Abstract
We investigated a cluster of 10 Burkholderia cepacia complex-positive cultures among ventilated patients and those with a tracheostomy in an acute care hospital. Isolates from 5 patients had outbreak-strain–related Burkholderia contaminans. Isolates of B. cepacia complex unrelated to the outbreak strain were cultured from a sink drain. The investigation identified practices that might have led to contamination of patient respiratory care supplies with tap water, which might have contributed to the cluster.
Keywords: Burkholderia cepacia complex, Infection control, Ventilation, Mechanical
Burkholderia cepacia complex (BCC) comprises opportunistic pathogens commonly causing respiratory colonization and infection among patients with cystic fibrosis (CF)1 and increasing their morbidity and mortality.2 Health care-associated outbreaks from intrinsically and extrinsically contaminated medical products or inadequate infection control practices also have been reported.3–7 Wet environments, sinks, and possibly tap water, are a frequent venue.5
During January–July 2011, a total of 8 BCC cases were identified among non-CF patients in intensive care units at an acute care hospital; 7 were from the surgical intensive care unit (SICU). All patients were ventilated or had had a tracheostomy before the infection occurred. Despite control measures, 2 additional cases were identified in September and October. Concerned about ongoing transmission and potential spread to other vulnerable patient populations, the hospital, the state health department, and the Centers for Disease Control and Prevention initiated an investigation.
METHODS
A case was defined as first detection of B cepacia in a clinical culture from a non-CF patient obtained >2 days after admission to Hospital A during the period January 1–October 31, 2011. An outbreak case met the case definition plus identification as Burkholderia contaminans genetically related to the outbreak strain by repetitive element-polymerase chain reaction (rep-PCR). BCC typing of non-CF patient isolates was not performed before 2011. To evaluate BCC incidence, identify additional cases, and establish background rates among non-CF patients at Hospital A, microbiology records were reviewed to find all B cepacia-positive cultures for January 1, 2009–October 31, 2011. Medical records reviews were conducted of patients with outbreak-strain–related BCC. Records were abstracted for health care exposures during the 2-week window before the first positive culture.
Previous to this investigation, Hospital A collected and tested specimens from patient ventilator components, bronchoscopes, and bronchoscope carts. Environmental specimens, including high-touch surfaces, tap water, sinks, faucet aerators, and chlorhexidine mouthwash, were collected from BCC-related SICU rooms. Surface samples were taken using Sponge-Sticks (3M, St Paul, MN) that are premoistened with neutralizing buffer. Water from taps and from in-room toilets was placed in water collection bottles containing sodium thiosulfate to neutralize any chlorine residuals present in the tap water.
Investigators interviewed and observed health care workers, environmental cleaning, and respiratory equipment disinfection procedures, and reviewed adherence to hand hygiene and isolation precautions.
Clinical isolates were sent to the University of Michigan’s Research Laboratory and Repository. Species-level isolates identification was performed (16S rRNA, recA PCR, and recA restriction fragment length polymorphism analysis), and isolates were genotyped (rep-PCR by BOX A1R primer). Environmental specimens from the investigation were sent to the Centers for Disease Control and Prevention for culture, isolation, and identification. Genetic relatedness of clinical and environmental BCC isolates were compared by pulsed field gel electrophoresis (PFGE); interpretations were based on Tenover’s criteria8 and analysis by BioNumerics software (Applied Maths, Austin, TX).4
RESULTS
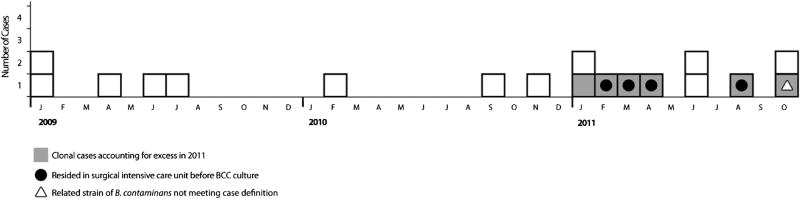
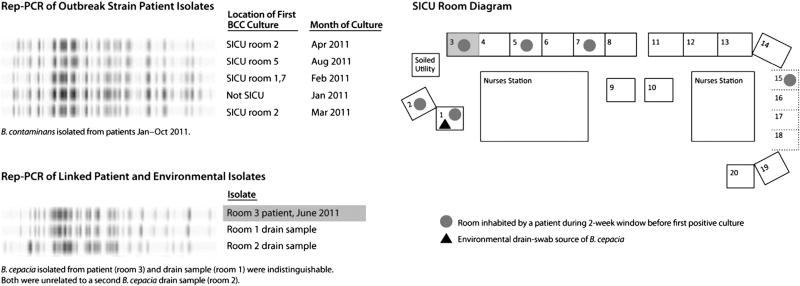
Eighteen BCC isolates were detected at Hospital A during the period January 2009–October 2011 (Fig 1). Before 2011, an average of 4 BCC-positive cultures per year occurred at Hospital A. Ten isolates were detected during 2011: 5were indistinguishable by rep-PCR and by PFGE, and identified as B contaminans outbreak strain; 1 additional isolate of related B contaminans was from an outpatient and did not meet the case definition; the remaining 4 were Burkholderia stabilis, Burkholderia cenocepacia, B cepacia, and unrelated B contaminans. All 5 cases of outbreak strain B contaminans (Fig 2) were detected in respiratory cultures, and patients had signs present at time of culture consistent with infection (fever [60%], tachycardia [100%], hypotension [60%], or leukocytosis [60%]). Four had been admitted to the SICU before the first positive B contaminans culture. SICU outbreak infections were among men aged 45–74 years who had an 87-day average length of stay, required mechanical ventilation (mean 8.8 days) before culture, and were exposed to surgery and bedside procedures (eg, bronchoscope-assisted tracheostomy placement). During the 2 weeks before the first positive culture, all 4 patients had a minimum of 2 central lines and 1 gastrointestinal tube; 3 received total parenteral nutrition. Environmental specimens from ventilator components and bronchoscopes were negative for BCC. B cepacia was cultured from 2 sink-drain swabs from rooms previously occupied by multiple patients with BCC. The environmental specimen isolated from room 1, obtained November 2011, was indistinguishable by rep-PCR (Fig 2) from B cepacia isolated from a patient residing in room3 during June 2011, andwas closely related by PFGE (94% similarity). An environmental isolate of B cepacia from room 2 was unrelated (>7 band differences) by PFGE or rep-PCR.
Fig 1.
Epidemiologic curve of cases, by month of first positive Burkholderia cepacia complex (BCC) culture. BCC isolate typing at Hospital A began in January 2011. Before January 2011, isolates from patients without cystic fibrosis were identified using BCC selective agar.
Fig 2.
Repetitive element-polymerase chain reaction (rep-PCR) profile of clinical and environmental isolates and room diagram of surgical intensive care unit (SICU), patient, and environmental sampling location.
Infection control review revealed nonadherence to hand hygiene and isolation precautions, lapses in respiratory therapy procedures, and in cleaning and disinfection of shared equipment. Practices included rinsing nebulizer cups with tap water and storing them wet. Because of limited counter space in SICU rooms, patient care products were observed beside the sink where they were at risk for splashes.
DISCUSSION
On the basis of our findings, immediate changes were implemented in respiratory therapy procedures and trainings, and hand hygiene protocols were examined. Although not required for immunocompetent patient areas, Hospital A removed all aerators from SICU manual and automatic sinks because of surgical patients’ vulnerability. In-room carts were provided to eliminate the need to store supplies near sinks. No further episodes of BCC were identified in the SICU during the period October 2011–May 2012.
CONCLUSIONS
We report 5 B contaminans outbreak-strain infections; 4 were among ventilated SICU patients, detected during a 10-month period. Outbreak-related infections appear to account for BCC excess during 2011. Indistinguishable (by rep-PCR) B cepacia isolates from an SICU patient and sink drain indicate tap water or patient material poured down the drain as possible sources for transmission. Inadequate drying of tap-water–rinsed nebulizer components used for medication administration and proximity of stored patient supplies to sinks might have facilitated exposure. In small-scale epidemiology studies, PFGE might be more discriminating, and rep-PCR might indicate more population-level relatedness. 9 Our PFGE results might reflect changes in the local population of B cepacia occurring during the 3 months between patient and environmental sampling. Limitations of this investigation include our inability to perform environmental sampling during the time the majority of cases were identified and the low sensitivity of environmental cultures.
Given investigator-observed lapses in hand hygiene and in shared equipment cleaning and disinfection, nosocomial transmission possibly occurred. In previous health care outbreaks, tap water has been implicated.5, 7 This investigation highlights the potential for safe practices to prevent BCC transmission. Diminishing tap water contamination of patient respiratory products and improved basic infection control practices were associated with cluster termination.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). This investigation underwent review by CDC’s Scientific Education and Professional Development Program Office human subjects’ protection coordinator and was determined to be public health response, which is nonresearch.
Footnotes
Conflicts of interest: None to report.
References
- 1.Mahenthiralingam E, Baldwin A, Dowson CG. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol. 2008;104:1539–51. doi: 10.1111/j.1365-2672.2007.03706.x. [DOI] [PubMed] [Google Scholar]
- 2.de Vrankrijker AM, Wolfs TF, van der Ent CK. Challenging and emerging pathogens in cystic fibrosis. Paediatr Respir Rev. 2010;11:246–54. doi: 10.1016/j.prrv.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Dolan SA, Dowell E, LiPuma JJ, Valdez S, Chan K, James JF. An outbreak of Burkholderia cepacia complex associated with intrinsically contaminated nasal spray. Infect Control Hosp Epidemiol. 2011;32:804–10. doi: 10.1086/660876. [DOI] [PubMed] [Google Scholar]
- 4.Kutty PK, Moody B, Gullion JS, Zervos M, Ajluni M, Washburn R, et al. Multistate outbreak of Burkholderia cenocepacia colonization and infection associated with the use of intrinsically contaminated alcohol-free mouthwash. Chest. 2007;132:1825–31. doi: 10.1378/chest.07-1545. [DOI] [PubMed] [Google Scholar]
- 5.Lucero CA, Cohen AL, Trevino I, Rupp AH, Harris M, Korkan-Kelly S, et al. Outbreak of Burkholderia cepacia complex among ventilated pediatric patients linked to hospital sinks. Am J Infect Control. 2011;39:775–8. doi: 10.1016/j.ajic.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Martin M, Christiansen B, Caspari G, Hogardt M, von Thomsen AJ, Ott E, et al. Hospital-wide outbreak of Burkholderia contaminans caused by prefabricated moist washcloths. J Hosp Infect. 2011;77:267–70. doi: 10.1016/j.jhin.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Nasser RM, Rahi AC, Haddad MF, Daoud Z, Irani-Hakime N, Almawi WY. Outbreak of Burkholderia cepacia bacteremia traced to contaminated hospital water used for dilution of an alcohol skin antiseptic. Infect Control Hosp Epidemiol. 2004;25:231–9. doi: 10.1086/502384. [DOI] [PubMed] [Google Scholar]
- 8.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Pershing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coenye T, Spilker T, Martin A, LiPuma JJ. Comparative assessment of genotyping methods for epidemiologic study of Burkholderia cepacia genomovar III. J Clin Microbiol. 2002;40:3300–7. doi: 10.1128/JCM.40.9.3300-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]