Abstract
The first examples of biologically active monocyclic 1,2-azaborines have been synthesized and demonstrated to exhibit not only improved in vitro aqueous solubility in comparison to the corresponding carbonaceous analogues, but in the context of a CDK2 inhibitor, also improved biological activity and better in vivo oral bioavailability. This proof-of-concept study establishes the viability of monocyclic 1,2-azaborines as a novel pharmacophore with distinct pharmacological profiles that can help address challenges associated with solubility in drug development research.
Keywords: azaborine, BN heterocycle, drug discovery, CDK2 inhibitor, solubility
COMMUNICATION
BOR-ing? NO!!! Monocyclic 1,2-azaborines can serve as a novel pharmacophore with improved in vitro aqueous solubility, improved bioactivity, and better in vivo oral availability compared to their carbonaceous analogues.
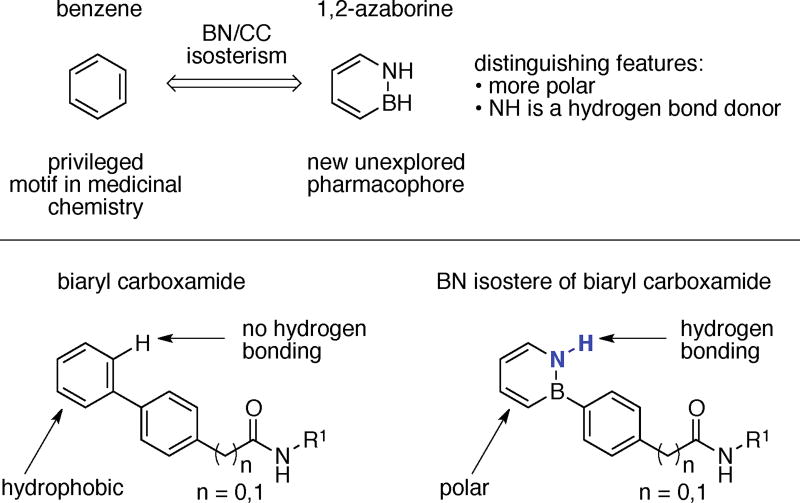
One of the main goals of synthetic chemistry is to create structural diversity – and as a consequence produce new functions and properties – beyond what Nature can achieve. For instance, a key impetus behind the laboratory syntheses of bioactive natural products is to diversify the original portfolio of structures to systematically investigate structure-function relationships and elucidate mechanism of action.1 This exploration of new chemical space facilitates the development of reagents that illuminate new biology and the development of therapeutics that can benefit society. BN/CC isosterism2 (i.e., the replacement of a carbon-carbon unit with a boron-nitrogen (BN) unit) has recently emerged as a strategy to increase the chemical space of compounds relevant to biomedical research.3 When applied to a “privileged” structural motif in medicinal chemistry,4 this approach can produce a new versatile pharmacophore. Aromatic rings are ubiquitous in medicinal chemistry, and arene-containing compounds prevail among topselling small-molecule drugs.5 BN/CC isosterism of arenes results in the so-called azaborine heterocycles where specifically 1,2-azaborines are designated as compounds with the boron and nitrogen atoms adjacent to each other (Scheme 1).6 It has been demonstrated that 1,2-azaborines can bind to aryl recognition pockets7 in biological targets and engage in hydrogen bonding inside those binding pockets.8 Furthermore, it has been shown that both the B- and N-Et BN isosteres of ethylbenzene are inhibitors of ethylbenzene dehydrogenase (EbDH), in contrast to ethylbenzene itself, which is the naturally evolved substrate for the EbDH.9 Despite the recent advances made in the area of azaborine chemistry,10 the progress toward evaluating these heterocycles in the context of medicinal chemistry has remained underexplored. BN isosteres of naphthalene have recently been profiled in vitro and in vivo in terms of biological activity and ADMET (absorption, distribution, metabolism, excretion, toxicity) properties.11,12 However, to the best of our knowledge, profiling of the arguably more versatile monocyclic 1,2-azaborine motif has not been reported. Thus, essential questions such as stability, biological activity, pharmacological properties of monocyclic 1,2-azaborines have remained unanswered. In our initial exploration in this area, we sought to investigate 1,2-azaborine isosteres of biologically active biphenyl carboxamides, the biphenyl motif being a “privileged” sub-motif of the arene family in drug discovery research.13,14 In this communication, we establish that 1,2- azaborine-based biphenyl carboxylic acids are compatible with the CDMT/NMM amide coupling conditions, and that the resulting amides 1) are air and water stable, 2) are more soluble in water than their carbonaceous counterparts, 3) exhibit better in vivo oral availability, and 4) can exhibit stronger biological activity due to hydrogen bonding.
Scheme 1.
BN/CC isosterism in the context of biologically active biphenyl carboxamides.
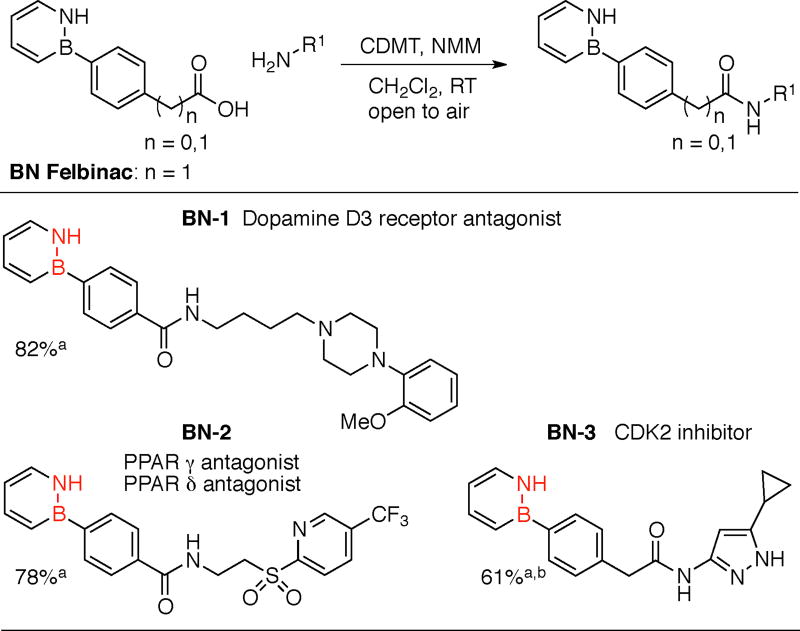
In 2013, we reported a functional-group tolerant Rh-catalyzed B-arylation of B-Cl-substituted 1,2-azaborines and as a demonstration synthesized the BN isostere of Felbinac, a nonsteroidal anti-inflammatory drug.15 Recognizing the versatility of the carboxylic acid functional group present in Felbinac, we sought to develop amide-coupling conditions to access BN isosteres of the ubiquitous biphenyl carboxamide family of biologically active compounds. Gratifyingly, the use of the 2-chloro-4,6-dimethoxy-1,3,5-triazine/N-methylmorpholine (CDMT/NMM) conditions16 furnished the desired amide coupling products in moderate to good yield (Table 1). We specifically chose three biphenyl carboxamides that inhibit a distinct set of biological targets (dopamine D3 (BN-1),17 PPAR γ and δ (BN-2),18 and CDK2 (BN-3)19 to evaluate the effects of BN/CC isosterism on their pharmacological properties. It is worth noting that the amide coupling can be conducted in air. Furthermore, stability studies reveal no decomposition when BN-1, BN-2, and BN-3 are exposed to air and water at 50 °C for 24 hours, demonstrating the viability of these BN heterocycles in medicinal chemistry applications.20
Table 1.
Synthesis of BN isosteres of biologically active biphenyl carboxamides
Yields are isolated yields.
Yield after amide coupling followed by N-BOC removal from the pyrazole group.
Table 2 shows the ADMET behavior of BN-1, BN-2, and BN-3 in direct comparison to their carbonaceous analogues CC-1, CC-2, and CC-3.21 A general trend can be observed in terms of the effect of BN/CC isosterism on aqueous solubility properties: the BN isosteres are more soluble both under buffered and FASSIF conditions. As a result, they have decreased membrane permeability (more negative PAMPA value) than their carbonaceous analogues. The better aqueous solubility behavior of 1,2-azaborine derivatives is consistent with reported electronic structure analysis that revealed a 2.1 D dipole moment for 1,2-dihydro-1,2-azaborine in contrast to benzene’s dipole moment of 0 D.22 Thus, the incorporation of the 1,2-azaborine motif renders the relatively hydrophobic biphenyl motif more hydrophilic. A majority of currently marketed drugs are poorly soluble.23 Thus, BN/CC isosterism can potentially be used as a design strategy to produce more soluble active pharmaceutical ingredients. No general trend can be discerned from the RLM Cl, CYP3A4, and hERG data. It appears that functional groups unrelated to BN/CC isosterism may be more responsible for the observed data. Overall, our ADMET data indicate that there is no particular red flag associated with the use of 1,2-azaborines as a pharmacophore in medicinal chemistry.
Table 2.
ADMET study of biphenyl carboxamides and BN-biphenyl carboxamides.
| cmpd | solubility pH 6.8 (mM) |
FASSIF (mM) |
logD | PAMPA | RLM CL (mL min−1 kg−1) |
CYP3A4 IC50 (mM) |
hERG IC50 (mM) |
|---|---|---|---|---|---|---|---|
| CC-1 | <0.005 | 0.021 | 4.2 | −3.9 | 51 | >20 | 0.042 |
| BN-1 | 0.046 | 0.22 | 4.1 | −4.3 | 53 | 11 | 0.17 |
| CC-2 | <0.004 | <0.004 | 3.5 | −4.2 | 39 | >20 | >30 |
| BN-2 | <0.004 | 0.006 | 3.5 | −4.5 | 53 | >20 | >30 |
| CC-3 | <0.004 | 0.006 | 4.4 | −3.9 | 48 | >20 | >30 |
| BN-3 | <0.013 | 0.03 | 3.9 | −4.5 | 48 | >20 | >30 |
FASSIF: Fasted and Fed State Simulated Stomach and Intestinal Fluids.
PAMPA: Parallel Artificial Membrane Permeability Assay.
RLM Cl: Rat Liver Microsome Clearance.
CYP3A4: Cytochrome P450 3A4 inhibition.
hERG: Human Ether-a-go-go Related Gene ion channel inhibition.
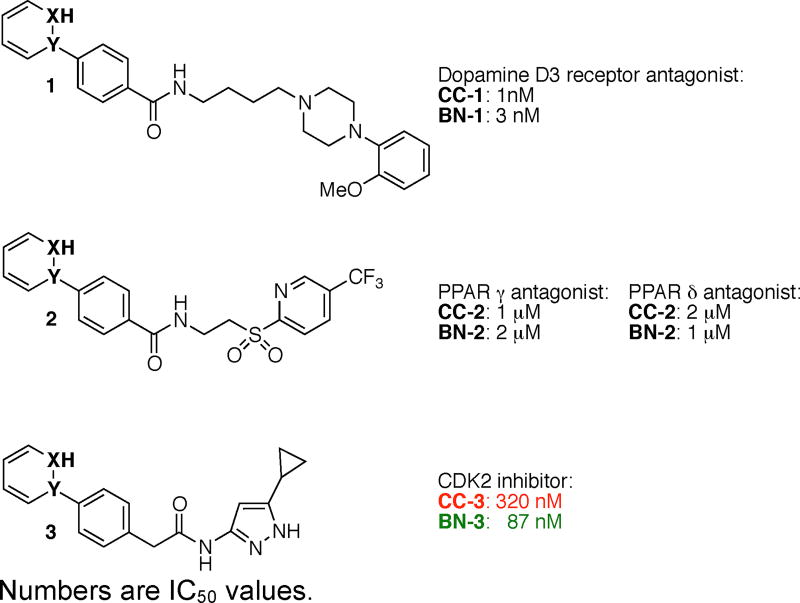
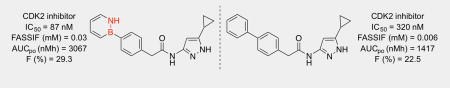
We then turned our attention to evaluating the biological activity of our BN isosteres in comparison to their all-carbon derivatives. Compound CC-1 was reported as a selective dopamine D3 antagonist,17 and in our analysis CC-1 exhibited an IC50 value of 1 nM (Scheme 2). Its BN isostere BN-1 is also biologically active although the activity is slightly attenuated with an IC50 value of 3 nM. CC-2 has been investigated as antagonists of PPAR γ and δ18 and in our assay we have determined IC50 values of 1 and 2 µM, respectively. Similarly, the corresponding BN isostere BN-2 also exhibits low micromolar activity against PPAR γ and δ (Scheme 2). Compound CC-3 was reported as a potent nanomolar antiproliferative agent in a CDK2 kinase assay.19 In our CDK2 assay CC-3 showed an IC50 of 320 nM. Interestingly, the BN derivative BN-3 (IC50 = 87 nM) showed improved potency than CC-3. Compound BN-3 is selective for CDK2. When tested against a panel of 29 kinases, BN-3 was found to be a more selective inhbitor of CDK2 than CDK1 (IC50 = 460 nM).
Scheme 2.
Effect of BN/CC isosterism on biological activity. Numbers are IC50 values.
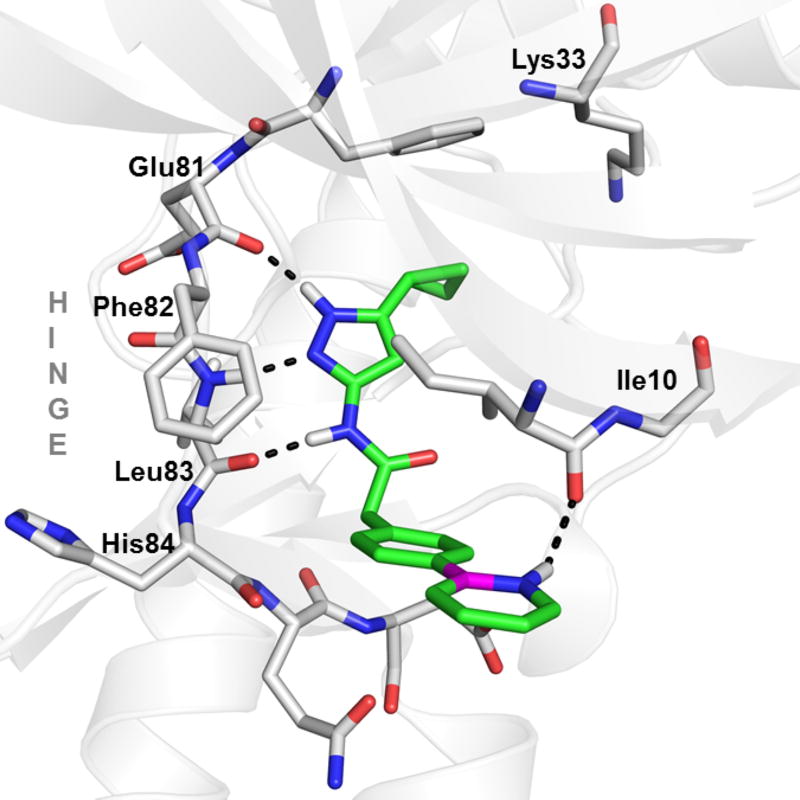
The improved biological activity of BN-3 vs. CC-3 was intriguing. To understand this improvement in potency, BN-3 and CC-3 were analyzed by docking24,25 in a high resolution crystal structure of CDK2/cyclin A (PDB entry 1VYW).19 Shown in Figure 1 is one of the three docking poses obtained for BN-3 in the active site of CDK2. In addition to the hydrogen bonding interaction between the pyrazole amide fragment and hinge residues Leu83, Glu81, an additional hydrogen bonding interaction was observed between the NH of the azaborine and the backbone carbonyl of Ile10. The 3–4 fold improvement in binding of BN-3 vs. CC-3 may be attributed to this NH…O=C(amide) hydrogen bonding which we have recently quantified to be ~ 1 kcal/mol in strength (in the context of binding to T4 Lysozymes).8
Figure 1.
Modeled binding mode of BN-3 (green) in the ATP binding site of CDK2 (white). Hydrogen bonding interactions are shown as black dotted lines. Boron in BN-3 is in magenta color.
Finally, we asked the question whether the observed improved in vitro solubility for BN-3 vs. CC-3 would translate into in vivo pharmacokinetic behavior. Gratifyingly, we determined that BN-3 exhibits pharmacokinetic properties that are superior to CC-3 in male Sprague Dawley Rat models (Table 3). When dosed intravenously, BN-3 showed lower clearance and a longer terminal half-life (t1/2) than CC-3. Additionally, BN-3 gave a two-fold increase in AUCpo (area under the curve per oral administration) relative to CC-3. This results from a combination of lower clearance and greater bioavailability. The maximum concentration (Cmax) of CC-3, 692 nM, is observed at 0.5 hour after oral dosing. BN-3 on the other hand, has maximum concentration of 746 nM at 1.5 hours after dosing, probably due to the increased solubility prolonging the precipitation time and allowing BN-3 to be absorbed further down the intestine than CC-3. Despite the slightly lower permeability of BN-3 relative to CC-3 in vitro, the improved solubility and lower clearance of BN-3 in vivo enabled an increase in oral exposure for BN-3 compared to CC-3.
Table 3.
Pharmacokinetic Parameters of CC-3 and BN-3 after Intravenous and Oral Administration to Male Sprague Dawley Rats.
| cmpd | IV dose (mg/kg) |
PO dose (mg/kg) |
AUCiv (nMh) |
AUCpo (nMh) |
F (%) |
Clearance (mL min−1 kg−1) |
t1/2 (h) |
tmax (h) |
MRT (h) |
Cmax (nM) |
|---|---|---|---|---|---|---|---|---|---|---|
| CC-3 | 0.5 | 5.0 | 1261 | 283 | 22.5 | 39.2 | 10.5 | 0.5 | 0.8 | 692 |
| BN-3 | 1.0 | 5.0 | 2092 | 613 | 29.3 | 23.7 | 11.6 | 1.5 | 2.7 | 746 |
PO dose: per os dose (oral). F: bioavailability. MRT: mean residence time.
AUCiv: area under the curve (intraveneous) normalized to 1 mg/kg dose.
AUCpo: area under the curve (oral) normalized to 1 mg/kg dose.
In summary, we have synthesized the first examples of biologically active monocyclic 1,2-azaborines and demonstrated that BN/CC isosterism in the context of biphenyl carboxamides leads to improvement in vitro aqueous solubility and better in vivo oral availability. The BN isosteres of biologically active biphenyl carboxamides are air and moisture stable, and they exhibit biological activity that is comparable to their carbonaceous counterparts. Furthermore, in the context of a CDK2 inhibitor, we have demonstrated that the presence of a 1,2-azaborine motif can lead to improved biological activity likely from an additional hydrogen bonding interaction associated with the NH of the 1,2-azaborine moiety. Overall, we have demonstrated the viability of the monocyclic 1,2-azaborine motif serving as a novel pharmacophore with a distinct pharmacological profile. In view of the solubility challenges associated with many aryl-based drug candidates, BN/CC isosterism may represent a new design principle in medicinal chemistry to address this challenge.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health NIGMS (R01-GM094541). We thank Dr. Donald Upson for helpful discussions. S.-Y. L. thanks the Camille Dreyfus Teacher-Scholar Awards Program for a Teacher- Scholar award and the Humboldt Foundation for the Friedrich Wilhelm Bessel Research Award.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.(a) Nicolaou KC, Hale CRH, Nilewski C, Ioannidou HA. Chem. Soc. Rev. 2012;41:5185–5238. doi: 10.1039/c2cs35116a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wright PM, Seiple IB, Myers AG. Angew. Chem. Int. Ed. 2014;53:8840–8869. doi: 10.1002/anie.201310843. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2014;126:8984–9014. [Google Scholar]; (c) Rodrigues T, Reker D, Schneider P, Schneider G. Nat. Chem. 2016;8:531–541. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 2.For an overview, see: Liu Z, Marder TB. Angew. Chem. Int. Ed. 2008;47:242–244. doi: 10.1002/anie.200703535.Angew. Chem. 2008;120:248–250.Bosdet MJD, Piers WE. Can. J. Chem. 2009;87:8–29.Campbell PG, Marwitz AJV, Liu S-Y. Angew. Chem. Int. Ed. 2012;51:6074–6092. doi: 10.1002/anie.201200063.Angew. Chem. 2012;124:6178–6197..
- 3.(a) Zhou H-B, Nettles KW, Bruning JB, Kim Y, Joachimiak A, Sharma S, Carlson KE, Stossi F, Katzenellenbogen BS, Greene GL, Katzenellenbogen JA. Chem. Biol. 2007;14:659–669. doi: 10.1016/j.chembiol.2007.04.009. [DOI] [PubMed] [Google Scholar]; (b) Ito H, Yumura K, Saigo K. Org. Lett. 2010;12:3386–3389. doi: 10.1021/ol1012058. [DOI] [PubMed] [Google Scholar]
- 4.Roughley SD, Jordan AM. J. Med. Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 5.McGrath NA, Brichacek M, Njardarson JT. J. Chem. Ed. 2010;87:1348–1349. [Google Scholar]
- 6.For select references on monocyclic 1,3- and 1,4-azaborines, see: Xu S, Zakharov LN, Liu S-Y. J. Am. Chem. Soc. 2011;133:20152–20155. doi: 10.1021/ja2097089.Xu S, Mikulas T, Zakharov LN, Dixon DA, Liu S-Y. Angew. Chem. Int. Ed. 2013;52:7527–7531. doi: 10.1002/anie.201302660.Angew. Chem. 2013;125:7675–7679.Braunschweig H, Damme A, Jimenez-Halla JOC, Pfaffinger B, Radacki K, Wolf J. Angew. Chem. Int. Ed. 2012;51:10034–10037. doi: 10.1002/anie.201205795.Angew. Chem. 2012;124:10177–10180.Liu X, Zhang Y, Li B, Zakharov LN, Vasiliu M, Dixon DA, Liu SY. Angew. Chem. Int. Ed. 2016;55:8333–8337. doi: 10.1002/anie.201602840.Angew. Chem. 2016;128:8473–8477.Schafer M, Beattie NA, Geetharani K, Schafer J, Ewing WC, Krahfuss M, Horl C, Dewhurst RD, Macgregor SA, Lambert C, Braunschweig H. J. Am. Chem. Soc. 2016;138:8212–8220. doi: 10.1021/jacs.6b04128..
- 7.Liu L, Marwitz AJV, Matthews BW, Liu S-Y. Angew. Chem. Int. Ed. 2009;48:6817–6819. doi: 10.1002/anie.200903390. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2009;121:6949–6951. [Google Scholar]
- 8.Lee H, Fischer M, Shoichet BK, Liu S-Y. J. Am. Chem. Soc. 2016;138:12021–12024. doi: 10.1021/jacs.6b06566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knack DH, Marshall JL, Harlow GP, Dudzik A, Szaleniec M, Liu S-Y, Heider J. Angew. Chem. Int. Ed. 2013;52:2599–2601. doi: 10.1002/anie.201208351. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2013;125:2660–2662. [Google Scholar]
- 10.For select references on the late-stage functionalization of monocyclic 1,2-azaborines, see: Baggett AW, Vasiliu M, Li B, Dixon DA, Liu S-Y. J. Am. Chem. Soc. 2015;137:5536–5541. doi: 10.1021/jacs.5b01916.Brown AN, Li B, Liu S-Y. J. Am. Chem. Soc. 2015;137:8932–8935. doi: 10.1021/jacs.5b05879..
- 11.(a) Vlasceanu A, Jessing M, Kilburn JP. Bioorg. Med. Chem. 2015;23:4453–4461. doi: 10.1016/j.bmc.2015.06.019. [DOI] [PubMed] [Google Scholar]; (b) Rombouts FJ, Tovar F, Austin N, Tresadern G, Trabanco AA. J. Med. Chem. 2015;58:9287–9295. doi: 10.1021/acs.jmedchem.5b01088. [DOI] [PubMed] [Google Scholar]
- 12.For select references on recent synthetic method development for BN isosteres of naphthalene, see: Wisniewski SR, Guenther CL, Argintaru OA, Molander GA. J. Org. Chem. 2014;79:365–378. doi: 10.1021/jo402616w.Amani J, Molander GA. Org. Lett. 2015;17:3624–3627. doi: 10.1021/acs.orglett.5b01750.Jouffroy M, Davies GH, Molander GA. Org. Lett. 2016;18:1606–1609. doi: 10.1021/acs.orglett.6b00466..
- 13.Horton DA, Bourne GT, Smythe ML. Chem. Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 14.In the statistical analysis by Roughley et al., biaryl compounds have an occurrence level of 38.6% across the analyzed data set, see reference 4.
- 15.Rudebusch GE, Zakharov LN, Liu S-Y. Angew. Chem. Int. Ed. 2013;52:9316–9319. doi: 10.1002/anie.201304443. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2013;125:9486–9489. [Google Scholar]
- 16.(a) Lee H-W, Kang TW, Cha KH, Kim E-N, Choi N-H, Kim J-W, Hong C., II Synth. Commun. 1998;28:1339–1349. [Google Scholar]; (b) Barnett CJ, Wilson TM, Kobierski ME. Org. Process Res. Dev. 1999;3:184–188. [Google Scholar]
- 17.Leopoldo M, Lacivita E, Colabufo NA, Contino M, Berardi F, Perrone R. J. Med. Chem. 2005;48:7919–7922. doi: 10.1021/jm050729o. [DOI] [PubMed] [Google Scholar]
- 18.Shearer BG, Wiethe RW, Ashe A, Billin AN, Way JM, Stanley TB, Wagner CD, Xu RX, Leesnitzer LM, Merrihew RV, Shearer TW, Jeune MR, Ulrich JC, Willson TM. J. Med. Chem. 2010;53:1857–1861. doi: 10.1021/jm900464j. [DOI] [PubMed] [Google Scholar]
- 19.Pevarello P, Brasca MG, Amici R, Orsini P, Traquandi G, Corti L, Piutti C, Sansonna P, Villa M, Pierce BS, Pulici M, Giordano P, Martina K, Fritzen EL, Nugent RA, Casale E, Cameron A, Ciomei M, Roletto F, Isacchi A, Fogliatto G, Pesenti E, Pastori W, Marsiglio A, Leach KL, Clare PM, Fiorentini F, Varasi M, Vulpetti A, Warpehoski MA. J. Med. Chem. 2004;47:3367–3380. doi: 10.1021/jm031145u. [DOI] [PubMed] [Google Scholar]
- 20.See Supporting Information for details.
- 21.See Supporting Information for experimental details.
- 22.Chrostowska A, Xu S, Lamm AN, Mazière A, Weber CD, Dargelos A, Baylère P, Graciaa A, Liu S-Y. J. Am. Chem. Soc. 2012;134:10279–10285. doi: 10.1021/ja303595z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Thayer AM. Chem. Eng. News. 2010;88(22):13–18. [Google Scholar]; (b) Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, Porte CJH. Pharmacol. Rev. 2013;65:315–499. doi: 10.1124/pr.112.005660. [DOI] [PubMed] [Google Scholar]
- 24.Docking poses were generated in a high resolution crystal structure of CDK2/cyclin A (PDB entry 1VYW)19 using the extra precision mode in Glide, version 6.6, Schrödinger, LLC, New York, NY, 2015.
- 25.For leading references on Docking, see: Kitchen DB, Decornez H, Furr JR, Bajorath J. Nat. Rev. Drug Discov. 2004;3:935–949. doi: 10.1038/nrd1549.Kolb P, Ferreira RS, Irwin JJ, Shoichet BK. Curr. Opin. Biotechnol. 2009;20:429–436. doi: 10.1016/j.copbio.2009.08.003.Irwin JJ, Shoichet BK. J. Med. Chem. 2016;59:4103–4120. doi: 10.1021/acs.jmedchem.5b02008..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.