Abstract
Background
We assessed the longitudinal hazard characteristics for death and progression in patients with glioblastoma, evaluated the impact of prognostic factors and treatment on the hazard within different time intervals to determine if effects are time varying, and quantified the influence of progression on survival.
Methods
Among patients randomized to Radiation Therapy Oncology Group trial 0525, which compared dose-dense with standard-dose temozolomide, we estimated the hazards of death and treatment failure (death or progression) over time and their interdependence.
Results
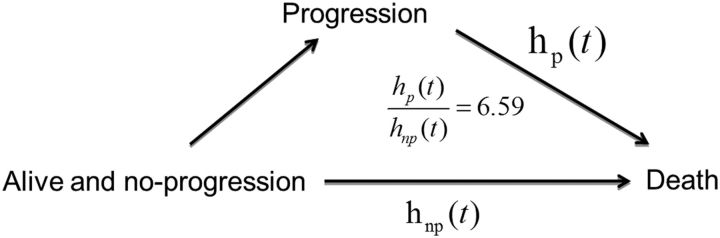
The peak hazard of death was reached at around 16 months with a slow decline after that; the hazard of progression/death reached a peak at around 6 months and decreased dramatically thereafter. The survival advantages for patients with MGMT gene promoter methylation and recursive partitioning analysis class III were substantial in the first 2 years, but lessened thereafter. The progression-free survival benefit of dose-dense over standard-dose temozolomide occurred in the first 6 months (hazard ratio: 0.70; 95% CI: 0.58–0.86; P < .001), although it diminished thereafter. After adjusting for recursive partitioning analysis class and MGMT methylation status, the hazard ratio of death for patients who had progressed over nonprogressors was 6.59 (95% CI: 5.15–8.43; P < .001).
Conclusion
After the peak hazard of death, a consistently high hazard remains, but it is lower than in the peak period. The progression hazard peak is earlier, and then hazard consistently declines. The rate of dying after disease progression is about 6.59 times the rate for nonprogressors, suggesting that progression-free survival may be a relevant clinical endpoint.
Keywords: glioblastoma, hazard of death, hazard of failure (progression or death)
There were 226 791 new cases of primary brain tumors during 2004–2007 in the United States, which approximates an annual average incidence of 56 698. Almost a third (31%) of these were gliomas, with glioblastoma (GBM) accounting for over one-half (53.7%) of all gliomas.1 The prognosis for patients with GBM remains poor: the median survival is only 12–15 months and <5% of patients survive 5 years post diagnosis.1,2 Approximately 90% of GBM patients experience disease progression prior to death; therefore, it is meaningful to quantify the impact of disease progression on patient survival. In order to do this, it is important to investigate the temporal pattern in progression events, and the association between progression and subsequent death. This information is best characterized by the failure hazard, which heuristically represents the rate of failure in any small interval of time. In this respect, the hazard importantly differs from more commonly used summaries such as survival curves, which are aggregated over time. The hazard can thus more readily identify change-points or peaks in risk of failure over time. Other prognostic and/or predictive variables may also have time-varying effects on failure, and it is of value to explore these varying effects. The objectives of this analysis, based on using data from the phase III randomized Radiation Therapy Oncology Group (RTOG) 0525 trial, were: (i) to determine characteristics of the long-term hazard for death and progression, evaluating whether the hazard itself is time variant or time invariant; (ii) to evaluate the effect of prognostic factors on the hazard within different time intervals to determine if these effects are time varying; and (iii) to quantify the influence of disease progression on survival.
Methods
Patient Population
RTOG 0525, an intergroup collaboration including also the European Organisation for Research and Treatment of Cancer (EORTC) and the North Central Cancer Treatment Group, opened in 2006 and completed accrual in 2008. One hundred and eighty-five facilities in North America and 24 facilities in Europe enrolled 1173 study participants. All patients provided written informed consent to participate in this institutional review board–approved trial. Key study eligibility criteria required that tumor tissue, obtained at the time of biopsy or surgery, be sent for central histopathologic confirmation and that an analysis of methylation of the O6-DNA methylguanine-methyltransferase gene (MGMT) be performed. Upon central confirmation of GBM histopathology, eligible patients were randomized into 1 of 2 treatment arms after completion of chemoradiotherapy: the standard arm (standard-dose temozolomide [TMZ] on days 1–5 every 28 d for ≤12 cycles/mo) or the experimental arm (dose-dense TMZ on days 1–21 every 28 d for ≤12 cycles/mo). Study participants were stratified by (i) recursive partitioning analysis (RPA) class (RPA III, IV, or V), based upon age, performance status, extent of pretreatment surgery, and neurologic function; (ii) MGMT status (methylated, unmethylated, or indeterminate); and (iii) radiation treatment (US standard or European). The study was designed to accrue 750 randomized patients, which would provide 80% power to detect a 25% increase in median survival from 14 months in the standard arm (estimated) to 17.6 months (hazard ratio [HR] for death = 0.80; type I error, 0.025 [one-sided]). Additional details of the design and conduct as well as primary findings of the trial have been presented.3
For this analysis, we excluded protocol-ineligible patients (n = 48); patients who were not randomized due to insufficient tissue (n = 144), disease progression prior to randomization (n = 48), death prior to randomization (n = 18), or other reasons (n = 82); and randomized patients without follow-up information (n = 2). The resultant cohort consists of 831 randomized patients. Results in this report reflect data reported to the RTOG statistical and data management center as of January 6, 2011. Median follow-up time is 31.9 months (range, 0.2–53.3 mo).
Statistical Methods
The hazard rate is defined mathematically as the probability of failure during a small time interval given that failure has not occurred previously,4,5 thus representing the “instantaneous risk” that the failure will occur at that time. In practice, the hazard rate is estimated as the fraction of patients at risk who fail in some discrete time-window through life table methods or other approaches, such as by using a Nelson–Aalen estimator, events/person-years in discrete intervals, etc.6,7 These approaches can be extended to allow the hazard to be estimated as a smooth function of time.8–14 The smoothing procedure, when applied appropriately, provides an accurate estimate and improves the statistical performance of the resulting hazard rate estimator. In this analysis, the “event” for failure (the progression-free survival [PFS] endpoint) is death or tumor progression, whichever occurs first. Smooth estimates of the underlying hazard function (hazard of death or failure) were estimated by using kernel-based smoothing methods,8–10 which are widely adopted to uncover structural features in the data with the appropriate choice of kernel and bandwidth. Cox proportional hazards (PH) models15 are commonly used to estimate hazard ratios as measures of effect of covariates (eg, treatment, prognostic factors); and in standard Cox PH models the covariate effects are assumed to be time invariant, which means that the influence of covariates do not vary over time. However, extensions of the standard Cox PH model have been developed to allow time-varying covariate effects.16,17 The comparisons of hazards between subgroups within time intervals were made using the piecewise Cox PH model.7 These subgroups were created on the basis of prognostic factors collected at study entry. To characterize how the prognostic effect of covariates might vary over time, the hazard ratios contrasting prognosis by subgroups were estimated separately in an early and a late follow-up interval. For these analyses, the time partition was chosen by visual inspection of hazard plots and numerical comparison of log partial likelihood from the models. For the latter, the exact values of the log partial likelihood were calculated under different possible cutoff points and the cutpoint leading to the largest value was chosen as a partition point. The majority of GBM patients experience progression prior to death; therefore we evaluated progression as a time-dependent covariate, using a time-varying covariate Cox PH model to assess the effect of progression on survival. A 2-sided test at a significance level of .05 was used for testing the time-varying covariate.
Results
Hazard of Death and Failure Over Time
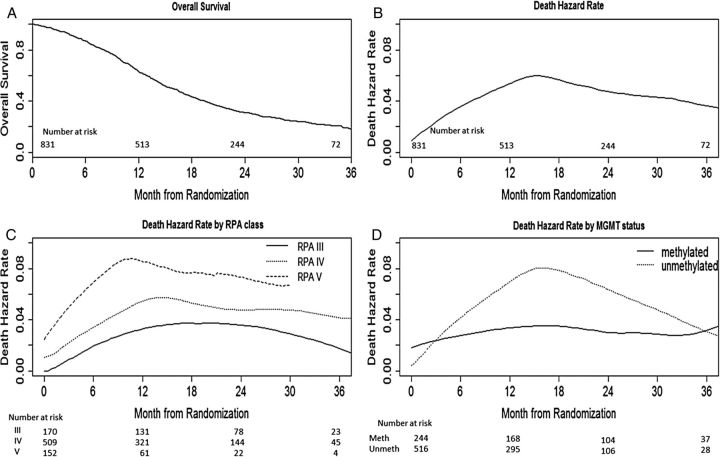
Figure 1A shows the overall survival (OS; from randomization) for the entire patient cohort (N = 831). The peak hazard of death occurred at around 16 months; after that, a consistently high hazard remained, but it was lower than at the peak (Fig. 1B). During the high risk period (9–24 mo), the monthly hazard rate for death was between 4.5% and 6%. Patients were divided into subgroups based on known or suspected prognostic factors: RPA class (III vs IV vs V), MGMT methylation status (methylated vs unmethylated), radiation type (European vs USA), and treatment options (standard-dose vs dose-dense). Figure 1B shows the hazards of death at a specific time point after patient randomization for the entire group, and Fig. 1C and D are for MGMT and RPA subsets. The shape of the death hazard for RPA class IV and MGMT unmethylated subsets is similar to that for the entire group (Fig. 1B). The hazard for MGMT methylated patients appeared more constant over time (Fig. 1C). Patients with better than expected prognosis (MGMT methylated tumor and RPA III) had a lower hazard at all intervals.
Fig. 1.
OS and death hazard over time.
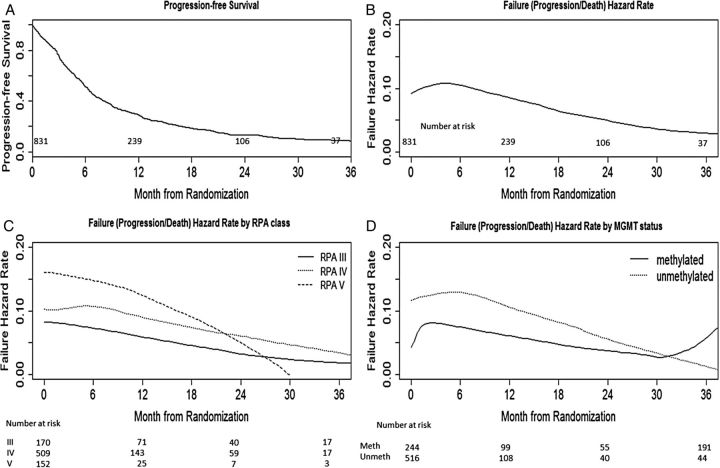
Figure 2A shows the PFS (from randomization) for the entire cohort. The hazard of progression/death (referred to as “failure”) reached a peak at around 6 months and decreased dramatically thereafter (Fig. 2B). During the high risk period (0–9 mo), the monthly hazard of failure was around 10%. Figure 2B shows the hazard of failure at a specific time point after patient randomization for the entire group, and Fig. 2C and D are for the MGMT and RPA subgroups. The shape of the failure hazard for RPA class IV and unmethylated subsets is similar to the graph for the entire group. For the methylated subset, the hazard of failure reached a peak at 3 months, decreased dramatically thereafter, and then rebounded at around 30 months, implying that peak rates of failure may occur in a biphasic manner in the methylated subpopulation. Patients with better than expected prognosis (MGMT methylated tumor and RPA III) had a lower failure hazard at all intervals.
Fig. 2.
PFS and failure hazard over time.
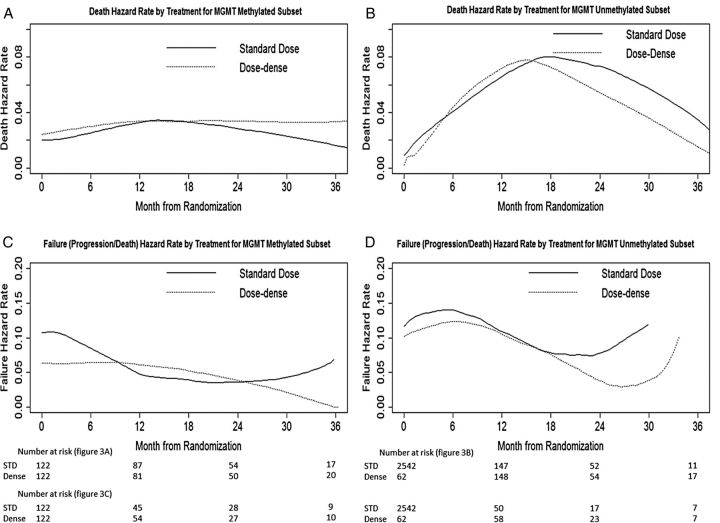
Figure 3 shows the hazard over time by treatment groups for both MGMT methylated and unmethylated subsets. For the MGMT methylated subset, death hazards appeared more constant over time for both treatment options (Fig. 3A), while for the MGMT unmethylated subset there was a peak in hazards for both treatments at around 15 months (Fig. 3B). For the MGMT methylated subset, the failure hazard for the dose-dense arm was relatively constant in the first 9 to 12 months and then decreased, while the failure hazard for the standard-dose arm appeared higher in the first 3 months and then decreased thereafter (Fig. 3C). For MGMT unmethylated patients, the failure hazard again showed an early peak in both treatment groups (Fig. 3D). As in Fig. 2, these analyses suggest that the negative prognostic effect of an MGMT unmethylated tumor manifests itself in the form of rapid progression after diagnosis followed by a period of high mortality rate, while for MGMT methylated tumors, progression and death risk are less dynamic over time. Dose-dense TMZ does not appear to alter this pattern substantively.
Fig. 3.
Hazard by treatment for MGMT methylated and unmethylated subsets.
Time-Varying Effect of Prognostic Factors and Treatment
A simple way to examine the time dependency of prognostic factors and treatment on clinical benefit (OS, PFS) is to partition time into discrete intervals and estimate the effect (in terms of HRs) within each interval. Beginning at ∼24 months from randomization, death hazard begins to appear relatively low compared with the initial 24 months (Fig. 1B); beginning at ∼6 months from randomization, failure hazard begins to decrease (Fig. 2B). Thus we chose these 2 cutoffs as relevant time points for partition and estimated effects of prognostic factors and treatment for the early interval (0–24 mo for OS, 0–6 mo for PFS) and late interval (>24 mo for OS, >6 mo for PFS). In addition, numerical comparison of log partial likelihood under semiparametric survival models also suggests that these 2 cutoff points are appropriate. While these cutpoints are not unique and do not universally apply without independent data validation, additional modeling studies showed that partition times slightly earlier or later led to similar results (data not shown). Table 1 gives the comparisons of hazards between subgroups within time intervals based on the piecewise Cox PH model. The time intervals were defined for OS as the first 24 months of follow-up, or beyond 24 months follow-up, and for PFS as the first 6 months or beyond 6 months follow-up.
Table 1.
Hazard ratios in different time intervals from randomization
| 0–24 mo |
>24 mo |
||||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| OS | RPA class | ||||
| III | – | – | – | – | |
| IV | 1.64 (1.30, 2.08) | <.0001 | 1.27 (0.79, 2.05) | .33 | |
| V | 2.91 (2.21, 3.82) | <.0001 | 1.69 (0.79, 3.65) | .18 | |
| Radiation type | |||||
| USA | – | – | – | – | |
| European | 0.98 (0.80, 1.22) | .88 | 0.88 (0.44, 1.76) | .71 | |
| MGMT status | |||||
| Methylated | – | – | – | – | |
| Unmethylated | 1.78 (1.46, 2.17) | <.0001 | 1.52 (0.94, 2.45) | .09 | |
| Treatment | |||||
| Standard dose | – | – | – | – | |
| Dose dense | 1.03 (0.88, 1.22) | .70 | 0.99 (0.65, 1.50) | .97 | |
| 0–6 mo |
>6 mo |
||||
| HR (95% CI) | P | HR (95% CI) | P | ||
| PFS | RPA class | ||||
| III | – | – | – | – | |
| IV | 1.31 (1.00, 1.71) | .05 | 1.68 (1.28, 2.21) | .0002 | |
| V | 1.89 (1.38, 2.59) | <.0001 | 2.35 (1.65, 3.36) | <.0001 | |
| Radiation type | |||||
| USA | – | – | – | – | |
| European | 0.82 (0.63, 1.07) | .14 | 0.98 (0.75, 1.29) | .90 | |
| MGMT status | |||||
| Methylated | – | – | – | – | |
| Unmethylated | 1.47 (1.17, 1.86) | .001 | 1.80 (1.42, 2.27) | <.0001 | |
| Treatment | |||||
| Standard dose | – | – | – | – | |
| Dose dense | 0.70 (0.58, 0.86) | .0005 | 1.12 (0.90, 1.38) | .32 | |
The relative benefits (longer survival) of the MGMT methylation subgroup (HR = 1.78 [unmethylated vs methylated], 95% CI: 1.46–2.17, P < .0001) and the RPA III subgroup (HR = 1.64 [class IV vs III], 95% CI: 1.30–2.08, P < .0001; HR = 2.91 [class V vs III], 95% CI: 2.21–3.82, P < .0001) were substantial in the first 2 years, but appeared weaker thereafter (HR = 1.52 [unmethylated vs methylated], 95% CI: 0.94–2.45, P = .09; HR = 1.27 [class IV vs III], 95% CI: 0.79–2.05, P = .33; HR = 1.69 [class V vs III], 95% CI: 0.79–3.65, P = .18). However, of note, the confidence intervals during these 2 time intervals overlapped; therefore, we should be cautious to not overinterpret the observed weakening effect over time. Neither radiation type (European vs USA) nor treatment (dose-dense vs standard-dose) has influence on death hazard in early (0–2 y) or later (>2 y) intervals.
Both RPA class and methylation status influenced progression/death hazard at both early (0–6 mo) and later (>6 mo) intervals, but radiation type had no influence on either of these intervals. None of these factors showed significant interactions with time. The relative benefit (longer PFS) of dose-dense over standard-dose TMZ occurred in the first 6 months (HR = 0.70, 95% CI: 0.58–0.86, P = .0005), although it diminished thereafter, and there was a significant interaction between time and treatment with respect to PFS (P = .015).
The early PFS benefit for dose-dense treatment was more heavily weighted toward MGMT methylated patients than MGMT unmethylated patients. The HRs of dose-dense over standard-dose TMZ for the first 6 months were 0.54 (95% CI: 0.36–0.81, P = .003) and 0.79 (95% CI: 0.63–1.00, P = .05) for MGMT methylated and unmethylated, respectively. After 6 months, the HRs of dose-dense over standard-dose TMZ were 1.37 (95% CI: 0.93–2.03, P = .12) and 1.01 (95% CI: 0.77–1.33, P = .95), respectively.
Relationship Between Progression and Survival
One important aspect of interest is the relationship between progression and OS. We evaluated progression as a time-dependent covariate, using a time-varying covariate Cox PH model to assess the effect of progression on survival. After adjusting for RPA class and MGMT methylation status, the HR of death for patients who had progressed over nonprogressors was 6.59 (95% CI: 5.12–8.43, P < .001). This indicates that there exists a strong relationship between progression and survival (Fig. 4).
Fig. 4.
Relationship between progression and survival.
Discussion
In this study, we examined the dynamics in the progression and death hazard from a cohort of over 800 randomized newly diagnosed GBM patients. Our data show that for patients with newly diagnosed GBM, the peak hazard of death occurs between 9 and 24 months, and thereafter consistently high hazard remains, which is only modestly lower than in the peak period. The progression hazard peak occurs earlier at 6 months and the hazard then consistently declines over time. This finding differs somewhat from the report of Ballman and colleagues18 based on 11 trials of newly diagnosed GBM with 1348 patients enrolled between 1980 and 2002, all treated without TMZ. They found that the hazard of progression was relatively flat between 4 and 12 months; it peaked at 6 months but without a dramatic drop afterward. In the Ballman report, the distribution of patients only undergoing a tumor biopsy, subtotal tumor resection, and gross total tumor resection was 20%, 60%, and 20%, respectively. Progression was determined by a combination of neurologic examination status and imaging results. However, in our study (RTOG 0525) the distribution of patients undergoing biopsy, subtotal resection, and gross total resection was 5%, 50%, and 45%, respectively; all patients received TMZ; progression was determined by clinical symptoms or imaging results. These may account for the difference between the 2 studies in terms of progression hazard over time.
The relative survival advantage of MGMT methylation and RPA class III was substantial in the first 2 years, but became somewhat weaker thereafter. These findings echo the conclusions from the EORTC/National Cancer Institute of Canada (NCIC) report:19 even though methylation of MGMT was the strongest predictor of clinical outcome, few patients with methylated MGMT live longer than 5 years. In the EORTC/NCIC trial, the OS rates for patients receiving the combination of radiotherapy and TMZ were 14.8%, 11.1%, 11.1%, and 8.3% at 2, 3, 4, and 5 years, respectively, for unmethylated patients, compared with 48.9%, 27.6%, 22.1%, and 13.8% for patients with methylated MGMT; expressed as a ratio (methylated vs unmethylated), this results in 2-, 3-, 4-, and 5-year values of 3.3, 2.5, 2.0, and 1.7, respectively. Both studies demonstrate that the difference in survival rate between methylated and unmethylated patients decreases beyond 2–3 years.
MGMT methylation status and RPA classes influenced progression hazard both early and late after diagnosis. The relative benefit (longer PFS) of dose-dense over standard-dose TMZ occurred in the first 6 months, but diminished thereafter. The effect on PFS associated with dose-dense TMZ may be explained by several possible mechanisms. Exposure to dose-dense TMZ may result in tumor growth arrest rather than cell death, thereby leading to early PFS benefit. Alternatively, crossover treatment at progression may have contributed to the lack of improvement in PFS with the dose-dense treatment translating to an OS benefit.
The rate of dying after progression is about 6.6 times the rate for nonprogressors; this is completely expected, underscoring that progression is an important clinical endpoint that strongly increases risk of death and that most patients with GBM die from progressive disease. Ballman18 and Lamborn20 and colleagues investigated the relationship between PFS and OS in high-grade gliomas through patient-level and study-level agreement using multiple trials. The Ballman report concluded that there was a strong association between PFS status and OS; the relationship was slightly stronger in patients with recurrent GBM than in patients with newly diagnosed disease, suggesting that progression at 6 months may be a reasonable endpoint for recurrent GBM, a conclusion that was also supported by Lamborn et al.20 In the study by Ballman et al,18 the pattern of progression hazard for recurrent GBM, peaking at 6 months and then dramatically dropping after that, is similar to the pattern of progression hazard over time in our study for newly diagnosed GBM; in addition, the HR for progression in recurrent GBM is 8.5, similar to what we found (6.6). It appears that the pattern of progression hazard over time and the influence of progression on survival for recurrent GBM based on the Ballman report are similar to what we found for newly diagnosed GBM patients. All these observations may suggest that PFS is a reasonable endpoint for newly diagnosed GBM patients. Of note, the method and frequency of progression detection may have impact on our observations, in that changing the detection time would alter the hazard estimates. Although our data are compelling for the parallel effects of treatment and markers on PFS and OS, declaring that PFS is a surrogate endpoint for OS will require a large number of randomized phase III trials. For example, Sargent et al21 completed a formal evaluation of disease-free survival versus OS based on 18 randomized trials. They concluded that in adjuvant colon cancer trials of fluorouracil-based regimens, disease-free survival after 3 years of median follow-up is an appropriate endpoint. The relationship between a potential surrogate endpoint and a true endpoint should be assessed on both trial and individual levels.22,23
There may be potential influence of postprogression therapy on our findings. Unfortunately, postprogression therapy data were not fully collected on RTOG 0525. Future analysis may consider whether use of salvage bevacizumab alters the relationship between PFS to initial therapy and OS.
Examination of hazards rather than quantities aggregated over time (ie, survival curves) may reveal information about the time-dependent dynamics of failure and factors related to it. By estimating hazards in separate time intervals, we effectively permit the effects of treatment and prognostic factors on OS and PFS to vary over time. This is in contrast to the clinical reports on this trial, where fixed treatment effect and effect of prognostic factors were estimated. However, even when the effects are not constant over time, the standard analysis of a Cox PH model with a fixed HR provides a reasonable summary that can be considered an average effect over time, unless the hazards are strongly nonproportional or crossing over time. However, this was not the case for this trial.
In summary, hazards over time reveal changes in the risk that may offer clues for both biological and therapeutic research to improve clinical outcomes. While methylated MGMT presents lower risk of death compared with unmethylated MGMT, there remains enough variation in outcomes over time to profile MGMT methylated patients for potential failure risk and identify candidates for adjuvant therapy efforts. Our data would suggest that certain patient subgroups are at considerably greater hazard of progressing early, as opposed to others that progress later. Recent molecular subtyping of GBM reveals 3 or 4 and possibly an even larger number of molecular subgroups; in future work we plan to correlate whether these molecularly defined subgroups are associated with differential hazard for progressing earlier versus later. It would be reasonable to consider different clinical trials for these subgroups of patients, based on molecular subtyping, which might possibly reveal specific pathways to be targeted.
Funding
This project was supported by RTOG grant U10 CA21661 and a Community Clinical Oncology Program grant U10 CA37422 from the National Cancer Institute. This study's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Acknowledgments
Conflict of interest statement. Minesh Mehta has or has had the following roles in the last 2 years: consultant: Abbott, Bristol-Myers Squibb, Elekta, Merck, Novelos, Novocure, Phillips, Roche, Vertex; stock options: Accuray, Pharmacyclics; board of directors: Pharmacyclics; speaker: Merck, Research to Practice. Meihua Wang is an employee of Merck at the time of manuscript submission. Mark Gilbert is a consultant for and received honoraria from Genentech, Merck, and EMD Serono. He received research support from Glaxo Smith Kline, Genentech, and Merck. No conflicts of interests are reported for J.D., M. Won, and W.C.
References
- 1. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007. Source: Central Brain Tumor Registry of the United States, Hinsdale, IL: website: www.cbtrus.org; 2011. [Google Scholar]
- 2. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;3595:492–507. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert M, Wang M, Aldape K, et al. Dose dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawless J. Statistical Models and Methods for Lifetime Data. New York: Wiley; 1982. [Google Scholar]
- 5. Aalen O, Gjessing H. Understanding the shape of the hazard rate: a process point of view. Stat Sci. 2011;16(1):1–22. [Google Scholar]
- 6. Andersen PK, Borgan O, Gill RD, et al. Statistical Models Based on Counting Processes. New York: Springer Verlag; 1993. [Google Scholar]
- 7. Klein J, Moeschberger M. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed.New York: Springer Verlag; 2003. [Google Scholar]
- 8. Muller HG, Wang JL. Locally adaptive hazard smoothing. Probab Theory Relat Fields. 1990;85(4):523–538. [Google Scholar]
- 9. Muller HG, Wang JL. Hazard rate estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. [PubMed] [Google Scholar]
- 10. Muller HG, Wang JL, Capra WB. From life tables to hazard rates: the transformation approach. Biometrika. 1997;84:881–892. [Google Scholar]
- 11. Silverman BW. Density Estimation for Statistics and Data Analysis. London: Chapman and Hall; 1986. [Google Scholar]
- 12. Singpurwalla ND, Wong MY. Estimation of the failure rate—a survey of nonparametric methods. Part I: non-Bayesian methods. Commun Statist-Theor Meth. 1983;12:559–588. [Google Scholar]
- 13. Gray R. Flexible methods for analyzing survival data using splines, with applications to breast cancer prognosis. J Am Statist Assoc. 1992;87:942–951. [Google Scholar]
- 14. Gray R. Hazard regression using ordinary nonparametric regression smoothers. J Comput and Graphical Statist. 1996;5:190–207. [Google Scholar]
- 15. Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 16. Sargent DA. Flexible approach to time-varying coefficients in the Cox regression setting. Lifetime Data Anal. 1997;3:13–25. [DOI] [PubMed] [Google Scholar]
- 17. Hess KR. Assessing time-by-covariate interactions in proportional hazards regression models using cubic spline functions. Stat Med. 1994;13:1045–1062. [DOI] [PubMed] [Google Scholar]
- 18. Ballman K, Buckner J, Brown P, et al. The relationship between six-month progression-free survival and 12-month overall survival endpoints for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 20. Lamborn K, Yung WKA, Chang S, et al. Progression-free survival: an important endpoint in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sargent DF, Wieand S, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. [DOI] [PubMed] [Google Scholar]
- 22. Molenberghs G, Buyse M, Geys H, et al. Statistical challenges in the evaluation of surrogate endpoints in randomized trials. Control Clin Trials. 2002;23:607–625. [DOI] [PubMed] [Google Scholar]
- 23. Buyse M, Molenberghs G. Criteria for the validation of surrogate endpoints in randomized experiments. Biometrics. 1998;54:1014–1029. [PubMed] [Google Scholar]