Abstract
Purpose of the Study
We evaluated the feasibility and reliability of commonly used clinical dementia assessments when administered via direct-to-home telemedicine videoconferencing. To date, few studies assessed the suitability of these measures when used in this setting.
Design and Methods
Sixty-six participants (33 patients with Alzheimer’s disease (AD) and their 33 caregivers) consented to assessment with a battery of tests in both the clinic setting and via telemedicine. We administered cognitive, behavior, and mood assessments to persons with mild, moderate, and severe AD both in the clinic setting and via direct-to-home telemedicine videoconferencing; test–retest reliability was assessed. We also explored how three caregiver measures performed when administered via telemedicine. Assessments were administered 2 weeks apart. Participant feedback about their experience was solicited.
Results
Twenty-eight dyads completed the assessments. Reliability was found to be good to excellent in all measures when used with direct-to-home telemedicine. For the most part, participants and clinicians found telemedicine to be a feasible option for assessing cognitive function and caregiver coping.
Implications
Findings indicate that these measures can be used to assess persons with AD, as well as their caregivers, across the telemedicine platform, directly to their homes. Use of this technology can expand access to care to the millions across the United States with AD and their caregivers.
Keywords: Telemedicine, Dementia, Caregiver
Innovative models of care are needed to meet the ever-increasing demand for quality professional care for the nearly 5.5 million Americans living with Alzheimer’s disease or a related dementia (ADRD) and their caregivers (Alzheimer’s Association, 2016). For many families, access to care for ADRD is limited by geographic, physical, and psychological barriers. Whether families live in rural or metropolitan areas, behavioral symptoms of ADRD, such as apathy or agitation, can make travel to a clinic site challenging even in the best of circumstances. The Alzheimer’s Association (2011) maintains that national priorities for care must address the needs of ill-equipped communities and unprepared caregivers. Telemedicine care has the potential to address these priorities and mitigate the challenges faced when families living with ADRD seek care.
Telemedicine is defined as the electronic exchange of medical information from one site to another with the goal of improving patient health (American Telemedicine Association, 2012). Since the 1960s, telemedicine has been used to provide health care to persons with a variety of neurological conditions, including stroke, epilepsy, and multiple sclerosis, to name only a few (Larner, 2011; Wittson, Affleck, & Johnson, 1961). The exploration of cognitive assessment with telemedicine began in the 1990s (C. J. Ball, Scott, McLaren, & Watson, 1993; Montani et al., 1997), and studies continue to examine the utility of this care model when used with persons with cognitive impairment (Martin-Khan et al., 2012; McEachern, Kirk, Morgan, Crossley, & Henry, 2008). Our study extends this work by examining the feasibility and reliability of direct-to-home, telemedicine-administered AD assessments.
Background
Telemedicine has been used to explore cognitive impairment since the 1990s. Pioneering work by Ball and colleagues (1993) explored the feasibility of administering the Mini-Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975), a brief cognitive screening test, via telemedicine. Subsequently, others around the world worked on establishing the reliability of the telemedicine-administered MMSE, and, for the most part, found robust agreement between the face-to-face MMSE and telemedicine-administered MMSE (C. Ball, Tyrrell, & Long, 1999; Ciemins, Holloway, Coon, McClosky-Armstrong, & Min, 2009; Cullum, Weiner, Gehrmann, & Hynan, 2006; Loh et al., 2004; McEachern et al., 2008; Montani et al., 1997; Saligari et al., 2002). However, to our knowledge, few studies have evaluated the reliability of other measures of cognitive impairment such as the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005). In addition, little work has been done on a key aspect to care delivery: assessment of caregiver status. Although awareness of the interplay between caregiver well-being and care recipient health has spurred the development of many caregiver strain assessments, the reliability of these assessments when used with telemedicine has not been addressed in depth.
Most prior studies used clinic or hospital-based tele medicine services, requiring patient transport to a tele medicine studio or site. Direct-to-home telemedicine is a newer approach that is gaining patient and family acceptance (Gardner et al., 2015), but a full understanding of the value of home-based telemedicine in dementia care is lacking in the current literature. Thus, the aim of our study, Alzheimer’s Care via Telemedicine for Oregon (ACT-ON), Phase I, was to establish the reliability and feasibility of commonly used measures of cognitive function, dementia severity, and mood in persons with Alzheimer’s disease (AD) when used with direct-to-home telemedicine technology. We also assessed the reliability of three measures of caregiver coping.
Methods
Participants
English-speaking men and women with AD (McKhann et al., 2011) and their caregivers were recruited from Oregon Health & Sciences University’s (OHSU) NIA-Layton Aging and Alzheimer’s Disease Center, and the community at large. To better understand the utility of direct-to-home telemedicine care in this population, we included patients across the spectrum of disease severity, from mildly to severely impaired. We recognized that this broad inclusion criteria may have affected measurement precision, but we were concerned that focusing on one stage (e.g., mild) would reduce the applicability of the measures in everyday practice. The AD diagnosis was confirmed via electronic medical record review. To be included in the study, participants were required to have adequate vision and hearing and access to a computer with reliable high-speed Internet access (see Equipment). Both patients and caregivers consented to this Institutional Review Board-approved study (00011476). To reduce family burden, we consented participants via telephone. In most cases, the caregiver served as the patient’s authorized representative for research and consented for them. Patients assented.
Procedures
Participant dyads (persons with AD and their caregivers) received an identical battery of tests (see Measures) both in the clinic setting and via telemedicine, approximately 2 weeks apart. The telemedicine and MoCA test–retest literature reports wide variability in test–retest intervals, from 60 minutes (Costa et al., 2012) to 7 months (Abdolahi et al., 2014). We determined that a 60-minute interval would be too taxing for the participants with ADRD, and the 7-month interval would allow for meaningful cognitive decline. Based on the literature and our clinical experience, we deemed 2 weeks to be an appropriate interval. To control for clinician effect, a counterbalanced design was used in which clinicians (one for each site) switched sites after half of the sample completed the study. We aimed to test the caregiver first in each setting, but recognized that factors such as care recipient anxiety and fatigue may dictate an order change. Thus, we did not counterbalance participant test order. To control for order effect, participant dyads were randomized to receive either the in-clinic battery first or the telemedicine visit first. To minimize cross-site variation, we used the same protocol for each site.
For the in-clinic assessment, each patient–caregiver dyad traveled to the university medical clinic where they met with the clinician who administered the battery of tests. The clinician assessed the caregiver first, in private. If needed, an assistant stayed with the patient in the waiting room to keep them occupied and safe. Then the clinician assessed the patient. The patient was given the option of having their caregiver stay with them during their assessments. No technology was used in the in-clinic visit.
Each dyad also participated in a telemedicine visit. Prior to the telemedicine visit, a research assistant (RA) with technical expertise met with each caregiver via telephone and telemedicine to test the family’s Internet connection, assist in downloading the secure telemedicine link, and resolve technical challenges. A telemedicine test was conducted prior to the telemedicine visit. During the test visit, the caregivers were given instructions on how to adjust room lightening for the best image, modify the environment to minimize distractions (e.g., turn off television), and maximize sound quality (e.g., remove barking dog from room). The test visits took about 20 to 120 minutes, depending on the caregivers’ computer expertise. Due to the lack of the clinicians’ telemedicine experience, OHSU’s Telehealth Department provided clinician training and support. Clinicians also received support from the senior study team member who has extensive telemedicine experience (D. Erten-Lyons).
For the telemedicine visit, each dyad, in their own home, connected with the clinician in our telemedicine office, a quiet, clutter-free office with a computer station. As in the clinic visit, we assessed the caregivers first. Once the caregiver battery was completed, the caregiver brought the patient to the computer for his or her assessment. Caregivers helped patients put on headphones, adjust volume, and engage with the telemedicine clinician.
After the initial visits were completed, some caregivers reported that they and the patients were distressed when conversations between the caregivers and the clinicians were overheard by the patient (e.g., discussions about hygiene). Thus, we asked caregivers to use headphones so that sensitive questions from the clinician would not be overheard. Because none of the telemedicine staff were at the dyads’ homes, we asked caregivers to find an activity to occupy the patients during the caregiver sessions. We encouraged caregivers to take breaks to check on the patients if needed.
Equipment
Real-time assessments were conducted remotely using direct-to-home videoconferencing technology. The mode of connectivity between the study participants and the clinicians was via Cisco’s Jabber TelePresence platform (Cisco, 2014). This technology allowed face-to-face, highly secure video connections to remote locations. Using their computer and a camera, participants in their homes were connected with the clinician at the university. Jabber is HIPAA compliant and secure as independently validated by the university’s information technology department. Using the Cisco Telepresence Content Server, the telemedicine visits were securely recorded and stored.
Participants were provided with cameras and headphones. Participants who did not have a computer, or did not have Internet service which accommodated the telemedicine visits were loaned an iPad. The iPads were preprogrammed so the only accessible function was a connection to our study interface (the live video feed) when the tablet was turned on. Cellular service was used when an Internet connection was unavailable. The iPads were mailed to the participants and postage was provided for their return. All iPads were successfully returned.
Measures
Feasibility was assessed by comparing the number of participants who attempted the measures with those who completed the measures. Feedback about telemedicine sessions was solicited by a co-investigator who observed the video-recorded visits and interviewed the participants and the clinicians, but did not perform any of the clinical assessments. Measure administration times were documented.
Patient cognitive function was measured with the MoCA (Nasreddine et al., 2005), and dementia stage was assessed using the caregiver report component of the Clinical Dementia Rating Scale (CDR) (Morris, 1993a). The MoCA is a 30-point cognitive assessment that measures visuospatial abilities, executive function, verbal learning and memory, attention, concentration, language, and orientation. It is a sensitive test for identifying persons at risk for dementia and was recently added to the National Alzheimer’s Coordinating Center’s (NACC) Uniform Data Set (UDS) battery of cognitive tests (ADC Clinical Task Force, National Alzheimer’s Coordinating Center, 2015; Nasreddine et al., 2005; Monsell et al., 2016).
Some modifications in the MoCA (Nasreddine et al., 2005) administration were required to accommodate the telemedicine milieu. To maximize consistency between test sites (in-clinic or telemedicine), we used the same modified MoCA for both sites. Three modifications were made and these bear further explanation.
First, to facilitate ease of scoring, the visuospatial/executive function items of the MoCA (trails, cube, clock) (Nasreddine et al., 2005) were enlarged, and one sheet of paper was used for each task. Prior to the telemedicine visit, the visuospatial/executive function section (containing the visual material needed for the test) was mailed to participants with instructions to open the envelope when instructed by the clinician. During the telemedicine visit, the clinician asked the patient to open the envelope and follow her instructions (Stillerova, Liddle, Gustafsson, Lamont, & Silburn, 2016). Caregivers were often present to assist with small tasks such as opening the envelope or finding a pen for the patient but were not allowed to assist the patients in completing the MoCA (Nasreddine et al., 2005). After each component of the visuospatial section was completed, the clinician asked the patient to hold the work up to their camera for the clinician to score. Caregivers held up the completed forms if the patient was unable. The papers were mailed back to the center to verify scoring. The same protocol was used in the clinic, but the papers were not mailed ahead of time and the telemedicine interface was not used.
Second, in the telemedicine visit, instead of having the patient view a paper version of the language (animal-naming) component of the MoCA (Nasreddine et al., 2005), we opened a file with the pictures on the clinician’s computer and shared it with the patient on his or her screen. The patient was asked to name the animals. The clinician used the computer mouse to “point” to each animal. The standard paper picture was used in the in-clinic setting.
Finally, a component of the MoCA (Nasreddine et al., 2005) has the patient tap the table each time he hears the letter “A” in a string of 29 letters. We asked the patients to clap their hands every time they heard the letter “A.” This provided a louder sound and visual feedback for the telemedicine clinician. In addition, the string of letters was printed on a small piece of paper and placed on the top of clinician’s computer near the camera, allowing the clinician to have better view of the patient while reading the string of letters.
We used the caregiver report component of the CDR (Morris, 1993a; Morris et al., 1997) to further assess patient function and rate dementia stage (0 = normal to 3 = severe). The CDR, also a part of the UDS (ADC Clinical Task Force, National Alzheimer’s Coordinating Center, 2015), asks caregivers to rate patient function across six categories (memory, orientation, judgment, home activities, community involvement, and personal care) (Morris, 1993a). The CDR worksheet was used to interview the caregivers (Morris, 1993b). In the telemedicine visit, the answer choices (e.g., yes/no) were shared with the caregivers on their computer screens, paper forms were used in the in-clinic visit. To minimize time demands on the patients, the patient component of the CDR was not performed in this study. The overall CDR score (0–3) was determined based on the caregivers’ worksheet answers.
The 15-item Geriatric Depression Scale (GDS; Almeida & Almeida, 1999; Yesavage et al., 1982) was used to assess patient mood. Each item was read aloud to the patient (in both the clinic and telemedicine setting), patients responded with “yes” or “no” to each stem. We did not use the GDS for caregivers because we did not want to put limitations on the age range of caregivers. To assess behavioral symptoms, caregivers were read a list of behaviors from the Revised Memory and Behavioral Problems Checklist (RMBPC) (Teri et al., 1992) and asked if the problem occurred in the last week. Caregivers responded with “yes” or “no.” After study start, an expert advisor noted that these two measures would provide a valuable foundation for our future work, they were thus added after the initial 7 dyads were assessed.
Caregiver burden and grief were assessed with the 4-item Zarit Burden Interview (ZBI; Bedard et al., 2001; Zarit, Anthony, & Boutselis, 1987), the RMBPC (Teri et al., 1992; caregiver reaction to behaviors), and the Marwit Meuser Caregiver Grief Index, Short Form (MMCGI-SF) (Marwit & Meuser, 2005). These measures were administered orally, in both the in-clinic and telemedicine visits. Clinicians read each item and caregivers responded with the aid of the visual response scale for each measure (a paper scale for the in-clinic visits, a shared-screen scale on their computers for the telemedicine visits).
A demographic form was completed (either in the in-clinic visit or the telemedicine visit) that asked for information about education, financial situation, and distance (in miles) from the university. Oregon is geographically large, but the population centers are in the northwest. By notating distance from the clinic, we could ascertain how telemedicine care could serve the larger region. The year of diagnosis was noted for the patients. Caregivers were asked how many hours per week they provided care (e.g., direct care and general supervision).
Statistical Analysis
Test–retest reliability was assessed using the intraclass correlation (ICC) for continuous variables, with the following standard performance parameters: excellent, >0.75; good, 0.40–0.75; marginal, <0.40 (Fleiss, 1981). Cohen’s Kappa coefficient was used for the categorical variables, with the same parameters (Cohen, 1960).
Results
Demographics
Thirty-three persons with AD and their 33 caregivers consented to our study, with 28 completing both an in-clinic visit and telemedicine visit (Table 1). The mean age of persons with AD was 71.6 (SD = 11.6) years, and 65.3 (SD = 9.6) years for their caregivers. Most were White and reported financial stability. One third of the sample lived more than 50 miles from the university. Of interest, 61% of persons with AD were women, and 61% were caregivers. This is consistent with national trends in which about two thirds of persons with dementia are women, while at the same time, two thirds of dementia caregivers are women (Alzheimer’s Association, 2016).
Table 1.
Demographics: ACT-ON Phase I (n = 28)
| Caregivers (% female) | 61 |
| Persons with AD (% female) | 61 |
| Age, Caregiver (mean, SD [range]) | 65.3, 9.6 [38–79] |
| Age, Person with AD (mean, SD [range]) | 71.6, 11.6 [51– 96] |
| Years caregiving (mean, SD [range]) | 3.5, 2.4 [<1–12] |
| Hours/week caregiving (mean, SD [range]) | 75.4, 72.8 [<1–168] |
| Years living with AD (mean, SD [range]) | 3.3, 3.7 [<1–15] |
| Distance from clinic (% >10 miles) | 75 |
| Education, patients (% with > 12 years) | 86 |
| Education, caregivers (% with >12 years) | 96 |
Feasibility
Of the 33 dyads who consented to the study, two dropped out due to technical difficulties, and three dropped out for other reasons (poor health and time limitations). Those who withdrew were not different in basic demographics and distance from clinic than those who completed the study. Of the 28 dyads who completed the visits, four patients (14%) were unable to complete the telemedicine MoCA (Nasreddine et al., 2005) due to frustration or problems with comprehension. One scoring error (clock) was noted after the MoCA telemedicine forms were mailed back to the center. All 28 caregivers completed the in-clinic and telemedicine batteries. Mean administration time for the in-clinic visits was 41.4 minutes (SD = 13) and 47.5 (SD = 12.6) minutes for the telemedicine visits.
There were technical challenges. First, some caregivers had limited experience with videoconferencing, necessitating extended learning sessions with the RA. However, at the time of the telemedicine visits, this paid off with few technical problems. Four families used the loaner iPad. Second, clinicians had to look into the telemedicine camera, at the top of the computer screen in order to give the impression of eye contact with the participants. Thus, clinicians had to divert their eyes away from participants’ faces, which at first was distracting. Finally, visual quality was good, but participants often had to be coached to close curtains, adjust lights, move chairs to maintain good quality. Sound quality was good.
It should also be noted that the telemedicine experience was truly dyadic in nature in that we relied on the caregivers to help with the telemedicine visits. This is similar to the findings of Radhakrishnan, Xie, and Jacelon (2015) that caregivers are needed, for the most part, when persons with AD are assessed via telemedicine.
Feedback from participants and clinicians indicated that they were satisfied with the effectiveness of the telemedicine platform for giving and receiving care. Although there was uncertainty that telemedicine visits could completely replace the traditional face-face visit, many enjoyed the convenience of the telemedicine visit, as noted by one participant: “I would prefer to have it (the visit) as a telemedicine and not waste my time, energy, and resources.”
Reliability
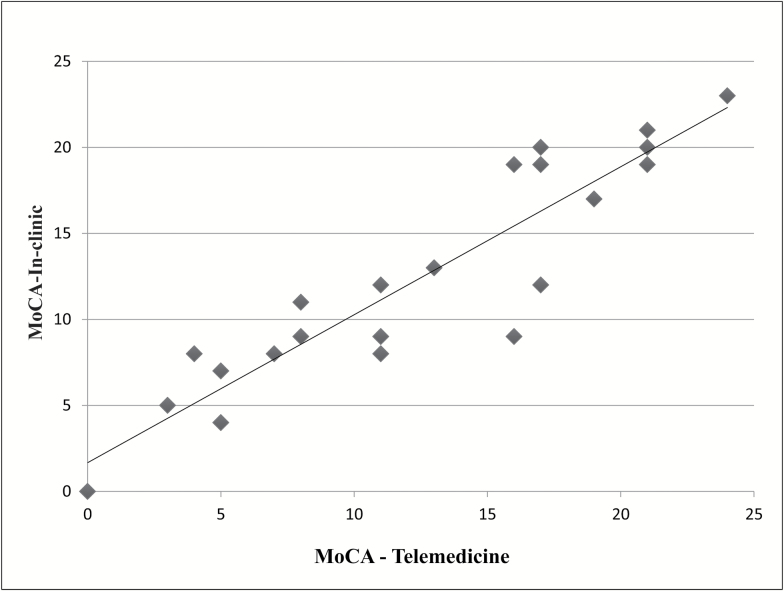
We found that most of the measures were suitable for telemedicine use with persons with mild-to-severe dementia (Table 2). The MoCA (Nasreddine et al., 2005) (Figure 1) had excellent reliability, ICC = 0.93. The two MoCA subsections we modified, visuospatial/executive section (trails, cube, clock) (ICC = 0.86) and letter clapping (k = 0.69) held up well. The RMBPC (occurrence of behaviors) (Teri et al., 1992) had excellent test–retest reliability (ICC = 0.77). The caregiver assessments of patients on the CDR (Morris et al., 1997) had good reliability (k = 0.75), as did the GDS (Almeida & Almeida, 1999; Yesavage et al., 1982) (ICC = 0.67).
Table 2.
Results, Measures of Dementia Status, ACT-ON Phase I (n = 28)
| Scale | In-clinic | Telemedicine | ICC/Kappa |
|---|---|---|---|
| Mean (range) | Mean (range) | ||
| MoCA | 12.2 (0–23) | 13.1 (0–24) | 0.93 (Excellent) |
| Visuospatial/Exe | 2.1 (0–5) | 1.9 (0–5) | 0.86 (Excellent) |
| Letter tapping | (categorical) | 0.69 (k, Good) | |
| CDR (range only) | 0.5–3 | 0.5–3 | 0.75 (Good) |
| RMBPCa (frequency of behaviors) | 9.5 (2–18) | 9.7 (2–18) | 0.77 (Excellent) |
| GDSa | 2.3 (0–9) | 3.0 (0–9) | 0.67 (Good) |
Note: CDA = Clinical Dementia Rating; MoCA = Montreal Cognitive Assessment; RMBPC = Revised Memory and Behavioral Problems Checklist.
aNot administered to first seven participants (n = 21).
Figure 1.
Linear relationship between in-clinic and telemedicine MoCA results. In-clinic range = 0–23, telemedicine range = 0–24; ICC = 0.93, indicating excellent correlation.
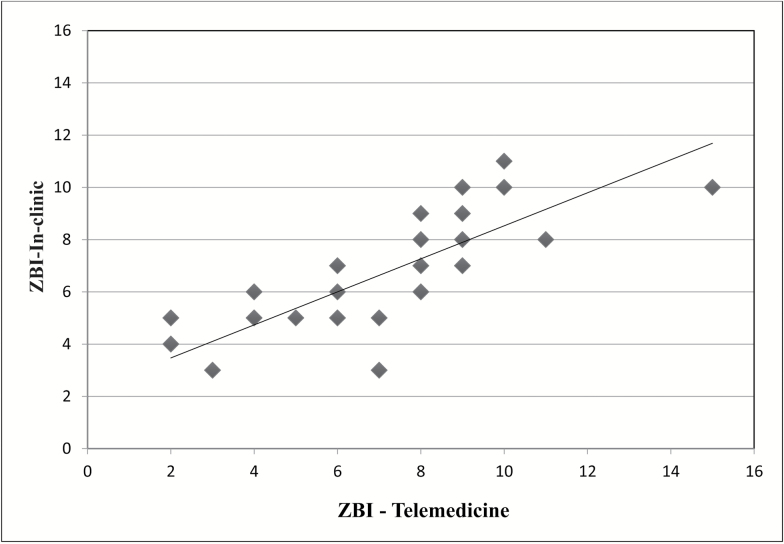
Caregiver assessments also proved to be reliable when used with the telemedicine interface (Table 3), with the MMCGI-SF (Marwit & Meuser, 2005) and the ZBI (Bedard et al., 2001) (Figure 2) having excellent reliability (ICC = 0.87 and 0.79, respectively). The RMBPC (caregivers’ reactions to identified bothersome behaviors) (Teri et al., 1992) also had excellent reliability (0.80).
Table 3.
Results, Caregiver Measures, ACT-ON Phase I (n = 28)
| Scale | In-clinic | Telemedicine | ICC/Kappa |
|---|---|---|---|
| Mean (range) | Mean (range) | ||
| MMCGI-SF | 47.3 (22–66) | 46.1 (23–61) | 0.87 (Excellent) |
| ZBI | 6.4 (3–11) | 6.7 (2–15) | 0.79 (Excellent) |
| RMBPCa (reaction to behaviors) | 11.1 (1–36) | 11.1 (1–43) | 0.80 (Excellent) |
Note: RMBPC = Revised Memory and Behavioral Problems Checklist.
aNot administered to first seven participants (n = 21).
Figure 2.
Linear relationship between in-clinic and telemedicine ZBI results. In-clinic range = 3–11, telemedicine range = 2–15; ICC = 0.79, indicating excellent test–retest reliability.
Discussion
Our study explored the feasibility of using telemedicine for assessing patients with AD and their caregivers. For the most part, patients, caregivers, and clinicians found the telemedicine modality feasible. We also assessed the reliability of the MoCA (Nasreddine et al., 2005), the CDR (Morris et al., 1997), the GDS (Yesavage et al., 1982), and the RMBPC (Teri et al., 1992) when used with the direct-to-home telemedicine, with patients with mild-to-severe dementia. We provide new data on the reliability of the caregiver burden and grief measures (the ZBI (Zarit et al., 1987), the RMBPC (Teri et al., 1992), and the MMCGI-SF (Marwit & Meuser, 2005)) when used with this methodology. The reliability of all measures in the study, for both patients and caregivers, ranged from good to excellent; none were considered marginal. The results suggest that these measures can be used with confidence in telemedicine dementia assessment and care.
Despite the fact that the MoCA is gaining acceptance in research and clinical practice (Monsell et al., 2016), we found only two studies establishing the feasibility and reliability of the MoCA (Nasreddine et al., 2005) when used with telemedicine, both for persons with Parkinson’s disease (PD). Abdolahi and colleagues (2014) examined the reliability of the MoCA when used with persons with PD via telemedicine and Stillerova and colleagues (2016) examined the feasibility of using the MoCA with telemedicine. We found both similarities and differences between our study and theirs.
Abdolahi and colleagues’ (2014) PD subsample had a higher mean face-to-face MoCA score than ours (27.0, SD = 1.93), but their reliability was lower (ICC = 0.37). Unlike our study, they assessed all patients face-to-face first, and then the PD subsample, with telemedicine, 7 months later. It is possible that the long lag time between visits accounted for the low reliability because the PD patients’ cognitive function may have changed over that interval.
Like Abdolahi and colleagues (2014), we grappled with how best to administer the visuospatial/executive section to our participants. Abdolahi and colleagues (2014) successfully e-mailed the visuospatial/executive sections to their participants who printed this section prior to test administration. Stillerova and colleagues (2016) used the paper and pen version, given to participants prior to telemedicine testing. Both approaches work well, but the e-mail version may be more cost-effective. A tablet form of the MoCA is now available (Nasreddine, 2016), however, data on testing the tablet with the telemedicine interface could not be found in the current literature.
Participants in Stillerova and colleagues’ (2016) study found the telemedicine option acceptable. Unlike our experience, all their participants (n = 11) completed all test items, however, only one of their participants was found to be cognitively impaired (average scores not reported). This may explain their high completion rate.
Saligari and colleagues (2002) were among the first to assess the GDS (Almeida & Almeida, 1999) via telemedicine. They found it was feasible to administer the GDS in both local clinical settings and in a rural satellite clinic, and reported the GDS was reliable, but no statistical data were reported. Loh and colleagues (2004) assessed the reliability of the GDS when used with telemedicine in their group of 20 inpatient participants. They assessed the patients both face-to-face in the hospital setting, and again via telemedicine, while the patients were still in the hospital. They found good reliability, with the ICC = 0.78. Unlike our work, these were not direct-to-home assessments and their participants’ average MMSE (24) (Folstein et al., 1975) was higher than our average MoCA (13.3, which approximates a MMSE score of 19 (Monsell et al., 2016)). Reliability of the GDS decreases with lower cognitive scores (Bedard et al., 2003), which may explain the higher ICC (0.78) in the Loh study (2004) when compared with ours (0.67).
Family caregivers are an essential component of the U.S. health care system, but many feel unsupported and overburdened (Reinhard, Levine, & Samis, 2012). Telemedicine caregiver assessment can literally meet caregivers where they are, providing needed support and guidance. Studies have explored the caregiver experience and the role telemedicine can play (e.g., Chi & Demiris, 2015; Griffiths, Whitney, Kovaleva, & Hepburn, 2016; Lee et al., 2000), but we could not identify any that assessed the reliability of measures of burden or predeath grief in the context of dementia caregiving. This may be because many of these paper-and-pencil tests are self-administered. However, we wanted to see how the assessments would hold up via telemedicine so they could be used in future studies and clinical applications. These assessments had excellent reliability. Furthermore, we found that oral administration of the caregiver assessments spurred caregiver conversation and catharsis, even though our administration was scripted.
Administering caregiver measures via telemedicine was advantageous because the data were collected at the time of the visits, instead of waiting for participants to mail their assessments back to the research center. All caregivers completed all measures, both in the clinic and telemedicine sites. Survey return rates challenge researchers and a variety of tactics have been tested to increase rates (Edwards et al., 2009). The telemedicine administration of the measures allowed for 100% participation of all caregiver measures, a rate not achieved when mail-in procedures are used. Our study suggests that other caregiver measures, such as the Screen for Caregiver Burden Scale (Vitaliano, Russo, Young, Becker, & Maiuro, 1991), could also be assessed for reliability with telemedicine. This, in turn, could widen the field of caregiver research and ultimately, support options.
Strengths and Limitations
The ACT-ON, Phase I study had strengths as well as limitations. Our strong reliability findings may be due to our careful attention to administering the same version of each measure at both the in-clinic and telemedicine visits. The clinicians used the same scripts for measure administration, resulting in extended testing sessions. To minimize participant burden, we limited the testing for the care recipients to two measures. We aimed to test the caregivers first, but we followed the caregivers’ advice on order of testing if needed for emotional or practical reasons. In our efforts to optimize the testing experience, it is possible that the results were positively biased.
Modification of the visual/spatial section of the MoCA (Nasreddine et al., 2005), to make telemedicine administration more feasible, could be considered a strength as well as a limitation. Aiming for consistent application, we used these modifications in the in-clinic visits as well, which may have affected the scores.
We did not collect quantitative data on participants’ comfort levels with telemedicine. These data were collected in Phase II of ACT-ON and will be reported in future publications.
A limitation to this study, like many in Oregon, is that most of our participants were White. Further, most came from higher socioeconomic backgrounds, had computers, and were willing to work with the RA to download the necessary programs to complete the visits. More work is needed to explore the feasibility of providing telemedicine care to financially marginalized families who do not own a computer or who are of lower educational attainment levels.
Similarly, a sample with better minority representation would have provided more insight into the feasibility of this medium. For example, George, Hamilton, and Baker (2012) found that some of their African American participants felt the telemedicine platform lacked the personal connection needed to establish clinician–patient trust.
Most of our caregivers were spouses, with the average age being 65.3 years. Participation of more sons and daughters (who may be younger) could affect feasibility and result in different reliability outcomes. However, few participants dropped out of the study due to lack of computer experience. This is consistent with the national trend, in which Internet usage among adults aged 65 and older has grown substantially over the last 2 years, with 58% of them now using the Internet (Pew Research Center, 2015). With the continuing drop in computer hardware costs, direct-to-home clinical video visits should become more accessible over time.
Implications for Practice
Previous work has found that it is possible to administer the MMSE, the GDS, and the MoCA through a standard telemedicine interface (Abdolahi et al., 2014; Loh et al., 2004; Montani et al., 1997; Stillerova et al., 2016). Our study extends this work by adding important reliability information on commonly used measures of cognitive function and caregiver coping when used with telemedicine. Uniquely, we provide new information on the feasibility of telemedicine assessment when used directly to participants’ home.
Taken together, the early foundational studies, coupled with our work, point to a promising future for telemedicine dementia care. A 40% increase in the number of people with AD is expected in the next 10 years (Alzheimer’s Association, 2016) and the traditional clinic-based assessment modality of care will be stretched thin. Telemedicine care can ease the demand on in-clinic care. However, despite the advances in modern telemedicine care, Medicare does not cover direct-to-home telemedicine care for all U.S. beneficiaries. Telemedicine visits are covered if the patient lives in a designated rural area and receives care at a medical facility known as an “originating site” (U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services, 2015). Thus, although direct-to-home AD care is a promising alternative to families, it is not available to many older American adults.
Although telemedicine care may not be an option for all, it has the potential to meet the needs of many families living with ADRD. Armed with knowledge that the measures in our study can be used remotely, directly to the homes of with persons with AD, gerontologists may expand their care across the geographic, economic, and psychological barriers that stymie easy access to dementia care. Our study also opens new avenues of caregiver assessment and support. Using the reliable measures from this study, our future work will examine the quality of dementia care using this direct-to-home telemedicine model (ACT-ON, Phase II), as well as the feasibility of providing caregiver support.
Funding
The project described was funded by the Oregon Health Authority’s State Innovation Model grant, supported by funding Opportunity Number CMS-1G1-12-001 from the U.S. Department of Health and Human Services, Centers for Medicare & Medicaid Services and the content provided is solely the responsibility of the authors and do not necessarily represent the official views of HHS or any of its agencies. This work was also supported by the National Institute on Aging (P30-AG008017, P30AG024978).
References
- Abdolahi A. Bull M. T. Darwin K. C. Venkataraman V. Grana M. J. Dorsey E. R., & Biglan K. M (2016). A feasibility study of conducting the Montreal Cognitive Assessment remotely in individuals with movement disorders. Health Informatics Journal, 22, 304–311. doi:1460458214556373 [DOI] [PubMed] [Google Scholar]
- ADC Clinical Task Force, National Alzheimer’s Coordinating Center (2015). NACC Uniform Data Set, initial visit packet, version 3.0, March 2015. Seattle, WA: University of Washington. [Google Scholar]
- Almeida O. P., & Almeida S. A (1999). Short versions of the Geriatric Depression Scale: A study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. International Journal of Geriatric Psychiatry, 14, 858–865. doi:10.1002/(SICI)1099-1166(199910)14:103.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association (2011). Alzheimer’s from the frontlines: Challenges a national Alzheimer’s plan must address Retrieved from http://www.alz.org/documents_custom/napareport.pdf
- Alzheimer’s Association (2016). 2016 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 12, 459–509. doi:10.1016/j.jalz.2016.03.001 [DOI] [PubMed] [Google Scholar]
- American Telemedicine Association (2012). What is telemedicine? Retrieved from http://www.americantelemed.org/about/telehealth-faqs-
- Ball C. Tyrrell J., & Long C (1999). Scoring written material from the mini-mental state examination: A comparison of face-to-face, fax and video-linked scoring. Journal of Telemedicine and Telecare, 5, 253–256. doi:10.1258/1357633991933819 [DOI] [PubMed] [Google Scholar]
- Ball C. J. Scott N. McLaren P. M., & Watson J. P (1993). Preliminary evaluation of a low-cost videoconferencing (LCVC) system for remote cognitive testing of adult psychiatric patients. British Journal of Clinical Psychology, 32, 303–307. doi:10.1111/j.2044-8260.1993.tb01060.x [DOI] [PubMed] [Google Scholar]
- Bedard M. Molloy D. W. Squire L. Dubois S. Lever J. A., & O’Donnell M (2001). The Zarit Burden Interview: A new short version and screening version. The Gerontologist, 41, 652–657. doi:10.1093/geront/41.5.652 [DOI] [PubMed] [Google Scholar]
- Bedard M. Squire L. Minthorn-Biggs M. Molloy D. W. Dubois S. O’donnell M., & Lever J. A (2003). Validity of self-reports in dementia research. Clinical Gerontologist, 26, 155–163. doi:10.1300/J018v26n03_13 [Google Scholar]
- Chi N. C., & Demiris G (2015). A systematic review of telehealth tools and interventions to support family caregivers. Journal of Telemedicine and Telecare, 21, 37–44. doi:10.1177/1357633X14562734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciemins E. L. Holloway B. Coon P. J. McClosky-Armstrong T., & Min S. J (2009). Telemedicine and the Mini-Mental State Examination: Assessment from a distance. Telemedicine Journal and E-Health, 15, 476–478. doi:10.1089/tmj.2008.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisco (2014). Cisco TelePresence server data sheet Retrieved from http://www.cisco.com/c/en/us/products/collateral/conferencing/telepresence-server/data_sheet_C78-7287571.pdf
- Cohen J. (1960). A coefficient of agreement for nominal scales. Educational and Psychological Measurement, 20, 37–46. doi:10.1177/001316446002000104 [Google Scholar]
- Costa A. S. Fimm B. Friesen P. Soundjock H. Rottschy C. Gross T., … Reetz K (2012). Alternate-form reliability of the Montreal Cognitive Assessment screening test in a clinical setting. Dementia and Geriatric Cognitive Disorders, 33, 379–384. doi:10.1159/000340006 [DOI] [PubMed] [Google Scholar]
- Cullum C. M. Weiner M. F. Gehrmann H. R., & Hynan L. S (2006). Feasibility of telecognitive assessment in dementia. Assessment, 13, 385–390. doi:10.1177/1073191106289065 [DOI] [PubMed] [Google Scholar]
- Edwards P. J. Roberts I. Clarke M. J. DiGuiseppi C. Wentz R. Kwan I., … Pratap S (2009). Methods to increase response to postal and electronic questionnaires. Cochrane Database of Systematic Reviews, MR000008. doi:10.1002/14651858.MR000008.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss J. L. (1981). Statistical methods for rates and proportions (2nd ed). Somerset, NJ: John Wiley. [Google Scholar]
- Folstein M. F. Folstein S. E., & McHugh P. R (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gardner M. R. Jenkins S. M. O’Neil D. A. Wood D. L. Spurrier B. R., & Sandhya P (2015). Perceptions of video-based appointments from the patient’s home: A patient survey. Telemedicine and E-Health, 21, 281–284. doi:10.1089/tmj.2014.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. Hamilton A., & Baker R. S (2012). How do low-income urban African Americans and Latinos feel about telemedicine? A diffusion of innovation analysis. International Journal of Telemedicine and Applications: Special Issue on Usability of Telehealth Technologies, 2012. doi:10.1155/2012/715194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P. C. Whitney M. K. Kovaleva M., & Hepburn K (2016). Development and implementation of tele-savvy for dementia caregivers: A department of veterans affairs clinical demonstration project. The Gerontologist, 56, 145–154. doi:10.1093/geront/gnv123 [DOI] [PubMed] [Google Scholar]
- Larner A. J. (2011). Teleneurology: An overview of current status. Practical Neurology, 11, 283–288. doi:10.1136/practneurol-2011-000090 [DOI] [PubMed] [Google Scholar]
- Lee J. H. Kim J. H. Jhoo J. H. Lee K. U. Kim K. W. Lee D. Y., & Woo J. I (2000). A telemedicine system as a care modality for dementia patients in Korea. Alzheimer Disease and Associated Disorders, 14, 94–101. [DOI] [PubMed] [Google Scholar]
- Loh P. K. Ramesh P. Maher S. Saligari J. Flicker L., & Goldswain P (2004). Can patients with dementia be assessed at a distance? The use of telehealth and standardised assessments. Internal Medicine Journal, 34, 239–242. doi:10.1111/j.1444-0903.2004.00531.x [DOI] [PubMed] [Google Scholar]
- Martin-Khan M. Flicker L. Wootton R. Loh P. K. Edwards H. Varghese P., … Gray L. C (2012). The diagnostic accuracy of telegeriatrics for the diagnosis of dementia via video conferencing. Journal of the American Medical Directors Association, 13, 487.e19–487.e24. doi:10.1016/j.jamda.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Marwit S. J., & Meuser T. M (2005). Development of a short form inventory to assess grief in caregivers of dementia patients. Death Studies, 29, 191–205. doi:10.1080/07481180590916335 [DOI] [PubMed] [Google Scholar]
- McEachern W. Kirk A. Morgan D. G. Crossley M., & Henry C (2008). Reliability of the MMSE administered in-person and by telehealth. Canadian Journal of Neurological Sciences, 35, 643–646. doi:10.1017/S0317167100009458 [DOI] [PubMed] [Google Scholar]
- McKhann G. M. Knopman D. S. Chertkow H. Hyman B. T. Jack C. R. Jr Kawas C. H., … Phelps C. H (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7, 263–269. doi:10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. E. Dodge H. H. Zhou X. H. Bu Y. Besser L. M. Mock C., … Neuropsychology Work Group Advisory to the Clinical Task Force. (2016). Results from the NACC Uniform Data Set neuropsychological battery crosswalk study. Alzheimer Disease and Associated Disorders, 30, 134–139. doi:10.1097/WAD.0000000000000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montani C. Billaud N. Tyrrell J. Fluchaire I. Malterre C. Lauvernay N., … Franco A (1997). Psychological impact of a remote psychometric consultation with hospitalized elderly people. Journal of Telemedicine and Telecare, 3, 140–145. doi:10.1258/1357633971931048 [DOI] [PubMed] [Google Scholar]
- Morris J. C. (1993a). The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology, 43, 2412–2414. doi:10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Morris J. C. (1993b). Clinical Dementia Rating worksheet Retrieved from http://alzheimer.wustl.edu/adrc2/Images/CDRWorksheet.PDF
- Morris J. C. Ernesto C. Schafer K. Coats M. M. S. N. Leon S. M. S. N. Sano M., … Woodbury P (1997). Clinical Dementia Rating training and reliability in multicenter studies: The Alzheimer’s disease cooperative study experience. Neurology, 48, 1508–1510. doi:10.1212/WNL.48.6.1508 [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S. (2016). MoCA test online Retrieved from http://www.mocatest.org/electronic-tests/
- Nasreddine Z. S. Phillips N. A. Bédirian V. Charbonneau S. Whitehead V. Collin I., … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. doi:10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Pew Research Center (2015). Americans’ internet access: 2000–2015 Retrieved from http://www.pewinternet.org/2015/06/26/americans-internet-access-2000-2015/
- Radhakrishnan K. Xie B., & Jacelon C. S (2015). Unsustainable home telehealth: A Texas qualitative study. The Gerontologist, 56, 830–840. doi:10.1093/geront/gnv050 [DOI] [PubMed] [Google Scholar]
- Reinhard S. C., Levine C., Samis S. (2012). Home alone: Family caregivers providing complex chronic care Retrieved from http://www.aarp.org/content/dam/aarp/research/public_policy_institute/health/home-alone-family-caregivers-providing-complex-chronic-care-rev-AARP-ppi-health.pdf
- Saligari J. Flicker L. Loh P. K. Maher S. Ramesh P., & Goldswain P (2002). The clinical achievements of a geriatric telehealth project in its first year. Journal of Telemedicine and Telecare, 8(Suppl. 3), 53–55. doi:10.1258/13576330260440862 [PubMed] [Google Scholar]
- Stillerova T. Liddle J. Gustafsson L. Lamont R., & Silburn P (2016). Could everyday technology improve access to assessments? A pilot study on the feasibility of screening cognition in people with Parkinson’s disease using the Montreal Cognitive Assessment via internet videoconferencing. Australian Occupational Therapy Journal, doi:10.1111/1440–1630.12288 [DOI] [PubMed] [Google Scholar]
- Teri L. Truax P. Logsdon R. Uomoto J. Zarit S., & Vitaliano P. P (1992). Assessment of behavioral problems in dementia: The Revised Memory and Behavior Problems Checklist. Psychology & Aging, 7, 622–631. doi:10.1037/0882-7974.7.4.622 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services (2015). Telehealth services (ICN 901705) Retrieved from www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN
- Vitaliano P. P. Russo J. Young H. M. Becker J., & Maiuro R. D (1991). The screen for caregiver burden. The Gerontologist, 31, 76–83. doi:10.1093/geront/31.1.76 [DOI] [PubMed] [Google Scholar]
- Wittson C. L. Affleck D. C., & Johnson V (1961). Two-way television in group therapy. Psychiatric Services, 12, 22–23. doi:10.1176/ps.12.11.22 [DOI] [PubMed] [Google Scholar]
- Yesavage J. A. Brink T. L. Rose T. L. Lum O. Huang V. Adey M., & Leirer V. O (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17, 37–49. doi:10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Zarit S. H. Anthony C. R., & Boutselis M (1987). Interventions with care givers of dementia patients: Comparison of two approaches. Psychology and Aging, 2, 225–232. doi:10.1037/0882-7974.2.3.225 [DOI] [PubMed] [Google Scholar]