Abstract
Latino immigrants that work on farms experience chronic exposures to potential neurotoxicants, such as pesticides, as part of their work. For tobacco farmworkers there is the additional risk of exposure to moderate to high doses of nicotine. Pesticide and nicotine exposures have been associated with neurological changes in the brain. Long-term exposure to cholinesterase-inhibiting pesticides, such as organophosphates and carbamates, and nicotine place this vulnerable population at risk for developing neurological dysfunction. In this study we examined whole-brain connectivity patterns and brain network properties of Latino immigrant workers. Comparisons were made between farmworkers and non-farmworkers using resting-state functional magnetic resonance imaging data and a mixed-effects modeling framework. We also evaluated how measures of pesticide and nicotine exposures contributed to the findings. Our results indicate that despite having the same functional connectivity density and strength, brain networks in farmworkers had more clustered and modular structures when compared to non-farmworkers. Our findings suggest increased functional specificity and decreased functional integration in farmworkers when compared to non-farmworkers. Cholinesterase activity was associated with population differences in community structure and the strength of brain network functional connections. Urinary cotinine, a marker of nicotine exposure, was associated with the differences in network community structure. Brain network differences between farmworkers and non-farmworkers, as well as pesticide and nicotine exposure effects on brain functional connections in this study, may illuminate underlying mechanisms that cause neurological implications in later life.
Keywords: Latino immigrant workers, pesticide, nicotine, resting state fMRI, brain network, mixed model
1. Introduction
Latino immigrant workers employed on farms experience chronic exposures to cholinesterase-inhibiting pesticides such as organophosphates and carbamates [1, 2]. Such exposure to pesticides could place this vulnerable population at greater risk for the development of neurological dysfunction [3–5]. A recent longitudinal study by Quandt et al. [6] showed that total cholinesterase, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities in farmworkers are decreased during the agricultural season compared to non-farmworkers. Although the role of long-term exposures to low to moderate levels of pesticides remains controversial [7], a growing body of studies indicates that chronic exposure to cholinesterase-inhibiting pesticides is significantly related to cognitive impairment [8, 9]. The long-term effects of exposures to pesticides may include an increased risk of developing depression [10] or neurodegenerative disorders such as Alzheimer’s disease [11] and Parkinson’s disease [12–14].
Tobacco farmworkers are not only exposed to pesticides, but also experience nicotine exposure through dermal absorption. Tobacco farmworkers can have systemic nicotine levels that are comparable to regular smokers [15, 16]. While it is known that large doses of nicotine are toxic [17, 18], many studies have indicated that lower, nontoxic doses of nicotine improve cognitive performance through modulating the release of several neurotransmitters including acetylcholine and dopamine [19, 20]. An interesting finding is that nicotine may actually be protective against the development of Parkinson’s disease [21, 22]. Co-exposure to pesticides and nicotine is particularly interesting since both cholinesterase-inhibiting pesticides and nicotine alter cholinergic neurotransmission. Pesticides increase cholinergic neurotransmission by blocking the degradation of the acetylcholine. Nicotine, on the other hand, increases cholinergic neurotransmission by directly binding to the acetylcholine receptor.
The basal forebrain cholinergic system has projections that broadly innervate the cerebral cortex and subcortical nuclei. Changes in cholinergic neurotransmission could therefore have far-reaching rather than local, brain effects. Thus, studies aimed at evaluating the neurobiological changes associated with exposure to pesticides and nicotine would benefit by techniques that examine the brain as an integrated system rather than a collection of isolated brain areas.
Functional brain network analyses that use resting-state functional magnetic resonance imaging (rs-fMRI) have demonstrated great promise in examining systemic brain changes across health and disease [23–25]. FMRI, as a non-invasive technique, is sensitive to changes in blood oxygenation that occur in response to changes in brain acidity. The blood-oxygenation level-dependent (BOLD) signal is sensitive to the changes in the relative amounts of blood oxyhemoglobin (higher) and deoxyhemoglobin (lower) that occur with increased neural activity [26]. A typical fMRI study is performed by collecting multiple (often hundreds) scans of the brain to identify the BOLD signal fluctuation that occur over time. Rs-fMRI measures the spontaneous fluctuations of the BOLD signal when the participant is not performing an explicit task [27, 28]. Approximately 95 percent of the brain metabolism occurs due to these spontaneous fluctuations [29].
The statistical association or dependency among BOLD signals from different parts of the brain image is referred to as functional connectivity [27], and represents the functional interactions among different brain areas. Brain network analyses are based on graph theory and evaluate the connectivity patterns across the entire brain rather than focusing on connectivity to and from a single brain area [30]. Brain networks and graph theory methods are growingly used in studies of the human brain because these methods examine the brain as an integrated system [31]. Within a systems view of the brain, circuits are critical for normal and abnormal neurological processes rather than individual brain areas. Brain network analyses are proving to be clinically meaningful in studies of neurodegenerative disorders such as Alzheimer’s [32–35] and Parkinson’s [36, 37] diseases, as well as for evaluating brain changes associated with smoking [38, 39].
This study used brain network analysis of rs-fMRI data and a mixed-effects modeling framework [40] to compare brain network connectivity patterns between Latino immigrant workers engaged in farm work to those not engaged in farm work. The network analysis was used to characterize global as well as local brain connectivity patterns. This study provides important evidence for the potential neurobiological impacts of pesticide and nicotine exposures on the brains of Latino farmworkers.
2. Materials and Methods
2.1. Participants
The analysis reported here is based on data collected as part of a larger research project, “Pesticide Exposures & Neurological Outcomes for Latinos: PACE4.” The PACE4 study is an ongoing community-based study with an initial size of 447 Latino male participants including migrant farmworkers and immigrant non-farmworkers. Farmworkers were recruited from east central North Carolina, and non-farmworkers were recruited from Forsyth County in west central North Carolina. Farmworkers were currently employed as agricultural laborers, and had worked in agriculture for at least 3 years. Non-farmworkers could not have worked in occupations in the past 3 years that exposed them to pesticides, such as farm work, forestry, landscaping, and lawn maintenance. The current study used the same population as in [41]. Briefly, 81 of the PACE4 participants were recruited to have a brain MRI scan. The current study focused on 74 participants (48 farmworkers and 26 non-farmworkers) with complete data, which including a brain image, at least had one blood sample for cholinesterase measurements, and one urine sample for cotinine measurements. Among the 7 excluded data, four participants had no blood sample, two had no urine sample, and one participant was missing both. All participants gave written informed consent for participation in the main study and consented again to participate in the imaging study. The study was approved by the Wake Forest School of Medicine Institutional Review Board.
Details of the parent study participant population [42] and this sub-population [41] have been previously reported. Participant characteristics for this study are included here for completeness. As shown in table 2.1, the farmworkers tended to be younger with less education. Neither difference achieved statistical significance, but both variables were included in the mixed statistical model. The smoking status was significantly different between the two groups. Due to this difference and our interests in the effects of nicotine on brain networks, smoking status and pack years were also included in the analyses. Note that there were significant differences in the country of origin with all farmworkers being from Mexico but 42% of non-farmworkers being from other countries. Despite this difference in country-of-origin, all participants in both groups identified as Latino.
Table 2.1.
Study Population Characteristics
| Participant Characteristics | Farmworkers (n = 48) |
Non-Farmworkers (n = 26) |
*p-value |
|---|---|---|---|
| Age | 40.33±6.99 (Min/Max: 31/71) | 43.61±10.49 (Min/Max: 30/58) | 0.1108 |
| Education | 0.0654 | ||
| 0–6 grade (Edu1) | 17 (35.4%) | 7 (26.9%) | |
| 7–11 grade (Edu2) | 24 (50.0%) | 9 (34.6%) | |
| 12 grade or more (Edu3) | 7 (14.6%) | 10 (38.5%) | |
| Country of birth | <0.0001 | ||
| Mexico | 48 (100%) | 15 (57.7%) | |
| Central America | 8 (30.8%) | ||
| South America | 3 (11.5%) | ||
| Occupation | N/A | ||
| Farmworker | 48 (100) | ||
| construction | 7 (26.9%) | ||
| Production | 6 (23.1%) | ||
| Food preparation/restaurant | 3 (11.5%) | ||
| Maintenance/cleaning | 3 (11.5%) | ||
| Sales | 1 (3.8%) | ||
| Mechanic | 2 (7.7%) | ||
| Other | 1 (3.8%) | ||
| Unemployed | 3 (11.5%) | ||
| Pack years smoked at baseline, yrs | 1.6695±4.64 | 0.6501 ±2.94 | 0.3617 |
| Smoking status | 18 smokers (37.5%) | 2 smokers (7.7%) | <0.0001 |
2.2. Cholinesterase and Cotinine measurements
Whole blood acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) activities (umole/min/ml), and urinary cotinine levels (ng/ml) were used as indirect measures of exposure to pesticide and nicotine, respectively. Cholinesterase activity was measured from blood samples that were collected June 2, 2013, to October 20, 2013. Average across the summer (for those with multiple samples) was used in our analyses. Most participants had three or four samples but five individuals (four non-farmworkers, one farmworker) had two samples and a single participant (farmworker) had one sample. Cholinesterase activities were assayed with a modification of the radiometric method of Johnson and Russell [43]. Urine samples that were assessed for cotinine levels were collected between June 2, 2013, and October 20, 2013. The assay was performed by Salimetrics LLC using standard procedures. Details about the assays can be found in works published by Quandt et al. [6] and Arcury et al. [15].
2.3. Image acquisition
All imaging was carried out at Wake Forest School of Medicine using a Siemens 3T Skyra scanner equipped with a 32-channel head coil. For the functional data, whole-brain gradient echo-planar imaging (EPI) sequence was employed to acquire the blood-oxygenation level-dependent (BOLD) contrast images with the following parameters: slice thickness = 4.0 mm, in-plane resolution = 4 mm × 4 mm, TR = 1700ms, and 157 volumes with 35 contiguous slices per volume. The first 8 image volumes were discarded to allow tissue magnetization to achieve steady-state. High-resolution (0.98mm×0.98mm×1.0mm) T1-weighted scans were acquired in the sagittal plane using a single-shot 3D MPRAGE GRAPPA2 sequence with the following parameters: acquisition time = 5.0min and 30s, TR = 2.3s, TE = 2.99ms, 192 slices. High-resolution images were used in preprocessing the functional images.
2.4. Image processing and network generation
The rs-fMRI data underwent a series of standard preprocessing steps using Statistical Parametric Mapping 8 (SPM8) software (Wellcome Trust Center, London, UK: www.fil.ion.ucl.ac.uk/spm/). T1-weighted structural images were first normalized to MNI standard space (www.mni.mcgill.ca) with 4×4×4 mm3 voxels. Functional scans were realigned to the first volume for head movement correction and slice-time corrected. Functional scans were then co-registered to their structural images, and transformed into the standard space using the registration transformation matrices. Low frequency drift and physiological noise were reduced through standard band pass filtering (0.009–0.08Hz), and regressing out motion parameters, and whole brain mean signal, mean white matter (WM), and mean cerebral spinal fluid (CSF) signals [44, 45] using in-house generated Matlab scripts.
Using the preprocessed data and the automated anatomical labeling (AAL) atlas [46], mean time series of 116 brain regions were extracted through averaging the time series of all voxels in each region. A functional brain network for each participant was constructed through computing the Pearson (full) correlation between all pairs of the time series. Each one of the 116 brain regions represents a node in the network and the correlation between the nodes quantifies the weighted edge between them. Negative correlations were set to zero for the subsequent analyses as multiple graph variables, clustering in particular, remain poorly understood in networks containing negative edges [47, 48]. This is a standard procedure for brain network analyses because most graph analysis algorithms that yield topological network properties cannot accommodate negative edges. We also generated networks using partial correlation due to its capability in distinguishing direct from indirect connections between brain regions [49]. Unfortunately, there were convergence problems in mixed model fits using partial correlations. Additionally, partial correlation may underperform full correlation in certain contexts due to the small fraction of indirect connections and the over removal of signal resulting from the large number of regressors [50]. Thus, all results presented here are for the full correlation analyses.
2.5. Mixed-effects modeling framework
Regression analysis is mainly used for two purposes: 1) predicting values of a dependent variable(s) from the values of a number of independent variables, and 2) determining which independent variables are associated with the dependent variable(s) and the magnitude of the association. This study used the regression analysis for the latter purpose. Since we had repeated measurements (i.e., thousands of functional connectivity measures between different brain regions for each person), a mixed-effects regression framework was employed to capture and account for the correlations between the repeated measurements of each participant. In other words, a functional connection between one pair of brain regions in a participant is not independent of the other pairs of connections within that participant’s brain. A mixed-effects regression framework allows dealing with this situation through including random effects for each participant.
We used a two-part, mixed-effects modeling framework [40] to statistically compare the global and local network properties between Latino immigrant workers employed on farms to those in working in other industries with low likelihood of pesticide exposure. The modeling framework allowed comparing network properties between the two groups through the inclusion of interaction covariates. The flexibility of the mixed model also allowed for the inclusion of non-network variables. This allowed us to include cholinesterase enzyme activity levels and urinary cotinine (a nicotine metabolite) levels to quantify the relationship between brain network patterns and cholinesterase and nicotine levels. We were also able to control for important confounding variables such as age, education, and smoking status. The approach models both the probability of having a connection (presence/absence) and the strength of a connection if it exists. The modeling framework is detailed below.
Let Tijk denote a variable specifying whether a connection exists between node j and node k for the ith participant, and Wijk (Wijk ≥ 0) denote the connection strength (i.e. the (j,k)th element in the ith participant’s correlation matrix); then, we can write:
| (2.1) |
| (2.2) |
where pijk is the probability of having a connection between node j and node k for the ith participant, β is a vector of parameters corresponding to the fixed effects, and bi is the random effects vector for participant i. Fixed effects represent the population-based parameters, and random effects are participant-specific parameters that capture correlation between repeated measurements of each participant (i.e., capture between-participant variations). Assuming that Sijk is a non-zero correlation value (i.e. Sijk = Wijk > 0), the two-part mixed-effects modeling framework can be defined as below (where the subscripts r and s denote parameters for the presence (Part 1) and strength (Part 2) modes, respectively):
| (2.3) |
| (2.4) |
Here Xijk and Zijk are design matrices for the fixed and random effects with the same set of covariates in both models, and εijk captures the random noise in the connection strength between node j and node k for the ith participant. Equation 2.3 is a logistic mixed-effects regression model that models the relationship between the probability (pijk) of having a connection and a set of covariates (Xijk). In general, logistic regression models are used for modeling the probability of binary outcomes (presence or absence of a functional connection in our case). Equation 2.4 quantifies the relationship between the strength of present connections and the same set of covariates. FZT is the Fisher’s Z-Transform applied to correlation values to ensure the normality assumption is met. Using equations 2.3 and 2.4 we evaluated the relationships between a set of covariates and probability and strength of brain connections. Including interactions of brain network properties with the covariate of interest in these models allowed finding brain network differences between farmworkers and non-farmworkers. To complement the mixed model findings, traditional univariate comparisons of brain network properties were also performed using a T-test.
2.6. Covariates
Farmworker Status (FWS)
FWS, our main covariate of interest, was a binary variable that distinguished farmworkers from non-farmworkers. We used farmworkers as the reference group. (FWS was set to zero for all connections corresponding to farmworkers and one for all connections corresponding to non-farmworkers.)
Network Covariates (Net)
Four metrics that represented different properties of the brain network were used. Network segregation, integration, and resilience were characterized by using the average clustering coefficient, average global efficiency, and degree difference in each nodal pair, respectively [51]. For degree, the difference was used to capture assortativity [52]. The overall modularity [53] was another utilized metric that quantified how much the network subdivides into interconnected communities (community structure).
Interaction Covariates (Int)
Interaction covariates included interactions of the four network metrics with FWS. These covariates determined if the relationships between the outcome variable (connection probability or strength) and network metrics (clustering coefficient, global efficiency, degree, and modularity) were different between farmworkers and non-farmworkers.
Exposure Covariates (Exp)
Blood acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities (umole/min/ml) were used as markers of pesticide exposure. Urinary cotinine level (ng/ml) was used as a marker of nicotine exposure.
Confounding covariates (Conf)
Six variables including age (as a continuous variable), educational level, pack years of smoking as a continuous variable (smok_years), smoking status (smok_status), spatial Euclidean distance (dist1) between nodes (i.e., brain regions) [54], and the square of spatial Euclidean distances (dist2) between nodes were used as confounding covariates. Smoking status was a binary variable that distinguished current smokers from non-smokers. Non-smoker group was used as the reference group in the modeling analysis. Pack years of smoking was calculated based on the National Cancer Institute definition:
Educational level was a categorical variable with three levels of educational attainment years including, level 1: 0–6, level 2: 7–11, and level 3: ≥12. These three levels are represented by Edu1, Edu2, and Edu3, respectively. The third educational level (Edu3) was used as the reference group in the modeling process. These variables were categorized as confounders because they were not the focus of this study, and they were assumed to affect the functional connections in the brain.
Thus, the fixed effects parameters βr and βs can be decomposed into βr = [βr,0 βr,Net βr,Exp βr,FWS βr,Int βr,Conf] and βs = [βs,0 βs,Net βs,Exp βs,FWS βs,Int βs,Conf]. βr,0 and βs,0 correspond to the intercepts in equations 2.3 and 2.4, respectively. Figure 2.1 shows different steps of our approach.
Figure 2.1. Schematic of different steps.
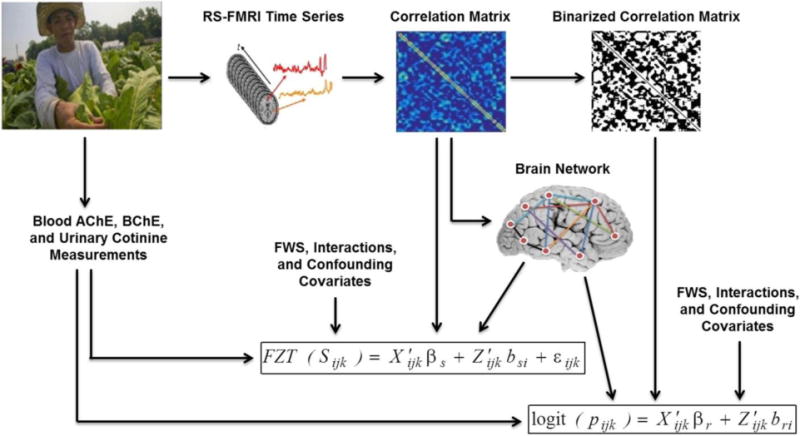
Rs-fMRI data were collected from each study participant. The average time series was determined from 116 anatomical brain regions as defined in the AAL atlas. Each region served as a network node. A correlation matrix was obtained through calculating the Pearson correlation between the average time series from every node pair, with negative correlation set to zero. The adjacency matrix was obtained via binarizing the correlation matrix. The four network metrics including nodal clustering coefficient, global efficiency, degree and overall modularity were extracted from the weighted brain network. These metrics along with exposure measurements including blood AChE and BChE activities, and urinary cotinine levels, farmworker status (FWS), and confounding variables were used as covariates in the two-part mixed-effects modeling framework to assess the relationship of farmworker status (and other covariates) with the probability and strength of functional brain connections. (2-column figure)
The random effects bri, bsi, and error εi were assumed to be normally distributed and mutually independent. We used an unstructured covariance matrix for random effects in terms of their Cholesky-root factors [55]. Unstructured covariance matrices are the most common forms of covariance structures for modeling data sets with small number of random effects. We also used a grouping effect that allowed modeling two different sets of covariance parameters for farmworkers and non-farmworkers. The parameters in equations 2.3 and 2.4 were estimated via a restricted pseudo-likelihood approach [56] with the residual denominator degrees of freedom approximation of the F-test for a Wald statistic used for inference. Analyses were conducted in SAS software v9.4.
To clarify how the modeling framework links the connectivity probability and strength to a set of covariates, it is important to note that each parameter presented in table 3.1 represents the change in the log odds of an edge existing (probability model) and the change in the average strength of that connection (strength model) for each unit change in the given covariate. The network metric parameters, βNet = [βClust βEglobe βDeg βModul], give the change in the log odds of an edge existing and the change in the average strength of that connection for each unit increase in the respective metrics for farmworkers. The interaction parameters, βInt = [βFWSxClust βFWSxEglobe βFWSxDeg βFWSxModul], give the additional change in the log odds of an edge existing and the additional change in the average strength of that connection for each unit increase in the respective metrics for non-farmworkers.
3. Results
3.1. Brain Network Metrics
Table 3.1 presents the parameter estimates, standard errors, and False Discovery Rate (FDR)-corrected p-values obtained for the probability (eq. 2.3) and strength (eq. 2.4) models. The table details the relationship between the covariates and the probability and strength of functional connections in the brain.
Table 3.1.
Parameter estimates, standard errors, and p-values for probability and strength models
| Probability (Presence) Model Outputs | Strength Model Outputs | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Parameter | Estimate | SE | *p-value | Parameter | Estimate | SE | *p-value |
| βr,0 | 0.01793 | 0.03245 | 0.5807 | βs,0 | 0.3205 | 0.006376 | <0.0001 |
| βr,Clust | 10.5373 | 0.6255 | <0.0001 | βs,Clust | 2.0462 | 0.07152 | <0.0001 |
| βr,Eglobe | −20.8878 | 1.3925 | <0.0001 | βs,Eglobe | 2.0806 | 0.1678 | <0.0001 |
| βr,Deg | 0.2360 | 0.02353 | <0.0001 | βs,Deg | −0.08181 | 0.001607 | <0.0001 |
| βr,Modul | 0.1502 | 0.4075 | 0.7124 | βs,Modul | −0.6123 | 0.09568 | <0.0001 |
| βr,Cotinine | −0.00585 | 0.01074 | 0.5862 | βs,Cotinine | −0.00312 | 0.002444 | 0.2018 |
| βr,AChE | 0.04860 | 0.02623 | 0.0639 | βs,AChE | −0.01949 | 0.005883 | 0.0009 |
| βr,BChE | −0.05218 | 0.07858 | 0.5067 | βs,BChE | −0.00675 | 0.01796 | 0.7072 |
| βr,FWS | −0.03901 | 0.04712 | 0.4077 | βs,FWS | 0.005605 | 0.006506 | 0.3890 |
| βr,FWS×Clust | 2.0876 | 0.9961 | 0.0361 | βs,FWS×Clust | 0.2481 | 0.1107 | 0.0250 |
| βr,FWS×Eglobe | −4.5325 | 2.5401 | 0.0744 | βs,FWS×Eglobe | −0.3895 | 0.2250 | 0.0835 |
| βr,FWS×Deg | 0.03329 | 0.04061 | 0.4123 | βs,FWS×Deg | 0.004114 | 0.002263 | 0.0690 |
| βr,FWS×Modul | −4.5484 | 0.7976 | <0.0001 | βs,FWS×Modul | −0.3499 | 0.1694 | 0.0388 |
| βr,dist | −0.00976 | 0.004572 | 0.0328 | βs,dist | −0.03500 | 0.000979 | <0.0001 |
| βr,dist2 | −0.05547 | 0.004820 | <0.0001 | βs,dist2 | 0.02335 | 0.000622 | <0.0001 |
| βr,Age | 0.000096 | 0.01109 | 0.9931 | βs,Age | −0.00508 | 0.002539 | 0.0455 |
| βr,Edu1 | 0.1340 | 0.03165 | <0.0001 | βs,Edu1 | −0.03231 | 0.007005 | <0.0001 |
| βr,Edu2 | 0.05476 | 0.02664 | 0.0398 | βs,Edu2 | −0.02192 | 0.006087 | 0.0003 |
| βr,smok_years | −0.01788 | 0.01153 | 0.1211 | βs,smok_years | −0.00680 | 0.002425 | 0.0050 |
| βr,smok_status | 0.08300 | 0.02627 | 0.0016 | βs,smok_status | 0.002705 | 0.005939 | 0.6488 |
Adjusted using the adaptive FDR procedure detailed in [80]
Bold p-value signify covariates of interest which are smaller than 0.05.
Clustering coefficient (pr,Clust < 0.0001, ps,Clust < 0.0001) and global efficiency (pr,Eglobe < 0.0001, ps,Eglobe < 0.0001), as measures of brain network segregation and integration, were significant factors in predicting both the connection probability and strength. Degree difference (pr,Deg < 0.0001, ps,Deg < 0.0001) was positively related to the connection probability and inversely related to the connection strength. Modularity (ps,Modul < 0.0001) was associated with the connection strength. Modularity was also associated with the connection probability in farmworkers (Note that modularity was related to the connection probability only as an interaction covariate (pr,FWS×Modul < 0.0001)). There were significant interactions with farmworker status such that the network metric effects differed between populations. These effects are described in section 3.2 below.
3.2. Farmworker Status (FWS) and Interaction Covariates
Functional connectivity patterns differed between farmworkers and non-farmworkers by nodal clustering coefficient and overall network modularity. Figure 3.1 was created for illustrative purposes to help understand these differences when clustering coefficient and modularity increase from their minimum to their maximum values (i.e., it exhibits how the significant interactions translate into different relationships for farmworkers and non-farmworkers). The results for probability and strength models are explained separately below.
3.2.1. Probability Model Results
The connection probability patterns were similar between farmworkers and non-farmworkers when network metrics were equal to their averages (pr,FWS = 0.4077).
Connection probability patterns differed between farmworkers and non-farmworkers as nodal clustering coefficient (pr,FWS×Clust = 0.0361) and overall modularity (pr,FWS×Modul < 0.0001) varied. The connection probability in non-farmworkers was higher and increased at a faster rate than in farmworkers as the clustering coefficient increased. Figure 3.1.A illustrates how the significant interaction results in the higher connection probability and its faster increase in non-farmworkers when clustering coefficient increases from its minimum to its maximum. Figure 3.1.B illustrates how the connection probability in non-farmworkers decreases as modularity increases from its minimum to its maximum; whereas, modularity in farmworkers did not affect the connection probability (i.e., the relationship between modularity and connection probability was significant only for non-farmworkers).
Figure 3.1. Connection Probability* and Connection Strength as functions of clustering coefficient and modularity.
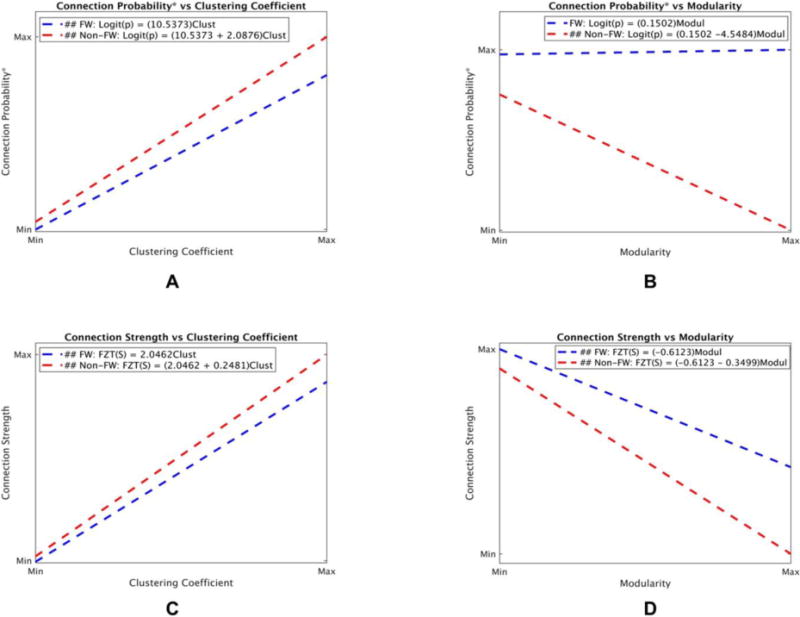
This figure was created using coefficients obtained from the probability and strength models (table 3.1) to illustrate how connection probability* (A and B) and connection strength (C and D) change in farmworkers and non-farmworkers as clustering coefficient and modularity increase from their minimum to their maximum values. A. Connection probability in non-farmworkers was higher and increased at a faster rate than in farmworkers when clustering coefficient increased. B. Connection probability in non-farmworkers decreased as modularity increased; however, connection probability did not have a significant relationship with modularity in farmworkers. C. Connection strength in farmworkers was higher and increased at a faster rate than in non-farmworkers when clustering coefficient increased. D. Connection strength in farmworkers was higher and decreased at a slower rate than in non-farmworkers as modularity increased. *It is important to note that the y-axis in all figures is the log-odds of connection probability. Any change in the log-odds of connection probability reflects a similar change in the connection probability, thus the y-axis was labeled as connection probability instead of log-odds of connection probability for simplicity. (##: Significant relationship.) (2-column color figure)
3.2.2. Strength Model Results
The connection strength patterns were similar between farmworkers and non-farmworkers when network metrics were equal to their averages (ps,FWS = 0.3890).
Connection strength patterns did differ between farmworkers and non-farmworkers as nodal clustering coefficient (ps,FWS×Clust = 0.0250) and overall modularity (ps,FWS×Modul = 0.0388) varied. The connection strength in non-farmworkers was higher and increased at a faster rate than in farmworkers as clustering coefficient increased. Figure 3.1.C shows the higher connection strength and its faster increase in non-farmworkers when clustering coefficient increases from its minimum to its maximum. The connection strength in non-farmworkers was lower and decreased at a faster rate than in farmworkers as modularity increased (figure 3.1.D).
As pointed out in section 2.5, to complement the mixed model findings, traditional univariate comparisons of brain network properties were also performed using a T-test. The results showed that the average clustering coefficient (p = 0.0108) and modularity (p = 0.0495) were both significantly different between farmworkers and non-farmworkers. As shown in figure 3.2, both clustering coefficient and modularity were higher in farmworkers compared to non-farmworkers.
Figure 3.2. Boxplots for clustering coefficient and modularity in farmworkers and non-farmworkers.
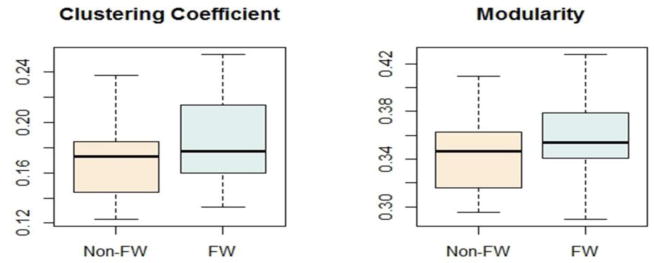
Both clustering coefficient (p = 0.0108) and modularity (p = 0.0495) were significantly higher in farmworkers. (single or 1.5-column figure)
3.3. Exposure Covariates
Figure 3.3 shows boxplots for AChE, BChE, and cotinine for farmworkers and non-farmworkers. While there were clear trends for lower cholinesterase activity in the farmworkers, the average AChE and BChE activities were not different among the two groups. It is important to note that in the full parent population there were significant differences between populations [6]. The modeling results showed that AChE had a significant inverse relationship with the connection strength (ps,AChE = 0.0009); however, urinary cotinine and blood BChE did not have significant main effects on the connection probability or strength.
Figure 3.3. Boxplots for urinary cotinine levels (ng/ml) and acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities (umole/min/ml) in farmworkers and non-farmworkers.
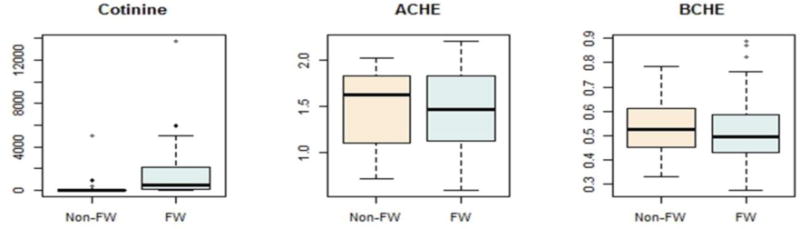
Urinary cotinine was significantly different between farmworkers and non-farmworkers (p = 0.0063). However, AChE (p = 0.6598) and BChE (p = 0.6209) were not different between the two groups. (1.5- or 2- column figure)
3.4. Confounding Covariates
Spatial distance between brain regions played an important role in predicting the probability (pr,dist = 0.0328, pr,dist2 < 0.0001) and strength (ps,dist < 0.0001, ps,dist2 < 0.0001) of brain connections. The connection probability and strength between two regions decreased as the spatial distance increased. Education (pr,Edu1 < 0.0001, pr,Edu2 = 0.0398) was another significant factor in determining the probability of brain connections. Participants with 0–6 or 7–11 years of educational attainment were more likely to have brain connections than participants with ≥ 12 years of educational attainment. The connection strength showed the reverse pattern with lower values in the less educated participants (ps,Edu1 < 0.0001, ps,Edu2 = 0.0003). Thus, less educated participants have more, yet weaker brain connections. Note that there is a trend for difference in education between farmworkers and non-farmworkers (table 2.1). Thus, including education in the model is important to control for the potential influence of this covariate on the results. Pack years of smoking (ps,smok_years = 0.0050) and smoking status (pr,smok_status = 0.0016) were also associated with brain connections. Pack years of smoking was inversely related to the connection strength; but, smokers were more likely to have brain connections than non-smokers. The results also showed age as an important covariate in predicting the connection strength (ps,age = 0.0455). Older participants had weaker brain connections. Inclusion of these covariates in the model allowed for the comparison of network properties between farmworkers and non-farmworkers while controlling for these potential confounding effects.
3.5. Parameter Estimates after excluding Cotinine, AChE and BChE
To test the biological hypothesis that exposure to pesticides and nicotine contribute to brain network differences between farmworkers and non-farmworkers, additional mixed models were evaluated. In separate model fits, we evaluated the effects of excluding cotinine or AChE and BChE on parameter estimates. As table 3.2 presents, removing AChE and BChE affected the relationships between connection probability and education, smoking status and modularity × FWS interaction. Both Edu1 and Edu2 lost their significant relationships with the connection probability (pr,Edu1 < 0.0001 → pr,Edu1 = 0.0692, pr,Edu2 = 0.0398 → pr,Edu2 = 0.2165), however, the estimate for Edu2 didn’t change much (βr,Edu1 = 0.1340 → βr,Edu1 = 0.06858, βr,Edu2 = 0.05476 → βr,Edu2 = 0.04325). Smoking status lost its significant relationship with the connection probability (pr,smok_status = 0.0016 → pr,smok_status = 0.1463), and its estimate became approximately half of the original estimate (βr,smok_status = 0.08300 → βr,smok_status = 0.0495). Modularity × FWS interaction was no longer related to the connection probability (pr,FWS×Modul < 0.0001 → pr,FWS×Modul = 0.6161), and its estimate was noticeably different (βr, FWS×Modul = −4.5484 → βr, FWS×Modul = −0.4990). Removing AChE and BChE did not affect the significant relationships with connection strength.
Table 3.2.
Parameter estimates, standard errors, and p-values for probability and strength models without AChE and BChE
| Probability (Presence) Model Outputs | Strength Model Outputs | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Parameter | Estimate | SE | *p-value | Parameter | Estimate | SE | *p-value |
| βr,0 | 0.05960 | 0.03753 | 0.1123 | βs,0 | 0.3188 | 0.006579 | <0.0001 |
| βr,Clust | 10.5987 | 0.6400 | <0.0001 | βs,Clust | 2.0456 | 0.07087 | <0.0001 |
| βr,Eglobe | −20.9550 | 1.4004 | <0.0001 | βs,Eglobe | 2.0804 | 0.1642 | <0.0001 |
| βr,Deg | 0.2360 | 0.02356 | <0.0001 | βs,Deg | −0.08170 | 0.001621 | <0.0001 |
| βr,Modul | −0.7681 | 0.5293 | 0.1467 | βs,Modul | −0.5761 | 0.1011 | <0.0001 |
| βr,Cotinine | −0.00100 | 0.01428 | 0.9440 | βs,Cotinine | −0.00248 | 0.002623 | 0.3452 |
| βr,FWS | −0.01165 | 0.04213 | 0.7821 | βs,FWS | 0.006449 | 0.006745 | 0.3390 |
| βr,FWS×Clust | 2.3873 | 1.1080 | 0.0312 | βs,FWS×Clust | 0.2569 | 0.1129 | 0.0228 |
| βr,FWS×Eglobe | −4.7013 | 2.6317 | 0.0740 | βs,FWS×Eglobe | −0.3938 | 0.2226 | 0.0769 |
| βr,FWS×Deg | 0.03677 | 0.04028 | 0.3613 | βs,FWS×Deg | 0.003998 | 0.002273 | 0.0787 |
| βr,FWS×Modul | −0.4990 | 0.9950 | 0.6161 | βs,FWS×Modul | −0.3757 | 0.1742 | 0.0310 |
| βr,dist | −0.00988 | 0.004600 | 0.0317 | βs,dist | −0.03501 | 0.000978 | <0.0001 |
| βr,dist2 | −0.05492 | 0.004720 | <0.0001 | βs,dist2 | 0.02336 | 0.000623 | <0.0001 |
| βr,Age | 0.01319 | 0.01442 | 0.3603 | βs,Age | −0.00650 | 0.002579 | 0.0117 |
| βr,Edu1 | 0.06858 | 0.03774 | 0.0692 | βs,Edu1 | −0.02498 | 0.006880 | 0.0003 |
| βr,Edu2 | 0.04325 | 0.03499 | 0.2165 | βs,Edu2 | −0.02298 | 0.006404 | 0.0003 |
| βr,smok_years | −0.00849 | 0.01433 | 0.5535 | βs,smok_years | −0.00702 | 0.002531 | 0.0055 |
| βr,smok_status | 0.04954 | 0.03410 | 0.1463 | βs,smok_status | 0.001006 | 0.006359 | 0.8743 |
Adjusted using the adaptive FDR procedure detailed in [80]. Bold parameters represent those that changed after removing AChE and BChE.
Removing cotinine (table 3.3) affected the relationships between connection probability and education, AChE, and modularity × FWS interaction. Edu2 was no longer significantly associated with the connection probability (pr,Edu2 = 0.0398 → pr,Edu2 = 0.2326); however its estimate didn’t change much (βr,Edu2 = 0.05476 → βr,Edu2 = 0.03472). AChE showed significant association with the connection probability after removing cotinine; however, there was some trend for this covariate to be associated with the connection probability in the original model (pr,AChE = 0.0639 → pr,AChE = 0.0001). The estimate for AChE was also different after removing the cotinine (βr,AChE = 0.04860 → βr,AChE = 0.1065). The modularity × FWS interaction lost its significant relationship with connection probability (pr,FWS×Modul < 0.0001 → pr,FWS×Modul = 0.1686), and had a noticeably different estimate after removing cotinine (βr, FWS×Modul = −4.5484 → βr, FWS×Modul = −1.3277). The modularity × FWS interaction was no longer associated with the connection strength; however, it showed to be associated with the connection strength (ps,FWS×Modul = 0.0388 → ps,FWS×Modul = 0.0553). The estimate for modularity × FWS interaction didn’t change much (βs, FWS×Modul = −0.3499 → βs, FWS×Modul = −0.3258). These changes show that cotinine confounds relationships with both connection probability and strength. Thus, cotinine, AChE, and BChE, as markers of nicotine and pesticide exposures, are important covariates to be included in both models, and play important roles in modifying the significant relationships between several covariates and connectivity patterns, especially between the modularity × FWS interaction and connection probability.
Table 3.3.
Parameter estimates, standard errors, and p-values for probability and strength models without cotinine
| Probability (Presence) Model Outputs | Strength Model Outputs | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Parameter | Estimate | SE | *p-value | Parameter | Estimate | SE | *p-value |
| βr,0 | 0.03116 | 0.03437 | 0.3646 | βs,0 | 0.3201 | 0.006424 | <0.0001 |
| βr,Clust | 10.5466 | 0.6262 | <0.0001 | βs,Clust | 2.0437 | 0.07064 | <0.0001 |
| βr,Eglobe | −20.8914 | 1.3899 | <0.0001 | βs,Eglobe | 2.0832 | 0.1656 | <0.0001 |
| βr,Deg | 0.2358 | 0.02353 | <0.0001 | βs,Deg | −0.08183 | 0.001606 | <0.0001 |
| βr,Modul | 0.1733 | 0.4075 | 0.6683 | βs,Modul | −0.6423 | 0.09318 | <0.0001 |
| βr,AChE | 0.1065 | 0.02779 | 0.0001 | βs,AChE | −0.01915 | 0.005857 | 0.0011 |
| βr,BChE | −0.08032 | 0.08261 | 0.3309 | βs,BChE | −0.00703 | 0.01796 | 0.6955 |
| βr,FWS | 0.001131 | 0.04319 | 0.9791 | βs,FWS | 0.006729 | 0.006505 | 0.3010 |
| βr,FWS×Clust | 2.4342 | 1.0989 | 0.0267 | βs,FWS×Clust | 0.2488 | 0.1094 | 0.0230 |
| βr,FWS×Eglobe | −4.7792 | 2.6218 | 0.0683 | βs,FWS×Eglobe | −0.3908 | 0.2226 | 0.0791 |
| βr,FWS×Deg | 0.03649 | 0.04035 | 0.3658 | βs,FWS×Deg | 0.004139 | 0.002263 | 0.0674 |
| βr,FWS×Modul | −1.3277 | 0.9643 | 0.1686 | βs,FWS×Modul | −0.3258 | 0.1700 | 0.0553 |
| βr,dist | −0.00956 | 0.004495 | 0.0334 | βs,dist | −0.03501 | 0.000979 | <0.0001 |
| βr,dist2 | −0.05513 | 0.004704 | <0.0001 | βs,dist2 | 0.02336 | 0.000622 | <0.0001 |
| βr,Age | 0.007865 | 0.01264 | 0.5338 | βs,Age | −0.00515 | 0.002539 | 0.0429 |
| βr,Edu1 | 0.1310 | 0.03418 | 0.0001 | βs,Edu1 | −0.03171 | 0.006908 | <0.0001 |
| βr,Edu2 | 0.03472 | 0.02908 | 0.2326 | βs,Edu2 | −0.02066 | 0.006035 | 0.0006 |
| βr,smok_years | −0.01398 | 0.01192 | 0.2408 | βs,smok_years | −0.00655 | 0.002402 | 0.0064 |
| βr,smok_status | 0.07979 | 0.02487 | 0.0013 | βs,smok_status | 0.000117 | 0.005508 | 0.9830 |
Adjusted using the adaptive FDR procedure detailed in [80]. Bold parameters represent those that changed after removing Cotinine.
4. Discussion
The current study was conducted to determine whether brain functional networks are different between Latino immigrant workers that do or do not engage in farm work. The contribution of pesticide and nicotine exposure to any population differences in brain network connectivity was also examined. We used resting-state fMRI due to its promise in detecting early brain changes in people at risk of developing neurological dysfunction [57–60], as well as being a relatively straightforward technique that removes the burdens of experimental design, subject compliance, task-related confounds, and training demands. Rs-fMRI could be especially useful for future studies that focus on prenatal pesticide and nicotine exposure effects on children, due to difficulties of performing experimental tasks in children. However, future studies can also focus on cognitive task studies to better understand how pesticide and nicotine exposure affect the brain in a cognitively relevant manner.
This study evaluated the whole-brain functional connectivity patterns, and did not identify specific brain regions that contributed to group differences. Unlike most other studies in this area, however, it allowed controlling for important confounding variables such as age, education, and smoking. Furthermore, interactions between various brain regions play a key role in cognitive processes and complex behaviors [47], and numerous studies suggest that in neurodegenerative disorders, interconnected brain regions are targeted rather than single regions [61]. Thus, functional network mapping approaches provide valuable insights about complex patterns of pesticide and nicotine exposure effects on the brain and their likely contribution to cognitive impairment.
Overall, network properties in Latino immigrant workers, as well as effects of pesticide and nicotine on brain connections, were investigated via a mixed-effects modeling framework. Our results indicated that both connection probability and strength differed between farmworkers and non-farmworkers as clustering coefficient and modularity changed. Both probability and strength of brain connections in non-farmworkers were higher and increased at a faster rate than in farmworkers when nodal clustering coefficient increased. This means that for networks with a comparable density (probability) and strength of connection, the clustering coefficient is higher in farmworkers compared to non-farmworkers. This can be visualized by adding a horizontal line to figures 3.1.A and 3.1.C and seeing that it intersects the farmworker curve at a higher clustering coefficient value. Figure 4.1.A shows this for the connection probability. Since neither connection strength nor connection probability was different between the two groups when network metrics were equal to their averages (pr,FWS = 0.4077, ps,FWS = 0.3890), the clustering coefficient, on average, should be higher in farmworkers. In fact, as shown in Figure 3.2, the unadjusted comparison of average clustering coefficients did show higher clustering coefficient in farmworkers. Modularity in farmworkers was also higher than in non-farmworkers for any strength value. Again, this can be visualized by adding a horizontal line to figure 3.1.D and seeing that it intersects the farmworker curve at a higher modularity value (figure 4.1.B). Thus, the average modularity should also be higher in farmworkers based on networks with comparable density and strength of connection. Again, as shown in Figure 3.2, an unadjusted comparison showed that the average modularity was higher among farmworkers.
Figure 4.1. Connection probability as a function of clustering coefficient (A) and connection strength as a function of modularity (B).
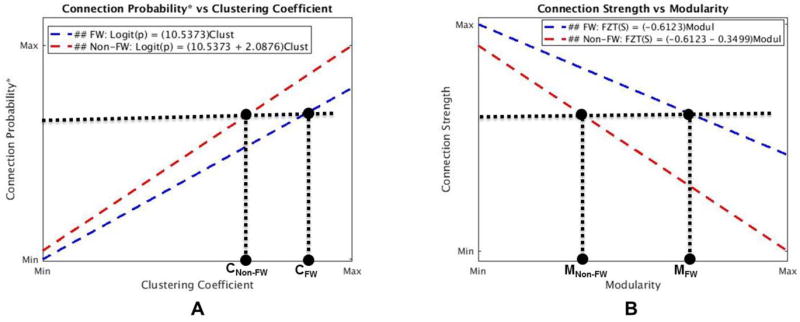
A. for any given connection probability, the clustering coefficient in farmworkers is higher than in non-farmworkers (CFW > CNon-FW). Similarly, for any given connection strength, clustering coefficient in farmworkers is higher than in non-farmworkers (not shown here). B. for any given connection strength, the overall modularity in farmworkers is higher than in non-farmworkers (MFW > MNon-FW). (2-column color figure)
Thus, our results suggest that brain networks in farmworkers are more clustered and modular when compared to non-farmworkers. Higher clustering coefficient and modularity in farmworkers indicate increased functional specificity (increased intra-modular connection probability and strength) and decreased functional integration across brain modules (decreased inter-modular connection strength) when compared to non-farmworkers. In other words, functional modules in farmworkers are more dense and have stronger interconnections than those in non-farmworkers. But, connections between functional modules in farmworkers are weaker than those in non-farmworkers (i.e., there are stronger distributed connections across functional modules in non-farmworkers’ brain networks). The potential implications for information processing is that farmworkers (with higher clustering and modularity) could have more segregated neural processing and less sharing of information between brain regions. Figure 4.2 shows two cartoon brain networks that visually depict the implications of the differences found between the brain networks in farmworkers and non-farmworkers.
Figure 4.2. Cartoon model of brain networks for farmworkers (A) and non-farmworkers (B).
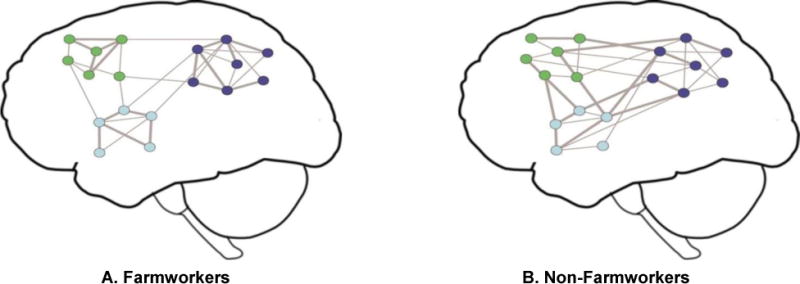
Each network node represents a brain region and the lines represent functional connections. Although the same brain areas are included in both networks, the overall network connectivity is different. The node color indicates the module membership and the edge thickness represents connection strength. The average connection probability (density) and strength are the same between farmworkers and non-farmworkers (i.e., the total number of brain edges and average strength of present edges are the same in A and B — Each network has 35 edges including 17 strong edges). However, brain networks of farmworkers are more modularly organized and have higher functional specificity and lower intermodular integrity when compared to non-farmworkers (stronger connections are shown with thicker edges). This cartoon model was created for illustrative purposes to better visualize the study results. (2-column color figure)
Modularity plays an important role in facilitating different types of cognitive tasks [62–65]. Many studies have also shown altered modularity in neurodegenerative disorders [66–70]. Increased modularity has been shown to be associated with effortful, high-level cognitive tasks. Vatansever et al. [63] showed that higher cognitive efforts were associated with a more globally integrated (i.e., less modular) brain network. They showed that changes in default mode network (DMN) connectivity are central in integrating information across brain modules. Flexible community formation in the DMN during demanding cognitive tasks facilitates integrating functional interactions across the brain. Shine et al., in another study in [71], showed that brain networks fluctuate between a state of specialized structure with greater intra-network connectivity (higher modularity and clustering) and a state of higher integrity with greater inter-network connectivity (lower modularity and clustering). They showed that brain networks reorganize toward the lower modularity state during challenging tasks. This finding is consistent with other works indicating that globally integrated brain networks support higher cognitive flexibility and control in complex tasks [64, 72]. Thus, increased whole-brain modularity in farmworkers might be associated with relative decreases in complex cognitive function and reduced performance in demanding tasks when compared to non-farmworkers. At the same time, increased modularity could support enhanced performance in simple cognitive tasks [73].
While it is important to reflect upon the findings within the context of the extant literature, the present results are all based on resting-state brain networks. How these findings are ultimately associated with cognitive function is open to further research. A meta-analysis [74] dichotomized pesticide exposed workers into long (≥10 years) and short (<10 years) exposure durations, and found lower cognitive and motor performances in workers with longer exposure duration. The farmworkers included in this current study had significantly higher exposure duration than the non-farmworkers [75]. Unfortunately, we were not able to include exposure years into our network analysis model at this time due to collinearity with farmworker status. It is important to note that resting-state brain network properties are strongly associated with brain function in health and disease [76–78]. Brain networks in individuals who have better cognitive performance are mostly in an integrated state during rest [76]. Furthermore, increased resting-state modularity has been associated with cognitive deficits in some neurodegenerative disorders [66, 67, 69]. Future studies with brain imaging on a larger sample of farmworkers would be able to examine the relationships between exposure years, cognition, and network organization.
The analyses that removed the cholinesterase and cotinine measures were intended to give further insight into the role of occupational chemical exposure in the population differences observed. When AChE and BChE were removed from the model there were important changes in the statistical significance and parameter estimates. The results indicated that AChE/BChE confound the population differences in the relationship between connection probability and community structure. A similar finding was observed when cotinine was removed from the model. However, neither AChE /BChE, nor cotinine had an effect on the population differences in the relationship between connection probability and clustering coefficient.
The comparable influence of AChE/BChE and cotinine is not overly surprising as both cholinesterase inhibiting pesticides and nicotine in tobacco can increase cholinergic neurotransmission [79]. Changes in AChE activity can lead to changes in acetylcholine at the synapse (i.e., reduced AChE associated with pesticide exposure can lead to decreased acetylcholine inactivation). The influence of BChE on acetylcholine metabolism is less clear. Nicotine, on the other hand, directly binds to nicotinic acetylcholine receptors and activates the cholinergic system. These findings indicate that alterations of the cholinergic nervous system from pesticide and/or nicotine exposure are at least associated with the population differences in modularity, but not clustering. After accounting for occupational exposure, the population differences for modularity highlighted in figure 4.1.B were eliminated. Thus, possible cognitive consequences (due to the modularity differences) that were pointed out above could be a result of chronic pesticide and/or nicotine exposures.
The current study is not without limitations. First, although the modeling approach allowed controlling for important confounding variables such as age, education, and smoking status, it did not provide detail about specific brain regions or subnetworks that differ, and only gave overall differences between connectivity patterns of farmworkers and non-farmworkers. Future studies can focus on functional connectivity patterns in specific brain subnetworks such as the basal ganglia or default mode network by incorporating regional covariates into the model. Second, farmworkers who participated in this study were from one area of the US. Thus, the pesticides used may be different from pesticides used in other parts of the country. Third, the average AChE activity in participants used in this study was not significantly different between farmworkers and non-farmworkers. Longitudinal assessments, however, have indicated decreased AChE and BChE activities in the larger inclusive population of Latino farmworkers evaluated in our previous study [14]. A longitudinal study on brain networks across the agricultural season could better reveal how the functional connections and brain network properties change as cholinesterase activities change in farmworkers. Finally, we only measured AChE and BChE in the blood, and how this reflects changes in brain enzymes is unclear.
5. Conclusion
The current study demonstrated that brain networks differed between Latino immigrant workers that did or did not engage in farm worker. Our results suggest that the farmworkers have more clustered and modular brain networks than the participants that did not engage in farm work. This finding is consistent with a higher number and strength of intra-modular connections in farmworkers, and a lower number and strength of inter-modular connections in farmworkers. Cholinesterase activity, as a marker of OP and carbamate pesticide exposure, and urinary cotinine, as a marker of nicotine exposure, were associated with the differences in brain network community structure (modularity). This could indicate that enhanced cholinergic neurotransmission may play an important role in modularity differences between farmworkers and non-farmworkers. However, the difference in clustering coefficient between the farmworkers and non-farmworkers was not associated with markers of pesticide or nicotine exposure. Thus, there are other population differences driving the clustering findings that cannot be accounted for by the variables measured in this study. While the neurobiological consequences of the topological brain network differences between farmworkers and non-farmworkers cannot be determined in the current study, the fact that some of the differences were associated with markers of occupational exposures is of concern. It is possible that the network differences observed could be acute or could be early indicators of brain changes in farmworkers that have neurological consequences in later life. Further studies are warranted to gain a deeper understanding of the implications of occupational chemical exposure for Latino immigrants working on farms.
Supplementary Material
Highlights.
-
➢
Functional brain networks are different between farmworkers and non-farmworkers.
-
➢
Farmworkers have more clustered and modular networks than non-farmworkers.
-
➢
Cholinesterase activity contributes to differences in brain network differences.
-
➢
Urinary cotinine contributes to differences in brain network differences.
-
➢
Cholinesterase activity is associated with whole brain functional connectivity.
Acknowledgments
Funding
This work was supported by NIEHS (grant number: R01 ES008739) and NIH award (K25 EB012236).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arcury TA, et al. Repeated Pesticide Exposure Among North Carolina Migrant and Seasonal Farmworkers. American Journal of Industrial Medicine. 2010;53(8):802–813. doi: 10.1002/ajim.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCauley L, et al. Oregon Indigenous Farmworkers Results of Promotor Intervention on Pesticide Knowledge and Organophosphate Metabolite Levels. Journal of Occupational and Environmental Medicine. 2013;55(10):1164–1170. doi: 10.1097/JOM.0b013e31829b28e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casida JE, Durkin KA. Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects. Annual Review of Entomology. 2013;58:99–117. doi: 10.1146/annurev-ento-120811-153645. Vol 58. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez AF, et al. Systematic reviews on neurodevelopmental and neurodegenerative disorders linked to pesticide exposure: Methodological features and impact on risk assessment. Environment International. 2016;92–93:657–679. doi: 10.1016/j.envint.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Kim KH, Kabir E, Jahan SA. Exposure to pesticides and the associated human health effects. Science of the Total Environment. 2017;575:525–535. doi: 10.1016/j.scitotenv.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Quandt SA, et al. Longitudinal Assessment of Blood Cholinesterase Activities Over 2 Consecutive Years Among Latino Nonfarmworkers and Pesticide-Exposed Farmworkers in North Carolina. Journal of Occupational and Environmental Medicine. 2015;57(8):851–857. doi: 10.1097/JOM.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nees F. The nicotinic cholinergic system function in the human brain. Neuropharmacology. 2015;96:289–301. doi: 10.1016/j.neuropharm.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Munoz-Quezada MT, et al. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review. International Journal of Occupational and Environmental Health. 2016;22(1):68–79. doi: 10.1080/10773525.2015.1123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayasinghe SS. Effects of acute organophosphate ingestion on cognitive function, assessed with the mini mental state examination. Journal of Postgraduate Medicine. 2012;58(3):171–175. doi: 10.4103/0022-3859.101374. [DOI] [PubMed] [Google Scholar]
- 10.Beseler CL, et al. Depression and Pesticide Exposures among Private Pesticide Applicators Enrolled in the Agricultural Health Study. Environmental Health Perspectives. 2008;116(12):1713–1719. doi: 10.1289/ehp.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parron T, et al. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicology and Applied Pharmacology. 2011;256(3):379–385. doi: 10.1016/j.taap.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicology and Applied Pharmacology. 2013;268(2):157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Moisan F, et al. Association of Parkinson’s Disease and Its Subtypes with Agricultural Pesticide Exposures in Men: A Case-Control Study in France. Environmental Health Perspectives. 2015;123(11):1123–1129. doi: 10.1289/ehp.1307970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouwer M, et al. Occupational exposures and Parkinson’s disease mortality in a prospective Dutch cohort. Occupational and Environmental Medicine. 2015;72(6):448–455. doi: 10.1136/oemed-2014-102209. [DOI] [PubMed] [Google Scholar]
- 15.Arcury TA, et al. Urinary Cotinine Levels Among Latino Tobacco Farmworkers in North Carolina Compared to Latinos Not Employed in Agriculture. Nicotine & Tobacco Research. 2016;18(6):1517–1525. doi: 10.1093/ntr/ntv187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quandt SA, et al. Environmental and behavioral predictors of salivary cotinine in Latino tobacco workers. Journal of Occupational and Environmental Medicine. 2001;43(10):844–852. doi: 10.1097/00043764-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Lavoie FW, Harris TM. Fatal nicotine ingestion. J Emerg Med. 1991;9(3):133–6. doi: 10.1016/0736-4679(91)90318-a. [DOI] [PubMed] [Google Scholar]
- 18.Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Archives of Toxicology. 2014;88(1):5–7. doi: 10.1007/s00204-013-1127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Froeliger B, Gilbert DG, McClernon FJ. Effects of nicotine on novelty detection and memory recognition performance: double-blind, placebo-controlled studies of smokers and nonsmokers. Psychopharmacology. 2009;205(4):625–633. doi: 10.1007/s00213-009-1571-y. [DOI] [PubMed] [Google Scholar]
- 21.Barreto GE, Iarkov A, Moran VE. Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson’s disease. Frontiers in Aging Neuroscience. 2015:6. doi: 10.3389/fnagi.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiyohara C, Kusuhara S. Cigarette smoking and Parkinson’s disease: a meta-analysis. Fukuoka Igaku Zasshi. 2011;102(8):254–65. [PubMed] [Google Scholar]
- 23.Sporns O, Betzel RF. Modular Brain Networks. Annu Rev Psychol. 2016;67:613–40. doi: 10.1146/annurev-psych-122414-033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems (vol 10, pg 186, 2009) Nature Reviews Neuroscience. 2009;10(4) doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 25.Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009;22(4):340–7. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa S, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa S, et al. Brain Magnetic-Resonance-Imaging with Contrast Dependent on Blood Oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dosenbach NUF, et al. Prediction of Individual Brain Maturity Using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raichle ME, Mintun MA. Brain work and brain imaging. Annual Review of Neuroscience. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 30.Simpson SL, Laurienti PJ. Disentangling Brain Graphs: A Note on the Conflation of Network and Connectivity Analyses. Brain Connect. 2016;6(2):95–8. doi: 10.1089/brain.2015.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci. 2013;15(3):247–62. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rombouts SARB, et al. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: An fMRI study. Human Brain Mapping. 2005;26(4):231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, et al. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. Neuroimage. 2006;31(2):496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, et al. Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28(10):967–78. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vemuri P, Jones DT, Jack CR. Resting state functional MRI in Alzheimer’s Disease. Alzheimers Research & Therapy. 2012;4(1) doi: 10.1186/alzrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szewczyk-Krolikowski K, et al. Functional connectivity in the basal ganglia network differentiates PD patients from controls. Neurology. 2014;83(3):208–214. doi: 10.1212/WNL.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tessitore A, et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79(23):2226–2232. doi: 10.1212/WNL.0b013e31827689d6. [DOI] [PubMed] [Google Scholar]
- 38.Janes AC, et al. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125(3):252–9. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vergara VM, et al. Alterations of resting state functional network connectivity in the brain of nicotine and alcohol users. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson SL, Laurienti PJ. A two-part mixed-effects modeling framework for analyzing whole-brain network data. Neuroimage. 2015;113:310–319. doi: 10.1016/j.neuroimage.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laurienti PJ, et al. Brain Anatomy in Latino Farmworkers Exposed to Pesticides and Nicotine. Journal of Occupational and Environmental Medicine. 2016;58(5):436–443. doi: 10.1097/JOM.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arcury TA, et al. Lifetime and current pesticide exposure among Latino farmworkers in comparison to other Latino immigrants. American Journal of Industrial Medicine. 2014;57(7):776–787. doi: 10.1002/ajim.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson CD, Russell RL. Rapid, Simple Radiometric Assay for Cholinesterase, Suitable for Multiple Determinations. Analytical Biochemistry. 1975;64(1):229–238. doi: 10.1016/0003-2697(75)90423-6. [DOI] [PubMed] [Google Scholar]
- 44.Biswal B, et al. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 45.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 47.Telesford QK, et al. The brain as a complex system: using network science as a tool for understanding the brain. Brain connectivity. 2011;1(4):295–308. doi: 10.1089/brain.2011.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraiman D, et al. Ising-like dynamics in large-scale functional brain networks. Physical Review E. 2009;79(6) doi: 10.1103/PhysRevE.79.061922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith SM. The future of FMRI connectivity. Neuroimage. 2012;62(2):1257–1266. doi: 10.1016/j.neuroimage.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 50.Smith SM, et al. Network modelling methods for FMRI. Neuroimage. 2011;54(2):875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 51.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Newman MEJ. Mixing patterns in networks. Physical Review E. 2003;67(2) doi: 10.1103/PhysRevE.67.026126. [DOI] [PubMed] [Google Scholar]
- 53.Newman MEJ, Girvan M. Finding and evaluating community structure in networks. Physical Review E. 2004;69(2) doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- 54.Friedman EJ, et al. Stochastic geometric network models for groups of functional and structural connectomes. Neuroimage. 2014;101:473–484. doi: 10.1016/j.neuroimage.2014.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gentle JE. Numerical linear algebra for applications in statistics. Springer Science & Business Media; 2012. [Google Scholar]
- 56.Wolfinger R, O’connell M. Generalized linear mixed models a pseudo-likelihood approach. Journal of statistical Computation and Simulation. 1993;48(3–4):233–243. [Google Scholar]
- 57.Sheline YI, et al. Amyloid Plaques Disrupt Resting State Default Mode Network Connectivity in Cognitively Normal Elderly. Biological Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh H, et al. beta-Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011;54(3):1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee MH, Smyser CD, Shimony JS. Resting-State fMRI: A Review of Methods and Clinical Applications. American Journal of Neuroradiology. 2013;34(10):1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seeley WW, et al. Neurodegenerative Diseases Target Large-Scale Human Brain Networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M, Deem MW. Development of modularity in the neural activity of children’s brains. Phys Biol. 2015;12(1):016009. doi: 10.1088/1478-3975/12/1/016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vatansever D, et al. Default Mode Dynamics for Global Functional Integration. Journal of Neuroscience. 2015;35(46):15254–15262. doi: 10.1523/JNEUROSCI.2135-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schultz DH, Cole MW. Integrated Brain Network Architecture Supports Cognitive Task Performance. Neuron. 2016;92(2):278–279. doi: 10.1016/j.neuron.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Braun U, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(37):11678–11683. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lebedev AV, et al. Large-scale resting state network correlates of cognitive impairment in Parkinson’s disease and related dopaminergic deficits. Movement Disorders. 2014;29:S79–S79. doi: 10.3389/fnsys.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baggio HC, et al. Functional Brain Networks and Cognitive Deficits in Parkinson’s Disease. Human Brain Mapping. 2014;35(9):4620–4634. doi: 10.1002/hbm.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chavez M, et al. Functional Modularity of Background Activities in Normal and Epileptic Brain Networks. Physical Review Letters. 2010;104(11) doi: 10.1103/PhysRevLett.104.118701. [DOI] [PubMed] [Google Scholar]
- 69.Gamboa OL, et al. Working memory performance of early MS patients correlates inversely with modularity increases in resting state functional connectivity networks. Neuroimage. 2014;94:385–395. doi: 10.1016/j.neuroimage.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Sun Y, et al. Disrupted Functional Brain Connectivity and Its Association to Structural Connectivity in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. Plos One. 2014;9(5) doi: 10.1371/journal.pone.0096505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shine JM, et al. The Dynamics of Functional Brain Networks: Integrated Network States during Cognitive Task Performance. Neuron. 2016;92(2):544–554. doi: 10.1016/j.neuron.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren S, et al. Dynamic Functional Segregation and Integration in Human Brain Network During Complex Tasks. IEEE Trans Neural Syst Rehabil Eng. 2016 doi: 10.1109/TNSRE.2016.2597961. [DOI] [PubMed] [Google Scholar]
- 73.Chen M, Deem MW. Development of modularity in the neural activity of children’s brains. Physical biology. 2015;12(1):016009. doi: 10.1088/1478-3975/12/1/016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meyer-Baron M, et al. Meta-analysis on occupational exposure to pesticides — Neurobehavioral impact and dose-response relationships. Environmental Research. 2015;136:234–245. doi: 10.1016/j.envres.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 75.Arcury TA, et al. Lifetime and current pesticide exposure among Latino farmworkers in comparison to other Latino immigrants. Am J Ind Med. 2014;57(7):776–87. doi: 10.1002/ajim.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schultz DH, Cole MW. Higher Intelligence Is Associated with Less Task-Related Brain Network Reconfiguration. Journal of Neuroscience. 2016;36(33):8551–8561. doi: 10.1523/JNEUROSCI.0358-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stevens AA, et al. Functional brain network modularity captures inter-and intra-individual variation in working memory capacity. PloS one. 2012;7(1):e30468. doi: 10.1371/journal.pone.0030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biswal B, et al. Functional connectivity in the motor cortex of resting human brain using echo‐ planar mri. Magnetic resonance in medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 79.Massoulié J, et al. Molecular and cellular biology of cholinesterases. Progress in neurobiology. 1993;41(1):31–91. doi: 10.1016/0301-0082(93)90040-y. [DOI] [PubMed] [Google Scholar]
- 80.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. Journal of educational and Behavioral Statistics. 2000;25(1):60–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


