Abstract
Alpha-7 nicotinic acetylcholine receptor (α7 nAChR) agonists attenuate pain and inflammation in preclinical models. This study tested whether systemic delivery of an α7 nAChR agonist attenuates neuropathic pain and associated immune-mediated pro-inflammation. Hind paw response thresholds to mechanical stimuli in male Sprague Dawley rats were assessed before and after sciatic chronic constriction injury (CCI) or sham surgery. Osmotic mini-pumps containing TC-7020, an α7 nAChR selective agonist, were implanted 10 to 14 days after surgery. TC-7020 (1, 3, and 10 mg/kg/d; s.c.) significantly attenuated CCI-induced allodynia, which lasted through 2 weeks of test compound administration. Spinal cords were collected after 2 weeks and processed for microglial and astrocyte activation markers within the ipsilateral L4-L6 dorsal horn. In addition, ipsilateral L4-5 dorsal root ganglia (DRGs) were processed for neuronal injury and satellite cell activation markers. CCI-induced central glial cell activation markers were not suppressed by TC-7020, even though TC-7020 is mildly blood-brain barrier permeable. However, TC-7020 downregulated the integrated density of activation transcription factor 3 (ATF3) but not the number of ATF positive cells. TC-7020 also downregulated phosphorylated extracellular signal kinase (p-ERK) and satellite cell activation in the CCI-affected DRGs. Therefore, systemic α7 nAChR agonist may be effective in treating neuropathic pain via reducing neuronal injury and immune cells activation occurring in the periphery.
Perspective
These studies demonstrated that TC-7020, an alpha7 nicotinic acetylcholine receptor agonist with partial blood-brain barrier permeability, reversed neuropathic pain in rats, likely via attenuation of inflammation in the DRG and/or the site of sciatic injury.
Keywords: Glial activation, dorsal root ganglia, chronic constriction injury, allodynia, antinociception, α7 nAchR agonist
Neuropathic pain remains a major health issue affecting at least 4 million people in the US alone with as yet no effective treatment available.58 Novel therapeutic targets for the treatment of neuropathic pain continue to be identified with a new line of development targeting neuroimmune interactions rather than targeting neurons only.
While the generation of pain from peripheral neuropathy originates from peripheral nerve injury/inflammation, immune cells present both in the periphery and within the central nervous system are increasingly being recognized as important contributors to neuropathic pain.3,17,64 Glial cells within the spinal cord significantly contribute to both the development and maintenance of neuropathic pain. Microglia and, to a lesser extent, astrocytes are the immunocompetent cells within the central nervous system.12,39,64 Upon activation, as occurs in neural insult, injury or inflammation, these glial cells become activated and release pro-inflammatory products including pro-inflammatory cytokines, reactive oxygen species, nitric oxide, and chemokines.64 Drugs that attenuate glial activation and their pro-inflammatory products have been shown to resolve neuropathic pain.14,38,39,47,55
Upon nerve injury or inflammation, peripheral immune cells are recruited to the site of injury and resident satellite cells within the affected dorsal root ganglia (DRG) become activated.40 These activated immune cells produce pro-inflammatory cytokines and may be critical in the development of chronic pain.40 Research is now recognizing the complex integration of both peripheral and central immune cells and neurons in the development and maintenance of neuropathic pain.3,12,52 One mechanism whereby multiple sites involved in pain pathways may be addressed in parallel is through alpha-7 nicotinic acetylcholine receptor (α7 nAChR) activation. There are 16 known subunits of nicotinic acetylcholine receptors found both in the periphery and within the central nervous system. Of these, α7 nAChRs are found abundantly within the central nervous system, in particular on microglia, and in the periphery on both neurons and on peripheral immune cells.48,62 In addition, agonism of these receptors results in reduction of pro-inflammatory products both within the periphery and within the central nervous system.6,7,30
Indeed,α7 nAChR agonists have shown good potential for suppressing numerous inflammatory-mediated pathologies in the periphery and centrally, ranging from ulcerative colitis, asthma, arthritis, and Parkinson's disease.4 Drugs that attenuate both peripheral and central inflammation, such as α7 nAChR agonists have been demonstrated to do, would be good therapeutic agents for the treatment of neuropathic pain.30,50,51
We have shown previously that intrathecal administration of a compound having α7 nAChR agonist activity attenuated intrathecal gp120-induced allodynia and attenuated pro-inflammatory cytokine mRNA and protein, most likely produced by glia.30 In addition, others have shown that systemic delivery of an α7 nAChR agonist attenuated inflammatory-mediated pain50,51,63 and intrathecal delivery reduced neuropathic pain.2,46 In order to determine whether systemic administration of a selective α7 nAChR agonist can also attenuate neuropathic pain, we administered a novel α7 nAChR agonist TC-702032 via subcutaneous osmotic mini-pump in rats with chronic constriction injury (CCI). Additionally, we aimed to identify if systemic TC-7020 would suppress glial activation within the spinal cord, which is known to occur in the CCI model of neuropathic pain.32,39 Since α7 nAChR agonists have demonstrated anti-inflammatory effects in the periphery,4 we evaluated the effect of the α7 agonist TC-7020 on DRG affected by CCI for both neuronal markers of stress and the surrounding satellite cells.
Methods
Subjects
Pathogen-free adult male Sprague Dawley rats (325–400 g; Harlan Labs, Madison, WI) were used in the behavioral experiment. Before experimental manipulations, rats were allowed 1 week to acclimate to the University of Colorado animal care facility. Rats were housed 2 per cage in temperature (23 ± 3°C)- and light (12:12 light:dark; lights on at 0700 hour)-controlled rooms with standard rodent chow and water available ad libitum. All procedures were performed during the lights on period of the day. A post hoc satellite study to determine pharmacokinetic/pharmacodynamic (PK/PD) correlations with the behavioral data generated under the 3 mg/kg/day TC-7020 dose condition at 1 day (24 hours), 3 days (72 hours), and 14 days (2 weeks) after mini-pump insertion was performed at Targacept, Inc. using adult male Sprague Dawley rats (325–340 g; Charles River Labs, Raleigh, NC). Acclimation, animal husbandry, and environmental controls were similar to those described above for the efficacy study. The procedures using live animals were approved by the Institutional Animal Care and Use Committees of the University of Colorado at Boulder and Targacept, Inc. for the pain and satellite PK studies, respectively. The care and use of the animals at each institution also conformed to guidelines of the Office of Laboratory Animal Welfare, the International Association for the Study of Pain, and applicable sections of the USDA Animal Welfare Act.
Drugs for In Vivo Behavioral Testing
The dose of TC-7020 was calculated as a free base equivalent and formulated in sterile saline the day of pump insertion. In order to deliver the required dose by the end of the study rather than at the beginning of the study, an estimated final weight for each rat was calculated based on a projected weight gain of 3 g per day (based on information from Harlan Laboratory growth curve) for the 2-week delivery regimen and averaged. TC-7020 was dissolved in sterile saline and administered via osmotic minipump (14 day, .5 (μL/h; Alzet, Cupertino, CA) at doses of 1 mg/kg/day, 3 mg/kg/, or 10 mg/kg/day. The doses used were based on pilot studies and previous experiments showing 3 mg/kg/day to be effective in attenuating hyperalgesia. Animals in the vehicle treatment group were implanted with mini-pumps containing only saline. The rats' final weight at the end of the study was within 10% of the estimated body weight.
Experimental Design for Neuropathic Pain Resolution and Tissue Analyses
Three doses of TC-7020 (1, 3, and 10 mg/kg/day) and a vehicle group were tested in CCI-induced allodynic rats and the 3 mg/kg/day dose was tested in sham rats (n = 6 per group). Ten to fourteen days after CCI surgery, the rats underwent brief isoflurane anesthesia and each had a preloaded, subcutaneous osmotic mini-pump implanted. Prior to mini-pump insertion, behavioral testing was conducted before CCI/sham surgery, 4 and 10 days after surgery. After pump insertion (# of days following surgery), behavior was assessed at 3 and 24 hours, and at 3, 7, and 14 days. Immediately after the last behavioral assessment on day 14, the rats were deeply anesthetized with sodium pentobarbital, cardiac blood was collected into an EDTA tube, and each rat was transcardially perfused with ice-cold saline, followed by fresh 4% paraformaldehyde. The spinal cord and DRG of L4-6 were dissected from each subject and post fixed for 6 hours in 4% paraformaldehyde. The tissue was then cryoprotected in 30% sucrose for at least 48 hours until processing. Transverse sections of the lumbar enlargement for the CCI + vehicle, CCI+3 mg/kg/day, and sham+3 mg/kg/day groups were processed for immunohistochemistry.
CCI of the Sciatic Nerve
Neuropathic pain was induced using the CCI model.5 CCI was performed at the midthigh level of the left hind leg as previously described.35 Briefly, the rats were anesthetized with isoflurane, the skin over the left hind limb was shaved, cleansed with SurgiScrub, and the surgery was performed under aseptic conditions. Four sterile chromic gut sutures (cuticular 4-0 chromic gut, FS-2; Ethicon, Somerville, NJ) were loosely tied around the gently isolated left sciatic nerve. Sham rats underwent the same surgical procedure except the nerve was isolated but no sutures were applied. The muscle was sutured and the skin was closed with surgical wound clips.
von Frey Testing for Mechanical Allodynia
All testing was conducted in a blinded fashion with respect to group assignment. Rats received 4, 40- to 60-minute habituation sessions in the test environment before the start of behavioral testing. The von Frey test was performed on the plantar surface of each hind paw within the region of sciatic nerve innervation, as described previously.36 A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (407 mg–15.136 g; Stoelting, Wood Dale, IL) was applied randomly to the left and right hind paws, each for 8 seconds at constant pressure. The stimulus intensity threshold for a given subject was determined as the filament intensity where 3 consecutive paw withdrawal responses were made. The stimulus intensity threshold to elicit a paw withdrawal response was used to calculate the 50% paw withdrawal threshold (absolute threshold) using the maximum likelihood fit method to fit a Gaussian integral psychometric function.22 This method normalizes the withdrawal threshold to parametric conditions.
Immunohistochemistry for Spinal Cord and DRG Sections
The L4-6 lumbar spinal cords (n = 4 per group) and ipsi-lateral L4/5 DRG (n = 6 per group) were sectioned (20 μm) and mounted onto gelatin-subbed slides. Spinal cord sections were permeabilized with .01M PBS and .1% TritonX-100 and then blocked for nonspecific binding with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 hour at room temperature. The sections were incubated overnight in primary antibodies for monoclonal mouse anti-rat OX-42 (1:100; Pharmingen, San Diego, CA) and polyclonal rabbit anti-rat glial fibrillary acidic protein (GFAP, 1:100; Dako, Carpinteria, CA). The DRG sections were incubated with phospho-ERK (1:100; Cell Signaling, Danvers, MA), activation transcription factor 3 (ATF3, a neuronal injury marker, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), monocyte chemoattractant protein-1 (MCP-1, 1:400; Cell Signaling) and GFAP (1:100). After washing, the sections were incubated with goat anti-rabbit IRdye680 or goat anti-mouse IR-dye680 conjugated secondary antibody (1:200; LI-COR Biosciences) for 2 hours at room temperature. The sections were then washed and allowed to dry before they were scanned on the Odyssey Infrared Imaging System (LI-COR Biosciences). The signal intensities for the dorsal horns were analyzed using the Odyssey infrared image system in a blinded fashion (LI-COR). For the fluorescence immunohistochemistry, the same procedures were used as described above except the secondary antibody was a goat anti-mouse or goat-anti rabbit Alexaflor 488 (Molecular Probes Inc, Eugene OR). The sections were then washed and cover-slipped with Vectorshield containing DAPI (Vector Laboratories, Burlingame, CA). Slides were viewed with an Olympus BX-61 microscope (Olympus America, Melville, NY) at 20× magnification using Olympus Suite software. For ATF3 and p-ERK total cells and positive cells were counted using the immunofluorescent images. Integrated density was calculated from the Odyssey imaging system for all stains in the DRG.
Study Plasma Collection Following 2-Week Osmotic Minipump Delivery
Cardiac blood at the time of cardiac perfusion was collected in EDTA tubes, centrifuged at 14,000 rpm for 10 minutes at 4°C. The plasma was collected and stored at −80°C until TC-7020 concentrations were measured.
Pharmacokinetic Study
A satellite study was conducted in order to determine potential pharmacokinetic correlations of the behavioral and histological findings. Briefly, similar to procedures used in the allodynia assessments, animals (n = 15) were implanted with s.c. minipumps containing either saline (n = 3) or 3 mg/kg/day TC-7020 (n = 12; free-base equivalent). At each of the following time points—24 hours, 3 and 14 days—after placement of the pumps, plasma, cerebrospinal fluid (CSF), spinal cord, and brain were collected from 1 saline- and 4 TC-7020-treated animals at the time of sacrifice. Blood was drawn via cardiac puncture from the anesthetized animal and placed in hematology tubes containing K2EDTA, then centrifuged to separate plasma. For collection of CSF, procedures similar to those described previously41 were employed where CSF was collected postmortem by placing the animal in a position that allowed the head to rest at an approximate 45° angle downward. A 25-gauge Vacutainer infusion set, attached to a 1-mL syringe, was then inserted into the underlying cisterna magna. CSF was withdrawn. The animals were decapitated and the brains were quickly removed by coarse dissection. For isolation and removal of the spinal cord, a laminectomy was performed using procedures similar to those described previously.27 The spinal cord was isolated and the meninges removed. All samples were placed in appropriately labeled containers and stored frozen at −80°C until analyses.
TC-7020 Extraction Procedure
Plasma and CSF samples were prepared for analyses in the same manner by the addition of 100 μL internal standard (in DI water) and 100 μL DI water to 200 μL of sample. Samples were mixed with a pipettor (3×) and centrifuged 10 minutes at 5,000 rpm. Brain and spinal cord samples were first homogenized in 2.0 and .5 mL 95:5 DI water: trifluoroacetic acid, respectively, using an ultrasonic homogenizer in an ice bath for 1 minute. Following centrifugation of the homogenate for 15 minutes at 20K rpm, a 200 μL aliquot of the supernatant was combined with 100 mL internal standard and 100 mL DI water. Samples were mixed with a pipettor (3×) and centrifuged 10 minutes at 5,000 rpm. Each matrix was quantitated against calibration standards prepared in matching control (untreated) matrix. Calibration and quality control standards were treated identically to samples with the exception of substituting 100 μL of standard solution (in DI water) for DI water.
TC-7020 Concentration Determination
Sample preparation is minimal due to the use of on-column cleanup during the liquid chromatography analyses. The concentrations of TC-7020 were determined using an AB Sciex 5500 LC-MS/MS instrument equipped with an ESI (Turbo Ion Spray) ionization source in the positive ion mode. For TC-7020, the MRM 342.0-201.0 was monitored. Ten μL injections were first loaded onto an Atlantis T3 column (4.6 × 20 mm, 5-μm particles; Waters, Milford, MA). The flow rate was 2.0 mL/minute with isocratic mobile phase of 100 mM NH4COOH in de-ionized water. Following the load, TC-7020 was back-flushed onto an XDB C-18 column (4.6 × 50 mm, 1.8-μm particles; Agilent, Santa Clara, CA) with an isocratic mobile phase of 80:20 100 mM NH4COOH:MeCN. Chromatographic separation was achieved using a gradient of 100 mM NH4COOH and MeCN flowing to the detector at 1.5 mL/minute. The calibration range for each matrix was .25 to 250 ng/mL, expressed as the homogenate for brain and spinal cord. The lower limit of quantitation (LLOQ) was affected by background interference in plasma and brain and raised to be the lowest standard greater than 3× the background matrix noise. LLOQs for each matrix were approximately 1.25 ng/mL for plasma, 1.0 ng/mL for brain, .5 ng/mL for spinal cord, and .25 ng/mL for CSF. All calculated residues were at least 6 times the approximated LLOQ; therefore, the baseline noise is considered to have minimal effect on the quantitation.
Statistical Analysis
Responses to behavioral testing were analyzed by repeated measures 2-way ANOVA (time, treatment) with Bonferroni post hoc comparisons. Immunohistochemical staining for the glial markers (microglia and astrocytes) and DRG markers (ATF3, GFAP, MCP-1, p-ERK) were analyzed using the LI-COR System where the densitometry for the ipsilateral dorsal horn or L4/5 DRG was evaluated and analyzed by 1-way ANOVA (treatment) with SNK post hoc comparisons. P < .05 was considered significant.
Results
Systemic α7 nAChR Agonist Reverses CCI-Induced Allodynia
α7 nAChR agonists have been identified to attenuate neuropathic pain, including CCI, via intrathecal administration.2,46,67 We aimed to determine whether systemic administration of a novel selective α7 nAChR agonist, TC-7020, was also able to reverse CCI-induced neuropathic allodynia in both the ipsilateral and contralateral hind paws. CCI resulted in significant allodynic response compared with sham-treated rats, which was stable 10 days after surgery and throughout the remainder of the study in the vehicle group. Rats were administered the α7 selective agonist TC-7020 or vehicle. Fig 1 shows the ipsilateral and contralateral hind paw behavioral response before CCI or sham surgery (BL), 4 and 10 days (pretreatment) after surgery and then 3 and 24 hours, and 3, 7, and 14 days after mini-pump insertion. There was no significant difference between CCI groups before drug administration. For the ipsilateral hind paw there was a significant interaction (F28,196 = 2.95, P < .0001) with post hoc comparisons showing that CCI rats receiving 1 mg/kg/day TC-7020 were significantly less allodynic compared to the CCI 1 vehicle group at 7 and 14 days after pump implantation (P < .05). 3 mg/kg/day and 10 mg/kg/day TC-7020 resulted in significantly less allodynia for these groups compared to vehicle at 3, 7, and 14 days after pump implantation (P < .05). There was a trend for the allodynia following all doses to be different to that of vehicle at 3 hours but it was not statistically significant. The behavioral responses from the contralateral hind paw showed a significant interaction (F28,197 = 4.21, P < .0001) with post hoc comparisons showing that CCI rats receiving 1 mg/kg/day TC-7020 were significantly less allodynic compared to the CCI + vehicle group from 1 to 14 days after pump implantation (P < .05). 3mg/kg/day and 10 mg/kg/day TC-7020 resulted in significantly less allodynia for these groups compared to vehicle at 3, 7, and 14 days after pump implantation (P < .05). TC-7020 (3 mg/kg/day) did not interfere with the behavioral response of the sham-operated animals (Fig 1).
Figure 1.
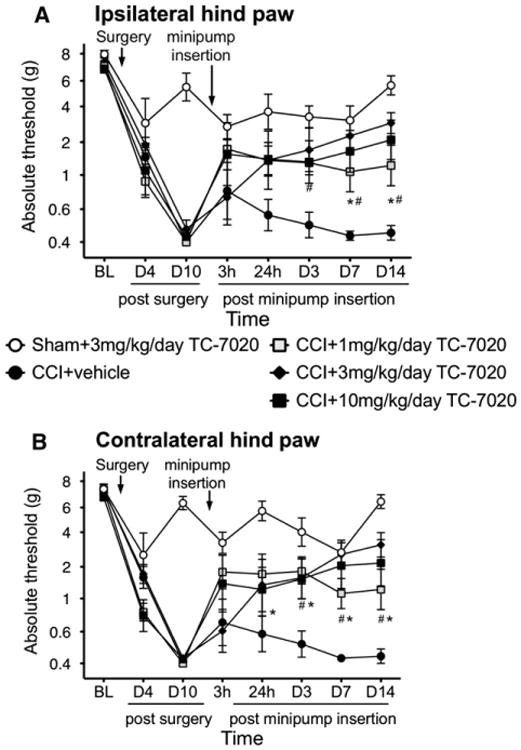
Mechanical allodynia is induced by CCI surgery but not in sham-operated animals. Von Frey testing to the hind paws assessed mechanical thresholds. Systemic administration of TC-7020, an α7 nAChR agonist, administered by a mini-pump subcutaneously implanted 10 to 14 days after surgery reversed the CCI-induced allodynia in both the ipsilateral (A) and contralateral (B) hind paw. 3 mg/kg/day had no effect on sham-operated animals. Data are presented as mean ± SEM (n = 6 per group). *P < .05 CCI + vehicle versus CCI+1 mg/kg/day TC-7020, #P < .05 CCI + vehicle versus CCI+3 and 10 mg/kg/day TC-7020.
Systemic α7 nAChR Agonist Has No Effect on CCI-Induced Glial Activation
α7 nAChRs are abundantly present on microglial cells and are found on astrocytes18; cell types that contribute to the maintenance of neuropathic pain via the continued release of pro-inflammatory cytokines and other mediators in the brain and spinal cord. Since TC-7020 is able to cross the blood-brain barrier to at least a limited degree, we sought to identify whether the reversal of the allodynia may potentially arise from a reduction in CCI-induced glial activation in the spinal cord. Fig 2 shows the integrated density of the ipsilateral dorsal spinal cord of the L4-6 region. There was a significant increase in both OX42 (F2,9 = 7.06, P < .05, Fig 2A) and GFAP (F2,8 = 5.41, P < .05, Fig 2B) for the CCI + vehicle group compared to sham-operated group on the ipsilateral side. There were no significant differences noted on the contralateral side (P > .05). There was no significant effect of 3 mg/kg TC-7020 on either GFAP or OX42 expression by immunohistochemistry. Fig 3 shows examples of images obtained and analyzed for changes in the integrated density of both OX-42 and GFAP within the ipsilateral dorsal horn of the lumbar spinal cord. The resolution obtained from the Odyssey images did not allow for separate analyses across laminae.
Figure 2.
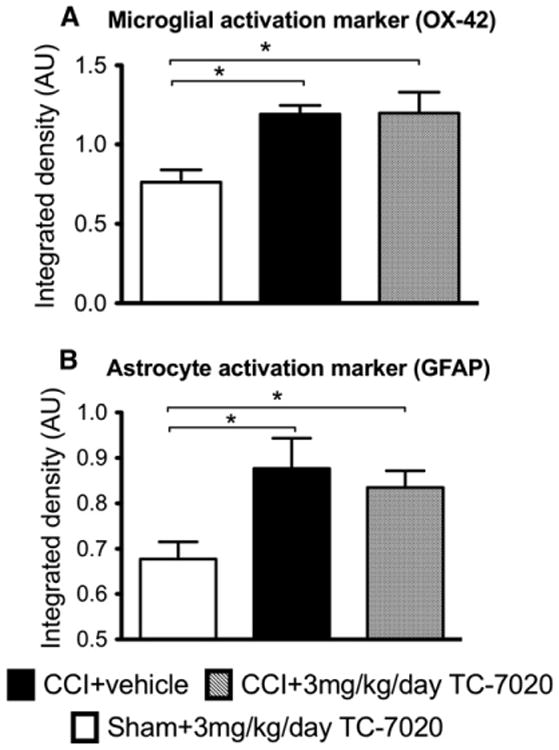
Three mg/kg/day of TC-7020 administered for 2 week in animals with CCI has no effect on either microglial activation marker (OX-42 immunohistochemistry, [A]) or astrocyte activation marker (GFAP immunohistochemistry, [B]) in the ipsilateral lumbar dorsal horn. There was significant increase in both OX42 and GFAP in the rats with CCI compared to sham rats. The data are presented as mean ± SEM (n = 4 per group). The average integrated density of 6 to 8 sections was calculated per rat. *P < .05 between groups.
Figure 3.
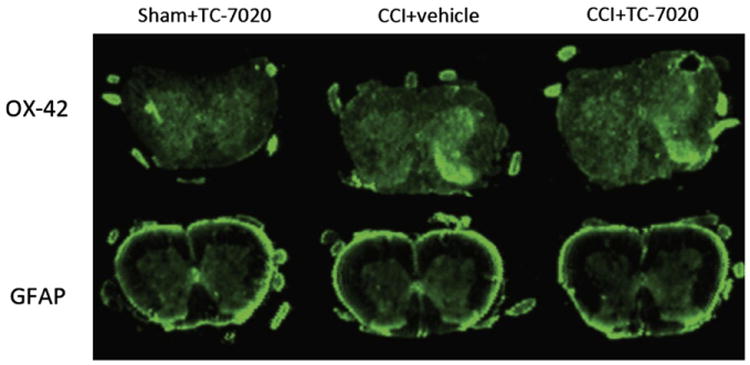
Example of the immunohistochemistry of OX42 and glial fibrillary acid protein, markers of microglial and astrocyte activation respectively, of the L4-6 spinal cord in CCI+vehicle, CCI+3 mg/kg/day of TC-7020 and sham+3 mg/kg/day TC-7020. The laminae I and II of the ipsilateral dorsal horn show increased staining in rats with CCI compared to sham-operated rats.
Systemic α7 nAChR Agonist Significantly Reduces Neuronal Marker of Injury Induced by CCI
Changes in both neuronal injury and satellite cell activation have previously been identified within DRGs that contain the cell bodies of sensory neurons whose peripheral axons are damaged by CCI.42 Given the distribution of TC-7020, DRGs located outside of the blood-brain barrier are more exposed to TC-7020 than spinal glial cells. Thus, in addition to more central sites of pain/allodynia mediation, we aimed to determine whether known CCI-induced changes in the ipsilateral L4 DRG were attenuated by TC-7020. Here, only tissues from vehicle versus the 3 mg/kg/day groups were analyzed. When measuring the number of positive cells relative to total cells for ATF3, there was a significant increase in the number of positive cells in the CCI rats compared to sham, regardless of drug administration (F2,12 = 5.89, P < .05). However, TC-7020 had no significant effect on the total number of ATF3 positive cells. For p-ERK, we did not find the positive cell counting reliable and have used integrated density only as been described previously.9 In the ipsilateral L4 DRG, there was a significant upregulation of integrated density measured for ATF3 (F2,14 = 6.77, P < .0001), MCP-1 (F2,12 = 8.77, P < .01), p-ERK (F2,13 = 7.32, P < .01), and GFAP (F2,10 = 24.44, P < .001) in rats with CCI compared to the sham-operated rats (Fig 4). ATF3, p-ERK and GFAP were significantly reduced by 3 mg/kg/day TC-7020 in rats with CCI compared to CCI 1 vehicle (P < .05).
Figure 4.
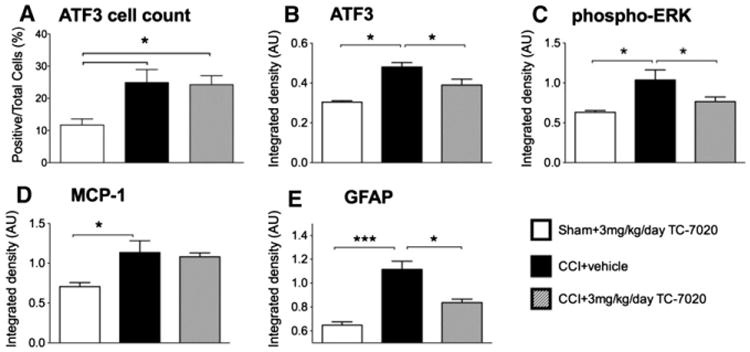
3 mg/kg/day of TC-7020 administered for 2 weeks in rats with CCI attenuated ATF3 (B), p-ERK (C) and GFAP (E) in the ipsilateral L4/5 dorsal root ganglia. The percentage of positive ATF3 cells were significantly increased in both CCI rats receiving vehicle and TC-7020 compared to sham (A). There was a significant increase in p-ERK (C) and MCP-1 (D) in rats with CCI compared to sham rats. The data are presented as mean ± SEM (n = 4–6 per group). The average integrated density of 3 to 4 sections per rats was calculated and included in the analysis. *P < .05 between groups. ***P < .001 between groups.
Plasma, CSF, Brain and Spinal Cord TC-7020 Concentrations
The mean plasma concentrations in plasma collected on day 14 of the study from the 0, 1, 3, and 10 mg/kg/day doses are found in Table 1. The mean plasma concentrations in the satellite study, which was dosed at 3 mg/kg/day, ranged between 73.3 and 79.3 ng/mL when measured at 24 and 72 hours and 14 days. Mean brain concentrations ranged from11.4 to 14.7 ng/g, mean CSF concentrations ranged from 2.04 to 2.75 ng/mL and mean spinal cord concentrations ranged from 5.97 to 7.75 ng/g across all timepoints, as shown in Table 2. Thus TC-7020 has consistent and measurable access to CNS and PNS tissues via the subcutaneous minipump administration utilized.
Table 1. Concentrations of TC-7020 in Study Plasma (n = 6/gp).
| Dose (mg/Kg/Day) | Mean ± SD Plasma (ng/mL) |
|---|---|
| 0 | BLOQ |
| 1 | 23.2 ± 5.2 |
| 3 | 55.4 ± 16.6 |
| 10 | 134 ± 22 |
Abbreviations: SD, standard deviation; BLOQ, below level of assay detection.
Table 2. Mean ± SD Tissue Concentrations of TC-7020 (s.c. Minipump) at 24 and 72 Hours and 14 Days (n = 4/gp).
| Time | Plasma (ng/mL) | Brain (ng/g) | CSF (ng/mL) | Spinal Cord (ng/g) |
|---|---|---|---|---|
| Vehicle | BLOQ | BLOQ | BLOQ | BLOQ |
| 24 hours | 73.3 ± 27.7 | 11.4 ± .9 | 2.04 ± .32 | 7.75 ± 4.03 |
| 72 hours | 78.8 ± 14.2 | 14.7 ± 3.2 | 2.09 ± .58 | 5.97 ± .96 |
| 14 days | 79.3 ± 11.2 | 14.1 ± 1.5 | 2.75 ± .50 | 7.02 ± 4.23 |
Discussion
This series of studies demonstrates that systemic administration of a novel selective α7 nAChR agonist, TC-7020, delivered by subcutaneous slow release mini-pump, significantly attenuated the allodynia induced by CCI in rats by 3 days and was maintained for the duration of the study (14 days). Hence, rapid pain reversal with no tachyphylaxis was observed in response to maintained TC-7020 treatment over the 2-week testing period. This effect of TC-7020 on neuropathic pain appeared to reflect an anti-allodynic rather than an analgesic action, as TC-7020 had no effect on the paw withdrawal threshold of sham-operated rats. As predicted, CCI induced significant upregulation of both a microglial activation marker (OX42) and an astrocyte activation marker (GFAP) within the ipsilateral dorsal horn of the L4-6 spinal region.64 Similarly, within the ipsilateral DRG whose distal axons were damaged by CCI, there was an increased activation of ATF3, MCP-1, p-ERK, and GFAP in rats with CCI compared to sham surgery, as anticipated by prior literature.40,42,43,66 Although TC-7020 was mildly blood-brain barrier permeable, it had no effect on the increased expression of the CCI-induced spinal glial activation markers OX42 and GFAP. In contrast, p-ERK, a marker of neuronal injury,42,43 and GFAP, a marker of satellite cell activation,40 were significantly attenuated by TC-7020. TC-7020 had no effect on MCP-1 in the DRG of CCI+TC-7020 compared to CCI + vehicle controls.
Taken together, these results indicate that systemic delivery of an α7 nAChR agonist, TC-7020, may be useful for treatment of neuropathic pain induced by peripheral nerve injury. The lack of effect on spinal glial cell activation markers was unexpected based on prior findings with compounds targeting α7 nAChRs.30 The results suggest that TC-7020 is not sufficiently blood-brain barrier permeable to exert a direct effect on the spinal glia. The results also indicate that TC-7020 sufficiently decreases signaling from damaged neurons through the DRG to spinal cord glia in a manner that attenuates the resultant proinflammatory response, which would normally amplify pain. As the intracellular signaling pathways leading to the expression of the glial activation markers studied here are generally independent of the intracellular signaling pathways leading to proinflammatory product production and release by these cells, additional studies are needed to define how the systemic delivery of α7 nAChR agonists like TC-7020 modulates the function of spinal cord glia beyond activation marker expression.4
Both generalized nicotinic receptor agonists and selective α7 nAChR agonists have been identified to effectively treat inflammatory and neuropathic pain in rats.2,46,67 Choline, a naturally occurring nutritional supplement and a constituent of acetylcholine, is a selective α7 nAChR agonist.50 Systemic administration of choline reduced hyperalgesia in the late phase of formalin63 and was found to be effective in a rat model of postoperative pain.50,51 Local application of α7 nAChR agonist into the plantar surface of a carrageenan-induced inflammation in the hind paw reduced TNFα and symptoms of inflammation including edema.19 In addition, intrathecal administration of choline both blocked and reversed central neuropathic pain.30 Interestingly, most studies including sham controls of various inflammatory or neuropathic pain models in both mice and rats show no antinociceptive or analgesic properties to α7 nAChR agonists, when administered systemically or intrathecally.30,33 However, some studies have shown that choline prolongs acute nociceptive responses to both thermal and mechanical challenges.13,63 Therefore, while numerous studies have shown good efficacy of α7 nAChR agonists in reversing neuropathic pain, most likely by reducing inflammatory cytokine synthesis, ours is the first to identify that systemic administration of a novel α7 nAChR agonist, TC-7020, reverses neuropathic allodynia for 2 weeks without the development of tolerance.
α7 nAChRs are found abundantly on microglia and also on astrocytes.18,49,62 The activation of glial cells within the spinal cord following nerve injury is now well recognized.12,52,64 Glial activation results in an increase in pro-inflammatory cytokines and the release of other pro-inflammatory mediators that maintain a pro-inflammatory milieu bathing the spinal neurons. This pro-inflammatory milieu assists in maintaining central sensitization and the persistence of neuropathic pain.15,64 Numerous labs have demonstrated that reducing the pro-inflammatory cytokines, or elevating the anti-inflammatory cytokine production, attenuates neuropathic allodynia.14,31,37,47 Application of choline, a selective α7 nAChR agonist, on microglia and peripheral macrophages in vitro decreases LPS-induced TNFα production.21,54,56 Also, intrathecal administration of an α7 nAChR agonist was shown to attenuate pro-inflammatory cytokine production and reduced the microglia activation marker, cd11b mRNA; both of which are upregulated following spinally induced neuroinflammation.30 Therefore, in the present study, the failure of TC-7020 to induce changes in the expression of spinal glial activation markers was surprising, but could be explained by the relatively low blood-brain barrier permeability of the compound, rendering attainable CNS concentrations at the doses tested insufficient to produce a significant effect. Whether higher doses of the compound are able to better penetrate into the CNS remains to be determined.
Unlike spinal glia, satellite cells within the DRG which express α7 nAChRs53 are outside of the blood-brain barrier.65 Thus, DRGs are exposed to systemic delivery of a drug or test compound, regardless of its ability to enter the CNS. Following nerve injury or inflammation, various markers are upregulated in the corresponding DRG neurons providing evidence of nerve injury. ATF3 is induced by stress or injury including in DRG neurons following nerve injury.20,42,45,60 Additionally, in various neuropathic pain models, there is an elevation of phosphorylated ERK (p-ERK), a stress kinase, within the satellite cells and large DRG neurons directly innervating the nerves that are injured.43 Interestingly, both ATF3 and p-ERK are activated in DRGs when TNFα is applied to the DRG.23,57 TNFα is also increased in the DRG and at the sight of nerve injury in models of neuropathic pain.34,40 However, to our knowledge, no prior study has explored whether preexisting ATF3 expression can be attenuated by drug administration. While TC-7020 did not alter the total number of ATF3 positive cells, when the tissue intensity of the ATF3-induced signal was evaluated, TC-7020 significantly reduced the signal of ATF3 in the CCI-associated DRGs. Since α7 nAChR agonists are capable of reducing TNFα production, it is possible that attenuation of ATF3 signal may be attenuated in a graded fashion rather than an on/off signal as measured by cell counts. Additionally, drugs attenuating the inflammation and/or injury may reduce preexisting ATF3 expression. It is also not known whether ATF3 expression or number of ATF3 positive cells continues to increase post-CCI surgery. Therefore, administration of TC-7020 may be preventing the continued increased CCI-induced ATF3 expression. Whether the attenuation of the satellite cell and neuronal markers occurs along the same temporal profile as that of the reversal of allodynia is still to be defined.
The effect of unilateral nerve injury inducing contralateral allodynia has been identified previously.10,38,61 The underlying phenomenon of mirror-image pain induced by unilateral injury is not the focus of the manuscript. However, it is of interest to note that systemic administration of TC-7020 reversed both the unilateral and contralateral allodynia. Previous studies using the sciatic inflammatory neuropathy model that can induce both unilateral and bilateral allodynia depending on the dose of zymosan,10,38,61 demonstrated that perisciatic administration of cytokine inhibitors was able to attenuate both unilateral and bilateral allodynia.61 Therefore, although the contralateral DRG was not measured in this study, it is a reasonable prediction that the contralateral pain would be attenuated because the unilateral pain was attenuated.
Another location of activated immune cells within the periphery that may be affected by systemic TC-7020 delivery is where the macrophages are recruited to the site of nerve injury or inflammation. When nicotine, a generalized nAChR agonist was administered perineurally on an injured sciatic nerve, there was a decrease IL-1b in the sciatic nerve.28 Since α7 nAChR agonists reduce pro-inflammatory cytokine production, it is possible that the reduction in nerve injury markers (ATF3 and p-ERK within the DRG) is the result of a reduction in pro-inflammatory cytokine synthesis at the injury site, thus reducing the extent of nerve injury.24
Peripheral immune cells are recruited to the site of injury by chemokines. Monocyte chemoattractant protein, or CCL2, is a potent chemokine for monocytes/macrophages and contributes to the maintenance of neuropathic pain following partial nerve injury.1 MCP-1 is elevated within the peripheral nervous system in particular the injured site and the DRG.25,26 After nerve injury, MCP-1 is induced in both small and large neurons and to some extent the surrounding satellite cells within the DRG.25,26,59,66 MCP-1 induced within the spinal cord and in DRG following nerve injury assists in maintaining the hyperexcitable state of neurons contributing to central sensitization.1,16 While there is no direct evidence indicating an effect of an α7 nAChR agonist on MCP-1, there is evidence that increased TNFα can induce MCP-1.16 In addition, α7 nAChR agonist applied to endothelial cells in vitro can effectively attenuate IL-6-induced MCP-1 production from endothelial cells.11 Therefore, we would expect that MCP-1 would be attenuated following α7 agonist administration. However, macrophages at the site of injury phagocytose debris from injured and axotomized axons8 and thus may be important in regeneration of peripheral nerves. Thus, reducing chemokines such as MCP-1, and subsequently decreasing the presence of macrophages, may not be beneficial to the healing process.44 In this study the levels of MCP-1 were unaltered by TC-7020 compared the CCI + vehicle groups. It is possible that the effect of TC-7020 to reduce nerve injury but not the levels of MCP-1 may prove useful for the reparative process.
In conclusion, we have provided evidence that a novel, selective α7 nAChR agonist, TC-7020, may be a suitable treatment for neuropathic pain. It effectively attenuated mechanical allodynia induced by nerve injury, possibly via actions on the DRG or site of injury. α7 nAChR agonists, when administered systemically in humans with mild endotoxemia, effectively attenuated elevated TNFα levels within plasma.29 Therefore, the safety profile and therapeutic benefit of systemic administration of α7 nAChR agonists remains positive. The effect of targeting the α7 nAChR on peripheral nerve and on immunocompetent cells both within the periphery and CNS make it a promising therapeutic target.
Acknowledgments
This work was supported by Targacept, Inc., Winston-Salem, North Carolina.
Jason D. Speake, Kristen G. Jordan, John W. James, Steven P. Wene, Robert C. Pritchard, and Sharon R. Letchworth are employees of and/or have stock in Targacept, Inc.
Granted patents/Pending applications related to TC-7020: U.S. 6,953,855 and foreign counterparts, U.S. 8,124,620 and foreign counterparts, and U.S. 2011/0124678 and foreign counterparts.
References
- 1.Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26:529–534. doi: 10.1016/j.it.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Abdin MJ, Morioka N, Morita K, Kitayama T, Kitayama S, Nakashima T, Dohi T. Analgesic action of nicotine on tibial nerve transection (TNT)-induced mechanical allodynia through enhancement of the glycinergic inhibitory system in spinal cord. Life Sci. 2006;80:9–16. doi: 10.1016/j.lfs.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68:931–949. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 6.Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, Tracey KJ. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–147. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 7.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 8.Bruck W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997;7:741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): Induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee PK, Al-Abed Y, Sherry B, Metz CN. Cholinergic agonists regulate JAK2/STAT3 signaling to suppress endothelial cell activation. Am J Physiol Cell Physiol. 2009;297:C1294–C1306. doi: 10.1152/ajpcell.00160.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 14.De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: Path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Fields RD. New culprits in chronic pain. Sci Am. 2009;301:50–57. doi: 10.1038/scientificamerican1109-50. [DOI] [PubMed] [Google Scholar]
- 16.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grace PM, Rolan PE, Hutchinson MR. Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain Behav Immun. 2011;25:1322–1332. doi: 10.1016/j.bbi.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Grybko M, Sharma G, Vijayaraghavan S. Functional distribution of nicotinic receptors in CA3 region of the hippocampus. J Mol Neurosci. 2010;40:114–120. doi: 10.1007/s12031-009-9266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurun MS, Parker R, Eisenach JC, Vincler M. The effect of peripherally administered CDP-choline in an acute inflammatory pain model: The role of alpha7 nicotinic acetylcholine receptor. Anesth Analg. 2009;108:1680–1687. doi: 10.1213/ane.0b013e31819dcd08. [DOI] [PubMed] [Google Scholar]
- 20.Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- 21.Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N. Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock. 2006;26:358–364. doi: 10.1097/01.shk.0000228168.86845.60. [DOI] [PubMed] [Google Scholar]
- 22.Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- 23.Inoue K, Zama T, Kamimoto T, Aoki R, Ikeda Y, Kimura H, Hagiwara M. TNFalpha induced ATF3 expression is bidirectionally regulated by the JNK and ERK pathways in vascular endothelial cells. Genes Cells. 2004;9:59–70. doi: 10.1111/j.1356-9597.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 24.Jancalek R, Dubovy P, Svizenska I, Klusakova I. Bilateral changes of TNF-alpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J Neuroinflammation. 2010;7:11. doi: 10.1186/1742-2094-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon SM, Lee KM, Cho HJ. Expression of monocyte chemoattractant protein-1 in rat dorsal root ganglia and spinal cord in experimental models of neuropathic pain. Brain Res. 2009;1251:103–111. doi: 10.1016/j.brainres.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 26.Jung H, Toth PT, White FA, Miller RJ. Monocyte chemo-attractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan IM, Yaksh TL, Taylor P. Ligand specificity of nicotinic acetylcholine receptors in rat spinal cord: Studies with nicotine and cytisine. J Pharmacol Exp Ther. 1994;270:159–166. [PubMed] [Google Scholar]
- 28.Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation. Pain. 2010;149:305–315. doi: 10.1016/j.pain.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Kox M, Pompe JC, Gordinou de Gouberville MC, van der Hoeven JG, Hoedemaekers CW, Pickkers P. Effects of the alpha7 nAChR Agonist GTS-21 on the Innate Immune Response in Humans. Shock. 2011;36:5–11. doi: 10.1097/SHK.0b013e3182168d56. [DOI] [PubMed] [Google Scholar]
- 30.Loram LC, Harrison JA, Chao L, Taylor FR, Reddy A, Travis CL, Giffard R, Al-Abed Y, Tracey K, Maier SF, Watkins LR. Intrathecal injection of an alpha seven nicotinic acetylcholine receptor agonist attenuates gp120-induced mechanical allodynia and spinal pro-inflammatory cytokine profiles in rats. Brain Behav Immun. 2010;24:959–967. doi: 10.1016/j.bbi.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, Maier SF, Rieger J, Watkins LR. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: A novel therapy for neuropathic pain. J Neurosci. 2009;29:14015–14025. doi: 10.1523/JNEUROSCI.3447-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marrero MB, Lucas R, Salet C, Hauser TA, Mazurov A, Lippiello PM, Bencherif M. An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther. 2010;332:173–180. doi: 10.1124/jpet.109.154633. [DOI] [PubMed] [Google Scholar]
- 33.Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, Chessell IP. Activation of the alpha7-nicotinic acetylcholine receptor reverses complete freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J Pain. 2008;9:580–587. doi: 10.1016/j.jpain.2008.01.336. [DOI] [PubMed] [Google Scholar]
- 34.Miao P, Madec K, Gong Y, Shen H, Eisenstat D, Melanson M, Gu X, Leong C, Klowak M, Namaka M. Axotomy-induced up-regulation of tumor necrosis factor-alpha in the dorsal root ganglia. Neurol Res. 2008;30:623–631. doi: 10.1179/174313208X289606. [DOI] [PubMed] [Google Scholar]
- 35.Milligan ED, Maier SF, Watkins LR. Sciatic inflammatory neuropathy in the rat: Surgical procedures, induction of inflammation, and behavioral testing. Methods Mol Med. 2004;99:67–89. doi: 10.1385/1-59259-770-X:067. [DOI] [PubMed] [Google Scholar]
- 36.Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- 37.Milligan ED, Soderquist RG, Malone SM, Mahoney JH, Hughes TS, Langer SJ, Sloane EM, Maier SF, Leinwand LA, Watkins LR, Mahoney MJ. Intrathecal polymer-based inter-leukin-10 gene delivery for neuropathic pain. Neuron Glia Biol. 2006;2:293–308. doi: 10.1017/S1740925X07000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers R, Wagner R, Sorkin L. Hyperalgesic actions of cytokines on peripheral nerves. In: Watkins L, Maier S, editors. Cytokines and Pain. Basel, CH: Birkhauser Verlag; 1999. pp. 133–156. [Google Scholar]
- 41.Nirogi R, Kandikere V, Mudigonda K, Bhyrapuneni G, Muddana N, Saralaya R, Benade V. A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. J Neurosci Methods. 2009;178:116–119. doi: 10.1016/j.jneumeth.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguchi K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:65–77. doi: 10.1016/s0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- 43.Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perrin FE, Lacroix S, Aviles-Trigueros M, David S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain. 2005;128:854–866. doi: 10.1093/brain/awh407. [DOI] [PubMed] [Google Scholar]
- 45.Peters CM, Ghilardi JR, Keyser CP, Kubota K, Lindsay TH, Luger NM, Mach DB, Schwei MJ, Sevcik MA, Mantyh PW. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol. 2005;193:85–100. doi: 10.1016/j.expneurol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 46.Rashid MH, Ueda H. Neuropathy-specific analgesic action of intrathecal nicotinic agonists and its spinal GABA-mediated mechanism. Brain Res. 2002;953:53–62. doi: 10.1016/s0006-8993(02)03270-5. [DOI] [PubMed] [Google Scholar]
- 47.Romero-Sandoval EA, Horvath RJ, DeLeo JA. Neuroimmune interactions and pain: Focus on glial-modulating targets. Curr Opin Investig Drugs. 2008;9:726–734. [PMC free article] [PubMed] [Google Scholar]
- 48.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15:195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth. 2010;105:201–207. doi: 10.1093/bja/aeq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowley TJ, Payappilly J, Lu J, Flood P. The antinociceptive response to nicotinic agonists in a mouse model of postoperative pain. Anesth Analg. 2008;107:1052–1057. doi: 10.1213/ane.0b013e318165e0c0. [DOI] [PubMed] [Google Scholar]
- 52.Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 53.Shelukhina IV, Kryukova EV, Lips KS, Tsetlin VI, Kummer W. Presence of alpha7 nicotinic acetylcholine receptors on dorsal root ganglion neurons proved using knockout mice and selective alpha-neurotoxins in histo-chemistry. J Neurochem. 2009;109:1087–1095. doi: 10.1111/j.1471-4159.2009.06033.x. [DOI] [PubMed] [Google Scholar]
- 54.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 55.Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi HK, Iwagaki H, Hamano R, Yoshino T, Tanaka N, Nishibori M. alpha7 Nicotinic acetylcholine receptor stimulation inhibits lipopolysaccharide-induced inter-leukin-18 and -12 production in monocytes. J Pharmacol Sci. 2006;102:143–146. doi: 10.1254/jphs.sc0060074. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi N, Kikuchi S, Shubayev VI, Campana WM, Myers RR. TNF-alpha and phosphorylation of ERK in DRG and spinal cord: Insights into mechanisms of sciatica. Spine (Phila Pa 1976) 2006;31:523–529. doi: 10.1097/01.brs.0000201305.01522.17. [DOI] [PubMed] [Google Scholar]
- 58.Taylor RS. Epidemiology of refractory neuropathic pain. Pain Pract. 2006;6:22–26. doi: 10.1111/j.1533-2500.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- 59.Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 60.Tsuzuki K, Kondo E, Fukuoka T, Yi D, Tsujino H, Sakagami M, Noguchi K. Differential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain. 2001;91:351–360. doi: 10.1016/S0304-3959(00)00456-5. [DOI] [PubMed] [Google Scholar]
- 61.Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, Marsh H, Maier SF, Watkins LR. Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats. Pain. 2004;110:299–309. doi: 10.1016/j.pain.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Su DM, Wang RH, Liu Y, Wang H. Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience. 2005;132:49–56. doi: 10.1016/j.neuroscience.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 64.Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: Implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. 2007;56:148–169. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watkins LR, Maier SF. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 66.White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv. 2009;9:188–195. doi: 10.1124/mi.9.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young T, Wittenauer S, Parker R, Vincler M. Peripheral nerve injury alters spinal nicotinic acetylcholine receptor pharmacology. Eur J Pharmacol. 2008;590:163–169. doi: 10.1016/j.ejphar.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


