Abstract
Background:
There is a two-way relationship between periodontal disease and diabetes. The purpose of this study was to evaluate the clinical and metabolic effects of a xanthan-based chlorhexidine (CHX) gel used as an adjunct to nonsurgical periodontal therapy in Type II diabetic patients with chronic periodontitis.
Materials and Methods:
Sixty-eight diabetic patients with moderate to advanced periodontitis and glycated hemoglobin (HbA1c) ≥6% were selected. The test group (n = 34) received scaling and root planning (SRP) plus xanthan-based CHX gel. The control group (n = 34) received single SRP. Fasting blood sugar (FBS) and HbA1c tests were done at the baseline and after 3 and 6 months. Data from the study were analyzed using descriptive statistics (mean ± standard deviation and frequency), ANOVA test by SPSS.15 software (SPSS Inc. Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results:
Patients in test group exhibited a decrease in FBS from the baseline (227 ± 64.97) to the 3 and 6 months follow-up (208 ± 61.95 and 201 ± 61.33; P < 0.001). HbA1cb levels decreased from 7.72 ± 0.99 to 6.20 ± 0.97 and 6.06 ± 1.04 after 3 and 6 months follow-up (P < 0.001), respectively. Reduction of FBS and HbA1c was statistically significant after 3 and 6 months in the control group (P < 0.001).
Conclusion:
Considering the limitations of this study, the application of CHX gel might improve the effects of nonsurgical periodontal treatment in diabetic patients with periodontitis.
Key Words: Chlorhexidine, chronic periodontitis, glycated hemoglobins, scaling, root planing, diabetes mellitus Type 2
INTRODUCTION
Periodontal disease is a periodontal inflammatory process, in which the primary etiologic factor may be microbiologic, systemic, or physical injury. It is a condition that affects and destroys the attachment apparatus.[1] Periodontal disease is best considered as the outcome of an ongoing host-parasite interaction between pathogenic microorganisms that have colonized the periodontal pocket and host tissues that resist such bacteria or their products.[2] Many systemic diseases and disorders have been introduced as risk indicator or risk factor in periodontal disease. One such example is diabetes mellitus.[3] Type 2 diabetes mellitus (DM2), the most common type of diabetes, is characterized by hyperglycemia, hyperlipidemia, and associated complications.[4]
One of the leading complication of diabetes is periodontitis, an infection of the periodontal supporting tissues.[5] There is an interacting, complex relationship between diabetes and periodontitis. Many studies have shown a greater incidence and severity of periodontitis in diabetic patients.[6] Meanwhile, a number of studies have suggested that periodontitis may actually be a risk factor for diabetic complications as well.[7,8] Southerland et al.[9] proposed some common pathogenesis involving an increased inflammatory response for periodontitis and diabetes. Indeed, patients with periodontitis have increased serum levels of inflammatory cytokines while diabetic patients have hyper-inflammatory immune cells that can aggravate the increased production of inflammatory cytokines.[10] This exacerbation can increase resistance to insulin and make it more difficult for patients to control their diabetes.[10,11]
Scaling and root planning (SRP) is an effective way for treatment and control of periodontal diseases; however, the ability of the clinician to access to deep pockets often results in a substantial changes in its effectiveness, and therefore to overcome this technical limitations, and to prevent early microbial recolonization, the adjunctive use of antimicrobials or anti-inflammatory agents may be indicated to achieve the best chance for clinical improvements.[12] The recent development of delivery systems placed subgingivally has provided the possibility of maintaining effective, intrapocket levels of antimicrobial agents for extended periods of time. Chlorhexidine (CHX) is one of the most effective local antimicrobial agents. It is an antiseptic, which adheres to organic agents when applied topically. Its efficacy as a topical rinse to inhibit dental plaque formation, and gingivitis has been well-established in study periods for up to 2 years without evidence of any bacterial resistance.[13] It has been demonstrated to be effective against subgingival bacteria when delivered through a sustained release device. CHX has been found to be an effective agent in plaque inhibition because it is well-retained in the oral cavity, reacting reversibly with receptors in the mouth due to its affinity for hydroxyapetite and acidic salivary protein.[14] It is generally accepted that the mode of action of CHX is dependent on initial adsorption to surfaces. CHX epitomizes the term substantivity, showing antimicrobial activity in the mouth for at least 7 h and probably more than 12 h.
Its systemic toxicity is very low in humans and has not produced any appreciable resistance to oral microorganisms and has not been associated with any teratogenic alterations. Short term use of CHX causes a significant reduction in the number of oral microorganisms.[15]
Advanced xanthan-based 1.5% CHX gel (CHLO-SITE®, Ghimas, Italy) is supplied in syringe (0.5 ml) with a special needle having a blunt tip and a lateral opening.[16] Chlosite is a xanthan-based syringable gel system. The gel is a combination of two CHX formulations: 0.5% CHX digluconate and 1.0% CHX dihydrochloride incorporated in a saccharidic polymer, xanthan. Cross-linking structure of xanthan controls the release of drugs, and it exhibits a near zero-order drug release. When in contact with water, it forms a three-dimensional pseudoplastic reticulum capable of holding and maintaining various substances in suspension. The CHX xanthan-based gel undergoes a progressive process of imbibition and is physically removed in 10–30 days. CHX digluconate is liberated in the first day and achieves a concentration >100 μg/ml which is maintained for an average of 6–9 days which is greater than the minimum inhibitory concentration for CHX (0.10 μg/ml). CHX dihydrochloride is released in the following days and maintains the bacteriostatic and bactericidal concentrations for at least 2 weeks and prevents recolonization.[17] The rationale for the adjunctive use of xanthan gum in a subgingival gel carrier relates to the increased viscosity of the carrier and in the bioadhesive properties of the polysaccharides, both of which may decrease the clearance and washing of CHX gel from the periodontal pockets.[16]
Findings that periodontal therapy appears to reduce periodontal infection and infflammation suggest that periodontal therapy may facilitate metabolic control of diabetes, improving insulin sensitivity by reducing peripheral infflammatory cytokine levels.[10] Indeed, among the earlier researches that investigated the effects of periodontal therapy on glycogenic control, a large number showed improved glycemic control after periodontal treatment[18] while some did not report a positive effect of periodontal therapy on glycemic control.[19] Stewart et al.[18] Previously found a decrease in glycated hemoglobin (HbA1c level) following nonsurgical periodontal treatment in diabetic patients.
Many studies clearly reported that the estimation of HbA1c can be an important and reliable parameter to evaluate the effective periodontal treatment on the level of glycemic control.[20] The purpose of this study is to investigate the effects clinical and metabolic effects of a xanthan-based CHX (Xan-CHX) gel used as an adjunct to SRP in the treatment of Type 2 diabetic patients with chronic periodontitis.
MATERIALS AND METHODS
This study was a randomized, controlled clinical trial conducted at the Department of Periodontics at the University of Tabriz, Tabriz, Iran, from June 2014 to August 2015. The study protocol was approved by the Research Council and the Ethical Committee of Tabriz University of Medical Sciences, Iran. This trial was registered at Iranian Registry of Clinical Trials database with the IRCT number of IRCT201411023690N5. After providing the patients adequate information about the study, informed consent was obtained. The patients were referred to the Department of Periodontics, Tabriz Dental School from the Diabetics Clinics and the Tabriz Diabetics Center. The patients' age range was 30–60 years. Sixty-eight patients matching the following inclusion criteria were selected:
Moderate-to-severe chronic periodontitis; having at least 20 teeth; minimum of 8 teeth with probing pocket depth (PD) of ≥5 mm; diagnosis of DM2 with HbA1c values over 6%;
No major diabetic complications;
Blood sugar controlled with glibenclamide and metformin, without insulin administration;
No systemic antibiotic administration or periodontal treatment within the last 6 months;
Teeth without large restorations and overhangs.
The following exclusion criteria were applied:
Presence of systemic diseases other than DM2 that could influence the course of periodontal disease
Smoking
Pregnancy or intention to become pregnant during the study period
Fixed orthodontic appliances
Refusal or inability to give informed consent.
The sample size was determined by a statistics expert according to the previous similar study.[21] Patients were randomly divided into control group (treatment by SRP) and test group (SRP + subgingival CHX gel) with the coin throw up (simple randomization, i.e., heads-control, tails-treatment). Clinical and metabolic parameters in baseline, 3 and 6 months after treatment were evaluated in both groups.
All subjects received a clinical examination by a single examiner who was an expert periodontist and blinded to the type of treatment. Intraexaminer reliability was tested by examining three patients in an identical manner one hour apart and observing more than 95% of recordings being within 1 mm. Periodontal parameters were evaluated using gingival index (GI), plaque index (PI), clinical attachment level (CAL), and probing PD. PI was recorded using the O'Leary index.[22] Gingival status was recorded for each tooth according to established GI criteria.[23] PD was measured from the gingival margin by UNC-15 (Hu-Friedy Instruments, Chicago, IL, USA) and CAL was considered as primary outcome and defined as the distance from the cementoenamel junction to the base of the pocket. HbA1c was the other primary outcome of this study and PI, GI, and PD were considered as the secondary outcomes.
Venous blood samples were taken from each participant and analyzed for fasting blood sugar (FBS) and HbA1c. Metabolic measurements were performed at baseline and 3 and 6 months later in both groups. All laboratory analyses were performed in a same laboratory. Reliability of biochemical assessments were confirmed through credentialed regulations and shown to be within acceptable standards. All periodontal treatments were performed by a dental student (SG) under the supervision of an expert periodontist (MF). Before the first treatment session, patients in both groups received standard oral hygiene instructions. In two groups, patients subjected to full-mouth SRP using an ultrasonic device (Various 350, NSK, Japan) and standard Gracey periodontal curettes (Hu-Friedy Instruments, Chicago, IL, USA) with no time limitation. Local anesthesia in the form of infiltration and spray was used when required. All patients received a second session of SRP after 2 weeks to ensure that no calculus was left.
For control patients, SRP was the only treatment modality given and eight teeth with 4–8 mm PD were selected for measuring the clinical parameters. For test patients, at least, eight teeth with 4–8 mm PD received subgingival application of CHX gel (chlosite). Chlosite is supplied in syringe (0.5 mL) with a special needle having a blunt tip and a lateral opening. Selected teeth were isolated and dried with cotton rolls. Blunt needle of prefilled syringe was inserted into the pockets in those sites that had been randomly assigned to receive it. The pockets were overfilled with gel, and periodontal dressing (coe-pak) was applied over test the sites to to reduce the chances of dilution and outflow of the drug by saliva and gingival crevicular fluid, and ensure gel remains in the site for the required period. We applied pressure on the tooth surface not on the external wall of the pocket during periodontal dressing placement, and after that, we checked if there is visible lack of gel from the pocket.
All the participated patients were asked to maintain meticulous oral hygiene. Dressing was removed after 7 days. Patients did not receive any further periodontal treatment for 6 months, and medical treatment for diabetes remained unchanged. Three and 6 months after the baseline examinations, all subjects were recalled for a second clinical examination and all the parameters were reassessed again. The clinician was totally blind at the reassessment session to the study groups.
Statistical methods
Data from the study were analyzed using descriptive statistics (mean ± standard deviation and frequency), ANOVA test by SPSS.15 software (SPSS Inc. Chicago, IL, USA). P < 0.05 was considered statistically significant.
RESULTS
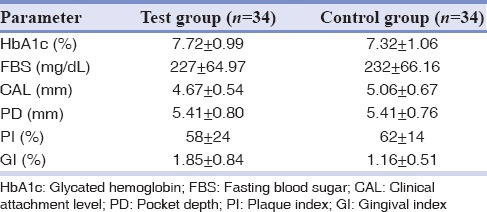
All patients completed the study period (6 months). The mean age of patients for test group was 52.7 ± 7.3 and for control group was 55.3 ± 8.8. No statically significant difference was revealed between groups in each demographic characteristic. Clinical and metabolic parameters of the patients at baseline are given in Table 1. The analysis of the result of baseline indicated that the two groups share the same profile for HbA1c, FBS, CAL, PD, PI, and GI and had no statistically significant difference.
Table 1.
Clinical and metabolic characteristics of the study subjects at baseline (mean±SD)
Periodontal therapy significantly improved all periodontal parameters in the two groups [Table 2]. Both the groups showed a significant change in PD after 1 and 6 months. The mean PD reductions from baseline to 3 and 6 months were found to be 1.74 ± 0.14 mm and 1.93 ± 0.26 mm, respectively, for control group, which was highly significant (P < 0.001); and 1.93 ± 0.33 mm and 2.03 ± 0.31 mm, respectively, for test group, which was highly significant (P < 0.001). The mean PD reduction, when compared from 3 to 6 months, was higher in test group, but this difference was not significant between groups. Alterations in CAL are reported in Table 2. Statistically significant clinical attachment gains were reported for all the groups at 3 and 6 months (P < 0.001). Comparing the mean clinical attachment gain from 3 to 6 months between groups, showed higher in test group, although not significant. As shown in Table 2, patients in test group exhibited a decrease in FBS from the baseline assessment (227 ± 64.97) to the 3 and 6 months follow-up assessment (208 ± 61.95 and 201 ± 61.33; P < 0.001). HbA1cb levels decreased from 7.72 ± 0.99 to 6.20 ± 0.97 and 6.06 ± 1.04 after 3 and 6 months follow-up assessment (P < 0.001). In addition, in the control group, reduction of FBS and HBA1c were statistically significant after 3 and 6 months (P < 0.001) [Table 2].
Table 2.
Measurements of clinical and metabolic parameters of the patients at baseline, 3 months and 6 months after treatment (mean±SD)
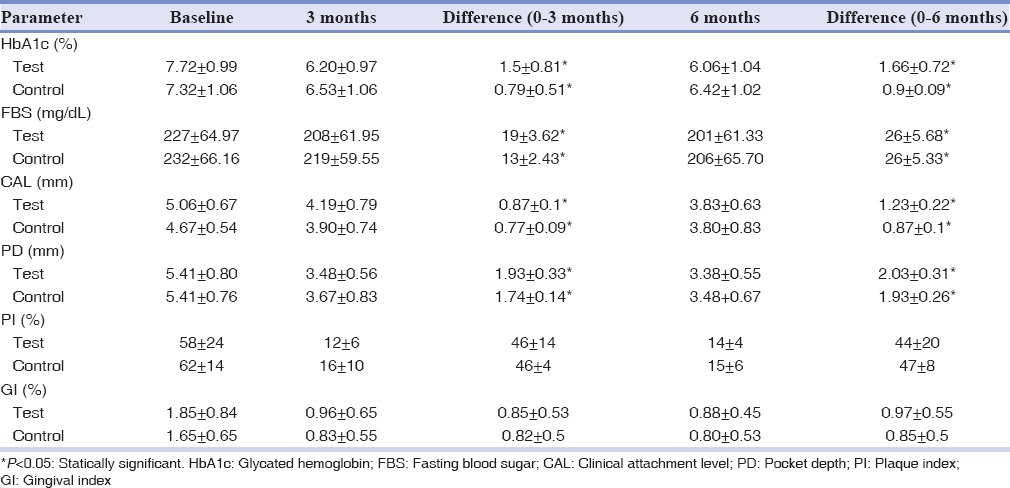
Comparison of FBS and HBA1c demonstrated that there was no significant difference in these parameters between the two groups. Although there was no significant difference, improvements in metabolic parameters in the test group were higher than control group [Table 2].
DISCUSSION
The aim of this preliminary investigation was to evaluate the effects of nonsurgical periodontal therapy, with and without the use of Xan-CHX gel as an adjunct to SRP on clinical and metabolic parameters in Type 2 diabetic patients with chronic periodontitis. The present study showed significant decreases in HbA1c and FBS 3 and 6 months after nonsurgical periodontal therapy with and without CHX in patients with DM2. However, improvements in clinical and metabolic parameters in CHX gel group were higher than the control group. These findings reveal that a reduction of periodontal infection can reduce HbA1c levels within a short period and thus may improve metabolic control in DM2 patients. The outcome of this study confirms prior evidence suggesting an interaction between periodontal status and diabetic metabolic control[4,7] and supports the hypothesis that a successful periodontal therapy can improve glucose metabolism. Our study results are consistent with prior studies positive responses to nonsurgical periodontal therapy in persons with DM2 reported by Westfelt et al.,[19] Kiran et al.,[24] and Moeintaghavi et al.[25]
Yang et al.[26] showed decreases in tumor necrosis factor (TNF) and HbA1c in DM2 patients after periodontal therapy, providing support for the possibility that periodontal therapy may reduce HbA1c values by reducing TNF concentrations in DM2 patients with periodontitis. Periodontitis is an infection that is twice in diabetic individuals compared to nondiabetics. Porphyromonas gingivalis, one of the pathogens responsible for this infection, can invade endothelial cells and is a potent signal for monocyte and macrophage activation. Thus, once established in the diabetic patient, this chronic infection complicates diabetes control and increases the occurrence and severity of microvascular and macrovascular complications. Periodontal disease may affect insulin signaling through pro-infflammatory mediators. The infflamed periodontium can be a source of infflammatory mediators, such as TNF-alpha, which can affect glucose and fat metabolism.[27] TNF impairs insulin signaling by increasing adipose secretion of free fatty acids. The research by Paolantonio et al.[16] revealed greater reductions in the percentages of sites positive for the eight periodontopathic bacteria for the Xan-CHX gel treatment group compared to SRP alone particularly up to 3 months after treatment. Higher improvements in metabolical parameters in our study can be related to antimicrobial effects of application of CHX gel. Our findings are inconsistent with those of Promsudthi et al.,[28] who showed a decrease in HbA1c levels after periodontal therapy with the use of systemic doxycycline; however, these decreases were reported to be not significant. There are several differences that could account for the discrepancy between the findings of the present study and those by Promsudthiet et al. The treatment group received mechanical periodontal treatment combined with systemic doxycycline in Promsudith et al.[28] study while the treatment group received mechanical periodontal treatment plus xanthan-based CHX gel in the present study. This study included a wide age range (30–60 years) of Iranian while Promsudth et al.[28] study was conducted on significantly older (age range: 55–80 years) Thai patients. The less than ideal reduction in plaque control scores in the treatment group may be another reason for the lack of significant reduction in HbA1c levels in Promsudthi et al.[28] study. One of the inevitable drawbacks of design of Promsudthi et al.[28] studies is that the provided periodontal treatment makes blinding procedures impossible for the examiners at 3-month evaluation point because of the absence of calculus and inflammation after periodontal treatment. Therefore, examiner bias, although unintentionally, could have impacted the periodontal measurements at the 3-month time point. Other additional sources of possible bias could be the lack of randomization of all subjects; the control group included patients who refused periodontal treatment.
The results of systematic review by Teeuw et al.[21] suggested that periodontal treatment leads to an improvement of glycemic control in Type 2 diabetic patients for at least 3 months.
Both SRP alone (control group) and adjunctive use of CHX gel (experimental groups) resulted in statistically significant reduction in the clinical periodontal parameters. PI and GI improved due to NSP treatment (SRP with or without CHX gel) modalities, along with improved oral hygiene practices commonly observed in the study subjects. Similar to our study, Chauhan et al.,[16] Gupta et al.[29] and Kranti et al.,[30] also reported significant improvement in the PPD and CAL after subgingival application of xanthan-based CHX gel as an adjunct to SRP. Dualstaged released antimicrobial property and enhanced gel structure and substantivity due to cationic charges of the CHX that can interact with the anionic charges of the bioadhesive xanthan gum polymer in xanthan-based CHX gel maybe responsible for better results as compared to SRP alone.[30] The same results have been reported with multiple uses of CHX gel and metronidazole gel in Perinetti et al.[31] study. Their results reveal that subgingival administrations of both metronidazole (1%) and CHX gels (1%) in 7 days intervals for 3 times may have a role in the management of persistent pockets. Bleeding on probing and PD significantly decreased in the metronidazole and CHX gel groups after treatment, in comparison with the baseline values at the 3 months interval. We find the same results about PD at the same interval. Our results are based on short term, small sample size, for clarifying the effect of nonsurgical periodontal therapy on glycemic control will require further studies with larger sample sizes and more longitudinal data.
CONCLUSION
The present study showed that nonsurgical periodontal therapy can effectively decrease FBS and HbA1c levels in diabetic patients. Higher improvements observed in metabolic parameters with application of CHX gel in our study can be attributed to antimicrobial effects of this adjective material. Periodontal therapy and follow-up might be, then, considered in the treatment plan of DM2 patients, especially those who have poor metabolic control despite administration of numerous medical interventions.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Van Dyke TE, Serhan CN. Resolution of inflammation: A new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 2.Dahlén G. Role of suspected periodontopathogens in microbiological monitoring of periodontitis. Adv Dent Res. 1993;7:163–74. doi: 10.1177/08959374930070020701. [DOI] [PubMed] [Google Scholar]
- 3.Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: An overview. Ann Periodontol. 2001;6:91–8. doi: 10.1902/annals.2001.6.1.91. [DOI] [PubMed] [Google Scholar]
- 4.Iacopino AM. Periodontitis and diabetes interrelationships: Role of inflammation. Ann Periodontol. 2001;6:125–37. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 5.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 6.Taylor GW, Borgnakke WS. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 7.Grossi SG. Treatment of periodontal disease and control of diabetes: An assessment of the evidence and need for future research. Ann Periodontol. 2001;6:138–45. doi: 10.1902/annals.2001.6.1.138. [DOI] [PubMed] [Google Scholar]
- 8.Lalla E, Lamster IB, Stern DM, Schmidt AM. Receptor for advanced glycation end products, inflammation, and accelerated periodontal disease in diabetes: Mechanisms and insights into therapeutic modalities. Ann Periodontol. 2001;6:113–8. doi: 10.1902/annals.2001.6.1.113. [DOI] [PubMed] [Google Scholar]
- 9.Southerland JH, Taylor GW, Offenbacher S. Diabetes and periodontal infection: Making the connection. Clin Diabetes. 2005;23:171–8. [Google Scholar]
- 10.Dag A, Firat ET, Arikan S, Kadiroglu AK, Kaplan A. The effect of periodontal therapy on serum TNF-alpha and HbA1c levels in type 2 diabetic patients. Aust Dent J. 2009;54:17–22. doi: 10.1111/j.1834-7819.2008.01083.x. [DOI] [PubMed] [Google Scholar]
- 11.Mealey BL. Periodontal disease and diabetes. A two-way street. J Am Dent Assoc. 2006;137(Suppl):26S–31S. doi: 10.14219/jada.archive.2006.0404. [DOI] [PubMed] [Google Scholar]
- 12.Cosyn J, Wyn I. A systematic review on the effects of the chlorhexidine chip when used as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J Periodontol. 2006;77:257–64. doi: 10.1902/jop.2006.050216. [DOI] [PubMed] [Google Scholar]
- 13.Schiott CR, Briner WW, Kirkland JJ, Löe H. Two years oral use of chlorhexidine in man. III. Changes in sensitivity of the salivary flora. J Periodontal Res. 1976;11:153–7. doi: 10.1111/j.1600-0765.1976.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 14.Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5:79–83. doi: 10.1111/j.1600-0765.1970.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 15.Davies RM, Jensen SB, Schiott CR, Löe H. The effect of topical application of chlorhexidine on the bacterial colonization of the teeth and gingiva. J Periodontal Res. 1970;5:96–101. doi: 10.1111/j.1600-0765.1970.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 16.Paolantonio M, D'Ercole S, Pilloni A, D'Archivio D, Lisanti L, Graziani F, et al. Clinical, microbiologic, and biochemical effects of subgingival administration of a Xanthan-based chlorhexidine gel in the treatment of periodontitis: A randomized multicenter trial. J Periodontol. 2009;80:1479–92. doi: 10.1902/jop.2009.090050. [DOI] [PubMed] [Google Scholar]
- 17.Rusu D, Benta A, Necker A. Non-surgical periodontal therapy using a novel chlorhexidine based xanthan gel; a split mouth study. Int Poster J Dent Oral Med. 2005;7:286–91. [Google Scholar]
- 18.Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001;28:306–10. doi: 10.1034/j.1600-051x.2001.028004306.x. [DOI] [PubMed] [Google Scholar]
- 19.Westfelt E, Rylander H, Blohmé G, Jonasson P, Lindhe J. The effect of periodontal therapy in diabetics. Results after 5 years. J Clin Periodontol. 1996;23:92–100. doi: 10.1111/j.1600-051x.1996.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 20.Hungund S, Panseriya BJ. Reduction in HbA1c levels following non-surgical periodontal therapy in type-2 diabetic patients with chronic generalized periodontitis: A periodontist's role. J Indian Soc Periodontol. 2012;16:16–21. doi: 10.4103/0972-124X.94598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: A systematic review and meta-analysis. Diabetes Care. 2010;33:421–7. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Leary TJ, Drake RB, Jividen GJ, Allen MF. The incidence of recession in young males: Relationship to gingival and plaque scores. SAM-TR-67-97. Tech Rep SAM-TR. 1967 Jul;:1–4. [PubMed] [Google Scholar]
- 23.Loe H, Silness J. Periodontal disease in pregnancy. I. prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 24.Kiran M, Arpak N, Unsal E, Erdogan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–72. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 25.Moeintaghavi A, Arab HR, Bozorgnia Y, Kianoush K, Alizadeh M. Non-surgical periodontal therapy affects metabolic control in diabetics: A randomized controlled clinical trial. Aust Dent J. 2012;57:31–7. doi: 10.1111/j.1834-7819.2011.01652.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang PS, Wang Y, Qi XM, Ren JM, Ge SH. The effect of periodontal initial therapy on circulating TNF-alpha and HbA1C in type 2 diabetes patients with periodontitis. Zhonghua Kou Qiang Yi Xue Za Zhi. 2003;38:364–6. [PubMed] [Google Scholar]
- 27.Cutler CW, Machen RL, Jotwani R, Iacopino AM. Heightened gingival inflammation and attachment loss in type 2 diabetics with hyperlipidemia. J Periodontol. 1999;70:1313–21. doi: 10.1902/jop.1999.70.11.1313. [DOI] [PubMed] [Google Scholar]
- 28.Promsudthi A, Pimapansri S, Deerochanawong C, Kanchanavasita W. The effect of periodontal therapy on uncontrolled type 2 diabetes mellitus in older subjects. Oral Dis. 2005;11:293–8. doi: 10.1111/j.1601-0825.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 29.Gupta R, Pandit N, Aggarwal S, Verma A. Comparative evaluation of subgingivally delivered 10% doxycycline hyclate and xanthan-based chlorhexidine gels in the treatment of chronic periodontitis. J Contemp Dent Pract. 2008;9:25–32. [PubMed] [Google Scholar]
- 30.Kranti K, Seshan H, Sameer Z. Clinical evaluation of topical subgingival application of biodegradable xanthan based 1.5% chlorhexidine gel for treatment on periodontal pockets. J Adv Dent Res. 2010;1:47–54. [Google Scholar]
- 31.Perinetti G, Paolantonio M, Cordella C, D'Ercole S, Serra E, Piccolomini R. Clinical and microbiological effects of subgingival administration of two active gels on persistent pockets of chronic periodontitis patients. J Clin Periodontol. 2004;31:273–81. doi: 10.1111/j.1600-051x.2004.00481.x. [DOI] [PubMed] [Google Scholar]


