Abstract
The host immune system is the first line of host defense, consisting mainly of innate and adaptive immunity. Immunity must be maintained, orchestrated, and harmonized, since overactivation of immune responses can lead to inflammation and autoimmune diseases, while immune deficiency can lead to infectious diseases. We investigated the regulation of innate and adaptive immune cell activation by Artemisia capillaris and its components (ursolic acid, hyperoside, scopoletin, and scopolin). Macrophage phagocytic activity was determined using fluorescently labeled Escherichia coli, as an indicator of innate immune activation. Concanavalin A (ConA)- and lipopolysaccharide (LPS)-induced splenocyte proliferation was analyzed as surrogate markers for cellular and humoral adaptive immunity, respectively. Neither A. capillaris water extract (WAC) nor ethanol extract (EAC) greatly inhibited macrophage phagocytic activity. In contrast, WAC suppressed ConA- and LPS-induced proliferation of primary mouse splenocytes in a dose-dependent manner. Similarly, EAC inhibited ConA- and LPS-induced splenocyte proliferation. Oral administration of WAC in mice decreased ConA- and LPS-induced splenocyte proliferation, while that of EAC suppressed LPS-induced splenocyte proliferation. Repeated administration of WAC in mice inhibited ConA- and LPS-induced splenocyte proliferation. Ursolic acid, scopoletin, and scopolin reduced ConA- and LPS-induced primary mouse splenocyte proliferation, while hyperoside did not show such activity. These results indicate that A. capillaris and its components, ursolic acid, scopoletin, and scopolin, suppress ConA- and LPS-induced adaptive immune cell activation. The results suggest that A. capillaris is useful as a regulator of adaptive immunity for diseases involving excessive immune response activation.
Keywords: Immunosuppression, Innate immunity, Adaptive immunity, Natural product, Immune therapy
INTRODUCTION
Innate immune responses are the first line of defense against antigen invasion and include phagocytic activity and antigen presentation, which guide and determine the adaptive immune response to antigen challenge. Host-pathogen interactions are typically initiated through host recognition of conserved molecular structures, known as pathogen-associated molecular patterns (PAMPs), which are essential for the pathogen life cycle. However, these PAMPs are deficient or compartmentalized in the host cell and are detected by the host’s germline-encoded pattern recognition receptors (PRRs), which are expressed on the surface of innate immune cells, such as dendritic cells, macrophages, and neutrophils (1). Effective PAMP detection facilitates pathogen eradication by rapidly inducing host immune responses through the activation of complex signaling pathways to induce cytokine and chemokine-mediated inflammatory responses (2,3). A characteristic of adaptive immunity is the induction of a target effector response in two steps using antigen-specific receptors in T and B cells. First, antigens are presented to and recognized by antigen-specific T or B cells, leading to cell priming, activation, and differentiation, which usually occur in specific lymphoid tissue environments. Secondly, the activated T cells leave the lymphatic tissue at the infected site and return to the disease site, or the effector reaction occurs due to antibody release from activated B cells (plasma cells) into the blood and tissue fluid (4). Overactivation of the immune system can lead to inflammation and autoimmune disease. On the contrary, when the immune system is suppressed, it becomes vulnerable to infection and tumor development. It is therefore important to maintain an immune balance.
Artemisia capillaris is an herbal plant well-known for its various beneficial properties. It has been widely used as a hepatoprotective, analgesic, and antipyretic agent (5). Many studies have shown that A. capillaris has various biological activities, such as hypoglycemic (6), hypolipidemic (7), anti-inflammatory (8), and anti-carcinogenic (9) effects. However, its immune-modulatory properties have not been fully examined. Therefore, we investigated whether a water or ethanol extract of A. capillaris and its components had immunosuppressive effects by modulating innate and adaptive immune cell activation using in vitro and ex vivo models. Our results may provide important information regarding immune balance regulation when consuming natural plant sources.
MATERIALS AND METHODS
Preparation of A. capillaris Thunb extracts
Whole A. capillaris plants were purchased from a local retailer and authenticated by Prof. J. H. Lee (Dongguk University, Kyeongju, Korea). The voucher specimen (No. 20090920) was deposited at the laboratory of Prof. J. S. Choi, a coauthor of this study. Whole plants were dried and ground into powder. The dried powder (each 100.0 g) was then separately refluxed with 70% (v/v) aqueous ethanol and water for 3 hr (2 × 0.5 L) at 95°C. The total filtrate was then concentrated to dryness in vacuo at 40°C to yield a 70% ethanol extract (EAC, 18.0 g, yield: 18.0%) and a water extract (WAC, 22.0 g, yield: 22.0%), respectively, which were then used in the pharmacological study.
Animals and cell culture
Male Balb/c mice, 7 weeks old, were obtained from Orientbio Inc (Seongnam, Gyeonggi, Korea). Animals were housed in groups of 5 per cage under standard animal housing conditions (23°C, 12 hr/12 hr light/dark cycle, light on at 08:00) in the animal facility at the Catholic University of Korea. Mice were allowed to acclimatize for at least 1 week before experiments. Cell culture procedures were performed as previously described (10). Briefly, primary splenocytes isolated from mice and a murine monocytic cell line (RAW264.7; ATCC TIB-71, American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained in a humidified atmosphere under 5% CO2 at 37°C.
Phagocytosis assays
This was performed as described previously (11). RAW264.7 cells were plated in 96-well plates at 1 × 105 cells/well and incubated overnight. To quantify phagocytosis, cells were incubated with fluorescently-labeled Escherichia coli (K-12) BioParticles (Life Technologies Inc., Grand Island, NY, USA). Engulfed particles were enumerated after 1 hr by measuring fluorescence emission per well at 480 nm.
Splenocyte proliferation assay
This was performed as described previously (11). Primary splenocytes were isolated from Balb/c mouse spleens. Cells were seeded in 96-well plates at 1 × 104 cells/well in complete DMEM. After stimulation with concanavalin A (ConA, 5 μg/mL) or lipopolysaccharide (LPS, 10 μg/mL), methylthiazol tetrazolium (MTT) assays (Sigma-Aldrich, St. Louis, MO, USA) were performed. MTT formazan product was determined using a microplate reader at an absorbance of 560 nm (Molecular Devices, San Francisco, CA, USA).
Histological assessment
Spleens were isolated from mice and fixed in 10% buffered formalin solution, dehydrated in ethanol, embedded in paraffin wax, sectioned, and stained with hematoxylin and eosin.
Statistical analyses
Data are presented as mean ± SEM for the indicated numbers. Data comparisons between groups were examined using a student’s t-test (significant when p < 0.05).
RESULTS
A. capillaris does not suppress macrophage phagocytic activity
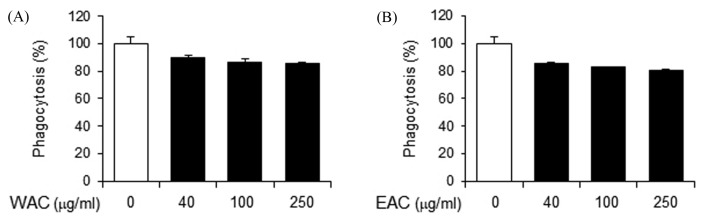
To investigate the effects of A. capillaris on innate immunity, WAC and EAC were prepared and their effects on macrophage phagocytic activity were determined as a representative indicator of innate immune response. Phagocytic activity was determined by the uptake of fluorescently-labeled E. coli in a mouse monocytic cell line (RAW 264.7). WAC treatment resulted in a slight decrease in phagocytic activity, by 20% maximum (Fig. 1A). EAC treatment reduced phagocytic activity to a similar degree (Fig. 1B). These results indicate that A. capillaris extracts do not significantly affect macrophage activity.
Fig. 1.
Artemisia capillaris does not suppress phagocytic activity of macrophages. RAW264.7 cells were treated with (A) a water extract (WAC) or (B) an ethanol extract (EAC) of A. capillaris. Phagocytic activity was measured as the uptake of fluorescently-labeled Escherichia coli (K-12). Data are mean ± SEM (n = 3).
A. capillaris suppresses ConA- and LPS-induced proliferation of primary mouse splenocytes
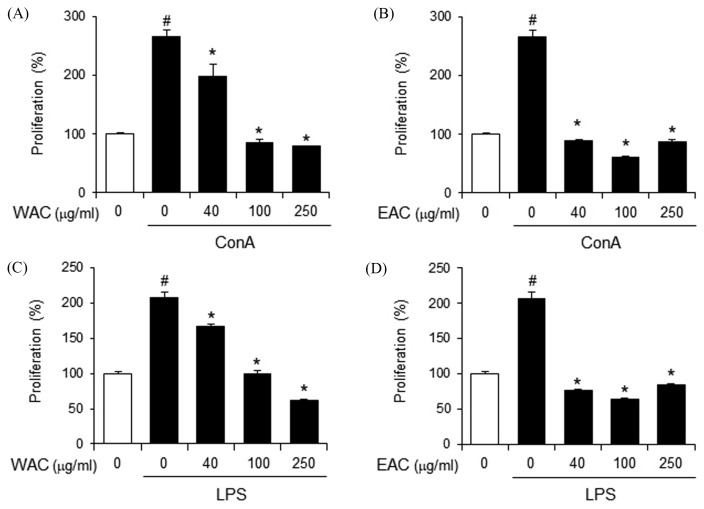
Next, we investigated the effect of A. capillaris on the activation of adaptive immune cells, such as T and B lymphocytes. ConA is known as a T lymphocyte division promoter whereas LPS is a B lymphocyte-activating factor. Therefore, ConA- and LPS-induced splenocyte proliferation was determined as parameters for cellular and humoral immunity, respectively. WAC decreased both ConA- and LPS-induced proliferation of primary mouse splenocytes in a dose-dependent manner (Fig. 2A and 2B). Similarly, EAC suppressed ConA- and LPS-induced splenocyte proliferation more potently than WAC (Fig. 2C and 2D). These results indicate that A. capillaris suppresses T and B lymphocyte activation, suggesting that A. capillaris treatment leads to a reduction in adaptive immune response, including cellular and humoral immune response.
Fig. 2.
Artemisia capillaris suppresses concanavalin A- and LPS-induced proliferation of primary mouse splenocytes. Primary splenocytes from Balb/c mice were stimulated with (A), (B). concanavalin A (ConA; 5 μg/mL) or (C), (D). lipopolysaccharide (LPS; 10 μg/mL) in the presence or absence of a (A), (C). water extract (WAC) or (B), (D). an ethanol extract (EAC) of A. capillaris for 24 hr. Cellular proliferation was measured by methylthiazol tetrazolium (MTT) assay. Data are mean ± SEM (n = 3). #Significantly different from vehicle alone, p < 0.05. *Significantly different from ConA or LPS alone, p < 0.05. Veh, vehicle.
Oral administration of A. capillaris extract suppresses ConA- and LPS-induced splenocyte proliferation
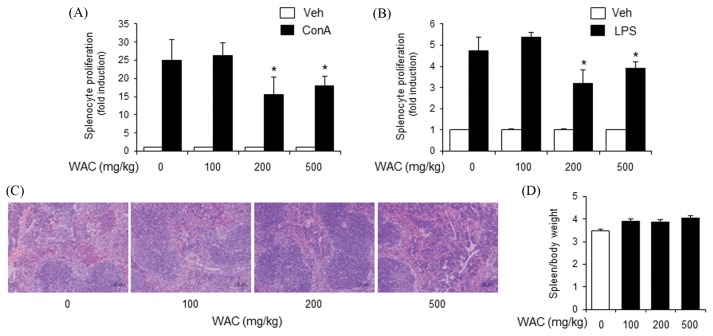
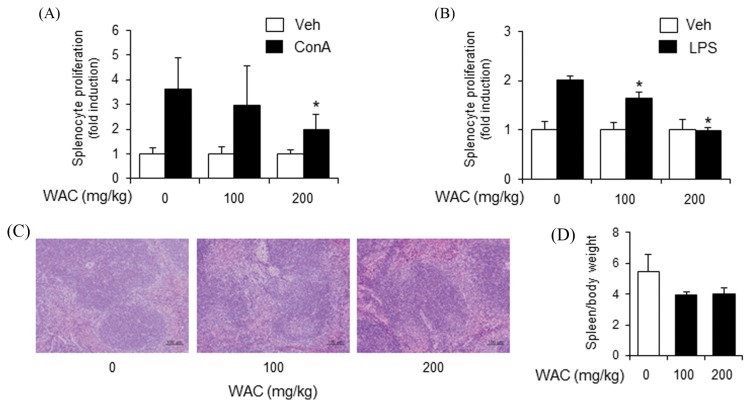
To confirm the inhibitory activity of A. capillaris on adaptive immunity, a single dose of A. capillaris extracts was orally administered to mice. Mouse splenocytes were isolated to examine whether A. capillaris intake affected ConA- or LPA-induced proliferation. WAC at 200 and 500 mg/kg reduced both ConA- and LPS-induced primary splenocyte proliferation, while WAC at 100 mg/kg did not inhibit splenocyte proliferation (Fig. 3A, 3B). Histological assessment of the mouse spleens did not show pathological changes in the WAC-treated groups (Fig. 3C). The spleen weight to body weight ratio slightly increased in the WAC-treated groups (Fig. 3D). However, no dose-response relationship was observed (Fig. 3D).
Fig. 3.
Oral administration of Artemisia capillaris water extract reduces concanavalin A- and LPS-induced proliferation of primary mouse splenocytes. Balb/c mice were orally administered a water extract (WAC) of A. capillaris. (A), (B). After 24 hr, splenocytes were isolated and stimulated with concanavalin A (ConA; 5 μg/mL) or lipopolysaccharide (LPS; 10 μg/mL). Cellular proliferation was measured by methylthiazol tetrazolium (MTT) assay. (C) Spleens were stained with hematoxylin and eosin. (D) Spleen weight per body weight. Data are mean ± SEM (n= 3). *Significantly different from ConA or LPS alone, p < 0.05. Veh, vehicle.
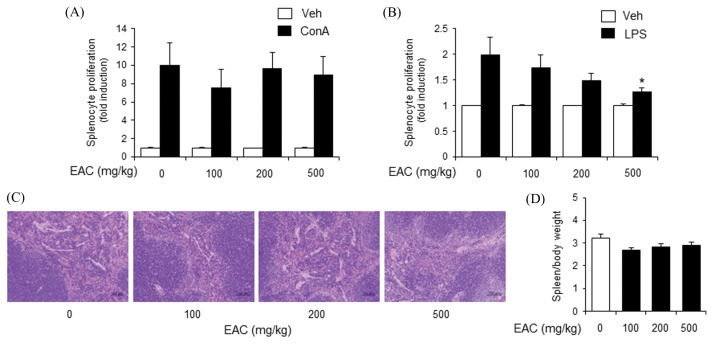
EAC treatment did not inhibit ConA-induced primary splenocyte proliferation, but did reduce LPS-induced splenocyte proliferation (Fig. 4A, 4B). There were no significant histopathological changes in mouse spleens from the EAC-treated groups (Fig. 4C). The spleen weight to body weight ratio slightly decreased in the EAC-treated groups (Fig. 4D). However, there was no observable dose-response relationship (Fig. 4D).
Fig. 4.
Oral administration of Artemisia capillaris ethanol extract decreases LPS-induced primary mouse splenocyte proliferation. Balb/c mice were orally administered an ethanol extract (EAC) of A. capillaris. (A), (B). After 24 hr, splenocytes were isolated and stimulated with concanavalin A (ConA; 5 μg/mL) or lipopolysaccharide (LPS; 10 μg/mL). Cellular proliferation was measured by methylthiazol tetrazolium (MTT) assay. (C) Spleens were stained with hematoxylin and eosin. (D) Spleen weight per body weight. Data are mean ± SEM (n = 3). *Significantly different from ConA or LPS alone, p < 0.05. Veh, vehicle.
We next investigated the effects of multiple A. capillaris exposures on adaptive immunity. Since inhibition was more potent with WAC treatment than with EAC treatment, WAC was orally administered to mice once per day for 14 days. Splenocytes were isolated and stimulated with ConA or LPS. ConA and LPS-induced proliferation was significantly attenuated by repeated administration of WAC (Fig. 5A, 5B). Histological evaluation of mouse spleen showed no noticeable changes in the treatment groups (Fig. 5C). The spleen weight to body weight ratio decreased in the EAC-treated groups (Fig. 5D). However, this decrease was not dose-dependent (Fig. 5D).
Fig. 5.
Repeated administration of Artemisia capillaris water extract suppresses concanavalin A- and LPS-induced primary mouse splenocyte proliferation. Balb/c mice were orally administered a water extract (WAC) of A. capillaris once per day for 14 days. (A), (B). Splenocytes were isolated and stimulated with concanavalin A (ConA; 5 μg/mL) or lipopolysaccharide (LPS; 10 μg/mL). Cellular proliferation was measured by methylthiazol tetrazolium (MTT) assay. (C) Spleens were stained with hematoxylin and eosin. (D) Spleen weight per body weight. Data are mean ± SEM (n = 3). *Significantly different from ConA or LPS alone, p < 0.05. Veh, vehicle.
These results indicate that orally administered A. capillaris results in the suppression of cellular and humoral adaptive immunity, without affecting spleen integrity.
Ursolic acid, scopoletin, and scopolin suppress ConA-and LPS-induced splenocyte proliferation
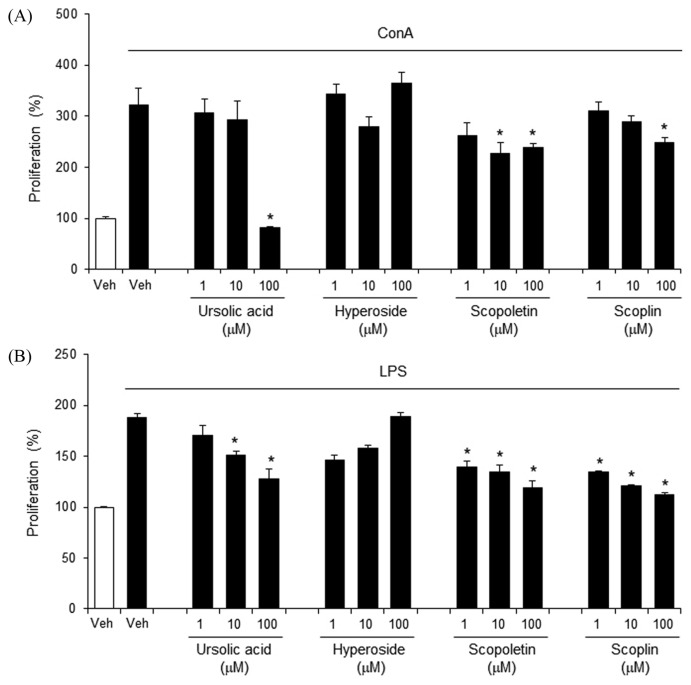
We investigated whether A. capillaris components would affect splenocyte proliferation. Ursolic acid, scopoletin, and scopolin inhibited ConA-induced primary splenocyte proliferation, whereas hyperoside did not (Fig. 6A). Similarly, ursolic acid, scopoletin, and scopolin suppressed LPS-induced splenocyte proliferation, whereas hyperoside did not show a dose-dependent inhibition (Fig. 6B). These results indicate that ursolic acid, scopoletin, and scopolin inhibit T and B lymphocyte activation, suggesting that these compounds inhibit cellular and humoral adaptive immune responses.
Fig. 6.
Ursolic acid and scopoletin suppresses concanavalin A- and LPS-induced proliferation of primary mouse splenocytes. (A), (B). Primary splenocytes isolated from Balb/c mice were stimulated with concanavalin A (ConA; 5 μg/mL) or lipopolysaccharide (LPS; 10 μg/mL) in the presence or absence of ursolic acid, hyperoside, scopoletin, and scopolin for 24 hr. Cellular proliferation was measured by methylthiazol tetrazolium (MTT) assay. Data are mean ± SEM (n = 3). *Significantly different from ConA or LPS alone, p < 0.05. Veh, vehicle.
DISCUSSION
Immunosuppressive agents are a mainstay treatment for organ graft patients, and are becoming increasingly important in the treatment of autoimmune diseases (12). The use of immunosuppressive drugs is essential in cases of solid organ transplantation because it prevents an immune response against the graft, or delays the appearance of de novo baseline disease. The most frequently used drugs act on pathways that inhibit T cell proliferation and activation, the main mechanisms involved in rejection (13). Calcineurin inhibitors, such as cyclosporin A and tacrolimus, are the most commonly used treatments. Cyclosporin A emerged as an alternative to azathioprine, triggering an important advance in medical transplantation (14). These drugs inhibit the calcineurin pathway, preventing nuclear factor dephosphorylation in active T lymphocytes and subsequent transfer to the nucleus, ultimately blocking upregulation of genes involved in T cell activation, and consequently, immune response (15). Immunosuppressive drug therapy, although necessary after transplantation, has many adverse consequences, including side effects induced by secondary metabolite formation. Calcineurin inhibitors are associated with nephrotoxicity, cardiotoxicity and neurotoxicity; moreover, they increase the risk of many diseases after transplantation (16). Therefore, novel immune suppressants sourced from widely-used, traditional medicinal plants could be beneficial in providing clinically useful and safe treatments, with fewer side effects (17). Our results indicate that A. capillaris and its components (ursolic acid, scopoletin, scopolin) inhibit adaptive immune cell activation. These results suggest the potential use of A. capillaris and its components as immunosuppressants for diseases involving excessive activation of the adaptive immune system.
The genus Artemisia (family Asteraceae) includes over 500 species, and is found in Europe, North America, and mainly in Asia (18). Among them, A. capillaris is known in Chinese medicine as “Yin Chen Hao” (medicinal term “Injin”), and is the most important herb in East Asia, especially in Korea and China (19). The active components identified in A. capillaris extracts are caffeic acid, chlorogenic acid, peroxides, isocuritol, isochlorogenic acid A, and scopolin. Among them, chlorogenic acid, an ester of caffeic acid and quinic acid, has been reported as a main component of A. capillaris extract (20). In addition, various compounds have been reported including coumarins (scoparone, scopolin), flavonoids (isorhamnetin, quercetin, isoquercitrin, hyperoside), chromones (capillarisin, 7-methylcapillarisin), phenylpropanoids (caffeic acid, chlorogenic acid, caffeoylquinic acids), lignans ((+)-sesamin, pluviatide, honokiol). and essential oils (β-pinene, β-caryophyllene, capillene) (5,21–24). Among these different constituents, we evaluated four compounds, including ursolic acid, hyperoside, scopoletin and scopolin (25–28), as modulators of adaptive immunity. Jang et al. (29) used response surface methodology to optimize the A. capillaris extraction parameters (extraction temperature, extraction time, and ethanol concentration) for obtaining an extract with high anti-inflammatory activity at the cellular level. The ranges used for determining the optimal extraction conditions were extraction temperatures of 57~65°C, ethanol concentrations of 45~57%, and extraction times of 5.5~6.8 hr (29). Yun et al. (30) investigated the 13-week subchronic toxicity and genotoxicity of the A. capillaris extract. In the 13-week toxicity study, using dosages of 25, 74, 222, 667, and 2,000 mg/kg body weight, oral A. capillaris extract administration in male and female rats did not result in any significant adverse effects on food/water consumption, body weight, mortality, hematology, serum biochemistry, organ weight, and histopathology. Accordingly, the no-observed-adverse-effect level in rats of both genders was established for the A. capillaris extract at 2,000 mg/kg/day, the highest dose level tested. Yun et al. (30) demonstrated that the A. capillaris extract is considered safe for human consumption.
Collectively, our results demonstrate that A. capillaris and its active components suppress adaptive immune cell activation in in vitro and ex vivo systems. These results suggest that A. capillaris and its active components may be useful as, or in the development of, novel immunosuppressive treatments.
ACKNOWLEDGMENTS
We thank Eunshil Jeong and Jae Hyun Jun for their technical assistance. This study was supported by a grant from Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2017R1A6A3A11032822 to G. Y.).
Abbreviations
- ConA
Concanavalin A
- LPS
lipopolysaccharide
- WAC
Artemisia capillaris water extract
- EAC
Artemisia capillaris water ethanol extract
- PAMPs
pathogen-associated molecular patterns
- PRRs
pattern recognition receptors
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- MTT
methylthiazol tetrazolium
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 5.Cha JD, Jeong MR, Jeong SI, Moon SE, Kim JY, Kil BS, Song YH. Chemical composition and antimicrobial activity of the essential oils of Artemisia scoparia and A. capillaris. Planta Med. 2005;71:186–190. doi: 10.1055/s-2005-837790. [DOI] [PubMed] [Google Scholar]
- 6.Twaij HA, Al-Badr AA. Hypoglycemic activity of Artemisia herba alba. J Ethnopharmacol. 1988;24:123–126. doi: 10.1016/0378-8741(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Chae K, Ha J, Park BY, Lee HS, Jeong S, Kim MY, Yoon M. Regulation of obesity and lipid disorders by herbal extracts from Morus alba, Melissa officinalis, and Artemisia capillaris in high-fat diet-induced obese mice. J Ethnopharmacol. 2008;115:263–270. doi: 10.1016/j.jep.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Kim EK, Kwon KB, Han MJ, Song MY, Lee JH, Lv N, Choi KB, Ryu DG, Kim KS, Park JW, Park BH. Inhibitory effect of Artemisia capillaris extract on cytokine-induced nitric oxide formation and cytotoxicity of RINm5F cells. Int J Mol Med. 2007;19:535–540. [PubMed] [Google Scholar]
- 9.Kim YS, Bahn KN, Hah CK, Gang HI, Ha YL. Inhibition of 7,12-dimethylbenz[a]anthracene induced mouse skin carcinogenesis by Artemisia capillaris. J Food Sci. 2008;73:T16–T20. doi: 10.1111/j.1750-3841.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Lee BH, Lee JY. Gambogic acid disrupts toll-like Receptor4 activation by blocking lipopolysaccharides binding to myeloid differentiation factor 2. Toxicol Res. 2015;31:11–16. doi: 10.5487/TR.2015.31.1.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang G, Seo EK, Lee JH, Lee JY. Suppression of splenic lymphocyte proliferation by Eucommia ulmoides and genipin. Chem Biodivers. 2015;12:538–546. doi: 10.1002/cbdv.201400376. [DOI] [PubMed] [Google Scholar]
- 12.Ader JL, Rostaing L. Cyclosporin nephrotoxicity: pathophysiology and comparison with FK-506. Curr Opin Nephrol Hypertens. 1998;7:539–545. doi: 10.1097/00041552-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 14.Williams D, Haragsim L. Calcineurin nephrotoxicity. Adv Chronic Kidney Dis. 2006;13:47–55. doi: 10.1053/j.ackd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Kuklina EM, Shirshev SV. Role of transcription factor NFAT in the immune response. Biochemistry Mosc. 2001;66:467–475. doi: 10.1023/A:1010238931555. [DOI] [PubMed] [Google Scholar]
- 16.Mika A, Stepnowski P. Current methods of the analysis of immunosuppressive agents in clinical materials: A review. J Pharm Biomed Anal. 2016;127:207–231. doi: 10.1016/j.jpba.2016.01.059. [DOI] [PubMed] [Google Scholar]
- 17.Cho KS, Lim Y-r, Lee K, Lee J, Lee JH, Lee I-S. Terpenes from Forests and Human Health. Toxicol Res. 2017;33:97–106. doi: 10.5487/TR.2017.33.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doh EJ, Paek S-H, Lee G, Lee M-Y, Oh S-E. Application of partial internal transcribed spacer sequences for the discrimination of Artemisia capillaris from other Artemisia species. Evid Based Complement Alternat Med. 2016;2016:7043436. doi: 10.1155/2016/7043436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim DW, Kim YT, Jang YJ, Kim YE, Han D. Anti-obesity effect of Artemisia capillaris extracts in high-fat diet-induced obese rats. Molecules. 2013;18:9241–9252. doi: 10.3390/molecules18089241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha H, Lee H, Seo CS, Lim HS, Lee JK, Lee MY, Shin H. Artemisia capillaris inhibits atopic dermatitis-like skin lesions in Dermatophagoides farinae-sensitized Nc/Nga mice. BMC Complement Altern Med. 2014;14:100. doi: 10.1186/1472-6882-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KM, Li Y, Kim B, Zhang H, Hwangbo K, Piao DG, Chi MJ, Woo MH, Choi JS, Lee JH, Moon DC, Chang HW, Kim JR, Son JK. High-performance liquid chromatographic analysis for quantitation of marker compounds of Artemisia capillaris Thunb. Arch Pharm Res. 2012;35:2153–2162. doi: 10.1007/s12272-012-1213-5. [DOI] [PubMed] [Google Scholar]
- 22.Sheu SJ, Chieh CL, Weng WC. Capillary electrophoretic determination of the constituents of Artemisiae Capillaris Herba. J Chromatogr A. 2001;911:285–293. doi: 10.1016/S0021-9673(01)00513-1. [DOI] [PubMed] [Google Scholar]
- 23.Wu TS, Tsang ZJ, Wu PL, Lin FW, Li CY, Teng CM, Lee KH. New constituents and antiplatelet aggregation and anti-HIV principles of Artemisia capillaris. Bioorg Med Chem. 2001;9:77–83. doi: 10.1016/S0968-0896(00)00225-X. [DOI] [PubMed] [Google Scholar]
- 24.Hong JH, Lee JW, Park JH, Lee IS. Antioxidative and cytoprotective effects of Artemisia capillaris fractions. Biofactors. 2007;31:43–53. doi: 10.1002/biof.5520310105. [DOI] [PubMed] [Google Scholar]
- 25.Jyoti MA, Nam KW, Jang WS, Kim YH, Kim SK, Lee BE, Song HY. Antimycobacterial activity of methanolic plant extract of Artemisia capillaris containing ursolic acid and hydroquinone against Mycobacterium tuberculosis. J Infect Chemother. 2016;22:200–208. doi: 10.1016/j.jiac.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Lee JY, Kwon YI, Jang HD. Anti-osteoclastic activity of Artemisia capillaris thunb. extract depends upon attenuation of osteoclast differentiation and bone resorption-associated acidification due to chlorogenic acid, hyperoside, and scoparone. Int J Mol Sci. 2017;18:322. doi: 10.3390/ijms18020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi BR, Kumar SK, Zhao C, Zhang LT, Kim CY, Lee SW, Jeon JH, Soní KK, Kim SH, Park NC, Kim HK, Park JK. Additive effects of Artemisia capillaris extract and scopoletin on the relaxation of penile corpus cavernosum smooth muscle. Int J Impot Res. 2015;2015;27:225–232. doi: 10.1038/ijir.2015.23. [DOI] [PubMed] [Google Scholar]
- 28.Ali MY, Jannat S, Jung HA, Choi RJ, Roy A, Choi JS. Anti-Alzheimer’s disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis. Asian Pac J Trop Med. 2016;9:103–111. doi: 10.1016/j.apjtm.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Jang M, Jeong SW, Kim BK, Kim JC. Extraction optimization for obtaining Artemisia capillaris extract with high anti-inflammatory activity in RAW 264.7 macrophage cells. Biomed Res Int. 2015;2015:872718. doi: 10.1155/2015/872718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun JW, Kim SH, Kim YS, You JR, Cho EY, Yoon JH, Kwon E, Ahn JH, Jang JJ, Che JH, Kang BC. A comprehensive study on in vitro and in vivo toxicological evaluation of Artemisia capillaris. Regul Toxicol Pharmacol. 2017;88:87–95. doi: 10.1016/j.yrtph.2017.05.010. [DOI] [PubMed] [Google Scholar]