Abstract
Objectives
This study sought to determine if obese patients had worse post-LVAD implantation outcomes and if the implantation of an LVAD allowed for weight loss.
Background
Obesity is a risk factor for cardiovascular disease including heart failure. Obese heart failure patients have better outcomes than those with normal weight; however obese patients have worse outcomes following heart transplantation.
Methods
Patients were identified in the UNOS database that underwent LVAD implantation as bridge to transplantation from May 2004 and April 2014, with follow-up through June 2014. Patients were grouped according to BMI based on the WHO classification
Results
Among 3,856 patients the risk of death or delisting was not significantly different between BMI groups (p=0.347). There was no increased risk of death (p=0.234) or delisting (p=0.918). The risk of complication requiring UNOS status upgrade was increased for those with Class II obesity or greater (HR 1.48, 95% CI 1.14–1.93, p=0.004), driven by increased infection and thromboembolism. Obese patients had worse post-transplant outcomes. Weight loss substantial enough to decrease BMI group was achieved by a small proportion of patients listed with Class I obesity or greater (9.6–15.5%).
Conclusions
Patients with obesity had similar freedom from death or delisting while on LVAD support. However, Class II obese or greater patients had an increased risk of complications requiring UNOS status upgrade compared with those with normal BMI during LVAD support and decreased post-transplant survival. Weight loss on device therapy was possible, but uncommon. Careful consideration is needed when a bridge to weight loss strategy is proposed.
Keywords: Left ventricular assist device, obesity, outcome
Introduction
Obesity is a worldwide epidemic with over one-third of adults in the United States obese (BMI>30). Obesity is a risk factor for heart failure and despite the “obesity paradox” where obese patients with heart failure have better outcomes than those of a normal BMI (1), many proceed to Stage D heart failure. Morbid obesity (BMI>35) is a barrier to candidacy for heart transplantation (HT) (2) and those who are obese and undergo transplantation have worse outcomes following HT (3). For these patients options include destination continuous-flow left ventricular assist device (LVAD), palliative care, weight loss, or a bridge to decision (weight loss) LVAD.
Less is known about the outcomes of obese patients following implantation of an LVAD. Studies have suggested increased device-thrombosis (4) and infections (5, 6), but the data on mortality for LVAD is limited. One registry and a number of small, single center studies have failed to demonstrate a statistically significant difference in post-implantation survival (5–9), though there has been up to a 12% difference in survival between groups raising the possibility that the studies were underpowered to show a true difference. This study sought to determine if obese patients had worse post-LVAD implantation outcomes and if the implantation of a LVAD allowed for weight loss.
Methods
The United Network for Organ Sharing (UNOS) database was analyzed for patients bridged to transplantation with a continuous-flow LVAD between May 2004 and April 2014. Follow-up data was collected through June 2014. This study included adult candidates (age ≥18 years) registered for single-organ, primary HT who received a Food and Drug Administration (FDA) approved CF-LVAD. Devices were limited to the Heartmate II (Thoratec/St. Jude, Pleasanton, CA) and Heartware HVAD (Heartware, Framingham, MA) which are contemporary durable devices. Patients who required temporary left sided mechanical circulatory support, BiVAD, or total artificial heart were excluded from the analysis. The primary endpoint was freedom from death or delisting while on device support. Secondary endpoints included death on LVAD support, delisting on LVAD support, complications (thromboembolism, device infection, device malfunction, or life-threatening ventricular arrhythmia) requiring UNOS listing status upgrade, change in BMI group while on device support, and post-transplant survival. By nature of the UNOS database, bridge to transplant was the ultimate strategy for all patients. Patients were grouped according to BMI based on the World Health Organization classification: Underweight (BMI<18.5), Normal (18.5–24.99), Overweight (25–29.99), Obese class I (30–34.99), and Obese class II or greater (BMI>35). Individual patient weight data was recorded at the time of listing for transplantation and at the time of waiting list removal. There were missing data for patients in this study: up to 15% for hemodynamic parameters, though no other variable had more than 1% missing. Imputation was not used for missing data. The study was submitted to the Institutional Review Board of Columbia University Medical Center and was determined to be exempt from review.
Statistical Analysis
Demographic and clinical variables were summarized with standard descriptive statistics and expressed as median (with interquartile range) for skewed continuous variables and count (with percentage) for categorical variables. Group comparisons were made with the Chi-squared and the Kruskal-Wallis test where appropriate. Kaplan-Meier survival analysis with Dunnett’s test applied for pairwise comparisons, univariate, and multivariable Cox proportional-hazards regression were performed to determine survival statistics. Cause-specific hazard models were created and cumulative incidence functions were calculated with death and delisting alternating as a competing event. A two-tailed p-value of less than 0.05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, North Carolina).
Results
During the study period, 3,856 patients met inclusion criteria, 3,245 (84.2%) with a Heartmate II and 611 (15.8%) with an HVAD. The distribution of BMI at time of listing was 2.2% Underweight, 25.5% Normal, 35.5% Overweight, 26.6% Class I obese, and 10.2% Class II obese or greater. Baseline characteristics were similar for all BMI groups except Underweight, which was more female, had fewer ischemic cardiomyopathies, increased HVAD use, higher GFR, less ICD use, a higher baseline PVR, and fewer were former smokers (Table 1).
Table 1.
Baseline characteristics of study population
| BMI <18.5 | 18.5 ≤ BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | BMI ≥ 35 | p-value | |
|---|---|---|---|---|---|---|
| N= 84 | N= 983 | N= 1,368 | N= 1,028 | N= 393 | ||
| Age | 43 (15.5–57) | 56 (45–63) | 57 (48–63) | 55 (45–61) | 49 (40–57) | <0.0001 |
| Male (%) | 44 (52.4) | 716 (72.8) | 1,086 (79.4) | 827 (80.5) | 281 (71.5) | <0.0001 |
| ICM (%) | 17 (20.2) | 390 (39.7) | 691 (43.2) | 425 (41.3) | 138 (35.1) | <0.0001 |
| Race (%) | <0.0001 | |||||
| White | 43 (51.2) | 618 (62.9) | 957 (70.0) | 674 (65.6) | 222 (56.5) | |
| Black | 28 (33.3) | 240 (24.4) | 289 (21.1) | 260 (25.3) | 144 (36.6) | |
| Device Type (%) | <0.0001 | |||||
| Heartmate II | 49 (58.3) | 797 (81.1) | 1,179 (86.2) | 887 (86.3) | 333 (84.7) | |
| Heartware HVAD | 35 (41.7) | 186 (18.9) | 189 (13.8) | 141 (13.7) | 60 (15.3) | |
| GFR (MDRD) | 101.1 (66.8–135.0) | 75.9 (52.8–89.0) | 64.8 (50.4–84.1) | 62.8 (47.8–78.7) | 61.7 (46.9–78.2) | <0.0001 |
| CrCl (Cockcroft-Gault) | 73.2 (54.3–98.8) | 73.2 (54.3–93.9) | 73.5 (56.7–95.0) | 66.8 (52.2–87.4) | 67.6 (51.8–87.4) | <0.0001 |
| Adj Body Wt CrCl | 73.2 (54.3–98.8) | 73.2 (54.3–93.9) | 76.7 (59.5–99.3) | 76.2 (59.4–97.9) | 82.1 (62.5–107.4) | <0.0001 |
| Creatinine (mg/dL) | 0.9 (0.7–1.1) | 1.1 (0.9–1.4) | 1.2 (0.9–1.5) | 1.2 (1.0–1.5) | 1.3 (1.0–1.6) | <0.0001 |
| Dialysis (%) | 2 (2.4) | 33 (3.4) | 32 (2.3) | 38 (3.7) | 12 (3.1) | 0.37 |
| Hemodynamics | ||||||
| mPAP (mmHg) | 30 (25–37) | 30 (22–37) | 30 (22–38) | 31 (23–39) | 33 (25–42) | <0.0001 |
| PCWP (mmHg) | 23 (15–28) | 20 (12–27) | 20 (13–27) | 20 (14–27) | 22 (16–29) | 0.0008 |
| PVR (WU) | 2.55 (1.79–4.23) | 2.45 (1.59–3.61) | 2.27 (1.45–3.33) | 2.24 (1.43–3.24) | 2.11 (1.37–3.01) | <0.0001 |
| CO (L/min) | 2.9 (2.4–3.8) | 3.8 (3.1–4.7) | 4.3 (3.5–4.2) | 4.6 (3.7–5.5) | 4.9 (4.0–5.8) | <0.0001 |
| CI (L/min/m2) | 1.87 (1.61–2.42) | 2.12 (1.73–2.56) | 2.13 (1.74–2.55) | 2.08 (1.70–2.50) | 2.09 (1.74–2.45) | 0.12 |
| Diabetes (%) | 6 (7.1) | 179 (18.3) | 438 (32.0) | 435 (42.5) | 173 (44.0) | <0.0001 |
| ICD (%) | 51 (60.7) | 726 (74.0) | 1,092 (79.9) | 833 (81.3) | 326 (83.0) | <0.0001 |
| Prior Smoker (%) | 26 (31.0) | 458 (46.8) | 749 (54.9) | 567 (55.5) | 193 (49.4) | <0.0001 |
| Prior Stroke (%) | 4 (4.8) | 54 (5.5) | 84 (6.2) | 60 (5.9) | 15 (3.8) | 0.28 |
| Transplanted (%) | 59 (70.2) | 742 (75.5) | 988 (72.2) | 706 (68.7) | 228 (58.0) | <0.0001 |
Data presented as Count (%) or Median (interquartile range). BMI=Body Mass Index; CrCl=Creatinine Clearance in mL/min; CI=Cardiac Index; GFR=Glomerular Filtration Rate in mL/min/1.73 m2; ICD=Implantable Cardioverter Defibrillator; ICM=Ischemic Cardiomyopathy; mPAP=Mean Pulmonary Artery Pressure; PCWP=Pulmonary Capillary Wedge Pressure; PVR=Pulmonary Vascular Resistance
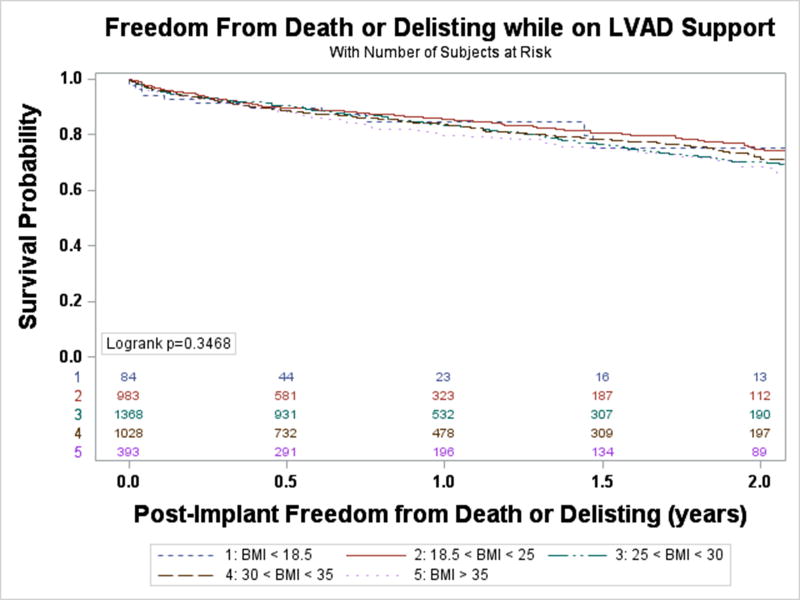
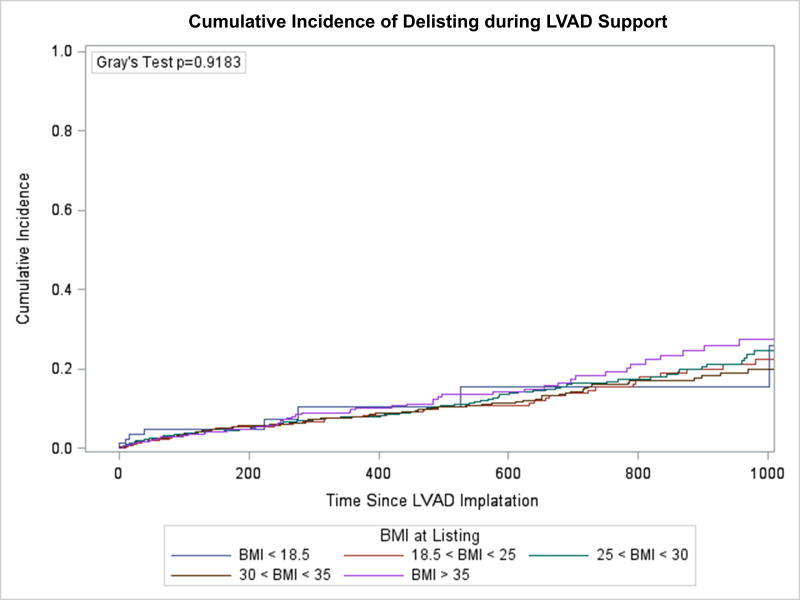
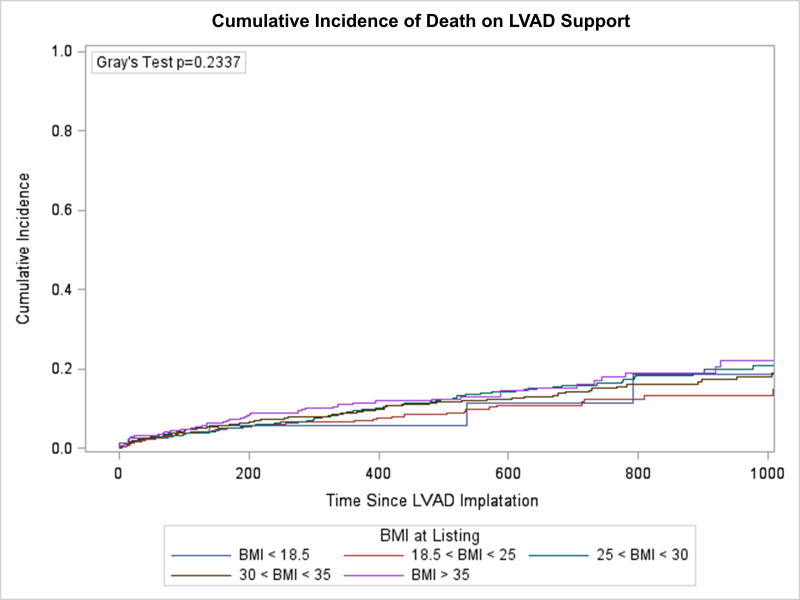
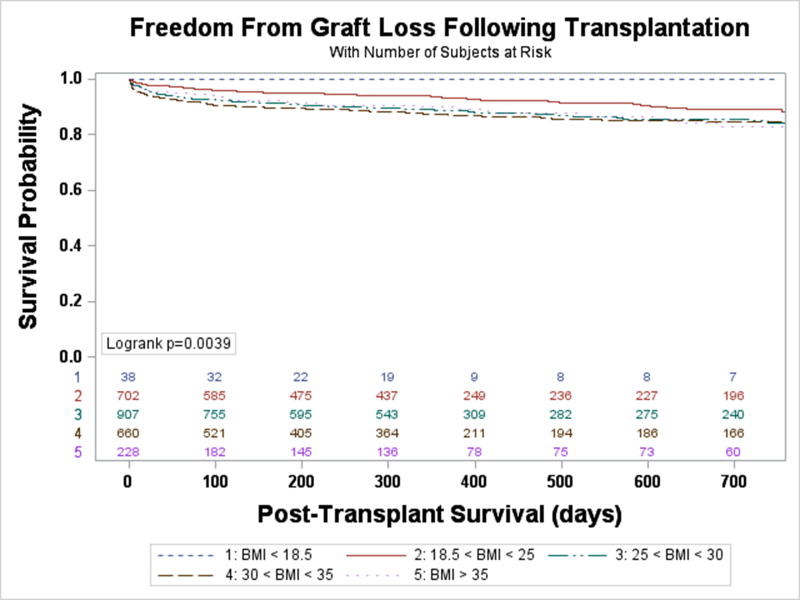
Freedom from death or delisting was analyzed based on BMI group. Between BMI groups there was no statistically significant difference in event-free survival while on LVAD support (Figure 1). An unadjusted Cox-proportional hazards model similarly demonstrated that when compared to those with a normal BMI, there was no significant difference between risk of death or delisting between BMI groups. There was a trend towards an increased risk of event for patients with Class II obesity or greater compared with patients with a normal BMI (HR 1.27, 95% CI 0.996–1.61, p=0.054), but this did not reach statistical significance. A competing risk analysis again failed to demonstrate a difference between BMI groups for risk of delisting while on LVAD support (Figure 2A, p=0.918) or death (Figure 2B, p=0.234). Analysis of BMI as a continuous variable found no association between BMI and the combined endpoint of death or delisting on unadjusted (HR 1.01, 95% CI 0.997–1.02, p=0.14) or multivariable analysis (HR 1.01, 95% CI 0.998–1.03, p=0.11).
Figure 1. Freedom from death or delisting while on LVAD Support.
Figure 2. Competing risk model of waitlist outcome.
A) Incidence of delisting on LVAD support, B) Incidence of death on LVAD support.
Adverse events were categorized as those that necessitated UNOS listing status upgrade while on device support. Using a combined endpoint of thromboembolism, device infection, device malfunction, or life-threatening ventricular arrhythmia and adjusting for time on LVAD support, those with Class II obesity or greater had an increased risk of event (HR 1.48, 95% CI 1.14–1.93, p=0.004, Table 2). There was a trend towards increased risk of infection among those with Class II obesity or greater (HR 1.59, 95% CI 0.99–1.94, p=0.058), but not among any other group. Thromboembolism was more common among those with greater BMI (Table 2). The risk of device malfunction or life-threatening ventricular arrhythmias was fairly uniform across all BMI categories.
Table 2.
Complications requiring UNOS listing status upgrade
| BMI < 18.5 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | BMI ≥ 35 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | p-value | HR | p-value | HR | p-value | HR | p-value | |
| Thromboembolism | 0.59 | 0.97 | 1.53 | 0.12 | 2.11 | 0.006 | 2.17 | 0.02 |
| Device Infection | 0.75 | 0.53 | 1.01 | 0.93 | 0.96 | 0.80 | 1.39 | 0.058 |
| Device Malfunction | 0.43 | 0.97 | 1.20 | 0.48 | 1.44 | 0.17 | 1.69 | 0.10 |
| Arrhythmia | 1.40 | 0.75 | 1.20 | 0.66 | 1.01 | 0.98 | 1.59 | 0.38 |
| Composite | 0.56 | 0.16 | 1.10 | 0.36 | 1.17 | 0.15 | 1.48 | 0.004 |
Reference Group: Normal BMI (18.5 ≤ BMI < 25)
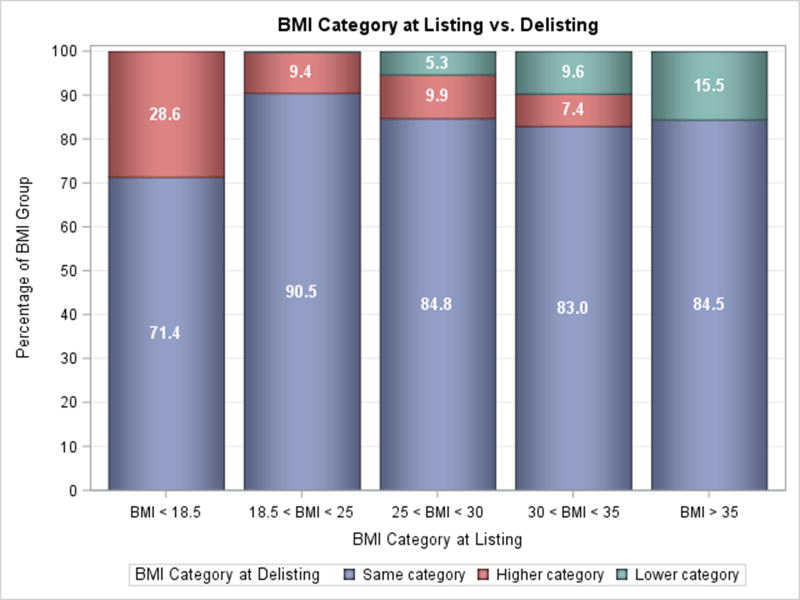
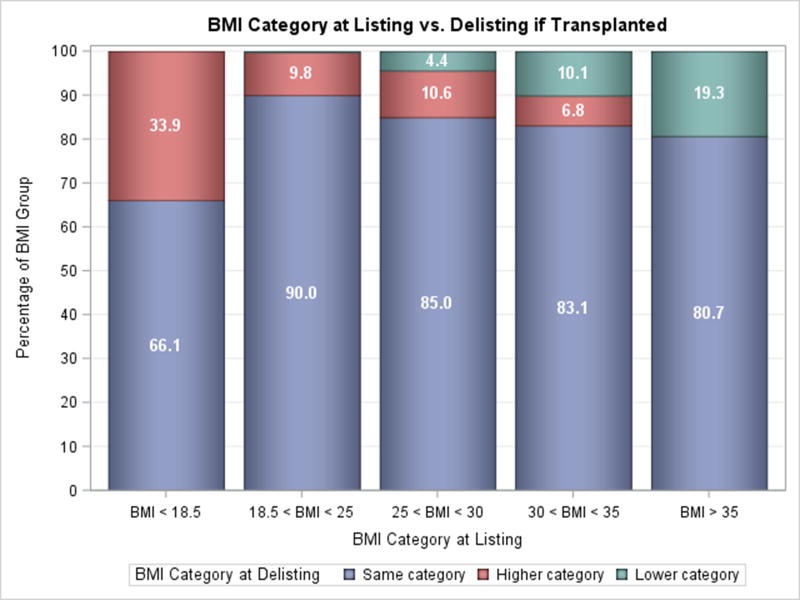
Changes in BMI group were uncommon following LVAD implantation. Only 15.5% of patients with Class II obesity or greater were able to lose enough weight to move to a lower BMI group at the time of transplantation or delisting (Figure 3A). Similarly, a small number of patients with Class I obesity were able to move to a lower group (9.6%), however a comparable amount moved to a higher BMI group (7.4%). Among patients who were obese and were successfully bridged to transplantation, the prevalence of lowering BMI while on LVAD support was similarly low (10.1% Class I obesity & 19.3% Class II and greater obesity, p=0.90, Figure 3B). Interestingly, more overweight patients (25≤BMI<30) patients gained weight than lost weight. Duration of LVAD support was not associated with the degree of weight loss in all patients as well as those with obesity (Supplementary Figures 1 & 2).
Figure 3. Weight Change on LVAD Support.
A) Change in BMI from Listing to Delisting for all Patients, B) Change in BMI from Listing to Delisting for Transplanted Patients.
During the study period, 2,535 patients underwent transplantation. Patients with a normal BMI had the greatest prospect of transplantation, while a greater BMI lowered the probability of transplantation following LVAD implantation (Supplementary Figure 3). After controlling for age, gender, complications requiring a UNOS listing status upgrade, blood type, and PRA>10% the probability of transplantation was lower for Class II obesity or greater (HR 0.52, 95% CI 0.44–0.60, p<0.0001), Class I obesity (HR 0.69, 95% CI 0.62–0.76, p<0.0001), and Overweight (HR 0.85, 95% CI 0.76–0.93, p=0.001). Survival following transplantation followed a similar pattern (Figure 4). When compared to those with a normal BMI, patients with Class I obesity at transplantation had 76% increase in risk of death (95% CI 1.25–2.47, p=0.001), followed by those with Class II obesity or greater (HR 1.57, 95% CI 0.99–2.48, p=0.057), and those overweight (HR 1.54, 95% CI 1.12–2.14, p=0.009). There was a small sample of Underweight patients transplanted and there were no mortalities during follow-up (Figure 4). Adjusting for age (10), renal dysfunction (10), device infection (11, 12), and duration of LVAD support (13, 14) (previously reported pre-transplant causes of decreased BTT post-transplant survival) in addition to ischemic time (15), donor age (15), allograft rejection requiring hospitalization (10), and post-transplant hospitalizations for infection (16) the increased risk of post-transplant mortality among those overweight remained: Class I obese had a 63% increase in risk of death (95% CI 1.15–2.30, p=0.006), Class II obesity or greater had a 53% increased risk (95% CI 0.95–2.45, p= 0.079), and overweight had a 50% increased risk (95% CI 1.08–2.08, p= 0.017). When BMI was treated as a continuous variable each one unit increase in BMI increased the risk of mortality by 5.4% (95% CI 1.03–1.08, p<0.0001) and similar in a multivariable model (HR 1.05, 95% CI 1.02–1.07, p<0.0001).
Figure 4. Freedom from death following transplantation.
Discussion
Obesity has reached epidemic proportions worldwide and is an independent risk factor for heart failure. This study reviewed the UNOS database seeking to determine if obese patients had worse post-LVAD implantation outcomes and if the implantation of a LVAD allowed for weight loss. There were four main findings of this study. First, the data demonstrated that a patient’s BMI at listing did not have an impact on freedom from death or delisting while on LVAD support. Second, patients with Class II obesity or greater have an increased risk of complication requiring UNOS listing status upgrade. Next, the probability of transplantation decreases as BMI increases. And lastly, despite similar survival while on LVAD support, obese patients have worse post-transplant survival, consistent with the data from the ISHLT registry (3).
LVADs have significantly improved survival in patients with Stage D heart failure, but their shortcoming remains complications. Complications requiring UNOS listing status upgrade were more common in those with Class II obesity or greater when compared to those with a normal BMI. Specifically, there were increases in thromboembolism and infection. These associations strengthen the prior reports of increased device thrombosis and infection among those with increased BMI (4–6). Both of these findings are biologically plausible as excess adipose tissue leads decreased immune surveillance, impaired chemotaxis, and altered macrophage differentiation (17). Furthermore, obesity has been demonstrated to promote thrombosis through inflammation, increased platelet activity, and impaired thrombolysis (18).
Weight loss while on LVAD support is possible, but occurred infrequently in this study. Among those with Class I obesity or greater only 9.6–15.6% of patients lost enough weight to decrease BMI group. The numbers were the similar among patients who went on to be transplanted, ranging from 10.1–19.3% of patients. This is disconcerting but not surprising. LVAD implantation has been successful in reversing high pulmonary vascular resistance (19), improving functional capacity, quality of life (20), and peak VO2 (21). The improvement in peak VO2 is only 3–4 ml/kg/min and as such patients on LVAD support never return to normal (age adjusted) exercise capacity, remaining with an exercise capacity similar to someone with mild heart failure (22).
Increased complications while on LVAD support did not lead to a statistically significant increase in the combined endpoint of death or delisting while on device support for Class II obese or greater patients (HR 1.27, 95% CI 0.996–1.61, p=0.054 compared with Normal BMI). When this trend is considered with the findings that in this study the probability of transplantation was 44% less for those with Class II obesity or greater and that only a small subset of Class II obese patients lose enough weight to lower their BMI group, clinicians should have pause using a bridge to transplantation strategy for a patient with Class II obesity or greater. An effort to identify patients who have the best opportunity to achieve weight loss and providing those patients with the necessary resources is vital. A strategy may involve engaging a multidisciplinary team including nutritionists, cardiac physical therapists, and an advanced heart failure cardiologists to help achieve weight loss. Otherwise the waiting list will continue to expand with patients with a decreased chance of transplantation and have worse outcomes after transplantation (23).
This study is not without limitations. First among them is the retrospective nature of the study. The UNOS dataset that was used is high-quality in that for all U.S. transplant centers data submission is mandatory by law, however it is limited to the data collected. As such, a number of covariates of interest including readmission, bleeding, and serum albumin were not available for analysis. Further, there were missing data in the data set though none had more than 1% aside from hemodynamic parameters where there was up to 15% missing. Imputation was not used for missing data; this introduces the potential for bias into the analysis. Patient weight was reported at the time of addition to the waiting list and at the time of removal from the waiting list. A limitation is that patients had the same weight at listing and waiting list removal, raising the possibility that the listing center did not update the individual patient’s weight and impacting on the findings of weight change. Additionally, not all complications were able to be captured given the limitations of the UNOS database. The most serious complications that required UNOS status upgrade were captured however. Lastly, correction for multiple comparisons was not performed in the analysis of this data and introduces the possibility of a Type I error.
In conclusion, patients with obesity have similar freedom from death or delisting while on LVAD support. However, Class II obese and greater patients had an increased risk of complications requiring UNOS listing upgrade compared with those with normal BMI while on LVAD support and decreased post-transplant survival. Weight loss on device therapy is possible, but not common. Careful consideration is needed when a bridge to weight loss strategy is proposed.
Supplementary Material
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE
Heart failure and obesity frequently co-exist. Obesity serves a barrier to cardiac transplantation, but less is known about its impact on mechanical circulatory support. This study found that obese patients have similar survival on LVAD support, but have increased complications and worse post-transplant survival. These results supplement the current body of data available to advanced heart failure cardiologists and should aid in decision making regarding the candidacy of obese patients for mechanical circulatory support and heart transplantation.
TRANSLATIONAL OUTLOOK
Further research is needed to elucidate the underlying biologic mechanisms that lead to worse outcomes post-transplant and increased complications during LVAD support for obese patients.
Acknowledgments
Financial Support: This study was supported by Lisa and Mark Schwartz and the Program to Reverse Heart Failure at New York Presbyterian Hospital/Columbia University. Dr. Clerkin is supported by National Institutes of Health Grant T32 HL007854-16.
Abbreviations List
- BMI
Body mass index
- BiVAD
Bi-ventricular assist device
- CF-LVAD
Continuous-flow left ventricular assist device
- ICD
Implantable cardiac defibrillator
- GFR
Glomerular filtration rate
- PRA
Panel reactive antibodies
- PVR
Pulmonary vascular resistance
- UNOS
United Network for Organ Sharing
- WHO
World Health Organization
Footnotes
Industry Relationships: Dr. Naka received consulting fees from Thoratec. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of Obesity and the Obesity Paradox on Prevalence and Prognosis in Heart Failure. JACC: Heart Failure. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. The Journal of Heart and Lung Transplantation. 35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Healy AH, Stehlik J, Edwards LB, McKellar SH, Drakos SG, Selzman CH. Predictors of 30-day post-transplant mortality in patients bridged to transplantation with continuous-flow left ventricular assist devices-An analysis of the International Society for Heart and Lung Transplantation Transplant Registry. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35:34–9. doi: 10.1016/j.healun.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirklin JK, Naftel DC, Pagani FD, et al. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. The Journal of Heart and Lung Transplantation. 34:1515–1526. doi: 10.1016/j.healun.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Brewer RJ, Lanfear DE, Sai-Sudhakar CB, et al. Extremes of body mass index do not impact mid-term survival after continuous-flow left ventricular assist device implantation. The Journal of Heart and Lung Transplantation. 31:167–172. doi: 10.1016/j.healun.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Zahr F, Genovese E, Mathier M, et al. Obese Patients and Mechanical Circulatory Support: Weight Loss, Adverse Events, and Outcomes. The Annals of Thoracic Surgery. 92:1420–1426. doi: 10.1016/j.athoracsur.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 7.Henderson C, Patel K, Sayer G, et al. Extremes of Obesity and LVAD Patient Morbidity and Mortality. Journal of Heart and Lung Transplantation. 2015;34:S189–S189. [Google Scholar]
- 8.McMenamy M, Arabia F, Czer L, et al. Breaking the Myth of Obesity as a Contraindication to Continuous Flow Left Ventricular Assist Devices. The Journal of Heart and Lung Transplantation. 34:S193. [Google Scholar]
- 9.Mohamedali B, Yost G, Bhat G. Obesity as a Risk Factor for Consideration for Left Ventricular Assist Devices. Journal of Cardiac Failure. 21:800–805. doi: 10.1016/j.cardfail.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report—2015; Focus Theme: Early Graft Failure. The Journal of Heart and Lung Transplantation. 34:1264–1277. doi: 10.1016/j.healun.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Healy AH, Baird BC, Drakos SG, Stehlik J, Selzman CH. Impact of Ventricular Assist Device Complications on Posttransplant Survival: An Analysis of the United Network of Organ Sharing Database. The Annals of thoracic surgery. 2013;95:870–875. doi: 10.1016/j.athoracsur.2012.10.080. [DOI] [PubMed] [Google Scholar]
- 12.John R, Pagani FD, Naka Y, et al. Post–cardiac transplant survival after support with a continuous-flow left ventricular assist device: Impact of duration of left ventricular assist device support and other variables. The Journal of Thoracic and Cardiovascular Surgery. 2010;140:174–181. doi: 10.1016/j.jtcvs.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Takayama H, Kalesan B, et al. Outcome of cardiac transplantation in patients requiring prolonged continuous-flow left ventricular assist device support. The Journal of Heart and Lung Transplantation. 2015;34:89–99. doi: 10.1016/j.healun.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Smedira NG, Hoercher KJ, Yoon DY, et al. Bridge to transplant experience: factors influencing survival to and after cardiac transplant. J Thorac Cardiovasc Surg. 2010;139:1295–305. 1305.e1–4. doi: 10.1016/j.jtcvs.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Hong KN, Iribarne A, Worku B, et al. Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. The Annals of thoracic surgery. 2011;92:520–7. doi: 10.1016/j.athoracsur.2011.02.086. discussion 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-first Official Adult Heart Transplant Report—2014;Focus Theme: Retransplantation. The Journal of Heart and Lung Transplantation. 2014;33:996–1008. doi: 10.1016/j.healun.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. International journal of obesity (2005) 2013;37:333–40. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 18.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122:3415–3422. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikus E, Stepanenko A, Krabatsch T, et al. Reversibility of fixed pulmonary hypertension in left ventricular assist device support recipients. European Journal of Cardio-Thoracic Surgery. 2011;40:971–977. doi: 10.1016/j.ejcts.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous Flow Left Ventricular Assist Device Improves Functional Capacity and Quality of Life of Advanced Heart Failure Patients. Journal of the American College of Cardiology. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 21.Benton CR, Sayer G, Nair AP, et al. Left Ventricular Assist Devices Improve Functional Class without Normalizing Peak Oxygen Consumption. Asaio Journal. 2015;61:237–243. doi: 10.1097/MAT.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 22.Mancini D, Goldsmith R, Levin H, et al. Comparison of exercise performance in patients with chronic severe heart failure versus left ventricular assist devices. Circulation. 1998;98:1178–83. doi: 10.1161/01.cir.98.12.1178. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson LW. The urgent priority for transplantation is to trim the waiting list. The Journal of Heart and Lung Transplantation. 32:861–867. doi: 10.1016/j.healun.2013.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.