Abstract
Background:
About 30% of stroke survivors clinically have depressive symptoms at some point following stroke and anxiety prevalence is around 20-25%.
Objective:
The purpose of this brief report is to evaluate a pilot trial of a constructive integrative psychosocial intervention (CIPI) over standard care in post-stroke depression or anxiety.
Methods:
Patients were randomly assigned to either CIPI (n = 23) or standard care (n = 19). Patients were assessed using the Hospital Anxiety and Depression Scale at the 1st, 3rd, and 6th months to monitor changes of mood.
Results:
A Wilcoxon signed-rank test indicated that compared to admission baseline, patients with the intervention had significantly normal post-stroke depression symptom levels at the 1st, 3rd, and 6th months (P < 0.005).
Conclusion:
CIPI appears to be of incremental value in treating depression as well as anxiety in subacute care.
Keywords: Anxiety symptoms, depressive symptoms, post-stroke anxiety, post-stroke depression
Introduction
Post-stroke depression (PSD) and post-stroke anxiety (PSA) are common serious occurrences with stroke experience.1 About 30% of stroke survivors clinically have PSD symptoms at some point following stroke2 and PSA prevalence is around 20-25%.3 PSD leads to 3.4-7 times higher mortality rate than is seen among patients without PSD.4
Furthermore, both PSD and PSA have a negative impact on recovery. Depression is associated with poor functional recovery in activities of daily living,4 physical function,5 cognitive recovery, i.e. memory, and problem solving in both the short- and long-term.6 Anxiety is associated with poor self-control, immobility, and fatigue.7 It can compromise rehabilitation outcome and negatively impact the quality of life after stroke.3 Comorbid depression and anxiety worsen the prognosis and the severity of depressive symptoms.8 Upon diagnosis, antidepressant pharmacotherapy is commonly used for intervention.9 By itself, it is not always adequate to address the psychosocial needs of stroke patients, whereas psychosocial interventions can help patients to learn strategies to moderate stress responses and overcome adjustment issues after stroke.10
Anxiety and depression may also co-occur in patient populations with stroke experience, and in two systematic literature reviews, PSD has been positively correlated with PSA.3 This would suggest a need for a value-added treatment that could address both conditions in patients to enhance functional recovery and ease the burden of patient care. A psychosocial intervention that addresses patient-oriented sensemaking of the stroke experience in the context of recovery supports would carry great potential for use in subacute rehabilitation care settings in managing both PSD and PSA symptoms.
Prospective psychosocial intervention for PSD and PSA
Psychosocial intervention may have a role in managing PSD and PSA through enhancing patient perceived control. The current evidence on its efficacy in reducing PSD symptom, however, is relatively limited.11-13 This also applies to PSA symptoms.14,15 PSA symptoms can be lowered by a multifactorial risk factor management program, as well as problem-solving therapy, cognitive behavioral therapy, or relaxation training.15-18
Considerable psychosocial factors have been found to increase the risk of depression.19 There is not perfect agreement on the list, but it includes: Psychiatric history, social factors such as “little social contact” and “living alone,” functional impairment,18 younger age (<59 years), being female,20 or a family history of psychiatric conditions.21 The complexity of the psychosocial factors indicates that a comprehensive psychosocial intervention may be effective in addressing those factors.
Comprehensive psychosocial interventions are those that result in a positive construction of experience of illness of illness by patients and significant others. This addresses their cognitions related to living with stroke and the related behavioral response to the stroke experience. The key qualities include evidence-supported components of psychosocial-behavioral intervention,22 life review,23 and education.24 The components that are known to enhance function recovery and coping after stroke are summarized in Table 1.
Table 1.
Components of a CIPI

This pilot study sought to establish the efficacy of a constructive integrative psychosocial intervention (CIPI) enhancement of standard care to reduce depression symptoms and/or anxiety symptoms in patients with PSD and PSA. We hypothesized that the CIPI enhanced care would reduce both PSD and PSA symptoms more than standard care alone. The following question guided the research: What is the prospective efficacy of CIPI compared to enhanced standard care in reducing post-stroke depression and anxiety symptoms?
Methods
Participants and setting
A total of 42 patients agreed to participate in the study including both male and female patients. The age range was from 30 to 75 years. Inclusion criteria were patients with depressive and anxiety symptoms who were (1) above 18 years old; (2) had a clinically diagnosed new stroke within a week (including hemorrhagic stroke, infarcts, and transient ischemic attack); (3) spoke either English or Mandarin; and (4) had satisfactory mental status. Exclusion criteria were (1) patients discharged to a nursing home and (2) other non-stroke-related neurological conditions such as brain tumor or traumatic brain injuries. Patients were randomized into CIPI intervention (n = 23) and standard care (n = 19). For all patients, this was the first episode of stroke. No patients in the study had a prior history of psychiatric impairment or prior neurological deficit. All participants’ age ranges from 30 to 75 years old (male patients: n = 25, 60%; female patients: n = 17, 40%).
All the participants were recruited from the acute stroke inpatient unit in one Singapore tertiary hospital, and the duration of the study was 1 year. Permission for the study was granted by SingHealth Centralized Institutional Review Board (reference number 2010/294/A). All patients gave individual consent for participation in the study.
Research design
The study design is outlined in Figure 1. It used a randomized control group in an acute stroke unit with pretest–posttest design. The data observation points of data based on previous related clinical studies22,24,25 were at 4 different times: Admission (baseline), the 1st month, the 3rd month, and the 6th month. At the 6th month, 7 (35%) and 5 (26%) had dropped out from CIPI, respectively, for patients with depressive symptoms and anxiety symptoms, but the dropout numbers were as high as 11 (65%) and 10 (56%) from standard care.
Figure 1.
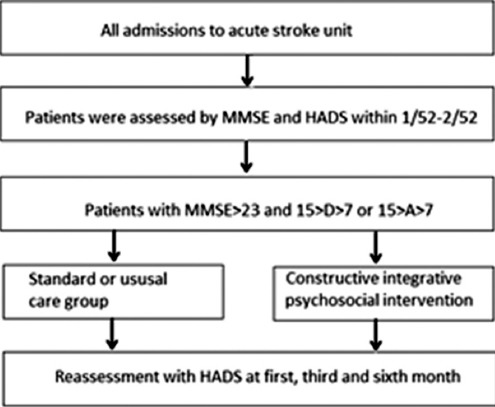
Study design - process and protocol for data collection
Instruments
The Mini-Mental Status Examination (MMSE) was utilized to screen for eligibility to participate in this study premised on adequate cognitive functioning (no or mild impairment). The MMSE is a brief 20-item measure commonly used to assess cognition impairment in stroke research.26 MMSE scores are categorized into three levels: 24-30 - no impairment; 18-23 - mild impairment; and 0-17 - severe impairment.27
Patients were assessed for anxiety and depressive symptoms using the Hospital Anxiety and Depression Scale (HADS). This is suitable for use in primary care settings with general population patients28 and has adequate reliability as a clinical screening tool.29 Patients with a score of 8 and above for depressive or anxiety symptoms were positive for PSD or PSA symptoms and selected for this current study. It was used to monitor mood changes before and after intervention.
Procedure
Human ethics approval for the study was granted by the Changi General Hospital, Singapore. The patients individually consented for the study in writing.
Analysis
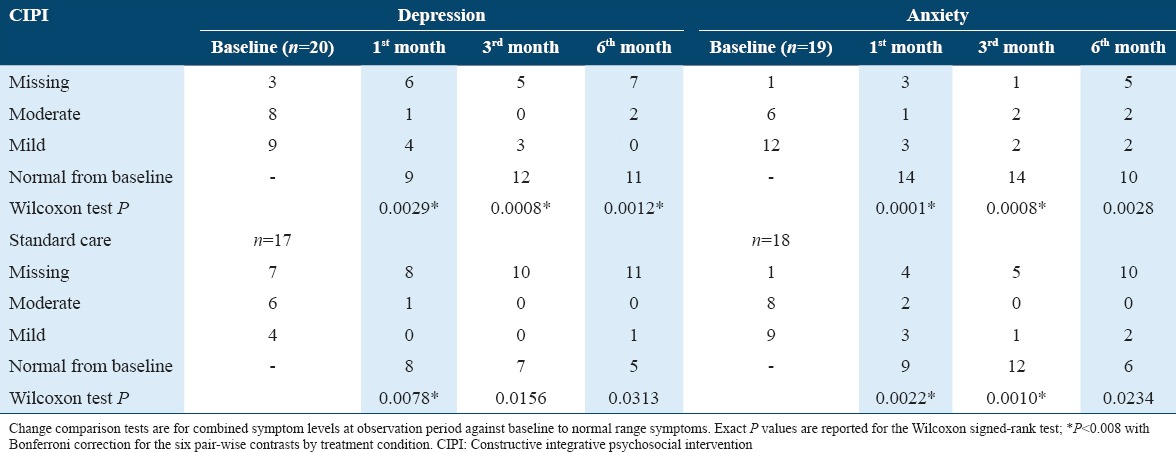
Variables such as age, gender, and activities of daily functioning were included in the analysis. Non-parametric statistical techniques are used to assess for changes in recovery from PSD or PSA with intervention or standard care against the baseline enrollment status. This experimental design allowed for six pair-wise comparisons of change in symptom status (moderate or mild to normal) by diagnosis (PSD vs. PSA) and treatment condition (CIPI enhanced standard care vs. standard care alone). A Wilcoxon signed-rank test was performed using GraphPad Prism (version 6.00 for Windows). P values were two-sided and P < 0.05 was considered as statistically significant, and P < 0.008 with Bonferroni correction was used for the six pair-wise contrasts by treatment condition.
Results
Within group comparison for depressive symptoms in CIPI
A Wilcoxon signed-rank test indicated that compared to admission baseline, patients in CIPI had significantly lower PSD symptoms at the 1st, 3rd, and 6th months (P < 0.005). This is illustrated in Figure 2. The result of Wilcoxon signed-rank test as well as the significance value was presented in Table 2.
Figure 2.
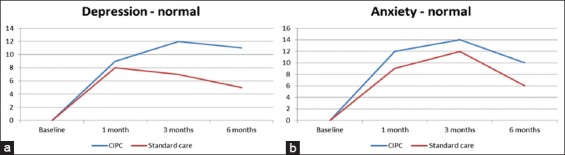
(a and b) Normal levels of depression and anxiety under standard care and constructive integrative psychosocial intervention at baseline, 1st, 3rd, and 6th months
Table 2.
Treatment outcomes with CIPI enhanced standard care compared to standard care over the treatment period
Within group comparison for anxiety symptoms in CIPI
Patients with CIPI enhanced care indicated significant changes in PSA over the 6-month treatment period compared to admission baseline: At 1st month and at 3rd month (P < 0.05) and more improvement in CIPI enhanced care (75%) comparing to standard care (64%) alone over the treatment period (P < 0.05) (Figures 2 and 3). Regardless of treatment group assignment, all patients eventually had similar level of the activities of daily living function at the 6th month.
Figure 3.
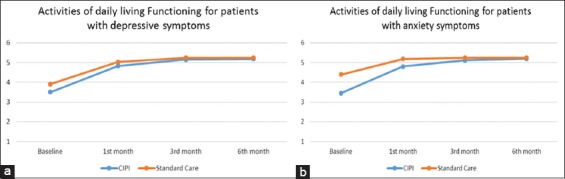
(a and b) Mean of activities of daily living functioning in standard care and constructive integrative psychosocial intervention over 6 months (raw data; 1 - dependent; 2 - maximum assistance; 3 - moderate assistance; 4 - minimal assistance; 5 - supervision; 6 - independence)
Between group comparisons CIPI versus standard care
Table 2 presents the PSD and PSA scores by treatment condition over the 6-month period. To assist interpretation of this table, an example is that at the 6th month of treatment, the percentage of improvement of patients with post-stroke depression treated with CIPI against baseline was small (11 out 13 or 85%) versus 5 out 6 (or 83%) treated with standard care. The proportional gains of patients with PSD recovery in CIPI at the 6th month compared with 1st-month status were higher than those in standard care (i.e., from 9 out of 14 to 11 out of 13 or a change of 21% in CIPI versus 8 out of 9 to 5 out of 6 or a change of −7% in standard care).
The change of depressive symptoms in standard care alone was significant at the 1st month (P = 0.0078), but not at the later stage, suggesting a relatively early plateauing of treatment effects with standard care alone. At the 6th month of treatment, the percentage of improvement of patients with PSD recovery against baseline was small (11 out 13, 85% vs. 5 out 6, 83%). However, the proportional gains of patients with PSD recovery at the 6th month were higher among patients with CIPI enhanced standard care compared to standard care only (i.e., from 9 out 14 to 11 out of 13 with a change of 21% vs. from 8 out 9 to 5 out of 6 with a change of −7%, respectively, P < 0.05), suggesting significant later onset treatment effects with CIPI enhancement.
Discussion and Conclusions
This is the first study to explore a holistic intervention – CIPI to manage PSD symptoms as well as PSA symptoms. Findings suggest possible enhanced recovery effects of CIPI in acute care rehabilitation from patients’ active engagement in treatment care such as importance of storytelling, illness self-management, and build up strength and coping.10
Findings of an early and marked decline in depression and anxiety symptoms in the 1st month suggest patient’s sensitivity to recovery effects in the immediate period post-morbidity.30 Nonetheless, adjustment after stroke is a process of adapting gradually to the changes in life after stroke. Patients can adjust better and improve over time.10,31-33 With a holistic approach like CIPI, patients are possible to continue to improve even after the 1st month.
The CIPI enhanced standard care has comparatively earlier recovery onset for PSA recovery compared to standard care alone. This suggests that patients with PSA may be more responsive to psychosocial-oriented intervention compared to those with PSD in the first 1 month. CIPI enhanced standard care appears to have superior patient recovery rates for both PSD and PSA post-discharge (or with outpatient status), compared to standard care alone. This effect would be expected with psychoeducation-focused psychosocial interventions aimed at reinforcing the patient’s perceived sense of control that can play an important role in stress and coping to manage living after stroke.34 Thus, what the findings from this study suggest is that CIPI enhanced care appears to be of incremental benefit to relief PSD and anxiety symptoms beyond what patients would experience with standard care in control group.
Limitations of this study include the small sample size per condition as well as high dropout rate observed in the study and which constrained its power to detect treatment effects that might be present. Future studies should seek to utilize a larger sample to detect any differences in treatment effects that may be masked with small sample sizes. Patients with both premorbid psychiatric conditions were excluded from the study. Future studies should include these patients to determine treatment effects on them. Furthermore, the fact that the internal consistency indices for both HADS-A and HADS-D were low to moderate in the study sample (0.42 and 0.62, respectively) suggests that there may be cultural influences in the reporting of symptoms by the Singapore patients which the HADS may not be sensitive to. Future studies should seek to use an outcome measure validated in the context of Singapore treatment setting. In the absence of such a measure, HADS scores should be supplemented with evidence from qualitative interview with the patients and activity records in so far as they may contribute to valid inferences regarding their recovery from depression or anxiety with stroke experience.
Another limitation was that the severity of neurological deficits was not monitored and recorded. Although the severity of neurological deficit was not monitor in this study, but the patients in intervention group require more assistance in ADL function comparing to patients in the intervention group. Similarly, the site of lesion was not taken into account. In a similar vein, there was no control for cerebrovascular risk factors such as hypertension. Recovery within types of stroke was not addressed by this study so that it remains unclear as to the efficacy of CIPI between and within stroke conditions. Despite these limitations, importantly, findings of the study suggest that a CIPI to augment standard care is of likely benefit PSD and PSA recovery in subacute rehabilitation care setting.
Acknowledgment
This study was preliminary to the first listed author’s PhD degree candidacy at the University of Sydney. The helpful comments of an anonymous reviewer are gratefully acknowledged.
Financial support
This research received research grants from Changi General Hospital, Singapore.
References
- 1.Kneebone II, Jeffries FW. Treating anxiety after stroke using cognitive-behaviour therapy: Two cases. Neuropsychol Rehabil. 2013;23:798–810. doi: 10.1080/09602011.2013.820135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis C, Zhao Y, Egede LE. Depression and increased risk of death in adults with stroke. J Psychosom Res. 2010;68:545–51. doi: 10.1016/j.jpsychores.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell Burton CA, Murray J, Holmes J, Astin F, Greenwood D, Knapp P. Frequency of anxiety after stroke: A systematic review and meta-analysis of observational studies. Int J Stroke. 2013;8:545–59. doi: 10.1111/j.1747-4949.2012.00906.x. [DOI] [PubMed] [Google Scholar]
- 4.Pohjasvaara T, Vataja R, Leppävuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol. 2001;8:315–9. doi: 10.1046/j.1468-1331.2001.00182.x. [DOI] [PubMed] [Google Scholar]
- 5.Schubert DS, Taylor C, Lee S, Mentari A, Tamaklo W. Physical consequences of depression in the stroke patient. Gen Hosp Psychiatry. 1992;14:69–76. doi: 10.1016/0163-8343(92)90028-9. [DOI] [PubMed] [Google Scholar]
- 6.Naess H, Lunde L, Brogger J, Waje-Andreassen U. Depression predicts unfavourable functional outcome and higher mortality in stroke patients: The bergen stroke study. Acta Neurol Scand Suppl. 2010;190:34–8. doi: 10.1111/j.1600-0404.2010.01373.x. [DOI] [PubMed] [Google Scholar]
- 7.Rachman S. Emotional processing. Behav Res Ther. 1980;18:51–60. doi: 10.1016/0005-7967(80)90069-8. [DOI] [PubMed] [Google Scholar]
- 8.Morrison V, Pollard B, Johnston M, MacWalter R. Anxiety and depression 3 years following stroke: Demographic, clinical, and psychological predictors. J Psychosom Res. 2005;59:209–13. doi: 10.1016/j.jpsychores.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Wannagat W, Zielasek J, Gaebel W. Therapy of post-stroke depression-a systematic review. Psychiatrie. 2013;10:108–29. [Google Scholar]
- 10.Kirkevold M, Bronken BA, Martinsen R, Kvigne K. Promoting psychosocial well-being following a stroke: Developing a theoretically and empirically sound complex intervention. Int J Nurs Stud. 2012;49:386–97. doi: 10.1016/j.ijnurstu.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Watkins CL, Auton MF, Deans CF, Dickinson HA, Jack CI, Lightbody CE, et al. Motivational interviewing early after acute stroke: A randomized, controlled trial. Stroke. 2007;38:1004–9. doi: 10.1161/01.STR.0000258114.28006.d7. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers J, Noreau L, Rochette A, Carbonneau H, Fontaine L, Viscogliosi C, et al. Effect of a home leisure education program after stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2007;88:1095–100. doi: 10.1016/j.apmr.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Lincoln NB, Flannaghan T. Cognitive behavioral psychotherapy for depression following stroke: A randomized controlled trial. Stroke. 2003;34:111–5. doi: 10.1161/01.str.0000044167.44670.55. [DOI] [PubMed] [Google Scholar]
- 14.Graham CD, Gillanders D, Stuart S, Gouick J. An Acceptance and Commitment Therapy (ACT)-based intervention for an adult experiencing post-stroke anxiety and medically unexplained symptoms. Clin Case Stud. 2014;4:83–97. [Google Scholar]
- 15.Ihle-Hansen H, Thommessen B, Fagerland MW, Oksengård AR, Wyller TB, Engedal K, et al. Effect on anxiety and depression of a multifactorial risk factor intervention program after stroke and TIA: A randomized controlled trial. Aging Ment Health. 2014;18:540–6. doi: 10.1080/13607863.2013.824406. [DOI] [PubMed] [Google Scholar]
- 16.Kneebone I, Walker-Samuel N, Swanston J, Otto E. Relaxation training after stroke: Potential to reduce anxiety. Disabil Rehabil. 2014;36:771–4. doi: 10.3109/09638288.2013.808275. [DOI] [PubMed] [Google Scholar]
- 17.Mikami K, Jorge RE, Moser DJ, Arndt S, Jang M, Solodkin A, et al. Prevention of post-stroke generalized anxiety disorder, using escitalopram or problem-solving therapy. J Neuropsychiatry Clin Neurosci. 2014;26:323–8. doi: 10.1176/appi.neuropsych.11020047. [DOI] [PubMed] [Google Scholar]
- 18.Ng KC, Chan KL, Straughan PT. A study of post-stroke depression in a rehabilitative center. Acta Psychiatr Scand. 1995;92:75–9. doi: 10.1111/j.1600-0447.1995.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 19.Ouimet MA, Primeau F, Cole MG. Psychosocial risk factors in poststroke depression: A systematic review. Can J Psychiatry. 2001;46:819–28. doi: 10.1177/070674370104600905. [DOI] [PubMed] [Google Scholar]
- 20.Poynter B, Shuman M, Diaz-Granados N, Kapral M, Grace SL, Stewart DE. Sex differences in the prevalence of post-stroke depression: A systematic review. Psychosomatics. 2009;50:563–9. doi: 10.1176/appi.psy.50.6.563. [DOI] [PubMed] [Google Scholar]
- 21.Tenev VT, Robinson RG, Jorge RE. Is family history of depression a risk factor for poststroke depression? Meta-analysis. Am J Geriatr Psychiatry. 2009;17:276–80. doi: 10.1097/JGP.0b013e3181953b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell PH, Teri L, Veith R, Buzaitis A, Tirschwell D, Becker K, et al. Living well with stroke: Design and methods for a randomized controlled trial of a psychosocial behavioral intervention for poststroke depression. J Stroke Cerebrovasc Dis. 2008;17:109–15. doi: 10.1016/j.jstrokecerebrovasdis.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis MC. Life review therapy as an intervention to manage depression and enhance life satisfaction in individuals with right hemisphere cerebral vascular accidents. Issues Ment Health Nurs. 2004;25:503–15. doi: 10.1080/01612840490443455. [DOI] [PubMed] [Google Scholar]
- 24.Kendall E, Catalano T, Kuipers P, Posner N, Buys N, Charker J. Recovery following stroke: The role of self-management education. Soc Sci Med. 2007;64:735–46. doi: 10.1016/j.socscimed.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulos GS, Wilkins VM, Marino P, Kanellopoulos D, Reding M, Sirey JA, et al. Ecosystem focused therapy in poststroke depression: A preliminary study. Int J Geriatr Psychiatry. 2012;27:1053–60. doi: 10.1002/gps.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narushima K, Chan KL, Kosier JT, Robinson RG. Does cognitive recovery after treatment of poststroke depression last? A 2-year follow-up of cognitive function associated with poststroke depression. Am J Psychiatry. 2003;160:1157–62. doi: 10.1176/appi.ajp.160.6.1157. [DOI] [PubMed] [Google Scholar]
- 27.Tombaugh TN, McIntyre NJ. The mini-mental state examination: A comprehensive review. J Am Geriatr Soc. 1992;40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 28.White D, Leach C, Sims R, Atkinson M, Cottrell D. Validation of the hospital anxiety and depression scale for use with adolescents. Br J Psychiatry. 1999;175:452–4. doi: 10.1192/bjp.175.5.452. [DOI] [PubMed] [Google Scholar]
- 29.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 30.Kirkevold M, Martinsen R, Bronken BA, Kvigne K. Promoting psychosocial wellbeing following stroke using narratives and guided self-determination: A feasibility study. BMC Psychol. 2014;2:4. doi: 10.1186/2050-7283-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyon BL. Psychological stress and coping: Framework for poststroke psychosocial care. Top Stroke Rehabil. 2002;9:1–15. doi: 10.1310/YA8Q-EQK9-00EF-EUG7. [DOI] [PubMed] [Google Scholar]
- 32.Mast BT, Vedrody S. Poststroke depression: A biopsychosocial approach. Curr Psychiatry Rep. 2006;8:25–33. doi: 10.1007/s11920-006-0078-z. [DOI] [PubMed] [Google Scholar]
- 33.Taylor GH, Todman J, Broomfield NM. Post-stroke emotional adjustment: A modified social cognitive transition model. Neuropsychol Rehabil. 2011;21:808–24. doi: 10.1080/09602011.2011.598403. [DOI] [PubMed] [Google Scholar]
- 34.Baltes MM, Baltes PB. The Psychology of Control and Aging (Psychology Revivals) Hove, UK: Psychology Press; 2014. [Google Scholar]


