Abstract
Breakthrough invasive fungal diseases (bIFDs) during voriconazole treatment are concerning, as they are associated with high rates of mortality and pathogen distribution. To evaluate the prevalence, incidence, patient characteristics, including IFD events, and overall mortality of bIFDs during voriconazole treatment for invasive aspergillosis (IA). We retrospectively analyzed the medical records of consecutive patients who had undergone voriconazole treatment for IA and who had bIFD events between January 2011 and December 2015. Eleven bIFD events occurred in 9 patients. The prevalence and incidence of bIFDs were 2.25% (9/368) and 0.22 cases per year, respectively. Overall mortality was 44.4% (4/9). The severity of the illness and persistence of immunodeficiency, mixed infection, and low concentration of the treatment drug at the site of infection were identified as possible causes of bIFDs. Seven of 11 events (63.6%) required continued voriconazole treatment with drug level monitoring. In 4 (36.3%) cases, the treatment was changed to liposomal amphotericin B. Two cases resulted in surgical resection (18.2%). Clinicians should be aware that bIFDs during voriconazole treatment for IA can occur, and active therapeutic approaches are required in these cases.
Keywords: Mycoses, Voriconazole, Aspergillosis, Breakthrough invasive fungal disease
Introduction
Voriconazole is a broad-spectrum antifungal agent that is effective against most Aspergillus species. It is now the initial standard treatment of choice for invasive aspergillosis (IA) in immunocompromised patients.1 A previous report suggested that voriconazole improves clinical responses and survival rates compared to initial treatment with amphotericin B deoxycholate.1 Invasive fungal diseases (IFDs) that occur during the ongoing use of systemic anti-fungal agents indicate an important, unresolved problem in the clinical management of immunocompromised patients.2 Breakthrough infections have been defined as the occurrence of IFDs while the patient is receiving antifungal agents, including agents administered for both prophylaxis and therapy.3 Some studies have reported breakthrough mucormycosis during voriconazole treatment, which is commonly used for mold prophylaxis or as a treatment for invasive IA.4–8 Kontoyiannis et al.8 suggested that previous prophylactic treatment with voriconazole was an independent risk factor for the development of mucormycosis, rather than IA, in patients with hematological malignancies. Another report demonstrated a high overall mortality rate (73%) associated with breakthrough mucormycosis during voriconazole treatment.6
As aforementioned, breakthrough IFDs (bIFDs) such as mucormycosis, candidiasis, and other rare fungal infections during voriconazole treatment are concerning, as they are associated with high rates of mortality and may affect pathogen distribution. There is currently a paucity of clinical study data regarding this topic, and prospective controlled clinical studies have not been feasible.2
Accordingly, the aims of the current study were to evaluate the prevalence, incidence, patient characteristics, and prognosis of bIFDs during voriconazole treatment for IA, and to assess the distributions of pathogens during each bIFD episode. Furthermore, we suggest some therapeutic approaches for the treatment of bIFDs during voriconazole treatment for IA based on our experience with a large cohort from a single center.
Subjects and methods
A cohort of patients aged over 18 years and diagnosed with IA between January 2011 and December 2015 were assessed. We retrospectively analyzed the medical records of consecutive patients who had received voriconazole treatment for IA at Seoul St. Mary's Hospital, The Catholic University, Korea, during in this period. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital, The Catholic University, Korea with a waiver for informed consent (KC16RISI0102).
Definitions
Classification of IA was based on the European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria, which requires positive diagnosis by host, radiologic and microbiologic assessments.9 A positive diagnosis of IA by microbiological assessment required a positive culture of Aspergillus sp. from clinically significant specimens10 and/or the detection of a galactomannan assay index cut-off of ≥0.5 during at least one time point using patient serum. Only probable or proven IFDs according to the revised EORTC/MSG criteria were included in the analysis.9
An IFD was considered to be a bIFD if the (1) causative organism was different from that originally detected before the commencement of voriconazole therapy, (2) occurrence was detected ≥3 days after the initiation of voriconazole therapy, or (3) subsequent infection occurred within 14 days after the discontinuation of voriconazole therapy.4,11 The day of diagnosis was defined as the day on which the first diagnostic procedure identifying the IFD was performed. If two species were cultured at the time of the secondary event but not concurrently, they were defined as two independent infections. A mixed infection was defined as an infection with two or more organisms detected concurrently at the time of the breakthrough event. Cases of worsening IA, which included immune reconstitution inflammatory syndrome (IRIS)12 or idiopathic pneumonitis syndrome,13 were excluded by two independent infectious disease specialists. Neutropenia was defined as an absolute neutrophil count <500 cells/mm3 or an absolute neutrophil count that was expected to be <500 cells/mm3 within 2–3 days.14 Other definitions for neutropenic fever according to Lee et al. were followed.14 Neutropenia duration was defined as the time from initial aspergillosis treatment to the time of bIFD. Overall mortality was defined according to survival within the 3-month follow-up period from when the bIFD was diagnosed.
Voriconazole therapeutic drug monitoring
Serum voriconazole trough levels were measured using high performance liquid chromatography (HPLC) coupled with tandem mass spectrometry (LC-MS/MS), as described previously with minor modifications.15 Following protein precipitation (10 μl of serum plus 40 μl of a zinc sulfate [100 mmol/l] solution, plus 100 μl of acetonitrile, which contained the internal standard, ketoconazole at 1 mg/l), 3 μl of the supernatant was injected into the LC-MS/MS system, which consisted of an Alliance 2795 HT HPLC device (Waters Corp., Milford, MA, USA) and a Quattro Premier XE tandem mass spectrometer (Waters Corp., Milford, MA, USA). Separation was achieved using a SecurityGuard C18 cartridge column (4.0 × 2.0 mm; Phenomenex, Macclesfield, UK). For HPLC, voriconazole was eluted by isocratic flow with 0.1% (v/v) formic acid, dissolved in 75% methanol containing 2 mM of ammonium acetate. The column flow rate was 0.25 ml/min, and the column temperature was maintained at 55 °C. The electrospray ionization source was operated in positive mode with a capillary voltage of 1 kV and a cone voltage of 40 V. Compounds were detected using the multiple reaction monitoring mode using ion transitions of m/z 350.0 → 224.1 for voriconazole and m/z 531.1 → 489.1 for ketoconazole. A 3-point set of lyophilized calibrators, and three concentrations of lyophilized quality control materials from Chromsystems were used, and the linearity of the standard curve ranged 0.1–10 mg/l. Blood sampling was performed at least 4 days after starting therapy, immediately before the subsequent voriconazole dose. The appropriate target range for the voriconazole trough levels was 1–5.5 mg/l.16–18
CYP2C19 genotyping
DNA extracted from buccal swabs was used for CYP2C19 genotyping. CYP2C19 genetic polymorphisms were determined using a Seeplex® CYP2C19 ACE Genotyping system (Seegene, Seoul, Korea) according to the manufacturer's instructions. Briefly, a multiplex polymerase chain reaction system was used to detect CYP2C19*2 and CYP2C19*3 alleles. The CYP2C19*17 allele was identified using polymerase chain reaction-restriction fragment length polymorphism analysis and sequencing as described previously.19 CYP2C19 phenotypic subgroups were classified as follows: homozygous extensive metabolizers (EMs), carriers of 2 wild-type alleles (CYP2C19*1) or 1 wild-type allele and 1 CYP2C19*17 allele; heterozygous extensive metabolizers (HEMs), carriers of 1 null allele and 1 wild-type allele; and poor metabolizers (PMs), carriers of 2 null alleles (most commonly CYP2C19*2 or CYP2C19*3).20,21
Fungus identification
Identification of fungus was performed by automated chemical tests with API 20C; bioMérieux, Marcy l’Etoile, France) (Vitek 2 system; Vitek 2 YST; bioMérieux), followed by visual inspection.22
Results
Patients’ characteristics
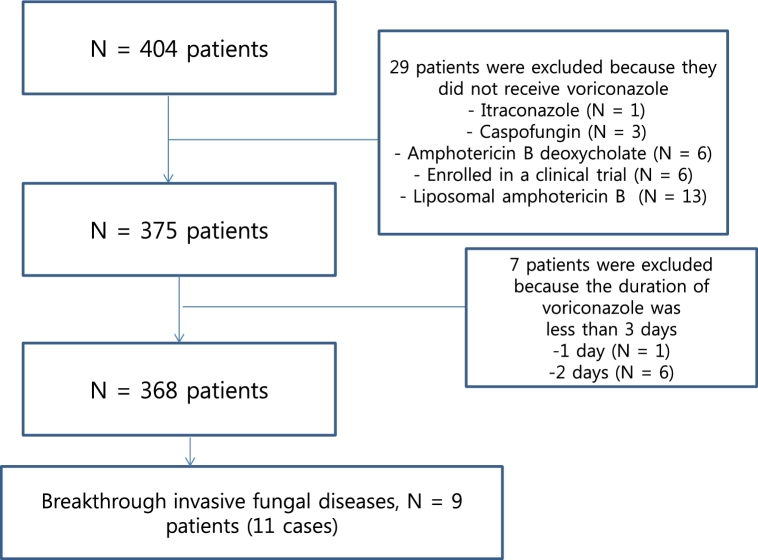
Four hundred and four patients underwent antifungal treatment for probable or proven IA between January 2011 and December 2015. Among them, 51 patients had proven IA, and the remainder (353 patients) had probable IA. Thirty-six patients were excluded, because voriconazole treatment was not provided (n = 29) (Fig. 1) or the duration of voriconazole treatment was less than 3 days (n = 7) (Fig. 1).
Figure 1.
Screening of patients with breakthrough invasive fungal diseases during voriconazole treatment.
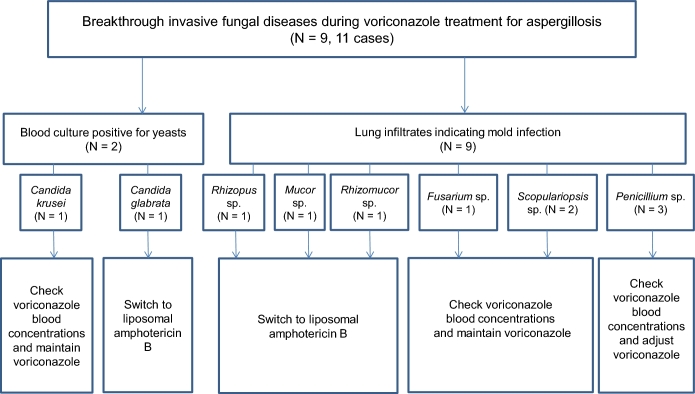
Nine patients had bIFD. Among them, considering the independent cases, total 11 cases of bIFD events occurred as shown in Fig. 2. The prevalence and incidence of bIFDs during the study period were 2.25% (9/368 patients) and 0.22 cases per year, respectively. The clinical and demographic characteristics of patients with bIFDs who received voriconazole for IA are shown in Table 1. The median patient age was 56 years (range 32–74 years), and 77.8% were male. Underlying hematologic diseases affecting these patients included acute myeloid leukemia (6/9 patients, 66.7%) and acute lymphoblastic leukemia (3/9, 33.3%). More than half of the affected patients (5/9, 55.6%) had undergone stem cell transplantation (SCT). Two patients (22.2%) had undergone matched sibling donor bone marrow transplantation (BMT), and this was followed by matched unrelated donor BMT (1/9, 11.1%), matched unrelated donor peripheral blood SCT (1/9, 11.1%) and/or double cord blood transplantation (1/9, 11.1%). Both of these patients received chemotherapy (2/9, 22.2%) and conservative care (2/9, 22.2%). Among the patients with SCT, 80% (4/5) experienced acute or chronic graft-versus-host-disease (GVHD). Of the total IFD patients, 77.8% (7/9) were diagnosed with neutropenia. The median duration of neutropenia was 21.5 days (range 14–52 days) continuously without an event of neutropenic recovery. Of the total IFD patients, 77.8% (7/9) received prednisolone (≥1 mg/kg/day). Voriconazole treatment was indicated due to proven and probable IA in 22.2% (2/9) and 77.8% (7/9) of patients, respectively. The median duration of breakthrough infection after commencement of voriconazole was 33 days (range 3–98 days). CYP2C19 genotyping revealed that 88.9% (8/9) of the total IFD patients had the heterozygous extensive metabolizer genotype. Therapeutic drug monitoring (TDM) analysis showed that almost all of the patients (7/9, 77.8%) received approximately 1–5 mg/L of voriconazole. The sites of bIFDs were the lung (7/9, 77.8%) and bloodstream (2/9, 22.2%).
Figure 2.
Diagram of pathogens and treatment for breakthrough invasive fungal diseases.
Table 1.
Clinical and demographic characteristics of patients with breakthrough invasive fungal diseases who receiving voriconazole for invasive aspergillosis.
| Characteristics | Breakthrough IFD (N = 9) |
|---|---|
| Age, median (range) years | 56 (32–74) |
| Male, no (%) | 7 (77.8%) |
| Underlying disease, no (%) | |
| AML | 6 (66.7%) |
| ALL | 3 (33.3%) |
| Treatment | |
| CTx. | 2 (22.2%) |
| SCT | 5 (55.6%) |
| Conservative care | 2 (22.2%) |
| GVHD, no (%) | 4 (4/5, 80%) |
| Neutropenia < 500/mm3, yes | 7 (77.8%) |
| Duration of neutropenia, median (range) days | 21.5 (14-52) |
| Corticosteroids; prednisolone ≥1 mg/kg/day, no (%) | 7 (77.8%) |
| Indication for voriconazole, no (%) | |
| Proven category | 2 (22.2%) |
| Probable category | 7 (77.8%) |
| Day of breakthrough infection after start of voriconazole, median (range) days | 33 (3-98) |
| CYP2C19 genotype | |
| Homozygous EM, no (%) | 0 (0%) |
| Heterozygous EM, no (%) | 8 (88.9%) |
| PM, no (%) | 0 (0%) |
| Not done, no (%) | 1 (11.1%) |
| Voriconazole TDM (mg/l) | |
| <1 | 1 (11.1%) |
| 1–5 | 7 (77.8%) |
| >5 | 1 (11.1%) |
| Site of breakthrough IFD | |
| Lung | 7 (77.8%) |
| Bloodstream | 2 (22.2%) |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CTx, chemotherapy; SCT, stem cell transplantation; GVHD, graft versus host disease; IA, invasive aspergillosis; EM, extensive metabolizer; PM, poor metabolizer; TDM, therapeutic drug monitoring; IFD, invasive fungal disease.
Possible causes of bIFD during voriconazole treatment
Reference to a previous report and analysis of individual cases23 enabled the determination of possible causes of bIFDs during voriconazole treatment. These were identified as host factor, mixed infection, and low concentration of the drug at the site of infection (Table 2). The host factor was divided into two categories: the severity of the illness and immunodeficiency. The severity of the illness was determined by SCT, which had been performed in 63.6% (7/9) of patients. Immunodeficiency was identified by prolonged neutropenia in 54.5% (6/11) of cases, GVHD in 36.4% (4/11) of cases, and corticosteroid use in 81.8% (9/11) of cases. Of the total IFD cases, 9.1% (1/11) involved mixed infection at the time of diagnosis with species such as Aspergillus sp. and Rhizomucor sp. Concerning the low concentration of the drug at the site of infection, 9.1% of the total cases (1/11) had a low TDM level (trough level <1 mg/l). Moreover, 9.1% of cases (1/11) were determined to involve a poor vascular supply. For example, patient 9 had an abscess and necrotic tissue, which originated from a liver abscess.
Table 2.
Possible causes of breakthrough Invasive fungal diseases during voriconazole treatment.
| Causes of antifungal therapy failure | N = 11 (%) |
|---|---|
| Host factor | |
| Severity of illness | |
| SCT, yes | 7 (63.6%) |
| Persistence of immunodeficiency (e.g. neutropenia or use of corticosteroids) | |
| Prolonged neutropenia | 6 (54.5%) |
| GVHD | 4 (36.4%) |
| Receipt of corticosteroids (prednisolone | 9 (81.8%) |
| ≥1 mg/kg/day) | |
| Mixed infection with other invasive fungal infection | 1 (9.1%) |
| Low concentration of the drug at the site of infection | |
| Pharmacokinetic and pharmacodynamic | 1 (9.1%) |
| (trough level <1 mg/l) | |
| Poor vascular supply (e.g., abscess and necrotic | 1 (9.1%) |
| tissue) |
Abbreviations: SCT, stem cell transplantation; GVHD, graft versus host disease.
Management and outcome
The management of all bIFD cases at our center is shown in Table 3. Seven of 11 cases (63.6%) required continued voriconazole treatment, and weekly TDM of voriconazole levels. Four cases (36.3%) required a change of medication to liposomal amphotericin B (3–5 mg/kg/day). Moreover, 2 of 11 cases (18.2%) underwent surgical resection, including lobectomy of the lung.
Table 3.
Management of breakthrough invasive fungal diseases.
| Management | Total (N = 11) (%) |
|---|---|
| Medical management | |
| Voriconazole maintenance with TDM, no (%) | 7 (63.6%) |
| Change to liposomal amphotericin B, no (%) | 4 (36.3%) |
| Surgical management | |
| Surgery (Lobectomy), no (%) | 2 (18.2%) |
Abbreviation: TDM, Therapeutic drug monitoring.
Detailed patient characteristics, including preexisting medical conditions, and progress after bIFD diagnosis, are shown in Table 4. The bIFD events per patient are described using patient numbers (i.e., 1, 2, 3, 4-1, 4-2, 5-1, 5-2, 6, 7, 8, and 9); numbers 4-1 and 4-2 represent two episodes of bIFD in the same patient. The primary site of IA was the lung (10/11 cases, 90.9%). There was a single case of infection primarily affecting the liver (1/11 cases, 9.1%). The causative bIFD pathogens varied and included Rhizopus sp., Fusarium sp., Penicillium sp., Scopulariopsis sp., Candida krusei, Rhizomucor sp., Candida glabrata, and Mucor sp. The treatment duration after bIFDs was 44 days (range 6–108 days). The overall mortality rate of patients within the 3 months following bIFDs was 44.4% (4/9). During the period of bIFDs, there were three incidences of bacterial coinfection with Enterococcus faecalis, Stenotrophomonas maltophilia, and Escherichia coli.
Table 4.
Outcome of breakthrough invasive fungal diseases.
| Medical condition before breakthrough IFD | Characteristics and progress of breakthrough IFD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt.-case | Gender/Age | Underlying disease and Tx. | Indication for VOR (organ) | Duration of VOR Tx. before breakthrough IFD (days) | Site of breakthrough IFD | Fungal Organism | Management | Tx. duration after breakthrough IFD (days) | Co-existing bacteremia | Outcome |
| 1 | M/52 | AML s/p MUD BMT | Probable (Lung) | 13 | Lung | Rhizopus sp. | Change to L-AMB | 6 | Death | |
| 2 | M/70 | AML, conservative care | Probable (Lung) | 4 | Lung | Fusarium sp. | Maintain VOR w/ TDM | 52 | Survival | |
| 3 | M/43 | ALL s/p MSD BMT | Proven (Lung) | 44 | Lung | Penicillium sp. | Maintain VOR w/ TDM | 65 | Survival | |
| 4-1 | M/47 | ALL s/p MUD PBSCT | Probable (Lung) | 22 | Lung | Scopulariopsis brevicaulis | Maintain VOR w/ TDM | 108 | Survival | |
| 4-2 | – | – | – | 98 | Lung | Penicillium sp. | Maintain VOR w/ TDM | 68 | – | |
| 5-1 | F/32 | ALL s/p MSD BMT | Probable (Lung) | 16 | Lung | Scopulariopsis sp. | Maintain VOR w/ TDM | 69 | Death | |
| 5-2 | – | – | – | 53 | Lung | Penicillium sp. | Maintain VOR w/ TDM | 7 | – | |
| 6 | M/74 | AML, conservative care | Probable (Lung) | 3 | Bloodstream | Candida krusei | Maintain VOR w/ TDM | 9 | Enterococcus faecalis | Death |
| 7 | M/37 | AML s/p DCBT | Probable (Lung) | 92 | Lung |
Rhizomucor sp. & Aspergillus sp. |
Change to L-AMB & lobectomy | 36 | Survival | |
| 8 | M/56 | AML s/p Consolidation CTx | Probable (Lung) | 33 | Bloodstream | Candida glabrata | Change to L-AMB | 21 |
Stenotrophomas maltophilia &
Escherichia coli |
Death |
| 9 | F/63 | AML s/p Consolidation CTx | Proven (Liver) | 90 | Lung | Mucor sp. | Change to L-AMB & lobectomy | 44 | Survival | |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblast leukemia; MUD, matched unrelated donor; MSD, matched sibling donor; BMT, bone marrow transplantation; PBSCT, peripheral blood stem transplantation; DCBT, double cord blood transplantation; sp, species, Pt, patient; CTx., chemotherapy; Tx., treatment; w/, with; TDM, therapeutic drug monitoring; VOR, voriconazole; L-AMB, liposomal amphotericin B.
Discussion
Breakthrough IFDs (bIFD) have previously been reported among immunocompromised patients who have received systemic antifungal agents, including azole drugs such as fluconazole, itraconazole, or echinocandin.2,6,24–28 However, current data concerning the prevalence, incidence, and patient characteristics of bIFD, especially due to IA, in those receiving voriconazole, are limited.
The present study describes the characteristics of patients and clinical management of bIFD episodes at a single medical center over a period of 5 years. There were several interesting findings in our retrospective study that will aid the implementation of improved treatment protocols for overall IFD in the future. The analysis demonstrated a bIFD prevalence of 2.25% and an incidence of 0.22 cases per year. The overall mortality after bIFDs was 44.4%. The overall mortality in our study was lower than that reported in a previous study assessing breakthrough mucormycosis during voriconazole treatment (73%).6
When Fusarium sp. or Penicillium sp. were grown as bIFD pathogens, TDM of voriconazole was useful for determining whether further treatment was needed. However, it was difficult to determine the optimal treatment in some cases. Two similar episodes occurred in patients 4 and 5, whereby the initial cultures grew Scopulariopsis sp. and the follow-up cultures grew Penicillium sp. Infection caused by Penicillium sp. is most commonly due to P. marneffei. In our study, Penicillium sp. was cultured from bronchoalveolar lavage and adequate sputum specimens during several independent assessments, and considering the patient's rapidly declining clinical situation, we regarded Penicillium sp. as a pathogen.29 Interestingly, patient 4 survived, but patient 5 died of post-operative bleeding after a heart-lung transplantation due to bronchiolitis obliterans, and this was independent of the bIFD. There were also two interesting cases where Candida sp. growth was observed (patients 6 and 8). C. krusei was cultured from a blood sample obtained from patient 6, and voriconazole was maintained using TDM. Subsequently, the patient died. However, he had coexisting E. faecalis bacteremia, and thus the cause of death was unclear. Based on this case, when a blood culture results in C. glabrata growth as bIFD, voriconazole should be changed to liposomal amphotericin B immediately. Patient 8 also died and had coexisting S. maltophilia bacteremia. Catheters were not removed in either of these cases. However, it was thought that catheter-related bacteremia was an unlikely cause, as the differential time to positivity was negative. Considering these candidemia episodes retrospectively, removal of the catheters may have been beneficial, regardless of the differential time to positivity. A respiratory specimen from patient 1 yielded Rhizopus sp. Although voriconazole was changed to liposomal amphotericin B treatment, his clinical status rapidly deteriorated, and he died within 3 days after starting treatment with liposomal amphotericin B. In contrast, Rhizomucor sp. was cultured from the respiratory tract of patient 7 after an onset period of over 90 days. Despite voriconazole being replaced with liposomal amphotericin B for the adequate treatment of bIFD, and treatment being administered for over 2 weeks, his lesion did not improve. Therefore, surgery, including lobectomy of the lung, was performed. Subsequently, he survived and received further liposomal amphotericin B treatment for more than 3 weeks. Specimen pathology confirmed IA. In this case, the aspergillosis persisted, and newly developed Rhizomucor sp. infection occurred. It was therefore thought that these fungi were concurrently infecting the patient. Patient 9's case was also of interest. Although she had IA affecting the liver and was treated with voriconazole for over 10 weeks, bIFD occurred in her lung after a prolonged period. Considering this rare pathogen, surgery, including lobectomy of the lung, was performed. Specimen pathology confirmed mucormycosis, and therefore, liposomal amphotericin B treatment was initiated. After 44 days, liposomal amphotericin B was discontinued. The patient required ongoing voriconazole treatment for hepatic IA, as at the time of the last consultation, her immunodeficiency and liver aspergillosis had persisted.
There were a number of limitations in this study. First, it was a small retrospective study performed only at a single center. Second, precise identification of the fungal pathogens using sequencing methods was not possible, and antifungal agent sensitivity tests were not performed. Third, the duration of some breakthrough events were short. In these cases, it was not possible to determine whether the newly detected fungal species had grown concurrently with Aspergillus sp., or whether Aspergillus sp. alone was present before confirmation by autopsy. Fusarium sp. breakthrough events were more difficult to confirm during this short time, as cross-reactivity with Aspergillus sp. can occur using galactomannan assays. Therefore, a consensus on the definition of bIFD like that for IFD established by the EORTC/MSG IFD is required.30
Nevertheless, this study has some important educational results. First, the persistence of poor patient immunity or immunodeficiency, despite prolonged treatment with voriconazole for IA using TDM, may be indicative of breakthrough IA. Immunodeficiency can commonly be identified by prolonged neutropenia, GVHD, or strong immunosuppression. Clinicians should be mindful of bIFD, instead of simply diagnosing the patient with IRIS or worsening IA. Second, our experiences support the use of TDM to adjust voriconazole doses, or alter treatment to a broad-spectrum agent such as liposomal amphotericin B when required. Third, further confirmation of the diagnosis may be required, in combination with consideration for surgery, such as lobectomy of the lung. The current Infectious Diseases Society of America guidelines recommend surgery for histological diagnoses, the removal of residual infiltrates prior to subsequent chemotherapy cycles, acute hemoptysis, the prevention of bleeding where fungal lesions have vessel involvement, and the reduction of fungal burden.31 We suggest adding an indication for surgery: symptoms suggestive of bIFDs.
Clinicians should be aware that bIFDs can occur during appropriate voriconazole treatment using TDM. Clinicians may need to employ active therapeutic approaches without hesitation to resolve bIFDs.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Herbrecht R, Denning DW, Patterson TF et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002; 347: 408–415. [DOI] [PubMed] [Google Scholar]
- 2. Maschmeyer G, Patterson TF. Our 2014 approach to breakthrough invasive fungal infections. Mycoses 2014; 57: 645–651. [DOI] [PubMed] [Google Scholar]
- 3. Nailor MD, Chandrasekar PH. Treatment of breakthrough fungal infections: is there one best drug strategy? Curr Fungal Infect Rep 2009; 3: 229–235. [Google Scholar]
- 4. Imhof A, Balajee SA, Fredricks DN et al. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis 2004; 39: 743–746. [DOI] [PubMed] [Google Scholar]
- 5. Marty FM, Cosimi LA, Baden LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Engl J Med 2004; 350: 950–952. [DOI] [PubMed] [Google Scholar]
- 6. Trifilio SM, Bennett CL, Yarnold PR et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant 2007; 39: 425–429. [DOI] [PubMed] [Google Scholar]
- 7. Vande Broek I, Schots R. Fatal cerebral zygomycosis breakthrough in a patient with acute lymphoblastic leukemia on voriconazole prophylaxis after cord blood SCT. Bone Marrow Transplant 2009; 44: 765–766. [DOI] [PubMed] [Google Scholar]
- 8. Kontoyiannis DP, Lionakis MS, Lewis RE et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis 2005; 191: 1350–1360. [DOI] [PubMed] [Google Scholar]
- 9. De Pauw B, Walsh TJ, Donnelly JP et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horvath JA, Dummer S. The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am J Med 1996; 100: 171–178. [DOI] [PubMed] [Google Scholar]
- 11. Lerolle N, Raffoux E, Socie G et al. Breakthrough invasive fungal disease in patients receiving posaconazole primary prophylaxis: a 4-year study. Clin Microbiol Infect 2014; 20: 952–959. [DOI] [PubMed] [Google Scholar]
- 12. Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11: 275–288. [DOI] [PubMed] [Google Scholar]
- 13. Shankar G, Cohen DA. Idiopathic pneumonia syndrome after bone marrow transplantation: the role of pre-transplant radiation conditioning and local cytokine dysregulation in promoting lung inflammation and fibrosis. Int J Exp Pathol 2001; 82: 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee DG, Kim SH, Kim SY et al. Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Intern Med 2011; 26: 220–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keevil BG, Newman S, Lockhart S et al. Validation of an assay for voriconazole in serum samples using liquid chromatography-tandem mass spectrometry. Ther Drug Monit 2004; 26: 650–657. [DOI] [PubMed] [Google Scholar]
- 16. Pascual A, Calandra T, Bolay S et al. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis 2008; 46: 201–211. [DOI] [PubMed] [Google Scholar]
- 17. Kim SH, Lee DG, Kwon JC et al. Clinical Impact of Cytochrome P450 2C19 Genotype on the treatment of invasive aspergillosis under routine therapeutic drug monitoring of voriconazole in a Korean population. Infect Chemother 2013; 45: 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim SH, Yim DS, Choi SM et al. Voriconazole-related severe adverse events: clinical application of therapeutic drug monitoring in Korean patients. Int J Infect Dis 2011; 15: e753–758. [DOI] [PubMed] [Google Scholar]
- 19. Baldwin RM, Ohlsson S, Pedersen RS et al. Increased omeprazole metabolism in carriers of the CYP2C19*17 allele; a pharmacokinetic study in healthy volunteers. Br J Clin Pharmacol 2008; 65: 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mikus G, Scholz IM, Weiss J. Pharmacogenomics of the triazole antifungal agent voriconazole. Pharmacogenomics 2011; 12: 861–872. [DOI] [PubMed] [Google Scholar]
- 21. Li-Wan-Po A, Girard T, Farndon P et al. Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol 2010; 69: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim MN, Shin JH, Sung H et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin Infect Dis 2009; 48: e57–61. [DOI] [PubMed] [Google Scholar]
- 23. Nucci M, Perfect JR. When primary antifungal therapy fails. Clin Infect Dis 2008; 46: 1426–1433. [DOI] [PubMed] [Google Scholar]
- 24. Kabbara N, Lacroix C, Peffault de Latour R et al. Breakthrough C. parapsilosis and C. guilliermondii blood stream infections in allogeneic hematopoietic stem cell transplant recipients receiving long-term caspofungin therapy. Haematologica 2008; 93: 639–640. [DOI] [PubMed] [Google Scholar]
- 25. Mann PA, McNicholas PM, Chau AS et al. Impact of antifungal prophylaxis on colonization and azole susceptibility of Candida species. Antimicrob Agents Chemother 2009; 53: 5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfeiffer CD, Garcia-Effron G, Zaas AK et al. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol 2010; 48: 2373–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Auberger J, Lass-Florl C, Aigner M et al. Invasive fungal breakthrough infections, fungal colonization and emergence of resistant strains in high-risk patients receiving antifungal prophylaxis with posaconazole: real-life data from a single-centre institutional retrospective observational study. J Antimicrob Chemother 2012; 67: 2268–2273. [DOI] [PubMed] [Google Scholar]
- 28. Kang SH, Kim HS, Bae MN et al. Fatal breakthrough mucormycosis in an acute myelogenous leukemia patient while on posaconazole prophylaxis. Infect Chemother 2015; 47: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lyratzopoulos G, Ellis M, Nerringer R et al. Invasive infection due to penicillium species other than P. marneffei. J Infect 2002; 45: 184–195. [DOI] [PubMed] [Google Scholar]
- 30. Tortorano AM, Esposto MC, Prigitano A et al. Cross-reactivity of Fusarium spp. in the Aspergillus Galactomannan enzyme-linked immunosorbent assay. J Clin Microbiol 2012; 50: 1051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mousset S, Buchheidt D, Heinz W et al. Treatment of invasive fungal infections in cancer patients-updated recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 2014; 93: 13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]