.7-5-458. [DOI] [PubMed] [Google Scholar]
- 10.Kaliberov SA, Buchsbaum DJ. Chapter seven--Cancer treatment with gene therapy and radiation therapy. Adv Cancer Res. 2012;115:221–63. doi: 10.1016/B978-0-12-398342-8.00007-0. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao L, Zhang J, Liu X. NDRG2: a Myc-repressed gene involved in cancer and cell stress. Acta Biochim Biophys Sin (Shanghai) 2008;40:625–35. doi: 10.1111/j.1745-7270.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 12.Tepel M, Roerig P, Wolter M, Gutmann DH, Perry A, Reifenberger G, et al. Frequent promoter hypermethylation and transcriptional downregulation of the NDRG2 gene at 14q11. 2 in primary glioblastoma. Int J Cancer 2008;123:2080–6. doi: 10.1002/ijc.23705. [DOI] [PubMed] [Google Scholar]
- 13.Liu N, Wang L, Liu X, Yang Q, Zhang J, Zhang W, et al. Promoter methylation, mutation, and genomic deletion are involved in the decreased NDRG2 expression levels in several cancer cell lines. Biochem Biophys Res Commun. 2007;358:164–9. doi: 10.1016/j.bbrc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 14.Lorentzen A, Vogel LK, Lewinsky RH, Saebo M, Skjelbred CF, Godiksen S, et al. Expression of NDRG2 is down-regulated in high-risk adenomas and colorectal carcinoma. BMC Cancer. 2007;7:192. doi: 10.1186/1471-2407-7-192. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi H, Jin H, Chu D, Wang W, Zhang J, Chen C, et al. Suppression of N-myc downstream-regulated gene 2 is associated with induction of Myc in colorectal cancer and correlates closely with differentiation. Biol Pharm Bull. 2009;32:968–75. doi: 10.1248/bpb.32.968. [DOI] [PubMed] [Google Scholar]
- 16.Golestan AM, Mojtahedi ZP, Ghalamfarsa GP, Hamidinia MM, Takhshid MAP. The Effects of NDRG2 Overexpression on Cell Proliferation and Invasiveness of SW48 Colorectal Cancer Cell Line. Iran J Med Sci. 2015;40:430–9. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SJ, Wang WY, Li B, Chen B, Zhang B, Wang X, et al. Expression of NDRG2 in human lung cancer and its correlation with prognosis. Med Oncol. 2013;30:421. doi: 10.1007/s12032-012-0421-7. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraji SN, Mojtahedi Z, Ghalamfarsa G, Takhshid MA. N-myc downstream regulated gene 2 overexpression reduces matrix metalloproteinase-2 and -9 activities and cell invasion of A549 lung cancer cell line in vitro. Iran J Basic Med Sci. 2015;18:773–9. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu XL, Liu XP, Lin SX, Deng YC, Liu N, Li X, et al. NDRG2 expression and mutation in human liver and pancreatic cancers. World J Gastroenterol. 2004;10:3518–21. doi: 10.3748/wjg.v10.i23.3518. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao L, Wu GJ, Liu XW, Zhang R, Yu L, Zhang G, et al. Suppression of invasion and metastasis of prostate cancer cells by overexpression of NDRG2 gene. Cancer Lett. 2011;310:94–100. doi: 10.1016/j.canlet.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Ren GF, Tang L, Yang AQ, Jiang WW, Huang YM. Prognostic impact of NDRG2 and NDRG3 in prostate cancer patients undergoing radical prostatectomy. Histol Histopathol. 2014;29:535–42. doi: 10.14670/HH-29.10.535. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Yu C, Jiang F, Gao L, Li J, Wang Y, et al. Overexpression of N-Myc downstream-regulated gene 2 (NDRG2) regulates the proliferation and invasion of bladder cancer cells in vitro and in vivo. PLoS One. 2013;8:e76689. doi: 10.1371/journal.pone.0076689. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz EM, Hanks GE. External beam radiation therapy for prostate cancer. CA Cancer J Clin. 2000;50:349–75; quiz 769. doi: 10.3322/canjclin.50.6.349. [DOI] [PubMed] [Google Scholar]
- 24.Mosleh-Shirazi MA, S Rahimi, S Karbasi, Medium-Term Stability of the Photon Beam Energy of An Elekta CompactTM Linear Accelerator Based on Daily Measurements of Beam Quality Factor. Iranian Journal of Medical Physics. 2016;12(4):230–234. [Google Scholar]
- 25.Zeng M, Cerniglia GJ, Eck SL, Stevens CW. High-efficiency stable gene transfer of adenovirus into mammalian cells using ionizing radiation. Hum Gene Ther. 1997;8:1025–32. doi: 10.1089/hum.1997.8.9-1025. [DOI] [PubMed] [Google Scholar]
- 26.Yaowen Z, Yongzhen C, Jin L, Qin W. Gene therapy and radiotherapy in malignant tumor. International Journal of Radiation Medicine and Nuclear Medicine. 2008;32:247–50. [Google Scholar]
- 27.Simons JW, Marshall FF. The future of gene therapy in the treatment of urologic malignancies. Urol Clin North Am. 1998;25:23–38. doi: 10.1016/S0094-0143(05)70430-4. [DOI] [PubMed] [Google Scholar]
- 28.Najafi M, Fardid R, Takhshid MA, Mosleh-Shirazi MA, Rezaeyan AH, Salajegheh A. Radiation-induced oxidative stress at out-of-field lung tissues after pelvis irradiation in rats. Cell J. 2016; 18(3): 340–345. doi: 10.22074/cellj.2016.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chhikara M, Huang H, Vlachaki MT, Zhu X, Teh B, Chiu KJ, et al. Enhanced therapeutic effect of HSV-tk+GCV gene therapy and ionizing radiation for prostate cancer. Mol Ther. 2001;3:536–42. doi: 10.1006/mthe.2001.0298. [DOI] [PubMed] [Google Scholar]
- 30.Lohr F, Hu K, Haroon Z, Samulski TV, Huang Q, Beaty J, et al. Combination treatment of murine tumors by adenovirus-mediated local B7/IL12 immunotherapy and radiotherapy. Mol Ther. 2000;2:195–203. doi: 10.1006/mthe.2000.0114. [DOI] [PubMed] [Google Scholar]
- 31.Kaliberov SA, Kaliberova LN, Buchsbaum DJ. Combined ionizing radiation and sKDR gene delivery for treatment of prostate carcinomas. Gene Ther. 2005;12:407–17. doi: 10.1038/sj.gt.3302432. [DOI] [PubMed] [Google Scholar]


