Abstract
Introduction
Donor specific anti-HLA antibodies (DSA) are common following heart transplantation and are associated with rejection, cardiac allograft vasculopathy (CAV), and mortality. Currently a non-invasive diagnostic test for pathologic AMR (pAMR) does not exist.
Methods
221 consecutive adult patients underwent heart transplantation from January 1st, 2010 through August 31th, 2013 and followed through October 1st, 2015. The primary objective was to determine whether the presence of DSA could detect AMR at the time of pathologic diagnosis. Secondary analyses included the association of DSA (stratified by MHC Class and de-novo status) during AMR with new graft dysfunction, graft loss (mortality or retransplantation), and development of CAV.
Results
During the study period 69 individual patients (31.2%) had DSA (24% had de-novo DSA) and there were 74 episodes of pAMR in 38 unique patients. The sensitivity of DSA at any MFI to detect concurrent pAMR was only 54.3%. The presence of any DSA during pAMR increased the odds of graft dysfunction (OR 5.37, 95% CI 1.34–21.47, p=0.018), adjusting for age, gender, and timing of AMR. Circulating Class II DSA after transplantation increased the risk of future pAMR (HR 2.97, 95% CI 1.31–6.73, p=0.009). Patients who developed de-novo Class II DSA had a 151% increase in risk of graft loss (contingent on 30-day survival) compared with those who did not have DSA (95% CI 1.11–5.69, p=0.027).
Conclusions
DSA were inadequate to diagnose pAMR, but Class II DSA provided prognostic information regarding future pAMR, graft dysfunction with pAMR, and graft loss.
Introduction
Donor-specific anti-HLA antibodies (DSA) develop in up to 50% of patients following solid organ transplantation. (1) It is known that DSA are detrimental following orthotopic heart transplantation (OHT), leading to increased cellular rejection (2), cardiac allograft vasculopathy (CAV) (3–5), antibody mediated rejection (AMR) (4), and mortality. (6–8) Prior to 2010, International Society for Heart and Lung Transplantation (ISHLT) guidelines for the diagnosis of AMR required the presence of DSA. (9) At a 2010 ISHLT consensus conference on AMR, DSA were removed as a requirement from the diagnostic criteria, though the panel strongly recommended screening for DSA at the time of AMR. (10) A recent study of pediatric heart transplant recipients concluded that DSA were sensitive to detect an episode of pathologic AMR (pAMR) grade 2 or 3 (AUC 0.75–0.79). (11) This study aimed to examine the role of DSA in AMR among an adult population.
Methods
This was a prospective cohort study that enrolled all 221 adult (age ≥ 18 years) patients who underwent heart transplantation at Columbia University Medical Center from January 1st, 2010 through August 31th, 2013. Patients were followed for clinical events through October 1st, 2015. The study protocol was approved by the Columbia University Medical Center Institutional Review Board.
Prior to transplantation, patients were screened with both complement-dependent cytotoxic enzyme-linked immunoassay analysis and a solid phase assay, Luminex LABScreen Single Antigen (One Lambda, Canoga Park, CA). A patient was considered sensitized pre-transplant if they had anti-HLA antibodies with an MFI greater than 5,000. Highly sensitized patients were treated pre-transplant with IVIG and/or plasmapheresis followed by B-cell depleting therapies (bortezomib, cyclophosphamide). A virtual cross-match was performed for all patients and a prospective cross-match was performed when technically feasible. If a prospective cross-match was not performed, a simultaneous cross-match was performed (concurrent with the transplant). No patients during the study period were transplanted with a positive cross-match, though highly sensitized patients were treated with perioperative IVIG.
Following OHT, routine surveillance endomyocardial biopsies (EMB) were performed weekly in the first month after transplantation, then every two weeks for one month, monthly for four months, bimonthly six months, every three months for six months, and then every six to twelve months. Thereafter EMB was performed annually unless clinical rejection was suspected. With each EMB, four to five pieces of the right ventricular endomyocardium were obtained. Each biopsy was graded for AMR according to the current ISHLT guidelines (pAMR 1h, 1i, 2, or 3). (12) C4d staining was performed by immunohistochemistry on formalin fixed paraffin embedded tissue. While there is no consensus on using C3d, our institution has also been staining for C3d routinely. The Cell Marque C4d (SP91) antibody was used following heat induced epitope retrieval at pH 6. Staining was performed with the Leica Biosystems BOND-III automated stainer with Bond Polymer Refine Detection with 3,3’-diaminobenzidine as the chromogen. Immunohistochemical staining was performed on all biopsies early post-transplant, for all histologically suspicious biopsies, for all biopsies graded ISHLT 1R/1B or greater, and when requested on clinical grounds (including symptoms of rejection, changes in EF or cardiac index [with or without new DSA], recent cellular rejection). Blood was drawn concurrently with each EMB to screen for DSA using complement-dependent cytotoxic analysis and solid phase assay, Luminex LABScreen Single Antigen (One Lambda, Canoga Park, CA). C1q testing was not regularly used. De-novo DSA were defined as DSA that were never detected prior to OHT.
Induction therapy with basiliximab was used unless a contraindication existed (infection, bleeding, or retransplantation). Standard immunosuppressive regimens included prednisone, a calcineurin inhibitor (tacrolimus or cyclosporine), and mycophenolate mofetil. Chronic immunosuppression was modified if patients developed renal insufficiency (creatinine >2.5 mg/dL) or CAV through the use of sirolimus or everolimus.
Screening for CAV was initially performed at one year post-OHT and then every other year thereafter with coronary angiograms. Additional angiograms were performed if there was unexplained graft dysfunction. Dobutamine stress echocardiography (DSE) was used to screen for CAV during non-angiogram years. If patients had a creatinine above 2.0 mg/dL, DSE was used in place of angiography. Angiograms were graded according to the 2010 ISHLT CAV recommended nomenclature by the diagnosing cardiologist (13).
The primary objective was to determine the ability (sensitivity, specificity, negative predictive value) of DSA to predict AMR at the time of pathologic diagnosis. Secondary analyses included the association of DSA (stratified by MHC Class and de-novo status) during AMR with graft dysfunction, graft loss (mortality or retransplantation), and subsequent development of CAV. We also investigated the future risk of AMR among those who develop DSA. Graft dysfunction was defined as at least two of the following: 33% decrease in cardiac index and a cardiac index of less than 2.2 L/min/m2, 33% increase in pulmonary capillary wedge pressure, or 33% decrease in left ventricular ejection fraction (14, 15).
Statistical Analysis
Demographic and clinical variables were summarized with standard descriptive statistics and expressed as median (with interquartile range) for skewed continuous variables and count (with percentage) for categorical variables. Group comparisons were made with the Chi-squared and the Kruskal-Wallis test where appropriate. Sensitivity, specificity, and predictive values were determined using 2x2 contingency tables. Receiver operator characteristic (ROC) curves were constructed and the areas under the curve (AUC) were calculated for the diagnostic performance of DSA to detect pAMR for mean fluorescence intensities (MFI) ranging from 1,000 to greater than 10,000. Kaplan-Meier survival analysis, unadjusted, and multivariable Cox proportional-hazards regression were performed to determine survival statistics. All statistical tests were 2-tailed with statistical significance defined to be at the 0.05 level. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
The cohort consisted of 221 consecutive patients who had a total of 3,790 DSA-EMB pairs. Sixty-nine individual patients (31.2%) had DSA detected following OHT, with a total of 494 DSA of any MFI detected. Among those, 340 were de-novo post-OHT DSA in a total of 53 patients (24%). Average age was similar among those with DSA compared to those without DSA, as was use of induction therapy. However, a greater proportion of those with DSA were female and mechanical circulatory support was used more frequently in those without DSA (Table 1). During a median of 3.5 years of follow-up AMR occurred 74 times (56 pAMR 1i, 10 pAMR 1h, 8 pAMR 2) in 38 unique patients (prevalence of 17.2% in the cohort), with a greater frequency among those with DSA. Cellular rejection was more frequent in those with DSA.
Table 1.
| DSA | No DSA | p-value | |
|---|---|---|---|
| n | 69 | 152 | |
|
| |||
| Age (y) | 53.8 (42.5–62.8) | 59.4 (47.0–64.3) | 0.17 |
|
| |||
| Sex | 0.08 | ||
| Male (%) | 47 (68.1) | 120 (79.0) | |
| Female (%) | 22 (31.9) | 32 (21.0) | |
|
| |||
| Donor Age (y) | 46 (33.0–60.0) | 50.5 (34.5–61.0) | 0.21 |
|
| |||
| MCS Bridge (%) | 33 (43.8) | 95 (62.5) | 0.04 |
|
| |||
| Induction (%) | 51 (73.9) | 102 (67.1) | 0.31 |
|
| |||
| AMR (%) n=210 | 27 (39.1) | 11 (7.8) | <0.0001 |
|
| |||
| ACR (%) n=210 | 41 (59.4) | 35 (24.8) | <0.001 |
|
| |||
| Sensitized Pretransplant (%) | 11 (15.9) | 7 (4.6) | 0.004 |
|
| |||
| MHC Class of DSA | |||
| Class I Only | 14 (20.3) | ||
| Class II Only | 34 (49.3) | ||
| Class I & II | 21 (30.4) | ||
MCS=Mechanical circulatory support; AMR=Antibody mediated rejection; ACR= Acute cellular rejection; DSA=Donor specific antibodies
Sensitivity and Specificity of DSA at the Time of Pathologic AMR Diagnosis
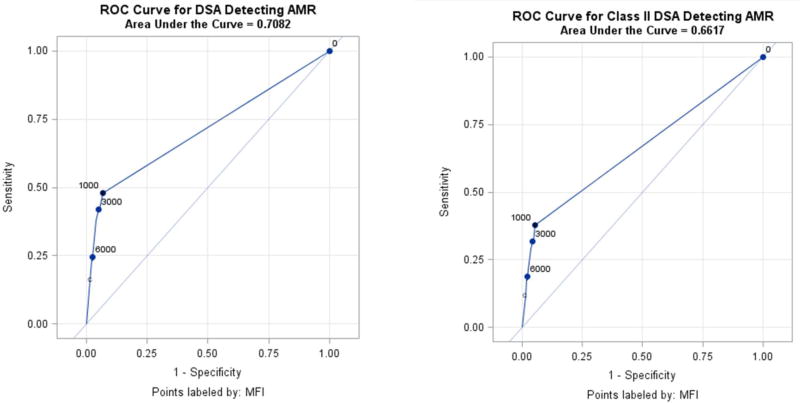
DSA were an unreliable test to screen for pAMR in this cohort. Analyzing DSA of any MFI, the sensitivity to detect concurrent pAMR was 54.3%, 44.4% for MFI>4,000, 23.5% for MFI>10,000 (Figure 1A). The sensitivity to detect pAMR remained poor for MHC Class II DSA (Figure 1B). Despite limited efficacy to rule out pAMR, the specificity of DSA for pAMR was acceptable. For DSA of any MFI the specificity was 93.4%, increasing to 98.1% when the MFI cutoff was 4,000. Similarly the negative predictive value (NPV) was 98.9%, but this was at least in part due to the low prevalence of pAMR on biopsy (2.0% of all biopsies) in this cohort.
Figure 1.
A. Receiver operator characteristic curve for donor specific antibodies detecting AMR B. Receiver operator characteristic curve for class II donor specific antibodies detecting AMR
Patients that had DSA present during pAMR had an increased risk of graft dysfunction. The presence of DSA of any MFI or MHC Class during an episode of pAMR led to a fivefold increase in the odds of concomitant graft dysfunction (OR 5.37, 95% CI 1.34–21.47, p=0.018), adjusting for age, gender, and timing of AMR (<1 year, >1 year). (14) Comparing the effect of MHC Class (adjusting for the same covariates), there was a nearly equivalent increase in the odds of accompanying graft dysfunction for those with only Class I DSA (OR 5.02, 95% CI 0.63–40.3, p=0.13) and Class II DSA (OR 5.44, 95% CI 1.3–22.6, p=0.02). Interestingly the odds of graft dysfunction did not correlate with MFI.
DSA and Risk of Future AMR
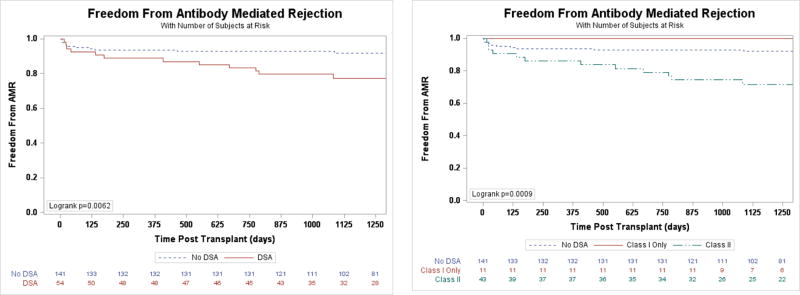
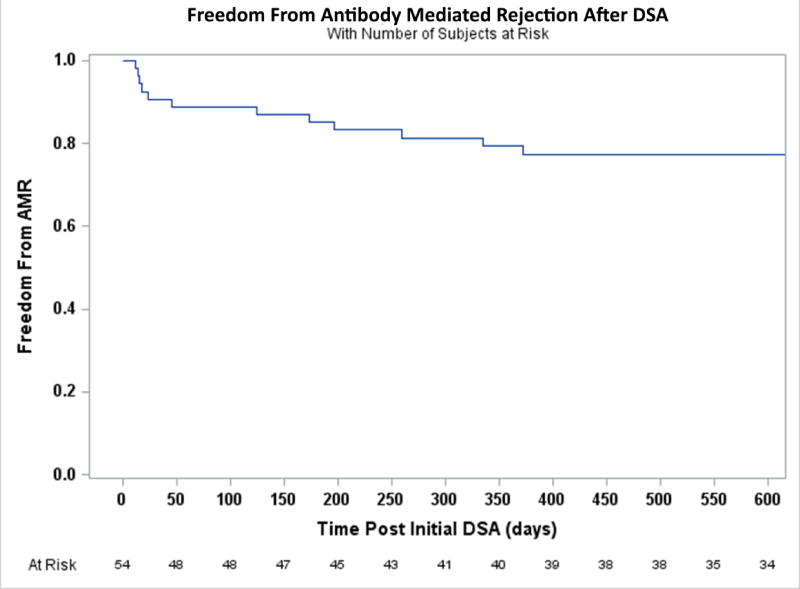
While DSA were unsuccessful in detecting concomitant pAMR in this study, they were predictive of future events. In a crude analysis, patients who made DSA at any time had more than seven times the odds of developing at least one episode of AMR during the entire follow-up period (OR 7.60, 95% CI 3.47–16.62, p<0.0001) compared with those who never made DSA. A time to event analysis of patients without previous AMR and who developed circulating DSA after transplantation (n=54) demonstrated that those patients had a nearly three-fold increased risk of future AMR (HR 2.97, 95% CI 1.31–6.73, p=0.009, Figure 2A) compared to those without prior AMR and who never developed DSA. Among those with DSA, the increased risk for AMR occurred only for patients with Class II DSA (Figure 2B). There was no interaction with DSA de-novo status (p=0.23). For those 54 patients the risk of AMR was the greatest in the first 30 days following DSA detection (Figure 3).
Figure 2.
A. Freedom from initial episode of AMR for based on DSA status B. Freedom from initial episode of AMR based on DSA class
Figure 3.
Freedom from initial episode of AMR following detection of DSA
DSA and Risk of Graft Loss
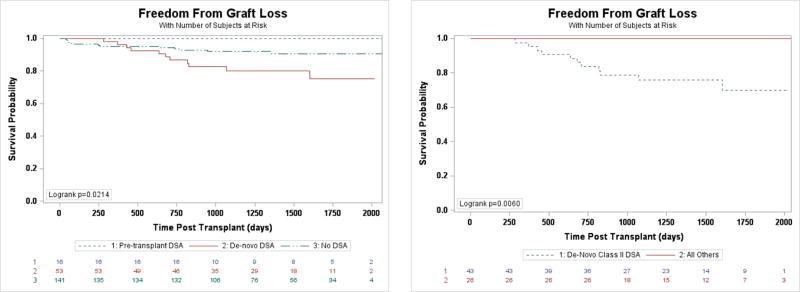
There was no evidence of an increased risk of graft loss between those with AMR with DSA, AMR without DSA, DSA alone, or neither DSA nor AMR (p=0.24). Notably, in every instance of graft loss associated with AMR, the DSA included Class II anti-HLA antibodies. De-novo DSA following transplantation have been shown to confer the greatest risk of post-transplant graft loss (8); our findings are consistent with this observation. Those with DSA that were present prior to transplantation had the lowest risk of graft loss (Figure 4A). Patients who developed de-novo DSA had a 151% increase in risk of graft loss (contingent on 30-day survival) compared with those who did not have DSA (95% CI 1.11–5.69, p=0.027). This risk remained after controlling for cellular rejection and recurrent AMR (HR 2.79, 95% CI 1.07–7.30, p=0.036). Notably, the risk of graft loss was attributable to de-novo Class II DSA (Figure 4B). As for Class I DSA, survival of those with pre-transplant and de-novo DSA was not diminished.
Figure 4.
A. Freedom from graft loss depending on de-novo DSA status. B. Freedom from graft loss based on de-novo DSA status and MHC class
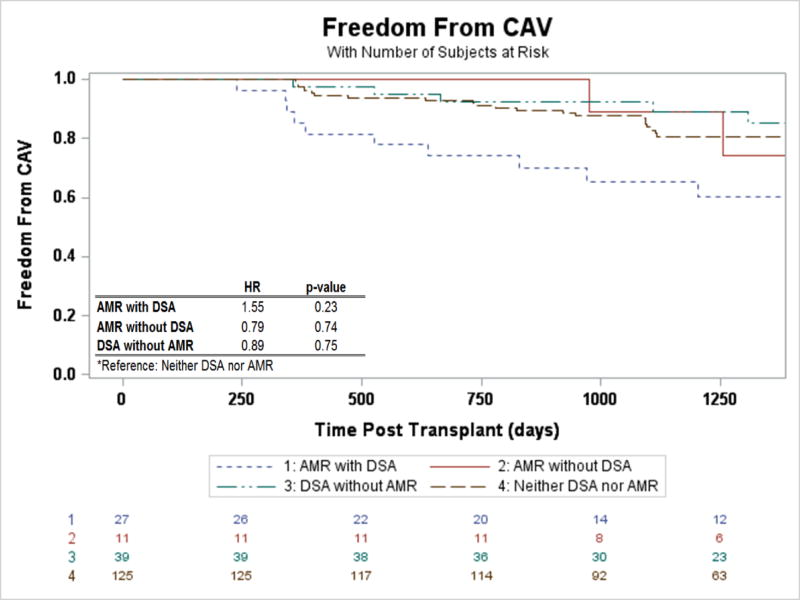
DSA and Risk of CAV
Analyzing the freedom from CAV (ISHLT CAV1 or greater) following transplantation, statistically there was no significant difference, though there was a suggestion that the CAV risk may be increased for those with AMR with DSA when compared to patients without DSA or AMR (Figure 5, HR 1.55, 95% CI 0.76–3.19, p=0.23). This risk did not differ by class of DSA (p=0.37). Freedom from CAV after the initial episode of AMR was also not different between groups (p=0.73).
Figure 5.
Freedom from CAV stratified by AMR and DSA status
Discussion
The existence of antibody mediated rejection has only been universally accepted for the last decade and not surprisingly the diagnosis and treatment continue to evolve. This prospective cohort study of 221 consecutive adult transplant patients with over 640 patient years of follow-up sought to determine the role that anti-HLA donor specific antibodies play in antibody mediated rejection and resulted in four primary findings. First, DSA were an inadequate screening test for pAMR. Next, the presence of DSA during an episode of AMR was associated with a fivefold increase the odds of graft dysfunction for both Class I & II DSA. Third, Class II DSA increased the risk of future AMR by three times, while Class I DSA did not. Lastly, post-transplant de-novo Class II DSA were associated with an increased risk of graft loss.
Following heart transplantation, patients undergo frequent endomyocardial biopsy during the first year; however afterwards routine biopsies are performed infrequently and often are prompted by clinical symptoms. Treated AMR in the first year after transplantation was shown to have good outcomes in one recent study (14), however late AMR with graft dysfunction has been shown to be associated with significant one year mortality (50–60%) despite treatment. (16–18) The poor outcomes in these studies were likely due to CAV and/or preceding subclinical AMR. Loupy et al. (19) recently described the presence of undiagnosed sub-clinical AMR in 40% of a cohort of patients that developed allograft failure. These studies demonstrate the importance of developing a reliable non-invasive screening test for AMR. The findings of this study suggest that measurement of DSA is not sensitive enough to be that indicator, as it performed only slightly better than a coin flip in this cohort. Similarly disappointing was a recent finding that the Total Allomap score in addition to gene subsets was not able to detect AMR. (20)
Complement deposition has been described as the “sine qua non” of AMR (21) and is needed (C4d) to diagnose immunopathologic AMR. (12) Production of C4d through the classical pathway requires antibody-antigen complex formation. However, in this study DSA were not found to be adequate to detect concomitant pAMR, though were found to have important prognostic significance. Patients with Class II DSA had three times the risk of future AMR when compared to those without DSA, the odds of significant hemodynamic and echocardiographic graft dysfunction when DSA were present during an episode of AMR were 437% greater, and Class II DSA were associated with an increased risk of mortality. These data signal that DSA (mostly Class II) were not innocent bystanders, though were also not sufficient to diagnose pAMR. A possible explanation may be that we are not screening for the correct antibody or enough antibodies. Antibodies to non-HLA antigens (MICA, MICB, endothelium, vimentin, myosin, angiotensin II type 1 receptor, and polyreactive) are capable of activating complement, though there is limited data about their role in AMR or in post-transplant care. Alternatively it may be that the episodes of anti-HLA DSA negative AMR do not require antibodies at all and are mediated by the antibody-independent lectin pathway. (22,23)
The ideal screening test for AMR remains unknown. The current gold standard is EMB and pathologic diagnosis. This method is not without problem as the disease may be focal and EMB itself may be inadequate to arrive at the diagnosis. This study has shown that DSA provide important information, but is not independently sensitive enough to make the diagnosis. Given the prevalence of pAMR (17% in this sample, with nearly half DSA negative) and the known deleterious effects of AMR, further collaborative study is needed focusing on other non-invasive means of diagnosing AMR such as biomarkers or non-HLA antibodies. An additional research question is whether treatment of DSA without concomitant pAMR is warranted. DSA have previously been shown to increase cellular rejection (2), CAV (3–5), and mortality (6–8). The additional findings of this study demonstrating Class II DSA increase the risk of future AMR, increase the risk of graft dysfunction with AMR, and that de-novo Class II DSA increase the risk of graft loss, suggest there is sufficient evidence to prompt a clinical trial for the treatment of Class II DSA in the absence of AMR.
This study was limited by the fact that it was conducted at only one large transplant center, which may potentially limit generalizability. While the number patients, biopsies, and DSA measurements in this cohort were substantial for a study of this nature, the absolute number of patients limited statistical power and subgroup analyses. Further, the follow-up was relatively short, ranging from two to five years, which limits the number of events. Additionally, CAV was diagnosed predominantly with angiography and not intracoronary imaging, which may have limited the ability to detect more subtle disease (e.g. intimal thickening preceding coronary artery luminal narrowing). Mean fluorescence intensity measurements have been known to vary between different HLA laboratories (24) and may limit the external validity of the findings related to MFI in this study; though the absence of findings for an MFI over 10,000 and graft dysfunction would suggest a true lack of association. Lastly, this study was observational and not interventional; therefore we are unable to comment on treatment strategies of asymptomatic DSA.
In conclusion, DSA were inadequate to diagnose pAMR, but Class II DSA provided prognostic information regarding future AMR, graft dysfunction with AMR, and graft loss.
Acknowledgments
KJC is supported by National Institutes of Health Grant T32 HL007854-16.
Footnotes
No other authors have disclosures pertinent to the contents of this study.
References
- 1.Valenzuela NM, Reed EF. Antibodies in Transplantation: The Effects of HLA and Non-HLA Antibody Binding and Mechanisms of Injury. Methods in molecular biology (Clifton, NJ) 2013;1034 doi: 10.1007/978-1-62703-493-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George JF, Kirklin JK, Shroyer TW, et al. Utility of posttransplantation panel-reactive antibody measurements for the prediction of rejection frequency and survival of heart transplant recipients. J Heart Lung Transplant. 1995;14:856–64. [PubMed] [Google Scholar]
- 3.Kaczmarek I, Deutsch MA, Kauke T, et al. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation. 2008;6:229–35. [PubMed] [Google Scholar]
- 4.Taylor DO, Yowell RL, Kfoury AG, Hammond EH, Renlund DG. Allograft coronary artery disease: clinical correlations with circulating anti-HLA antibodies and the immunohistopathologic pattern of vascular rejection. J Heart Lung Transplant. 2000;19:518–21. doi: 10.1016/s1053-2498(00)00095-4. [DOI] [PubMed] [Google Scholar]
- 5.Topilsky Y, Gandhi MJ, Hasin T, et al. Donor Specific Antibodies to Class II Antigens Are Associated With Accelerated Cardiac Allograft Vasculopathy – A 3-D volumetric IVUS study. Transplantation. 2013;95:389–396. doi: 10.1097/TP.0b013e318273878c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith JD, Banner NR, Hamour IM, et al. De Novo Donor HLA-Specific Antibodies after Heart Transplantation Are an Independent Predictor of Poor Patient Survival. American Journal of Transplantation. 2011;11:312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 7.Tambur AR, Pamboukian SV, Costanzo MR, et al. The presence of HLA-directed antibodies after heart transplantation is associated with poor allograft outcome. Transplantation. 2005;80:1019–25. doi: 10.1097/01.tp.0000180564.14050.49. [DOI] [PubMed] [Google Scholar]
- 8.Ho EK, Vlad G, Vasilescu ER, et al. Pre- and posttransplantation allosensitization in heart allograft recipients: Major impact of de novo alloantibody production on allograft survival. Human Immunology. 2011;72:5–10. doi: 10.1016/j.humimm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Heart Rejection. The Journal of Heart and Lung Transplantation. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Kobashigawa J, Crespo-Leiro MG, Ensminger SM, et al. Report from a consensus conference on antibody-mediated rejection in heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30 doi: 10.1016/j.healun.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware AL, Malmberg E, Delgado JC, et al. The use of circulating donor specific antibody to predict biopsy diagnosis of antibody-mediated rejection and to provide prognostic value after heart transplantation in children. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35:179–85. doi: 10.1016/j.healun.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. The Journal of Heart and Lung Transplantation. 32:1147–1162. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:717–27. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Clerkin KJ, Restaino SW, Zorn E, Vasilescu ER, Marboe CC, Mancini DM. The effect of timing and graft dysfunction on survival and cardiac allograft vasculopathy in antibody-mediated rejection. The Journal of Heart and Lung Transplantation. 2016 doi: 10.1016/j.healun.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coutance G, Ouldamar S, Rouvier P, et al. Late antibody-mediated rejection after heart transplantation: Mortality, graft function, and fulminant cardiac allograft vasculopathy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1050–7. doi: 10.1016/j.healun.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Coutance G, Ouldamar S, Rouvier P, et al. Late antibody-mediated rejection after heart transplantation: Mortality, graft function, and fulminant cardiac allograft vasculopathy. The Journal of Heart and Lung Transplantation. 34:1050–1057. doi: 10.1016/j.healun.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Hodges AM, Lyster H, McDermott A, et al. Late antibody-mediated rejection after heart transplantation following the development of de novo donor-specific human leukocyte antigen antibody. Transplantation. 2012;93:650–6. doi: 10.1097/TP.0b013e318244f7b8. [DOI] [PubMed] [Google Scholar]
- 18.Clerkin KJ, Restaino SW, Zorn E, Vasilescu ER, Marboe CC, Mancini DM. The impact of timing and graft dysfunction on survival and cardiac allograft vasculopathy in antibody mediated rejection. The Journal of Heart and Lung Transplantation. 2016 doi: 10.1016/j.healun.2016.04.007. Accepted, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loupy A, Toquet C, Rouvier P, et al. Late Failing Heart Allografts: Pathology of Cardiac Allograft Vasculopathy and Association With Antibody-Mediated Rejection. American Journal of Transplantation. 2016;16:111–120. doi: 10.1111/ajt.13529. [DOI] [PubMed] [Google Scholar]
- 20.Adatya S, Delijkich E, Hiller D, Sayer G, Yee J, Uriel N. Utility of Gene Expression (Allomap Score) in Antibody Mediated Rejection Detection. The Journal of Heart and Lung Transplantation. 35:S33–S34. [Google Scholar]
- 21.Colvin MM, Cook JL, Chang P, et al. Antibody-Mediated Rejection in Cardiac Transplantation: Emerging Knowledge in Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation. 2015;131:1608–1639. doi: 10.1161/CIR.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 22.Murata K, Baldwin WM. Mechanisms of complement activation, C4d deposition, and their contribution to the pathogenesis of antibody mediated rejection. Transplantation reviews (Orlando, Fla) 2009;23:139–150. doi: 10.1016/j.trre.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata K, Fox-Talbot K, Qian Z, et al. Synergistic deposition of C4d by complement-activating and non-activating antibodies in cardiac transplants. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7:2605–14. doi: 10.1111/j.1600-6143.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 24.Reed EF, Rao P, Zhang Z, et al. Comprehensive Assessment and Standardization of Solid Phase Multiplex-Bead Arrays for the Detection of Antibodies to HLA. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:1859–1870. doi: 10.1111/ajt.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]