Abstract
Objective. The increasing incidence of cancer survivorship has shifted treatment of cancer-related pain from short-term analgesia to long-term chronic pain management. As a result, alternatives to oral analgesics, such as intrathecal therapy, may be beneficial for patients with cancer-related pain. The authors review the use of intrathecal therapy in the management of cancer-related pain.
Methods. The Medline database was searched for English-language articles that included “ziconotide” or “morphine” AND (“cancer” OR “malignant”) AND “intrathecal” in title or abstract. Available abstracts from scientific congresses in the areas of neuromodulation and oncology were also reviewed.
Results. Intrathecal therapy provides pain relief with reduced systemic concerns in patients with cancer-related pain. Patients should undergo multidisciplinary evaluation and, in most cases, drug trialing before intrathecal pump implantation. Morphine, an opioid (µ-opioid receptor antagonist), and ziconotide, a nonopioid (selective N-type calcium channel inhibitor), are both approved for intrathecal analgesia; however, tolerance and safety concerns may deter the use of intrathecal morphine. Ziconotide has also shown efficacy for reduction of cancer-related pain; however, proper dosing and titration must be used to prevent adverse events. There is little information available on use of intrathecal therapies specifically in cancer survivors.
Conclusions. Treatment of cancer-related pain has shifted toward chronic pain management strategies, especially among cancer survivors. Intrathecal therapy provides an alternate route of administration of chronic pain medications (e.g., morphine and ziconotide) for cancer patients with and without active disease, although additional research is needed to support effectiveness in cancer survivors.
Keywords: Cancer Survivors, Chronic Pain, Intrathecal Implantable Drug Delivery System, Morphine, Opioid, Ziconotide
Introduction
Pain is one of the most common, onerous, and distressing symptoms for patients with cancer [1] and is a significant factor underlying impairment of quality of life [2]. A recent systematic review and meta-analysis reported that 52% of patients with cancer experienced pain, regardless of the stage of their disease [1]. High rates of pain were reported by patients with advanced, metastatic, or terminal disease (64%), and among those receiving anticancer treatment (59%). In addition, 33% of patients continued to report pain even after curative treatment [1]. Patients diagnosed with cancer may have pain prior to their cancer diagnosis, which can then be worsened by cancer-related treatment (e.g., surgery) [3,4]. To counteract this, prehabilitation before and/or during cancer treatment, such as exercise and relaxation techniques, may be valuable in reducing pain [5–8] and improving treatment completion [9]. Moreover, pain should be adequately addressed throughout cancer treatment, as inadequately controlled pain during this time period can predispose patients to development of chronic pain after treatment cessation [10].
The number of cancer patients receiving life-prolonging or curative treatment continues to increase in the United States, from an estimated 3.0 million survivors in 1971 to 9.8 million in 2001 and 13.7 million in 2012 [11,12]. In addition, cancer survivors are living longer after diagnosis, which means that the duration of survivorship is increasing [11,12]. According to the National Coalition for Cancer Survivorship definition, a person is considered a cancer survivor from the time of diagnosis through the rest of his or her life [13,14]; however, survivorship research typically focuses on the subgroup of patients with a history of cancer who are beyond the acute diagnosis and treatment phase of illness [13]. These patients have multiple medical and psychosocial needs that should be addressed to optimize outcomes, including adequate control of chronic pain [15,16]. Cancer survivors are prone to chronic pain syndromes such as neuropathy, lymphedema, myalgia, arthralgia, post-surgical pain, and genital pain [7]. Cancer survivors with chronic pain include patients with pain related to active disease progression and those in whom the disease has been arrested or eradicated. Unlike individuals with chronic noncancer pain, cancer survivors may have identifiable tissue damage underlying the pain and must contend with the prospect of possible recurrence of the cancer [17]. However, the cause of chronic pain in cancer survivors without active disease is often related to treatment rather than the cancer itself [17,18]. Surgery, radiation therapy, chemotherapy, hematopoietic cell transplantation, and hormonal therapy can all lead to chronic pain conditions in cancer survivors [17–19]. The mechanisms underlying the association between chronic pain and cancer-related treatments remain largely elusive; however, nerve damage may be the consequence of surgery and radiation therapy [10]. Chemotherapeutic agents appear to elicit peripheral neuropathic pain by inducing axonal damage and mitochondrial dysfunction and increased oxidative stress that results in heightened inflammation and altered neurotransmission [18,20].
Because the number and duration of cancer survivorship is increasing, cancer survivors with chronic pain may benefit from treatment strategies that are used in the management of chronic pain of noncancer origin [10,17]. In cancer survivors, chronic neuropathic and nociceptive (or skeletal) pain is typically managed with systemic pain medications, with opioids traditionally recommended as first-line therapy [21]. Evolving concerns regarding long-term use of systemic opioids, including lack of effectiveness, adverse events, abuse/dependence, and tolerance, are similar for patients with cancer-related and noncancer-related chronic pain [22], as well as opioid-induced immunosuppression [23], have resulted in consideration of nonopioid [10,17,22] and nonsystemic opioid (i.e., intrathecal [IT] drug delivery) strategies to control chronic pain.
IT drug delivery bypasses the blood-brain barrier and delivers medication directly into the IT space of the spinal column via an indwelling catheter connected to an implanted reservoir that is controlled by a programmable pump, which may be implanted or external [24–26]. Although IT treatment has traditionally been viewed as salvage therapy for patients who are unresponsive to or intolerant of high-dose opioids [25], it is increasingly recognized that IT therapy can improve pain care and increase functioning in patients who do not obtain adequate analgesia after a reasonable course of systemic opioid therapy, and in those intolerant of normal doses of systemic opioids [25,27].
Two medications, morphine (an opioid) and ziconotide (a nonopioid), are currently approved by the US Food and Drug Administration (FDA) for IT analgesia [28–30]. Morphine was approved for IT administration in the treatment of intractable chronic pain [30] based on efficacy data obtained from other routes of administration, and from retrospective studies of IT use. Based on the results of three randomized, placebo-controlled trials [31–33], ziconotide is indicated for the management of severe chronic pain in patients for whom IT therapy is warranted, and who are intolerant of or refractory to other treatment, such as systemic analgesics, adjunctive therapies, or IT morphine [29]. Morphine and ziconotide are the only agents recommended as first-line IT therapies for both neuropathic and nociceptive pain by the Polyanalgesic Consensus Conference (PACC); however, several other opioids (i.e., hydromorphone, fentanyl, sufentanil) and nonopioids (bupivacaine, clonidine, and baclofen), used alone or as combination therapy, are also included in the PACC recommendations for IT analgesia (Table 1) [27,28]. The aim of this review is to provide a clinically relevant summary of the use of IT therapy in the management of cancer-related pain and the two US FDA-approved analgesics (morphine and ziconotide).
Table 1.
| Drug | Mechanism of Action | PACC Recommendation | Common Adverse Effects | Notes |
|---|---|---|---|---|
Opioids
|
μ-opioid receptor agonist |
|
Sedation, respiratory depression, urinary retention, nausea, pruritus, cognitive impairment |
|
| Ziconotide | N-type voltage-sensitive calcium channel antagonist | First-line for neuropathic and nociceptive pain | Cognitive effects, psychiatric effects, ataxia, nausea, elevated creatine kinase, hypotension | IT ziconotide is FDA approved |
| Local anesthetics Bupivacaine | Blocks neural sodium channels | Neuropathic: first-line in combination with morphine | Motor weakness, hypotension, urinary retention | Chemical sympathectomy caused by IT bupivacaine may promote GI motility |
| Clonidine | α2-adrenergic agonist |
|
Ataxia, sedation, auditory disturbance | Abrupt discontinuation associated with serious withdrawal syndrome |
| Baclofen | GABA receptor agonist | Neuropathic: fifth-line Not recommended for nociceptive pain | Puncture headache, seizure, delirium, transient global amnesia |
|
FDA = US Food and Drug Administration; GABA = gamma-aminobutyric acid; GI = gastrointestinal; IT = intrathecal; PACC = Polyanalgesic Consensus Conference.
Adapted with permission from Brogan S, Junkins S. J Support Oncol 2010;8(2):52-9.[27].
Methods
All searches of the Medline database were conducted on June 19, 2015. A search for “ziconotide” AND (“cancer” OR “malignant”) in title or abstract yielded 31 English-language articles; review of article titles/abstracts identified 24 articles for further evaluation. A search for “morphine” AND (“cancer” OR “malignant”) AND “intrathecal” in the title or abstract yielded 213 English-language articles; of these, 24 articles were evaluated. Available abstracts from scientific congresses in the areas of neuromodulation and oncology were also reviewed. The selection of articles was not based on a scoring system; instead, articles were included in this review if they reported research results or provided other clinically relevant information regarding the use of IT therapy in the management of cancer-related pain. Articles were evaluated for the quality of study design: randomized, controlled trials; prospective, cohort, or observational studies; and retrospective analyses.
Patient Selection for Intrathecal Therapy for Cancer-Related Pain
The majority of patients with cancer-related pain can be adequately managed with conservative medical therapies such as systemic opioids [34]. However, implantation of an intrathecal implantable drug delivery system (IDDS) and IT therapy should be considered in patients with intractable focal pain or those intolerant of the side effects of systemic opioids [34]. Patients undergoing long-term toxic chemotherapy regimens may particularly benefit from IT therapy, rather than systemic analgesics, due to the lower additive risk of adverse events [35]. However, careful patient selection is required to increase the probability of success [34].
First, it should be confirmed that the chronic cancer-related pain is refractory to more conservative therapies and that comorbid psychiatric disorders and psychosocial issues that could negatively affect the treatment outcome have been adequately addressed [36]. This often requires comprehensive multidisciplinary evaluation by a variety of medical and sociological professionals, including oncologists, neurologists, psychiatrists, and social workers [34]. Psychological assessment is vital for identifying trait-related factors (e.g., personality, cognitive functioning), psychiatric conditions (e.g., anxiety, depression), and psychosocial issues (e.g., inadequate social support) that may hinder the patient’s ability to comply with the requirements of IT therapy [28,34,37,38]. Such psychological assessment may be foregone in patients with end-stage cancer but should be conducted in cancer survivors with chronic cancer-related pain [34,39]. In addition, existential and spiritual concerns should be addressed by providing counseling [40].
Once it has been established that the patient is a candidate for IDDS, PACC guidelines indicate that a trial of IT therapies may be considered before actual implantation of the IDDS to evaluate medication tolerability, response, and patient acceptance of the IT delivery method [37]. Trialing may be performed using a bolus injection or continuous infusion via the IT pump, if already implanted for use with other treatments, or an extramural pump (in patients without an IT pump implanted) [28]. Trialing may be contraindicated in patients with medical comorbidities related to cancer treatment, such as coagulopathies and immunodepression, but careful coordination with other members of the treatment team (e.g., oncologists) may allow for trialing [39,41]. Trialing may also be foregone in patients with cancer-related pain and limited life expectancy in order to avoid delaying initiation of IT therapy [37,39]. For such patients, an equation was developed to identify the appropriate initial dose of IT opioid therapy based on the patient’s systemic opioid dose (in morphine equivalents) prior to pump implant [42]. Otherwise, for cancer survivors without active, progressive disease, a standard trial of IT therapy is recommended [39]. The authors now recommend conservative IT opioid dosing for all patients except those with end-stage cancer.
It should be noted that the role of the trial continues to be debated by experts in the field; there is no consensus definition of outcomes to evaluate success, and well-designed studies on the utility of trialing are lacking [37]. The definition of a “successful” trial has not been standardized and may vary depending on patients’ abilities and goals [37]. During development of its consensus guidelines, the PACC regarded trial success as achievement of a ≥50% reduction from baseline in visual analog scale (VAS) or numeric analog scale (NAS) pain scores [37]; however, the guidelines concede that no official definition of success was reached [37]. Failure to achieve an arbitrary definition of success may prevent access to treatment for patients who might obtain benefits from longer-term IT therapy. Furthermore, in some cases, an improvement in functional ability—not just pain—might signify efficacy and justify IDDS implantation [34].
Several IT therapies are available for use in patients with cancer pain, but only morphine, a µ-opioid receptor antagonist, and ziconotide, a selective N-type calcium channel antagonist, have been approved by the FDA for IT therapy in patients with chronic pain, which includes cancer pain [28]. Trialing is typically performed for both medications to determine dosage and tolerability [28]. Most types of cancer-related pain respond to IT treatment, although the overall efficacy may vary by cancer type [39]. Patients with a defined pain etiology, as is typically the case in cancer-related pain [17,19], are considered good potential candidates for IT therapy. IT opioid therapy is appropriate for visceral and somatic nociceptive pain such as that experienced by patients with soft-tissue cancers (e.g., liver cancer) and bone metastases, respectively; both pain types seem to respond to IT opioid therapy [39]. Neuropathic pain associated with plexopathies may also benefit from IT opioid delivery [39]. Patients with mixed nociceptive/neuropathic pain may require combination IT therapy for optimal analgesic response [39]. IT ziconotide monotherapy may be useful for patients who are opioid resistant, have opioid-induced hyperalgesia, or are sensitive to opioid side effects [38]. In addition, ziconotide may be more appropriate than opioids for patients with obstructive sleep apnea, lung disease, or reduced pulmonary reserve; patients who have an increased risk of catheter-tip granulomas; and younger patients who have a long life expectancy [38]. In general, patients with high tumor mass burden, chronic postsurgical pain, and diffuse metastatic disease should be considered for IT therapy. Patients with cancer-related pain who responded to systemic opioids but were unable to tolerate the doses required for sufficient pain relief may receive benefit from IT opioids [34]. However, it is unlikely that patients with a poor response to systemic opioids will respond to IT opioid administration [34]; those patients may benefit not only from a change in route of analgesic administration but also a change in mechanism of action.
For patients considered candidates for IT therapy, practical placement considerations must be addressed before IDDS implantation. Patients with pain in the lower portions of the body (i.e., below the upper thoracic region) are most appropriate for IDDS implantation as placement may be more complicated in the rostral spinal column [34]. Catheter placement at a spinal location congruent with the primary site of pain origin is important, due to the limited bulk flow of cerebrospinal fluid, which restricts the rostral spread of IT agents [25,34].
Efficacy and Safety of Approved Intrathecal Therapy in Patients With Cancer-Related Pain
Very few randomized, controlled trials have investigated the efficacy and safety of IT therapy in the management of cancer-related pain. Prospective, observational studies and retrospective analyses are relatively more common. The higher-quality studies available on this topic (randomized, controlled trials and prospective, observational studies) are summarized in Table 2.
Table 2.
Randomized, controlled trials and prospective, observational studies of intrathecal therapy for cancer-related pain
| Study | Participants | Follow-up | Intervention | Efficacy | Conclusion |
|---|---|---|---|---|---|
| Randomized, Controlled Trials | |||||
| Patients with advanced cancer and refractory pain were randomized to receive either CMM (n = 99) or IDDS plus CMM (n = 101); all participants had a mean VAS score of ≥5 despite 200 mg/d of oral morphine or the equivalent | 4 weeks |
|
|
Adding IT therapy to CMM for patients with advanced cancer and refractory pain improved pain management and alleviated common opioid-related side effects | |
|
Patients with refractory pain due to cancer or AIDS were randomized to receive ziconotide (n = 71) or placebo (n = 40); all participants had a mean VASPI score of ≥50 mm despite a regimen of systemic or IT analgesics | 11 days | IT ziconotide was titrated over 5-6 days followed by a 5-day maintenance phase for responders and crossover of nonresponders to the opposite cohort | Mean VASPI scores improved by 53% in the ziconotide group compared with 18% in the placebo group (P < 0.001); 53% of patients in the ziconotide group reported moderate to complete pain relief compared with 18% in the placebo group (P < 0.001) | IT ziconotide demonstrated significant improvements in patients with cancer- or AIDS-related refractory pain |
| Prospective, Observational Studies | |||||
| Rauck et al, 2003 [54] | 119 patients with refractory cancer pain (inadequate efficacy and/or intolerable adverse effects with conventional pharmacologic treatment) | Up to 16 months | Patients received IT morphine sulfate via a patient-activated drug delivery system |
|
Use of a patient-activated drug delivery system resulted in effective analgesia and fewer adverse effects |
| Mercadante et al, 2007 [49] | 55 patients with advanced cancer and refractory pain (≥3 opioids and 2 routes of administration, including IV) | Up to 6 months or until death | IT morphine + levobupivacaine |
|
IT combination therapy with morphine + levobupivacaine provided rapid, sustained analgesia with a decrease in AEs and oral opioid consumption until death in patients with refractory pain due to advanced cancer |
| Mitchell et al, 2015 [53] | 22 patients with refractory cancer-related pain received IDDS implant | Up to 6 months | IT levobupivacaine + morphine |
|
IT therapy provided long-term pain control in patients with difficult-to-control cancer-related pain |
| Sjoberg et al, 1991 [50] | 52 patients with refractory cancer pain | Up to 6 months | IT morphine + bupivacaine |
|
IT combination therapy with morphine + bupivacaine was a useful tool in the treatment of refractory cancer pain in patients with advanced disease |
| Sjoberg et al, 1994 [51] | 53 patients with refractory cancer pain | Up to 6 months | IT morphine + bupivacaine |
|
IT combination therapy with morphine + bupivacaine (dose ratio of 1:10) was highly effective for managing refractory cancer-related pain |
| Alicino et al, 2012 [76] | 20 patients with disseminated cancer with bone metastasis involving vertebral bodies and refractory pain (VASPI score at rest >70 mm or severe AEs with systemic opioid treatment) | 28 days | IT ziconotide + morphine |
|
IT combination therapy with ziconotide + morphine allowed safe and rapid control of cancer-related pain that was refractory to oral opioids |
| Dupoiron et al, 2012 [77] | 77 patients with refractory pain due to incurable cancer; all patients had a NAS score of >6 despite 200 mg/d of oral morphine or the equivalent | 90 days | IT ziconotide + morphine, ropivacaine, and/or clonidine | Mean NRS pain score decreased from 8.1 at baseline to 4.1 after 30 days; improvement sustained at Month 2 (4.3) and Month 3 (4.1) | Ziconotide, in combination with other intrathecal agents, improved pain management in patients with refractory cancer-related pain |
| Brogan et al, 2015 [84] | 98 patients with refractory cancer-related pain received IDDS implant; 58 patients had follow-up data | 14-82 days (mean, 42 d) | IT agents included morphine, hydromorphone, fentanyl, bupivacaine, clonidine, baclofen, and ziconotide (91% of patients received either morphine or hydromorphone; 16% received ziconotide) |
|
IT therapy that included PCA provided improved pain control and reduced systemic opioid consumption |
AE = adverse event; BPI = Brief Pain Inventory; CCM = conventional medical management; DB = double-blind; IDDS = intrathecal drug delivery system; IT = intrathecal; IV = intravenous; NAS = numeric analog scale; NRS = numeric rating scale; OL = open-label; PBO-C = placebo-controlled; PCA = patient-controlled analgesia; R = randomized; VAS = visual analog scale; VASPI = visual analog scale of pain intensity.
Adapted with permission from Deer TR, et al. Pain Physician 2011;14(3):E283-E312.[39].
Morphine
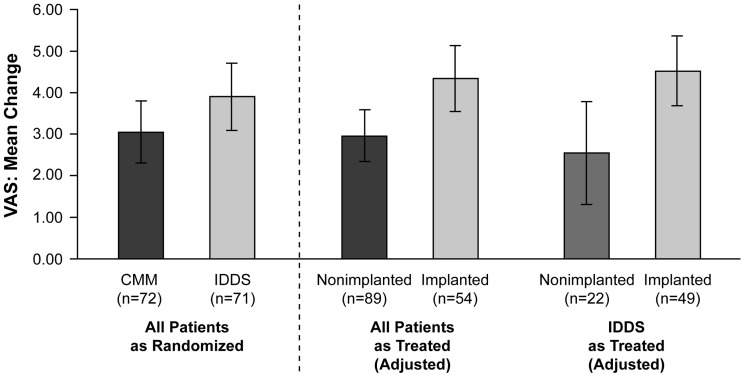
Morphine has been administered intrathecally for analgesia for more than 30 years [43], and one randomized, controlled trial has specifically assessed its efficacy and safety in patients with cancer-related pain (Table 2) [44]. In this trial, 202 patients with refractory cancer-related pain were randomized to receive an IDDS plus conventional medical management (CMM) or CMM alone [44]. All patients who received an IDDS were started on IT morphine; baseline median systemic morphine equivalent dose (MED) was similar among both groups (IDDS, 250 mg; CMM, 272 mg). Other IT analgesics were permitted for patients in whom morphine did not provide adequate pain relief [44]; such regimens included hydromorphone, morphine combined with bupivacaine or droperidol, and combined therapy with clonidine [44]. Some patients received a different treatment than the one to which they were randomized; therefore, as treated analyses were also conducted for assessment of pain (based on VAS score, range 0 [no pain] to 10 [worst pain imaginable]) and opioid-related side effects [44]. Drug tolerability was determined based on the sum of 15 preselected drug toxicity scores from the Common Toxicity Criteria. Improvement in VAS score was significantly greater in patients who received IT opioid therapy compared with those who did not (Figure 1) [44]. Similarly, patients who received IT opioids experienced a significantly greater reduction in opioid-related side effects relative to patients without an IDDS (P < 0.001) [44]. Follow-up analyses indicated that improvements in pain and opioid-related side effects among patients who received IT opioid therapy were sustained through Week 12 [45], and that the benefits of IDDS extended to patients with the most refractory pain [46]. However, a number of methodologic criticisms (e.g., unclear definition of refractory pain, between-group differences in disease severity at baseline, inconsistent IDDS trialing methods, cross-over between randomized groups, and failure to control for other treatments with the potential to provide analgesia) have called the conclusions of this study into question [47,48]. Thus, study findings suggesting that IDDS may prolong survival in patients with cancer-related pain [44,45] require replication.
Figure 1.
Mean reduction in VAS score with CMM alone versus CMM and IDDS [44].
Patients with cancer-related pain that was refractory to systemic morphine (200 mg/d) received CMM alone or IDDS plus CMM. CMM was based on previously published guidelines; IDDS was initiated with morphine but could be switched to other agents if necessary for pain reduction. Reduction in VAS pain score from baseline to Week 4 (as randomized and as treated) is presented. In the analysis of all patients as treated, the difference between nonimplanted and implanted patients is significant (P = 0.007). In the analysis of patients randomized to IDDS as treated, the difference between nonimplanted and implanted patients is also significant (P = 0.01).
Error bars are ±2 standard errors.
CMM = conventional medical management; IDDS = intrathecal drug delivery system; VAS = visual analog scale.
Reprinted with permission from Smith TJ, et al. J Clin Oncol 2002;20(19):4040-9.[44]
Several prospective studies have shown improvement of pain with IT morphine in combination with other agents (Table 2) [49–52]. Results of the largest prospective, observational study of IT morphine monotherapy in patients with refractory cancer-related pain (n = 119) are detailed in the section below on “Intrathecal Patient-Controlled Analgesia” [53]; in this trial, IT morphine significantly reduced pain from baseline through >1 year of follow-up with minimal side effects [53]. A smaller, prospective, observational study evaluated the use of combination IT therapy with morphine and levobupivacaine in 55 patients with advanced cancer and chronic refractory pain [49]. Patients were assessed at baseline, hospital discharge, and at months 1, 3, and 6. The final assessment was defined as the last observation made at least 1 week before the patient’s death [49]. Mean pain intensity score was 8.0 (out of 10) at baseline and was significantly reduced from baseline at Month 1 (3.9; P < 0.0001), Month 3 (3.9; P = 0.0002), and the last assessment prior to death (3.9; P < 0.0001) [49]. Mean systemic opioid consumption (in MED) was 566 mg/d at baseline and significantly decreased to 70 mg/d at Month 1 (P < 0.0001), 60 mg/d at Month 3 (P = 0.0002), and 184 mg/d at the last assessment prior to death (P = 0.29) [49]. Few complications were reported with IT therapy. Complications that occurred during the hospital stay following device implantation included urinary retention requiring bladder catheterization (six patients), headache (four patients), and bleeding (two patients) [49]. Two patients died due to reasons unrelated to IT implantation. After hospital discharge, local infection necessitated removal and replacement of the catheter in one patient; an additional patient experienced infection but the device was not replaced due to the patient’s short life expectancy [49]. One patient developed spinal cord compression that required discontinuation of IT drug administration [49]. In another prospective, observational study in 22 patients who were refractory to or intolerant of oral analgesia, IT morphine and levobupivacaine for an average of 178 days [52] significantly reduced “worst pain” score (as assessed by the Brief Pain Inventory [BPI]) from baseline to Day 1 and Week 1 and through 6 months (P ≤ 0.05 for all time points) [52]. A 66% improvement in average BPI score was observed after only 1 week of treatment, with sustained reductions through the 6-month study period [52]. No infections or hematomas were reported, but headache was common (27% of patients; 6/22) [52].
Similar results have been demonstrated in observational studies examining combination IT morphine and bupivacaine [50,51,54]. In a retrospective analysis of 17 patients who did not obtain adequate pain relief with IT morphine therapy, the addition of IT bupivacaine provided marked relief of pain in 10 patients and a moderate effect in another four patients [54]. In a prospective study of 52 patients with preterminal cancer pain who received IT morphine and bupivacaine (1:1 ratio), continuous pain relief (i.e., VAS of 0–2) was achieved in 85% of patients with no significant increase in morphine or bupivacaine dose [50]; however, the separate contribution of morphine versus bupivacaine was not determined. A separate prospective study of 53 patients with refractory cancer pain demonstrated significant reduction of daily pain (as assessed by VAS score) from baseline through 6 months with constant infusion of IT morphine 0.5 mg/mL and bupivacaine 4.75 mg/mL (1:10 ratio) [51]. Based on the results of the last two studies, it appears that a higher relative bupivacaine dose may provide more stable pain relief to a greater number of patients and reduce morphine-related side effects compared with lower doses [51].
Though lower in quality of evidence, four retrospective chart reviews also evaluated the efficacy of IT morphine in the relief of cancer-related pain [55–58]. Review of charts of 76 patients who received neuraxial therapy for analgesia at an academic cancer treatment center identified 56 patients in whom IT administration was used and 23 patients who received epidural analgesia [59]. The most common IT medications were morphine (28 patients), hydromorphone (11 patients), and morphine plus bupivacaine (seven patients) [59]. Severe pain was significantly reduced from baseline with IT and epidural analgesia, with no difference between groups [59]. Overall mean numeric rating scale (NRS) pain scores decreased from 7.9 (out of 10) at baseline to 4.1 at Week 8 (P < 0.001), with a concomitant reduction in mean overall opioid consumption [59]. A separate retrospective chart review of 43 patients with refractory cancer-related pain also demonstrated reduction of pain with IT morphine, but noted that pain reduction differed according to type and location of pain (neuropathic or nociceptive) and cancer progression [55]. Early in the course of patients’ disease, median best pain reduction was 78% for nociceptive pain versus 61% for patients with neuropathic pain; as the disease progressed, alleviation of pain was much greater for nociceptive pain (67%) compared with neuropathic pain (11%) [55]. In addition, the extent of pain relief varied with pain location, with pain in the trunk area, thorax, abdomen, pelvis, and spinal column being the most responsive to IT therapy [55]. Additional prospective studies are necessary to confirm these preliminary results.
Intrathecal implantable drug delivery systems involve risk for procedure- and device-related adverse events (e.g., bleeding, infection, catheter displacement or kinking, pump malfunction) regardless of the agent in the pump [60]. In addition, there are some adverse events of particular concern with IT morphine including respiratory depression, granuloma formation, endocrine disruption, peripheral edema, immunosuppression, constipation, urinary retention, pruritus, and hyperalgesia, as well as tolerance/physical dependence [41,61,62]. Pruritus is the most common IT morphine–related side effect, with prevalence estimates ranging from 0% to 100%; however, most incidences are mild [62]. Because morphine can interact with opioid receptors in the central respiratory centers [61], late (>2 hours after administration) respiratory depression has been reported in the literature with extradural and IT morphine therapy. Respiratory depression is the most feared adverse event, but it is rare (incidence, 0.1%–0.4%) and can be reversed with administration of mixed opioid receptor antagonist [62,63]. The risk of catheter-tip granuloma increases with longer duration and higher dose/concentration of IT opioid therapy [64]. When a granuloma is detected, noninvasive remediation (e.g., IT opioid discontinuation) is often sufficient; however, a neurosurgical consult is warranted if neurological symptoms are severe or there is evidence of spinal cord compression [64,65]. Opioid-induced hyperalgesia is also an uncommon but important side effect of opioid therapy that can occur with IT administration [62,66]. Opioid receptors can become desensitized to morphine (regardless of administration route) over time, leading to tolerance and dependence [61]. A retrospective investigation of the development of tolerance in 159 patients with refractory cancer-related pain who received IT morphine found moderate increases in the daily dose of IT morphine (2 to 3 times the initial dose), which reflected the development of tolerance without disruption of adequate analgesia [67]. Low or “microdosing” has been suggested as a strategy to potentially reduce adverse events associated with IT morphine use [68,69], but has not yet been studied in patients with cancer-related pain. In addition, some adverse events (e.g., withdrawal symptoms) can be the result of mechanical dysfunction of the pump (e.g., after repeated magnetic resonance imaging [70]) and would likely be unaffected by such dose adjustments [71].
Ziconotide
The first randomized, placebo-controlled trial of IT ziconotide was conducted in patients with chronic pain related to cancer (95 patients) or AIDS (13 patients; Table 2) who were refractory to conventional treatment [31]. In this study, ziconotide demonstrated efficacy for pain reduction, despite the brief study duration (5- to 6-day titration period followed by a 5-day maintenance phase for responders and a 5- to 6-day crossover period for nonresponders) [31]. Mean VAS of pain intensity (VASPI) scores (ranging from 0 [no pain] to 100 [worst pain imaginable]) improved by 53% in ziconotide-treated patients compared with 18% in the placebo group (P < 0.001), with sustained efficacy among ziconotide responders during the maintenance phase [31]. Pain relief was moderate to complete in 53% of patients in the ziconotide group and 18% of patients in the placebo group [31]. Five patients in the ziconotide group reported complete pain relief compared with none of the patients who received placebo [31]. Treatment response (defined as ≥30% decrease in mean VASPI score and no increase in concomitant opioid use or change in opioid type) was observed in 50% of patients receiving ziconotide versus 18% of patients receiving placebo (P = 0.001) [31]. Opioid use decreased by 9.9% in the ziconotide group and increased by 5.1% in the placebo group [31]. In patients randomized to placebo who crossed over to ziconotide, mean reduction in VASPI score was 45% at the end of the 5- to 6-day crossover period [31]. However, the ziconotide dosing and titration schedule initially used in this study (0.4 mcg/h starting dose with upward titration every 12 hours to the maximum tolerated dose) resulted in problems with tolerability, prompting a change to a more tolerable dosing regimen (to a starting dose of ≤0.1 mcg/h with upward titration once every 24 hours to a maximum dose of 2.4 mcg/h) [31]. The most common adverse events (≥20%) reported with ziconotide treatment in this study were dizziness, postural hypotension, nystagmus, nausea, fever, somnolence, and confusion, and were typically dose related [31].
In order to assess the potential tolerability of a slower ziconotide titration, a subsequent randomized, placebo-controlled trial in patients with primarily noncancer-related pain was conducted [33]. Five patients reported malignant pain in this study [72]. This trial used a slow ziconotide titration schedule (starting dose of 0.1 mcg/h with upward titration by 0.05 to 0.1 mcg/h no more than once every 24 hours). The maximum dose used by any ziconotide-treated patient was 0.8 mcg/h (19.2 mcg/d) in this study [33] compared with 2.4 mcg/h in the previous trial [31]. After 3 weeks of treatment, significant mean improvement from baseline in VASPI scores were observed in the ziconotide group (12% improvement) compared with the placebo group (5% improvement; P < 0.05) [29]. Discontinuations of medication were similar in both treatment groups (ziconotide, 5.4%; placebo, 4.6%; P = 0.8) [33]. Based on these results, it is currently recommended to initiate IT ziconotide at no more than 2.4 mcg/d (0.1 mcg/h) with upward titration by up to 2.4 mcg/d (0.1 mcg/h) at intervals of no more than 2 to 3 times per week, to a maximum of 19.2 mcg/d (0.8 mcg/h) [29]. However, two case reports have indicated that rapid dose escalation (e.g., from 8 mcg/d to 25 mcg/d during a 72-hour period) may be used to quickly improve cancer-related neuropathic pain without serious drug-related side effects [73,74]. In addition, a novel dosing strategy—bolus flex dosing—has been developed to potentially improve the tolerability and efficacy of IT ziconotide [75], but it has not yet been studied in patients with cancer-related pain.
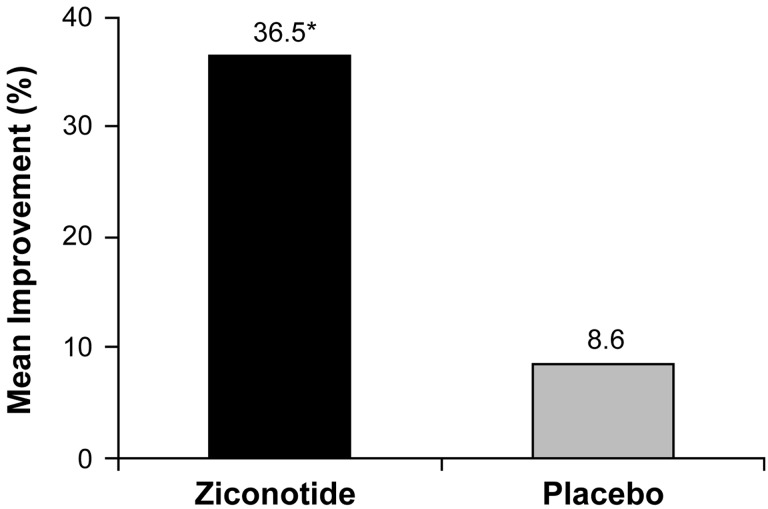
A post hoc pooled analysis of the aforementioned randomized trials (i.e., patients from the final [slower-titration, lower-dosing] regimen within the first ziconotide randomized, controlled trial [31] and the slower-titration ziconotide study [33]) examined the efficacy of ziconotide for relieving pain specifically in patients with cancer-related pain [76]. Of 67 patients with cancer-related pain, 51 (35 of whom received ziconotide and 16 of whom received placebo) had VASPI scores assessed at both baseline and the end of titration assessments [76]. Mean baseline VASPI scores were similar between the two groups (75.5 mm and 75.8 mm for ziconotide and placebo, respectively), and mean percentage improvement in VASPI score was significantly greater in the ziconotide group compared with the placebo group (Figure 2) [76].
Figure 2.
Mean percentage change in VASPI score: pooled analysis of patients with cancer-related pain in two placebo-controlled clinical trials of ziconotide [76].
Patients with pain related to cancer or AIDS received either a “fast” or “slow” titration schedule of ziconotide. The fast titration regimen consisted of a ziconotide 0.4 mcg/h starting dose with upward titration every 12 hours to the maximum tolerated dose over 5 to 6 days [31,76]. The slow titration schedule used a starting ziconotide dose of 0.1 mcg/h with upward titration by 0.05 to 0.1 mcg/h no more than once every 24 hours to a maximum allowable dose of 0.9 mcg/h over 21 days [33,76]. VASPI score was assessed at baseline and at the end of the titration period.
*P = 0.023 vs placebo.
VASPI = visual analog scale of pain intensity.
Consistent with the results from the post hoc pooled analysis, a retrospective chart review of 40 patients with cancer pain who received a bolus trial of ziconotide, either alone or in combination with hydromorphone or bupivacaine, demonstrated that ziconotide (mean effective dose of 2.77 mcg for ziconotide monotherapy or 2.47 mcg for ziconotide in combination) reduced pain by ≥30% within 24 hours in the majority (66%) of patients with minimal side effects [77]. A subsequent retrospective chart review over a 3-year period of 35 patients with cancer-related pain who had received a bolus trial at a tertiary academic cancer pain management center also observed significant pain reduction with minimal side effects using IT ziconotide. After the trial, pain reduction of ≥30% was reported for 27 patients (77%); IT ziconotide infusion was initiated (alone or with co-analgesics) in 21 patients of whom 19 had sufficient data available for review. Of the 19 patients, 53% achieved a stable ziconotide dose (mean, 3.0 ± 0.4 mcg/d) after approximately 6 weeks, at which time “average” pain was significantly reduced from baseline by 33% (P = 0.04) [78]. Only 7% of patients required dose reduction and discontinuation occurred in only one patient [78]. It should be noted that bolus trialing of ziconotide in patients with cancer-related pain is supported only by congress abstracts and not by published articles, although there are two published protocols in patients with noncancer-related pain [75,79].
A prospective observational study of 20 patients, all of whom had refractory pain related to disseminate cancer with bone metastasis involving vertebral bodies, evaluated IT combination therapy with ziconotide and morphine [80]. Patients had experienced pain refractory to oral opioids (mean oral morphine equivalent, 320 mg/d) for a mean of 6 months [80]. Patients received combination IT therapy with ziconotide 2.4 mcg/d and morphine 0.82 mg/d [80]. The dose of both medications was allowed to be increased depending on analgesic effect; if necessary ziconotide dose was increased by 1.2 mcg/d every 7 days [80]. A significant reduction in pain (as measured by VASPI score) from baseline with an IT combination of ziconotide and morphine was observed as early as 2 days (mean percentage change from baseline, 39 ±13%; P < 0.001) after treatment initiation [80]. After 28 days of ziconotide and morphine (maximum dose, 4.8 mcg/d and 3 mg/d, respectively), a 62% reduction in pain from baseline was observed (VAS 90 mm at baseline vs 34 mm at Day 28) [80]. The slow-dose titration (1.2 mcg/d every week if needed) of ziconotide allowed for tolerability; only four patients developed mild adverse events judged to be related to study medication (one person each with dizziness, asthenia, confusion, and ataxia) [80]. A separate observational study in 77 patients with chronic intractable pain due to incurable cancer demonstrated that a slightly more aggressive dose titration (starting dose of 1.0 mcg/d, titrated by 0.25 to 0.5 mcg/d every 2 days) of ziconotide in combination with other IT agents (morphine, ropivacaine, and clonidine) reduced pain (from an NRS score of 8.1 at baseline [out of 10] to 4.1 after 1 month) and resulted in few incidences (9% of patients) of treatment discontinuation [81].
A report from the Italian registry of ziconotide, a long-term, retrospective observational database, included 72 patients with noncancer pain and 32 patients with cancer-related pain [82]. Most patients with cancer-related pain (63%) had ziconotide as their first IT agent; morphine had previously been received in 38% of patients [82]. Most patients with cancer-related pain (77%) reported ≥50% reduction in pain intensity as assessed by VAS score, with a mean ziconotide dose of 5.5 mcg/d. Among the 10 patients with cancer-related pain who received IT ziconotide for >6 months (mean dose at 6 months, 4.9 mcg/d), pain reduction was maintained during the entire 6-month period [82]. Dose reductions were not reported in this study, but the main reasons for interruption of ziconotide therapy were adverse events (18%; 0 cancer patients, 19 noncancer patients) and uncontrolled pain (7%; one cancer patient, six noncancer patients) [82]. The most common adverse events overall were psychomotor disorders (34%), asthenia (22%), and balance disorders (20%) [82]. Unlike morphine, doses of ziconotide remained fairly constant once titrated, suggesting that tolerance was not an issue [82].
Selection of Intrathecal Medication for Cancer-Related Pain: Morphine vs Ziconotide
Both morphine and ziconotide have demonstrated efficacy in the treatment of cancer-related pain, although the number of prospective or randomized, controlled studies is limited for both agents. Both are recommended as first-line IT therapies for neuropathic and nociceptive pain [28]. Trialing of a medication may be considered, although there is some debate over its usefulness given the difficulty in extrapolating long-term effect from a short duration of drug exposure [28] and the need for rapid pain relief in cancer patients with short-life expectancy [37,39]. Trialing of morphine may be problematic due to route conversion issues and the potential need for dose reduction of systemic opioids, both of which may pose safety concerns for some patients [38]. A trial of ziconotide may be considered in patients in whom systemic opioid exposure has not provided adequate response and those who are opioid intolerant [28,38]. However, because the side effect profile of the medication is related to titration rate and not overall dosage, trialing of ziconotide presents some challenges [28].
There is little available information regarding the efficacy of IT analgesia in cancer survivors, as most studies of cancer-related pain enrolled patients with late-stage disease. Cancer survivors who receive an IDDS are likely to receive long-term IT therapy, and therefore, consideration of potential adverse events (e.g., neuropsychiatric side effects of ziconotide, granuloma formation, hypogonadism, immunosuppression, and development of tolerance and/or opioid-induced hypersensitivity with IT morphine) is of increased importance. Opioids are associated with several life-threatening adverse events (e.g., respiratory depression, granulomas) and incur tolerance [38], which may be of particular concern for younger patients (aged 18–50 years) in whom there is a greater risk for opioid tolerance compared with their older counterparts [83]. In addition, withdrawal symptoms may occur with morphine therapy if drug delivery is disrupted due to dysfunctions such as catheter disruption, pump malfunction, or an empty reservoir [38]. There was no indication of respiratory depression in clinical trials of IT ziconotide, even in patients who received doses greater than the maximum recommended dose, and ziconotide did not potentiate morphine-induced respiratory depression in animal studies [29]. IT ziconotide therapy may be interrupted or discontinued abruptly without evidence of withdrawal effects [29].
Intrathecal Patient-Controlled Analgesia for Cancer-Related Pain
Patient-controlled analgesia (PCA) is widely used in the intravenous administration of opioids for managing cancer-related pain [84,85]. Development of the Personal Therapy Manager (PTM) for the SynchroMed® Infusion System (Medtronic, Minneapolis, MN) extended the availability of patient-controlled analgesia to IT medication delivery [86,87]. This technology allows patients the freedom (within physician-set limits) to self-administer boluses of pain medication either in place of a continuous infusion pump or in response to breakthrough pain during use of a continuous pump system [88]. Similar patient-controlled IT bolus dosing is available for the Prometra® II Programmable Pump System (Flowonix Medical, Mt Olive, NJ) [89].
Use of PCA with Intrathecal Morphine
A prospective study investigated the safety and efficacy of patient-activated delivery of noncontinuous IT morphine boluses in patients with refractory cancer-related pain [53]. Of 149 enrolled patients, 119 completed screening and received the IDDS. Patients were experiencing chronic pain related to the disease (e.g., lung, breast, colorectal, or prostate cancer) or antineoplastic therapies. Mean NAS pain ratings decreased from 6.1 (out of 10) at baseline to 4.2 at Month 1 (31% reduction; P < 0.01); a similar magnitude of pain improvement was sustained through Month 13 (P < 0.05) [53]. During the 13-month period, 70% of patients reported a ≥50% reduction from baseline in the use of systemic opioids [53]. Overall success (≥50% reduction in NAS pain score, use of systemic opioids, and/or opioid complication severity index) was reported in 83%, 90%, 85%, and 91% of patients at Months 1, 2, 3, and 4, respectively [53]. Overall, PCA delivery of IT morphine was fairly well tolerated, although procedure- and device-related complications were frequent (55 events and seven events, respectively) [53].
A retrospective chart review that included 31 patients with refractory cancer-related pain who received IT therapy with morphine or hydromorphone (28 patients also received bupivacaine) with IT PCA for breakthrough pain also reported significant analgesic benefits with patient-controlled delivery of IT morphine [90]. At a 4- to 6-week follow-up, mean NRS score had decreased from 6.5 at baseline to 3.1 (P < 0.001) [90]. In addition, the proportion of patients with severe pain (score ≥7) was greatly reduced (3% at follow-up vs 65% at baseline), and overall non-IT opioid consumption (in MED) had decreased from 796 mg/d at baseline to 64 mg/d (P = 0.003) [90]. The only significant complication observed was an increase in intense pain in one patient due to catheter movement out of the IT space; the catheter was replaced and pain reduction was achieved [90]. Mild, transient, lower extremity weakness, and urinary hesitancy were reported by several patients, but did not require significant intervention [90].
A recent prospective study that evaluated the use of PCA during IT therapy with a number of agents (i.e., morphine, hydromorphone, fentanyl, bupivacaine, clonidine, baclofen, and ziconotide) in the management of patients with refractory cancer-related pain further demonstrated beneficial effects of IT PCA therapy [88]. Most patients (91%) in the trial received either morphine or hydromorphone in their IT regimen; nine patients (16%) received ziconotide [88]. In the 58 patients with available follow-up data, numerical rating scale score for “worst” pain decreased from 8.3 (out of 10) at baseline to 5.0 with IT therapy (average duration of follow-up, 42 days); 56% of patients obtained ≥30% pain reduction and 44% of patients obtained ≥50% pain reduction; the proportion of patients with severe pain (NRS score ≥7) decreased from 84% to 35% [88]. Most patients (61%) indicated that IT PCA was “a lot better” at controlling breakthrough pain than their previous medication [88]. Mean oral opioid consumption (in MED) decreased from 805 mg/d to 128 mg/d, and 66% of patients had discontinued all non-IT opioid medications at follow-up [88]. Complications in the 98 patients who received an IT pump implant included pump infection (one patient), postdural puncture headache (three patients), and lower extremity weakness (which was managed by decreasing or eliminating the bupivacaine dose) and urinary hesitancy (that did not necessitate bladder catheterization), which occurred in several patients [88].
Use of PCA with Intrathecal Ziconotide
According to the Medtronic PTM information for prescribers, use of PTM is contraindicated for IT ziconotide because of the medication’s defined titration schedule [91]. However, bolus dosing with IT ziconotide has been shown to be effective in reducing pain, which suggests that pain relief may be achievable through PTM administration [75]. Indeed, an approach for using the PTM in patients receiving continuous infusion of ziconotide alone or in combination with opioids has been proposed [92]. The use of PTM with IT ziconotide has not yet been evaluated in controlled clinical trials; however, there is preliminary evidence of efficacy from small uncontrolled studies [88,92]. In a case series of patients with cancer-related pain (N = 3; two with metastatic breast cancer and one with metastatic pancreatic cancer), continuous IT ziconotide (1.0 mcg/d to 6 mcg/d) and hydromorphone (1.2 mg/d to 1.5 mg/d) with PTM doses of IT ziconotide and hydromorphone (0.1 mcg to 0.25 mcg and 0.15 mg to 0.25 mg, respectively) every 3 to 8 hours also improved functionality and pain, although pain in one patient remained high [92]. A recent prospective study by Brogan et al. evaluated the use of PCA during IT therapy with several agents (i.e., morphine, hydromorphone, fentanyl, bupivacaine, clonidine, baclofen, and ziconotide), used either as IT monotherapy or in combination, in the management of patients with refractory cancer-related pain [88]. Four patients received ziconotide as the sole IT agent. In these four patients, opioid dose decreased from baseline to follow-up in two patients and remained the same in two patients [88]. These initial findings, although inconclusive, provide support for additional research to establish the efficacy, safety, and tolerability of PTM administration of IT ziconotide [92].
Clinical Implications
Most data regarding the use of IT therapy for reduction of cancer-related pain have been generated in cancer patients with advanced disease [28,34]. IT treatment in patients with end-stage cancer-related pain may add several additional challenges, although IT therapy has been shown to provide pain relief in these patients [93]. The decision to implant an IDDS must be based on an appropriate risk/benefit ratio that weighs the possible benefits (i.e., pain relief) and harms (e.g., surgery risk, drug management issues) of IT treatment against palliative care options (e.g., hospice) [28]. Placement of the IDDS device may be constrained by the need to access body regions required for radiation and chemotherapy treatments and the location of metastatic disease [34,39]. Exposure to radiation may cause failure of the IDDS device and epidural metastases may impair diffusion of IT medication within the spinal column and reduce analgesia [39]. In addition, timing of IDDS placement is dependent on patients’ attainment of acceptable white blood cell and platelet counts and requires communication and coordination with patients’ oncologists [39]. Patients with cancer-related pain may be taking a variety of medications (e.g., anticoagulants) in addition to chemotherapy agents, and may have additional comorbidities that impact the decision to implant an IDDS device and, potentially, the selection of IT treatments [39].
Cancer survivors (i.e., any person who has had a cancer diagnosis, particularly those beyond the acute diagnosis and treatment phase of illness) represent a unique patient population for IT therapy for chronic pain [13,14]. Chronic pain may be underreported by cancer survivors for fear of learning of a recurrence, unwillingness to be perceived as a “drug seeker,” or desire to be a “good patient” [19]. Survivors may have less comorbidity than their counterparts undergoing cancer treatment, and therefore, may require less intensive treatment (i.e., monotherapy instead of combination treatment, lower medication doses, more gradual dose escalation, and trialing). Unfortunately, there is little to no evidence regarding treatment regimens in this specific patient population; therefore the use of IT therapy for pain management must be extrapolated from studies of patients with chronic noncancer-related pain. For example, reduced IT medication doses and lower reliance on oral opioids (i.e., opioid-sparing and microdosing) [69,94] have been shown to be effective in the IT management of noncancer pain and may also be considered for cancer survivors. Finally, IT therapy choice should take into account the impact of long-term, chronic administration (e.g., efficacy and tolerance concerns with morphine vs ziconotide) that may be required by young cancer survivors.
In general, the use of IT analgesia in long-term cancer survivors should follow the chronic pain algorithm for IT therapy [28]. For patients with active disease, IT pain management should be individualized, based on disease progression and discussion with the oncology team [39]. Typically, advanced disease with progression warrants faster IT therapy titration and more liberal use of IT combination therapies [34,39], whereas the cancer survivor in long-term remission requires slower titration dependent on side effects and efficacy.
Conclusions
IT therapy for patients with cancer pain associated with advanced disease has been studied, but the optimal timing of implantation, selection of IT medications, and specific strategies for dosing and administration have not been well defined. Increases in cancer survivorship [10,17] are prompting a paradigm shift in the treatment of cancer-related pain from a short-term, palliative care approach toward long-term management of chronic pain. IT administration of pain medications (e.g., morphine and ziconotide) is a therapeutic option for cancer patients with and without active disease, although additional research is needed to support effectiveness in cancer survivors. In the absence of research on IT therapy in cancer survivors, optimal treatment regimens should integrate applicable findings from research in patients with advanced cancer with current best practices for patients with noncancer-related pain, with consideration for the particular medical and psychological needs of cancer survivors.
Acknowledgment
We thank Nancy Holland, PhD, and Jillian Gee, PhD, of Synchrony Medical Communications, LLC, West Chester, PA, for their medical editorial assistance with this manuscript.
References
- 1. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol 2007;18(9): 1437–49. [DOI] [PubMed] [Google Scholar]
- 2. Carr D, Goudas L, Lawrence D, et al. Management of cancer symptoms: Pain, depression, and fatigue. Evid Rep Technol Assess 2002;(61): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacobs M, Macefield RC, Elbers RG, et al. Meta-analysis shows clinically relevant and long-lasting deterioration in health-related quality of life after esophageal cancer surgery. Qual Life Res 2014;23(4): 1155–76. [DOI] [PubMed] [Google Scholar]
- 4. Kenny PM, King MT, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J Clin Oncol 2008;26(2): 233–41. [DOI] [PubMed] [Google Scholar]
- 5. Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev 2012;8: CD007566.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carvalho AP, Vital FM, Soares BG. Exercise interventions for shoulder dysfunction in patients treated for head and neck cancer. Cochrane Database Syst Rev 2012;4: CD008693.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Syrjala KL, Jensen MP, Mendoza ME, et al. Psychological and behavioral approaches to cancer pain management. J Clin Oncol 2014;32(16): 1703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA Cancer J Clin 2013;63(5): 295–317. [DOI] [PubMed] [Google Scholar]
- 9. Cheville AL, Alberts SR, Rummans TA, et al. Improving adherence to cancer treatment by addressing quality of life in patients with advanced gastrointestinal cancers. J Pain Symptom Manage 2015;50(3): 321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burton AW, Fanciullo GJ, Beasley RD, et al. Chronic pain in the cancer survivor: A new frontier. Pain Med 2007;8(2): 189–98. [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Cancer survivors–United States, 2007. MMWR Morb Mortal Wkly Rep 2011;60(9): 269–72. [PubMed] [Google Scholar]
- 12. Henley SJ, Singh SD, King J, et al. Invasive cancer incidence and survival–United States, 2011. MMWR Morb Mortal Wkly Rep 2015;64(9): 237–42. [PMC free article] [PubMed] [Google Scholar]
- 13. National Cancer Institute. Office of Cancer Survivorship. Survivorship Definition. 2014. Available at: cancercontrol.cancer.gov/ocs/statistics/definitions. html (accessed February 9, 2016).
- 14. National Coalition for Cancer Survivorship. NCCS Mission. 2015. Available at: http://www.canceradvocacy.org/about-us/our-mission (accessed February 9, 2016).
- 15. Hewitt M, Greenfield S, Stovall E, et al. , editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academy of Sciences; 2006. [Google Scholar]
- 16. Moryl N, Coyle N, Essandoh S, et al. Chronic pain management in cancer survivors. J Natl Compr Canc Netw 2010;8(9): 1104–10. [DOI] [PubMed] [Google Scholar]
- 17. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol 2014;32(16): 1739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paice JA. Chronic treatment-related pain in cancer survivors. Pain 2011;152(3 suppl): S84–9. [DOI] [PubMed] [Google Scholar]
- 19. Levy MH, Chwistek M, Mehta RS. Management of chronic pain in cancer survivors. Cancer J 2008;14(6): 401–9. [DOI] [PubMed] [Google Scholar]
- 20. Han Y, Smith MT. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front Pharmacol 2013;4: 156.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Denlinger CS, Ligibel JA, Are M, et al. Survivorship: Pain version 1.2014. J Natl Compr Canc Netw 2014;12(4): 488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paice JA, Von Roenn JH. Under- or overtreatment of pain in the patient with cancer: How to achieve proper balance. J Clin Oncol 2014;32(16): 1721–6. [DOI] [PubMed] [Google Scholar]
- 23. Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care 2008;2(1):14–8. [DOI] [PubMed] [Google Scholar]
- 24. Hayek SM, Deer TR, Pope JE, et al. Intrathecal therapy for cancer and non-cancer pain. Pain Physician 2011;14(3): 219–48. [PubMed] [Google Scholar]
- 25. Pope JE, Deer TR. Guide to implantable devices for intrathecal therapy. Pract Pain Manag 2013;3(8): 1–11. [Google Scholar]
- 26. Bottros MM, Christo PJ. Current perspectives on intrathecal drug delivery. J Pain Res 2014;4(7): 615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brogan S, Junkins S. Interventional therapies for the management of cancer pain. J Support Oncol 2010;8(2): 52–9. [PubMed] [Google Scholar]
- 28. Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference 2012: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery: Report of an interdisciplinary expert panel. Neuromodulation 2012;15(5): 436–64. [DOI] [PubMed] [Google Scholar]
- 29. Prialt (ziconotide) Solution, Intrathecal Infusion [package insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc; 2013. [Google Scholar]
- 30. INFUMORPH 200, INFUMORPH 500 (Preservative-Free Morphine Sulfate Sterile Solution) [package insert]. Eatontown, NJ: West-Ward Pharmaceuticals; 2011. [Google Scholar]
- 31. Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: A randomized controlled trial. JAMA 2004;291(1): 63–70. [DOI] [PubMed] [Google Scholar]
- 32. Wallace MS, Charapata SG, Fisher R, et al. The Ziconotide Nonmalignant Pain Study 96-002 Group. Intrathecal ziconotide in the treatment of chronic nonmalignant pain: A randomized, double-blind, placebo-controlled clinical trial. Neuromodulation 2006;9(2): 75–86. [DOI] [PubMed] [Google Scholar]
- 33. Rauck RL, Wallace MS, Leong MS, et al. Ziconotide 301 Study Group. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage 2006;31(5): 393–406. [DOI] [PubMed] [Google Scholar]
- 34. Gulati A, Puttanniah V, Hung J, et al. Considerations for evaluating the use of intrathecal drug delivery in the oncologic patient. Curr Pain Headache Rep 2014;18(2): 391.. [DOI] [PubMed] [Google Scholar]
- 35. Stearns L, Boortz-Marx R, Du PS, et al. Intrathecal drug delivery for the management of cancer pain: A multidisciplinary consensus of best clinical practices. J Support Oncol 2005;3(6): 399–408. [PubMed] [Google Scholar]
- 36. Deer TR, Caraway DL, Wallace MS. A definition of refractory pain to help determine suitability for device implantation. Neuromodulation 2014;17: 711–5. [DOI] [PubMed] [Google Scholar]
- 37. Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference–2012: Recommendations on trialing for intrathecal (intraspinal) drug delivery: Report of an interdisciplinary expert panel. Neuromodulation 2012;15(5): 420–35. [DOI] [PubMed] [Google Scholar]
- 38. Saulino M, Kim PS, Shaw E. Practical considerations and patient selection for intrathecal drug delivery in the management of chronic pain. J Pain Res 2014;7: 627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deer TR, Smith HS, Burton AW, et al. Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician 2011;14(3): E283–312. [PubMed] [Google Scholar]
- 40. Deer T, Winkelmuller W, Erdine S, et al. Intrathecal therapy for cancer and nonmalignant pain: Patient selection and patient management. Neuromodulation 1999;2(2): 55–66. [DOI] [PubMed] [Google Scholar]
- 41. Prager J, Deer T, Levy R, et al. Best practices for intrathecal drug delivery for pain. Neuromodulation 2014;17(4): 354–72. [DOI] [PubMed] [Google Scholar]
- 42. Malhotra VT, Root J, Kesselbrenner J, et al. Intrathecal pain pump infusions for intractable cancer pain: An algorithm for dosing without a neuraxial trial. Anesth Analg 2013;116(6): 1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Newsome S, Frawley BK, Argoff CE. Intrathecal analgesia for refractory cancer pain. Curr Pain Headache Rep 2008;12(4): 249–56. [DOI] [PubMed] [Google Scholar]
- 44. Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J Clin Oncol 2002;20(19): 4040–9. [DOI] [PubMed] [Google Scholar]
- 45. Smith TJ, Coyne PJ, Staats PS, et al. An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM). Ann Oncol 2005;16(5): 825–33. [DOI] [PubMed] [Google Scholar]
- 46. Smith TJ, Coyne PJ. Implantable drug delivery systems (IDDS) after failure of comprehensive medical management (CMM) can palliate symptoms in the most refractory cancer pain patients. J Palliat Med 2005;8(4): 736–42. [DOI] [PubMed] [Google Scholar]
- 47. Davis MP, Walsh D, Lagman R, et al. Randomized clinical trial of an implantable drug delivery system. J Clin Oncol 2003;21(14): 2800–1. [DOI] [PubMed] [Google Scholar]
- 48. Ripamonti C, Brunelli C. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J Clin Oncol 2003;21(14): 2801–2. [DOI] [PubMed] [Google Scholar]
- 49. Mercadante S, Intravaia G, Villari P, et al. Intrathecal treatment in cancer patients unresponsive to multiple trials of systemic opioids. Clin J Pain 2007;23(9): 793–8. [DOI] [PubMed] [Google Scholar]
- 50. Sjoberg M, Appelgren L, Einarsson S, et al. Long-term intrathecal morphine and bupivacaine in “refractory” cancer pain. I. Results from the first series of 52 patients. Acta Anaesthesiol Scand 1991;35(1): 30–43. [DOI] [PubMed] [Google Scholar]
- 51. Sjoberg M, Nitescu P, Appelgren L, et al. Long-term intrathecal morphine and bupivacaine in patients with refractory cancer pain. Results from a morphine: Bupivacaine dose regimen of 0.5:4.75 mg/ml. Anesthesiology 1994;80(2): 284–97. [DOI] [PubMed] [Google Scholar]
- 52. Mitchell A, McGhie J, Owen M, et al. Audit of intrathecal drug delivery for patients with difficult-to-control cancer pain shows a sustained reduction in pain severity scores over a 6-month period. Palliat Med 2015;29(6): 554–63. [DOI] [PubMed] [Google Scholar]
- 53. Rauck RL, Cherry D, Boyer MF, et al. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain 2003;4(8): 441–7. [DOI] [PubMed] [Google Scholar]
- 54. Van Dongen RT, Crul BJ, De BM. Long-term intrathecal infusion of morphine and morphine/bupivacaine mixtures in the treatment of cancer pain: A retrospective analysis of 51 cases. Pain 1993;55(1): 119–23. [DOI] [PubMed] [Google Scholar]
- 55. Becker R, Jakob D, Uhle EI, et al. The significance of intrathecal opioid therapy for the treatment of neuropathic cancer pain conditions. Stereotact Funct Neurosurg 2000;75(1): 16–26. [DOI] [PubMed] [Google Scholar]
- 56. Onofrio BM, Yaksh TL. Long-term pain relief produced by intrathecal morphine infusion in 53 patients. J Neurosurg 1990;72(2): 200–9. [DOI] [PubMed] [Google Scholar]
- 57. Follett KA, Hitchon PW, Piper J, et al. Response of intractable pain to continuous intrathecal morphine: A retrospective study. Pain 1992;49(1): 21–5. [DOI] [PubMed] [Google Scholar]
- 58. Penn RD, Paice JA. Chronic intrathecal morphine for intractable pain. J Neurosurg 1987;67(2): 182–6. [DOI] [PubMed] [Google Scholar]
- 59. Burton AW, Rajagopal A, Shah HN, et al. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med 2004;5(3): 239–47. [DOI] [PubMed] [Google Scholar]
- 60. Upadhyay SP, Mallick PN. Intrathecal drug delivery system (IDDS) for cancer pain management: A review and updates. Am J Hosp Palliat Care 2012;29(5): 388–98. [DOI] [PubMed] [Google Scholar]
- 61. Webster LR. The relationship between the mechanisms of action and safety profiles of intrathecal morphine and ziconotide: A review of the literature. Pain Med 2015;16(7): 1265–77. [DOI] [PubMed] [Google Scholar]
- 62. Ruan X. Drug-related side effects of long-term intrathecal morphine therapy. Pain Physician 2007;10(2): 357–66. [PubMed] [Google Scholar]
- 63. Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 2010;112(1): 226–38. [DOI] [PubMed] [Google Scholar]
- 64. Hassenbusch S, Burchiel K, Coffey RJ, et al. Management of intrathecal catheter-tip inflammatory masses: A consensus statement. Pain Med 2002;3(4): 313–23. [DOI] [PubMed] [Google Scholar]
- 65. Zacest AC, Carlson JD, Nemecek A, et al. Surgical management of spinal catheter granulomas: Operative nuances and review of the surgical literature. Neurosurgery 2009;65(6):1161–4. [DOI] [PubMed] [Google Scholar]
- 66. Wilson GR, Reisfield GM. Morphine hyperalgesia: A case report. Am J Hosp Palliat Care 2003;20(6): 459–61. [DOI] [PubMed] [Google Scholar]
- 67. Sallerin-Caute B, Lazorthes Y, Deguine O, et al. Does intrathecal morphine in the treatment of cancer pain induce the development of tolerance? Neurosurgery 1998;42(1): 44–9. [DOI] [PubMed] [Google Scholar]
- 68. Grider JS, Harned ME, Sloan PA. Patient selection and trialing techniques utilizing low-dose intrathecal morphine for chronic nonmalignant pain: A report of two cases. J Opioid Manag 2010;6(5): 371–6. [DOI] [PubMed] [Google Scholar]
- 69. Hamza M, Doleys D, Wells M, et al. Prospective study of 3-year follow-up of low-dose intrathecal opioids in the management of chronic nonmalignant pain. Pain Med 2012;13(10): 1304–13. [DOI] [PubMed] [Google Scholar]
- 70. Medtronic, Inc. Medical Device Correction. Important Information on Potential MRI Effects; August 2008. Available at: https://professional.medtronic.com/pt/neuro/idd/ind/product-advisories/WCM_PROD082428#.Vpll2fkrJD8 (accessed February 9, 2016).
- 71. Kosturakis A, Gebhardt R. SynchroMed II intrathecal pump memory errors due to repeated magnetic resonance imaging. Pain Physician 2012;15(6): 475–7. [PubMed] [Google Scholar]
- 72. Burton AW, Lemus B, Fletcher R, et al. Effectiveness of intrathecal ziconitide in malignant pain: A combined analysis of two controlled trials. Poster presented at: 2006 Annual Meeting of American Society of Clinical Oncology; June 2–6, 2006; Atlanta, GA. Abstract # 8552.
- 73. Bruel BM. Rapid titration of ziconotide for the treatment of servere intractable back pain from metastatic spinal anaplastic ependymoma. Eur J Pain 2007; S142–3. [Google Scholar]
- 74. Mohammed S, Brookes ME, Eldabe S. Ziconotide for severe neuropathic pain in metastatic breast cancer. J Pain Palliat Care Pharmacother 2012;26(3): 286–8. [Google Scholar]
- 75. Pope JE, Deer TR. Intrathecal pharmacology update: Novel dosing strategy for intrathecal monotherapy ziconotide on efficacy and sustainability. Neuromodulation 2015;18(5): 414–20. [DOI] [PubMed] [Google Scholar]
- 76. Burton AW, Lemus B, Fletcher R, et al. Effectiveness of intrathecal ziconotide in malignant pain: A combined analysis of two controlled trials [abstract]. J Clin Oncol 2006;24(18 suppl): 8552. [Google Scholar]
- 77. Engle MP, Uzodinma O, Bruel BM. Intrathecal bolus trials of ziconotide for cancer-related pain. Poster presented at: 16th Annual Meeting of North American Neuromodulation Society; December 6–9, 2012; Las Vegas, NV. Poster 389.
- 78. Bruel B, Kaur G, Engle MP. Ziconotide single shot trial and subsequent dosing for cancer-related pain. Poster and oral presentation at: International Neuromodulation Society 11th World Congress; June 13, 2013; Berlin, Germany.
- 79. Mohammed SI, Eldabe S, Simpson KH, et al. Bolus intrathecal injection of ziconotide (Prialt®) to evaluate the option of continuous administration via an implanted intrathecal drug delivery (ITDD) system: A pilot study. Neuromodulation 2013;16(6): 576–81. [DOI] [PubMed] [Google Scholar]
- 80. Alicino I, Giglio M, Manca F, et al. Intrathecal combination of ziconotide and morphine for refractory cancer pain: A rapidly acting and effective choice. Pain 2012;153(1): 245–9. [DOI] [PubMed] [Google Scholar]
- 81. Dupoiron D, Bore F, Lefebvre-Kuntz D, et al. Ziconotide adverse events in patients with cancer pain: A multicenter observational study of a slow titration, multidrug protocol. Pain Physician 2012;15(5): 395–403. [PubMed] [Google Scholar]
- 82. Raffaeli W, Sarti D, Demartini L, et al. Italian registry on long-term intrathecal ziconotide treatment. Pain Physician 2011;14(1): 15–24. [PubMed] [Google Scholar]
- 83. Hayek SM, Veizi IE, Narouze SN, et al. Age-dependent intrathecal opioid escalation in chronic noncancer pain patients. Pain Med 2011;12(8): 1179–89. [DOI] [PubMed] [Google Scholar]
- 84. Ruggiero A, Barone G, Liotti L, et al. Safety and efficacy of fentanyl administered by patient controlled analgesia in children with cancer pain. Support Care Cancer 2007;15(5): 569–73. [DOI] [PubMed] [Google Scholar]
- 85. Sousa AM, de Santana NJ, Guimaraes GM, et al. Safety profile of intravenous patient-controlled analgesia for breakthrough pain in cancer patients: A case series study. Support Care Cancer 2014;22(3): 795–801. [DOI] [PubMed] [Google Scholar]
- 86. Ilias W, le Polain B, Buchser E, et al. Patient-controlled analgesia in chronic pain patients: Experience with a new device designed to be used with implanted programmable pumps. Pain Pract 2008;8(3): 164–70. [DOI] [PubMed] [Google Scholar]
- 87. Maeyaert J, Buchser E, Van Buyten JP, et al. Patient-controlled analgesia in intrathecal therapy for chronic pain: Safety and effective operation of the Model 8831 Personal Therapy Manager with a pre-implanted SynchroMed Infusion System. Neuromodulation 2003;6(3): 133–41. [DOI] [PubMed] [Google Scholar]
- 88. Brogan SE, Winter NB, Okifuji A. Prospective observational study of patient-controlled intrathecal analgesia: Impact on cancer-associated symptoms, breakthrough pain control, and patient satisfaction. Reg Anesth Pain Med 2015;40(4): 369–75. [DOI] [PubMed] [Google Scholar]
- 89. Flowonix Medical. Flowonix Medical Inc. announces FDA approval of new product. 2015. Available at: http://www.prnewswire.com/news-releases/flowonix-medical-inc-announces-fda-approval-of-new-product-300125380.html (accessed February 9, 2016).
- 90. Brogan SE, Winter NB. Patient-controlled intrathecal analgesia for the management of breakthrough cancer pain: A retrospective review and commentary. Pain Med 2011;12(12): 1758–68. [DOI] [PubMed] [Google Scholar]
- 91. Medtronic Inc. Personal Therapy Manager for SynchroMed II: Physician Manual. 2007. Available at: http://professional.medtronic.com/wcm/groups/mdtcom_sg/@mdt/@neuro/documents/documents/idd-ptm8835-manl.pdf (accessed February 9, 2016).
- 92. McDowell GC. Use of the personal therapy manager with Prialt® (ziconotide intrathecal infusion) for patient-controlled analgesia: A case series highlighting techniques and outcomes. Presented at: North American Neuromodulation Society 14th Annual Meeting. Las Vegas, NV; 2010. Available at: http://www.slideserve.com/uri/use-of-the-personal-therapy-manager-with-prialt-ziconotide-intrathecal-infusion-for-patient-controlled-analgesia-case (accessed February 9, 2016).
- 93. Kim JH, Jung JY, Cho MS. Continuous intrathecal morphine administration for cancer pain management using an intrathecal catheter connected to a subcutaneous injection port: A retrospective analysis of 22 terminal cancer patients in Korean population. Korean J Pain 2013;26(1): 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Grider JS, Harned ME, Etscheidt MA. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain Physician 2011;14(4): 343–51. [PubMed] [Google Scholar]