Abstract
Purpose
The purpose of this study was to test efficacy of a family-based, culturally tailored intervention for Hispanics with type 2 diabetes and their family members.
Methods
Hispanic patients with type 2 diabetes and their family members recruited from community clinics and ethnic churches were assigned to groups (N=186). The intervention group received an 8-week culturally tailored diabetes educational program delivered in Spanish while the attention control group received 8-week sessions on general health information and two sessions on diabetes after completion of the study. Data were collected at baseline, after intervention and at 1- and 6-month follow-ups for both patients and families. Comparisons of change over time were performed using growth curve analyses after propensity score adjustment.
Results
Intervention patients improved in diabetes knowledge and diabetes self-efficacy over time (but did not sustain at 6-month follow-up). A1C was lower at 1-month follow-up. Family members had improvements in diabetes knowledge and physical health-related quality of life.
Conclusions
Including families in the interventions may improve glycemic control, diabetes knowledge, self-efficacy and physical health-related quality of life. However, strategies for sustaining improvements are need.
Keywords: Hispanics, Type 2 diabetes, Diabetes self-management, Family-based intervention
The United States has the third largest number of persons in the world suffering from type 2 diabetes (T2DM)1; and minority groups in the U.S., including Hispanics, suffer disproportionately from the disease. In 2012, 12.8% of Hispanics in this country had diagnosed diabetes, compared to 7.6% of non-Hispanics Whites2, and in recent years, diabetes has sharply increased in Hispanics aged 45 to 75 and older.2 Complications associated with diabetes, such as neuropathy, amputation, end-stage renal disease and stroke, are also higher in Hispanics than in Whites3, they have significantly higher rates of hospitalization for uncontrolled diabetes and complications and are 1.9 times more likely to die from diabetes than Whites.4
Following a healthy diet and exercise program, taking diabetes medications, and monitoring glucose can improve diabetes outcomes.3 Thus diabetes self-management is key to achieving glycemic control and preventing complications.4 However, only 36.8% of Hispanics with T2DM have controlled A1C.5 Less than half perform self-monitoring of glucose and meet recommendation for physical activity and diet6. Many challenges to diabetes self-care have been found in Hispanics7, including social and cultural influences.8 Family value plays an important role in Hispanic culture, and thus focusing on family involvement and family centeredness may be important in interventions for Hispanics with diabetes.9 Indeed, social support has been related to improved healthy eating and physical activity10, better glycemic control as measured by A1C11, improved knowledge12, improved self-efficacy13 and better self-management of diabetes among Hispanics.14 Research has shown the behavioral influence of family members on diabetes self-management of patients with diabetes15 (Chesla, Fisher, 2003). Thus, including family members in the diabetes education programs may improve patients’ adherence to diabetes self-management regimens16(Denham, Ware, Raffle & Leach, 2011). Providing accurate information on diabetes and risk factors associated with diabetes to family members may enhance family support and develop healthy behaviors for the entire family Denham, 2003;16,17 Denham, Ware, Raffle & Leach, 2011). However, few intervention studies have included family members or friends in efforts to improve diabetes self-management.18 This study therefore examined the efficacy of a family-based, culturally-tailored intervention for Hispanics with T2DM and their family members.
Hypothesis
The study tested the following hypotheses that patients in the 8-week family-based diabetes intervention group would show significantly greater improvements than an 8-week attention control group immediately after the intervention period (T2), and at 1 (T3) and 6-months (T4) after the intervention in: 1. the behavioral influences of diabetes knowledge, diabetes self-efficacy, and family support; 2. the behavioral outcomes of self-reported management of diabetes including physical activity, diet, and medications; 3. the physiological outcome of glycemic control (A1C %) and, 4. the psychological outcomes of physical and mental health-related quality of life.
Methods
Study Design
A quasi-experimental design was used to examine the effects of the 8-week intervention program for participants with diabetes and their family members. This design has the advantage of being able compare an intervention group to a prospectively followed group of participants who do not receive the intervention. However, because participants are not randomized to intervention or control, the design has the disadvantage that effects of selection bias could occur. Much research has been conducted to counter, where possible, such potential bias using methodological approaches such as propensity score adjustment19. An attention control group received general health promotion information for 8 weeks and two-session diabetes self-management education was provided at the completion of the study.
Participants and Setting
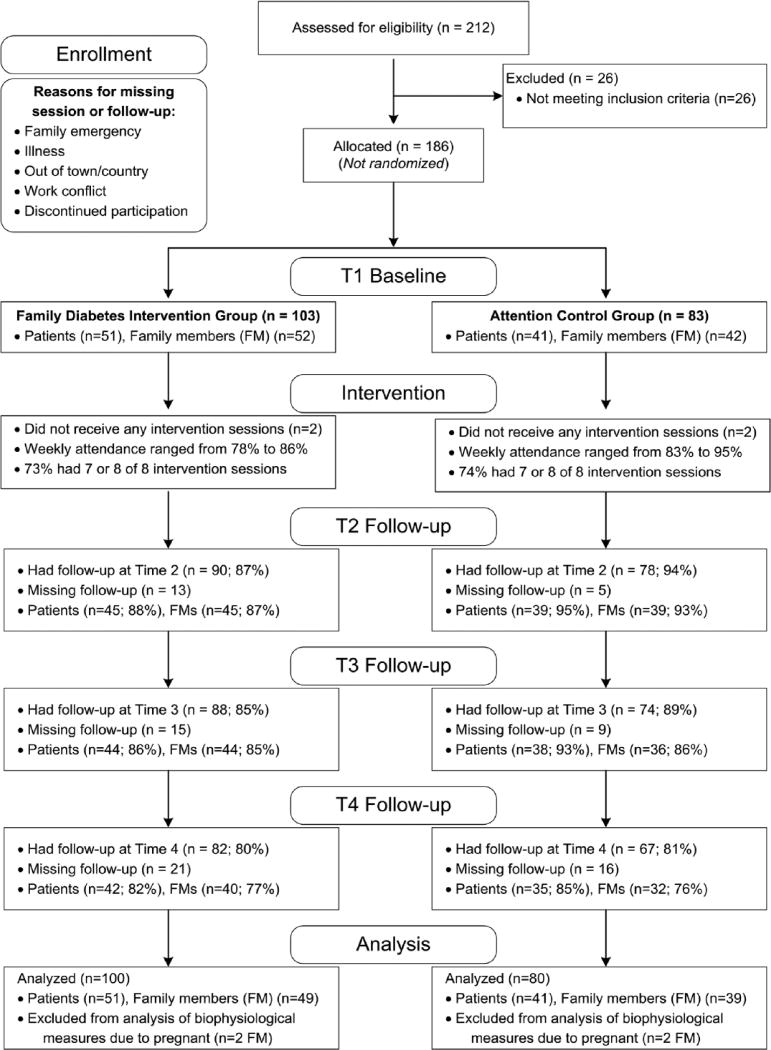
A convenience sample (N=186) of participants who had T2DM and their family members were recruited at six sites including clinics, physician offices, and churches in rural counties in central North Carolina between June 2012 and May 2015. There were 7 to 12 participants with T2DM per site. Recruitment included posted signs, flyers distributed by clinic staff, waiting room conversations with bilingual and bicultural research team members, by participants and clinic patients. Criteria for inclusion of individuals with diabetes were a) community-dwelling, b) self-identification as Hispanic, c) age 18 years or older, d) self-identification as having a medical diagnosis of T2DM, and e) an adult family member willing to participate in the study. Inclusion criteria for family members/relatives were a) residence in the same household as the participant with diabetes and b) age 18 years or older. Eligible participants with diabetes brought the same family member or members to each of the intervention and data collection sessions. A total of 92 Hispanic patients with T2DM (Intervention (I) n=51, Control (C) n=41) and their families (n=94) (Intervention (I) n=52, Control (C) n=42) participated in the study. Figure 1 describes the follow-up rates by group. All participants provided signed informed consent and the study was approved by the university IRB. Participants were assigned in groups to either intervention or attention control groups depending on the geographical locations of the clinical sites and churches. Face-to-face interviews in Spanish were conducted at baseline before intervention and post intervention, and at 1-month and 6-month follow-ups.
Figure 1.
Study flow chart
Intervention and Attention Control Conditions
Intervention group
The intervention consisted of eight weekly group sessions in clinics and churches for participants with diabetes and family members and two sessions (baseline and T4) with the family for data collection. Bilingual and bicultural registered nurses with team members provided the intervention. The eight weekly interactive modules, total of 12 hours (1.5 hours each week), were based on our pilot intervention for Hispanics and families and modified from a family-based diabetes program.20 The intervention was designed to increase knowledge of diabetes and self-efficacy, promote family support, decrease barriers to self-management, enhance self-management, improve glycemic control, and improve health-related quality of life. Intervention components included information on diabetes risk factors, symptoms and complications, facilitation of family values and beliefs and family support on diabetes, identification of barriers to diabetes self-management, discussion of the relationships among physical activity, food choices, medications, diabetes control, and problem solving skills, and goal setting for healthy behaviors. Each of the modules was tailored for those with low literacy and integrated cultural beliefs and values. A variety of teaching methods and pictorial food/activity logs, ethnic food models, pictorial food books, video, self-monitoring demonstrations, use of modified ethnic food recipes and culturally relevant activities were used.
Attention Control group
Attention control group was used in the study and participants in the attention control group received similar amount of time and dose as those in the intervention group. However, information on general health and diabetes routine care were provided to the participants in the attention control group. Eight sessions were offered by trained bilingual, bicultural RNs on topics such as age and gender appropriate regular physical exams, dental health, immunizations for adults and children, cancer screening, safety (driving, walking, water, and chemicals), depression, and injury prevention. When participants asked questions about diabetes, brief factual answers were given and routine care was provided to all patients with T2DM. Two diabetes self-management educational sessions were provided at 9-month after the completion of the study to attention control participants. All participants in both the intervention and attention control groups were given a pedometer during the 8 weeks of educational sessions to encourage physical activity.
Measures
Hemoglobin A1C was tested using finger stick blood, with a glycosylated hemoglobin A1CNow+ test. The test is valid and reliable.21 Diabetes self-efficacy, family support, and diabetes self-care activities were collected for patients but not for family members.
Diabetes knowledge was measured by the Spoken Knowledge of Diabetes in Low Literacy Patients with Diabetes (SKILLD)22, which assesses knowledge of glucose management, lifestyle modifications, recognition and treatment of acute complications, and activities to prevent long-term consequences of the disease. Upon approval by Dr. Rothman, the SKILLD was translated into Spanish and back-translated by bilingual/bicultural translators. This is a verbally administered scale consisting of 10 items with scores ranging from 0–10 where higher scores indicate better diabetes knowledge. Participants received an additional point towards their score if a given question was responded to correctly verbally.22 Reliability and validity of the SKILLD have been established22. KR-20 in the current study was 0.73.
Family support was examined using the brief Chronic Illness Resources Survey (CIRS), Spanish version. The CIRS asks items on a 5-point Likert scale that assesses social-environmental support of family members and friends, neighborhood and community to the person with T2DMs in medication taking, exercise and diet in the past 6 months. Higher scores indicate stronger perceived family support.23 Reliability of the Spanish version has been established.23 Coefficient alpha in the current study was 0.63.
The Stanford Diabetes self-efficacy (DSE), Spanish version, is an 8-item scale that measures the confidence of a person with diabetes to manage diet, exercise, and blood sugar and maintain control over the diabetes.24 Each of the 8 items are measured on a 10-point Likert-type rating response scale and a summed mean score was calculated. Higher scores indicate higher self-efficacy. Coefficient alpha was 0.85 for the current study.
Diabetes self-management was measured by the Revised Summary of Diabetes Self-Care Activities (SDSCA), Spanish version25, which assessed diet, exercise, glucose testing, medication and foot care over the past 7 days. The mean number of days per week of diabetes self-care activities were calculated with higher scores indicating better diabetes self-management. The validity and reliability of the Spanish version of the SDSCA have been reported for Hispanics with diabetes.25
Physical Activity (PA) was measured using the Short International Physical Activity Questionnaire (IPAQ) form, Spanish version, Last 7 Days Recall.26 The IPAQ short form provides information on time spent walking, time in vigorous and moderate intensity activity, and time in sedentary activities in the last 7 days. Total physical activity metabolic equivalent of minutes/week are estimated (MET min/wk.). Diet was assessed similarly to Hu et al.20 using questions about frequency (daily, weekly, monthly, never) of fruits and vegetables consumption within the Behavioral Risk Factor Surveillance Survey (BRFSS).27
Health-related quality of life was measured by the Spanish version of the Medical Outcomes Study Short Form Health Survey Version 2.28 SF-12v2® assessed self-reported health-related quality of life through physical (PCS) and mental (MCS) component summaries. The SF-12v2® uses Likert-type response rating scales. PCS and MCS scores were normed with 2009 General U.S. population scores with a mean of 50 and SD of 1041, with higher scores corresponding to better perceived health-related quality of life. The SF-12v2® has been used with both genders, and multiple ages and ethnic populations in a variety of setting.28 Reliability was 0.82 for the PCS and 0.86 for the MCS in the present study.
Data Analysis
Based on an a priori power analysis, a sample size of 35 patients per group at the end of the study allowed detection of a decrease in A1C of at least 10% with 80% power, based on pilot data from Hu et al.20 and assuming a two-sided Type I error of 0.05. Attendance, follow-up rates, and patient and family member characteristics were summarized using descriptive statistics. Baseline characteristics were compared between groups using Chi-square or Fisher’s exact tests for categorical measures, and t-tests or Wilcoxon rank-sum tests for continuous characteristics.
Analyses were performed separately for patients and family members. Four family members became pregnant over the study period and were excluded in the analysis of physiological measures (but analyzed for other measures). Attrition rates were compared between groups using Chi-square testing. Multiple imputation for missing data was performed in sensitivity analyses with 20 imputations using predictive mean matching with a fully conditional specification method.29
To account for the quasi-experimental study design19, inverse probability weighting (IPW) using propensity scores was employed in outcome modeling.30 The goal of incorporating propensity scores is to approximate the comparable distribution of variables that might be expected when beginning a randomized controlled trial.31 Outcome-specific propensity scores were estimated using pre-intervention baseline measures related to outcomes, as recommended by Brookhart32 and current literature. Findings from models unadjusted for propensity scores as well as adjusted for propensity scores using IPW were compared (e.g.,33). Standardized differences were compared before and after weighting for assessing whether adequate balance had been achieved.30 Robust sandwich standard errors were additionally specified to account for propensity score estimation.34
Longitudinal comparisons of change over time between groups were performed using growth curve modeling35, which allows for parsimonious modeling of change while using all available data and accommodating unequally spaced repeated measure.20 Model-based time-point specific differences were interpreted if omnibus tests for any differences in change over time between groups (i.e., group by time interaction) were significant.35
Results
Attendance, Attrition, and Missing Data
The median times of data collection from pre-intervention (baseline) were 2.5 months for post-intervention (max = 3.9), 3.5 months for 1-month post-intervention follow-up (max = 5.3) and 8.8 months for 6-month follow-up (max = 10.2). There were approximately two weeks on average between baseline and the first week of the intervention sessions. Forty-two of 51 patients in the intervention group (82%) remained in the study at the 6-month follow-up, compared to 35 of 41 patients in the control group (85%). Attrition rates did not differ by group for patients (χ2 = 0.081, df = 3, p = 0.994) and family members (χ2 = 0.079, df = 3, p = 0.994). Overall, there were 0% missing data at pre-intervention, 8.7% at immediate post-intervention, 10.9% to 12.0% at 1-month follow-up, and 16.3% for 6-month follow-up across variables. Similar conclusions for all analyses described below were found after multiple imputation.
Session attendance over the 8 weeks was comparable between groups. In the intervention group, 73% attended at least 7 of 8 sessions compared to 74% of the control group. In any given week, session attendance was between 78% to 86% for the intervention group and 83% to 95% for controls. Reasons for not attending sessions or missing follow-up data collection included work conflict, illness, family emergency, being out of town/country, and discontinuing participation in the study.
Groups at Baseline
Patient baseline characteristics are presented in Table 1. Groups were comparable on all characteristics except for receiving printed materials regarding diabetes in past year (I 37% vs. C 76%, p < 0.001), household annual income (p < 0.001), systolic blood pressure (I mean (M) = 128.6 mmHg vs. C M = 133.1 mmHg, p = 0.031), A1C (I M = 8.5% [69 mmol/mol] vs. C M = 9.5% [80 mmol/mol], p = 0.021), CIRS family support scores (I M = 1.4 vs. C M = 1.7, p = 0.002), and SKILLD diabetes knowledge scores (I M = 2.9 vs, C M = 4.2, p = 0.011).
Table 1.
Characteristics of Hispanic diabetic patients at pre-intervention (N = 92)
| Characteristic N (%) or Mean ± SD (Min, Max) | Overall (n = 92) | Family-Based Education Intervention (n = 51) | Attention Control (n = 41) | P-value |
|---|---|---|---|---|
|
| ||||
| T2DM Duration (years) | 8.1 ± 7.1 (0, 35) |
7.2 ± 6.7 (0, 35) |
9.2 ± 7.5 (0, 30) |
0.1576 |
|
| ||||
| Taking medicines for diabetes/blood sugar | 89 (97) | 48 (94) | 41 (100) | 0.2507 |
|
| ||||
| Use herbs, plants, licuados, or herbal medicines to treat DM | 44 (48) | 23 (45) | 21 (51) | 0.5591 |
|
| ||||
| Parent has Diabetes | 65 (71) | 32 (63) | 33 (80) | 0.0632 |
|
| ||||
| Ever attended diabetes class | 26 (28) | 12 (24) | 14 (34) | 0.2610 |
| Received printed materials regarding diabetes in past yr. | 50 (54) | 19 (37) | 31 (76) | 0.0002 |
|
| ||||
| Age (years) | 49.4 ± 11.3 (19, 78) |
49.9 ± 12.6 (19, 78) |
48.9 ± 9.6 (30, 71) |
0.7808 |
|
| ||||
| Gender | 0.6905 | |||
| Female | 54 (59) | 29 (57) | 25 (61) | |
| Male | 38 (41) | 22 (43) | 16 (39) | |
|
| ||||
| Country of origin | 0.4000 | |||
| United States | 2 (2) | 0 | 2 (5) | |
| Mexico | 74 (80) | 42 (82) | 32 (78) | |
| Other | 16 (17) | 9 (18) | 7 (17) | |
|
| ||||
| Language | 0.0849 | |||
| Spanish | 89 (97) | 51 (100) | 38 (93) | |
| English | 0 | 0 | 0 | |
| Other | 3 (3) | 0 | 3 (7) | |
|
| ||||
| Marital status | 0.6440 | |||
| Married/live with | 72 (78) | 41 (80) | 31 (76) | |
| Single/live alone | 12 (13) | 7 (14) | 5 (12) | |
| W/D/S* | 8 (9) | 3 (6) | 5 (12) | |
|
| ||||
| Education | 0.0693 | |||
| Less than 12 years | 69 (75) | 42 (82) | 27 (66) | |
| ≥High School to Some college | 23 (25) | 9 (18) | 14 (34) | |
|
| ||||
| Length in U.S. (years) | 18.4 ± 8.6 (0.2, 51.0) |
19.4 ± 9.3 (0.2, 42.0) |
17.3 ± 7.5 (7.0, 51.0) |
0.2998 |
|
| ||||
| Household income | <0.0001 | |||
| Less than $10,000 | 49 (53) | 40 (78) | 9 (22) | |
| $10,000 – $19,999 | 20 (22) | 8 (16) | 12 (29) | |
| $20,000 or more | 15 (16) | 2 (4) | 13 (32) | |
|
| ||||
| Health insurance | 27 (29) | 18 (35) | 9 (22) | 0.1624 |
|
| ||||
| Co-morbidities (CMs) | ||||
| DX heart trouble | 14 (15) | 9 (18) | 5 (12) | 0.4693 |
| Heart attack | 7 (8) | 6 (12) | 1 (2) | 0.1262 |
| High BP | 56 (61) | 28 (55) | 28 (68) | 0.1417 |
| Stroke | 5 (5) | 2 (4) | 3 (7) | 0.6528 |
| Kidney trouble | 24 (26) | 13 (25) | 11 (27) | 0.8290 |
| Sores | 9 (10) | 7 (14) | 2 (5) | 0.2896 |
| Limb nerve damage | 46 (50) | 28 (55) | 18 (44) | 0.2943 |
| No. of Comorbidities | 1.8 ± 1.3 (0, 6) | 1.8 ± 1.5 (0, 6) | 1.7 ± 1.2 (0, 4) | 0.7658 |
|
| ||||
| A1C (%) | 8.9 ± 2.1 (5.7, 13.1) |
8.5 ± 2.1 (5.7, 13.1) |
9.5 ± 2.0 (5.7, 13.1) |
0.0211 |
| 4.3 to 5.2% | 0 | 0 | 0 | 0.0713 |
| 5.3 to 5.6% | 0 | 0 | 0 | |
| 5.7 to 6.4% | 9 (10) | 7 (13) | 2 (5) | |
| 6.5 to 6.9% | 12 (13) | 10 (20) | 2 (5) | |
| 7.0 to 7.9% | 18 (20) | 11 (22) | 7 (17) | |
| 8.0 to 8.9% | 10 (11) | 4 (8) | 6 (15) | |
| 9.0 to 9.9% | 16 (17) | 7 (14) | 9 (22) | |
| 10.0 to 10.9% | 8 (9) | 3 (6) | 5 (12) | |
| 11.0 to 11.9% | 9 (10) | 6 (12) | 3 (7) | |
| 12.0 to 13.0% | 4 (4) | 0 | 4 (10) | |
| >13.0% | 6 (7) | 3 (6) | 3 (7) | |
|
| ||||
| A1C (mmol/mol) | 74 ± 23 (39, 120) |
69 ± 23 (39, 120) |
80 ± 21.9 (5.7, 13.1) |
0.0211 |
| 23 to 33 | 0 | 0 | 0 | 0.0713 |
| 34 to 38 | 0 | 0 | 0 | |
| 39 to 47 | 9 (10) | 7 (13) | 2 (5) | |
| 48 to 52 | 12 (13) | 10 (20) | 2 (5) | |
| 53 to 63 | 18 (20) | 11 (22) | 7 (17) | |
| 64 to 74 | 10 (11) | 4 (8) | 6 (15) | |
| 75 to 85 | 16 (17) | 7 (14) | 9 (22) | |
| 86 to 96 | 8 (9) | 3 (6) | 5 (12) | |
| 97 to 107 | 9 (10) | 6 (12) | 3 (7) | |
| 108 to 119 | 4 (4) | 0 | 4 (10) | |
| >119 | 6 (7) | 3 (6) | 3 (7) | |
|
| ||||
| BMI (kg/m2) | 31.2 ± 5.2 (22.5, 48.1) |
30.9 ± 5.7 (22.6, 48.1) |
31.6 ± 4.6 (22.5, 42.1) |
0.2417 0.3962 |
| <18.5 kg/m2 | 0 | 0 | 0 | |
| 18.5 – 24.9 kg/m2 | 7 (8) | 5 (10) | 2 (5) | |
| 25 – 29.9 kg/m2 | 38 (41) | 23 (45) | 15 (37) | |
| ≥30 kg/m2 | 47 (51) | 23 (45) | 24 (59) | |
|
| ||||
| SBP (mmHg) | 130.6 ± 15.6 (100, 190) |
128.6 ± 18.3 (100, 190) |
133.1 ± 11.0 (110, 160) |
0.0311 0.1130 |
| <120 | 17 (18) | 13 (25) | 4 (10) | |
| 120 – 129 | 22 (24) | 13 (25) | 9 (22) | |
| 130 – 139 | 31 (34) | 15 (29) | 16 (39) | |
| 140 – 159 | 17 (18) | 6 (12) | 11 (27) | |
| ≥160 | 5 (5) | 4 (8) | 1 (2) | |
|
| ||||
| High BP (mmHg) | 0.4879 | |||
| SBP≥140 or DBP≥90 | 28 (30) | 14 (27) | 14 (34) | |
| SBP<140 & DBP<90 | 64 (70) | 37 (73) | 27 (66) | |
|
| ||||
| Activity (IPAQ) Total MET-min/wk |
4514.9 ± 6425.5 (0, 23940) |
4983.1 ± 6906.2 (0, 23940) |
3932.0 ± 5871.2 (0, 23136) |
0.3724 |
|
| ||||
| BFRSS fruits/vegetables | 2.6 ± 0.4 (1.3, 3.7) |
2.6 ± 0.4 (1.3, 3.7) |
2.7 ± 0.4 (1.7, 3.5) |
0.7251 |
|
| ||||
| Supportive Resources (CIRS) (α=.63) | 1.6 ± 0.4 (1.0, 2.7) |
1.4 ± 0.3 (1.0, 2.5) |
1.7 ± 0.4 (1.0, 2.7) |
0.0018 |
|
| ||||
| Diabetes self-efficacy (DSE) (α=.85) | 6.6 ± 2.1 (1, 10) |
6.4 ± 1.8 (1.4, 10) |
6.8 ± 2.4 (1, 10) |
0.1849 |
|
| ||||
| Diabetes self-management: Diet (SDSCA) (α=.63) |
3.1 ± 1.4 (0, 6.8) |
3.0 ± 1.5 (0, 6.8) |
3.2 ± 1.4 (0.8, 6.0) |
0.5646 |
|
| ||||
| Diabetes self-management: General Diet (SDSCA) (α=.87) | 3.1 ± 2.0 (0, 7) |
3.0 ± 1.9 (0, 7) |
3.3 ± 2.1 (0, 7) |
0.5119 |
|
| ||||
| Diabetes self-management: Blood (SDSCA) (α=.84) |
1.7 ± 2.3 (0, 7) |
1.8 ± 2.3 (0, 7) |
1.7 ± 2.1 (0, 7) |
0.9120 |
|
| ||||
| Diabetes self-management: Foot (SDSCA) (α=.58) |
3.3 ± 2.5 (0, 7) |
2.6 ± 2.4 (0, 7) |
4.1 ± 2.5 (0, 7) |
0.0042 |
|
| ||||
| Diabetes self-management: Meds (SDSCA) |
6.3 ± 1.8 (0, 7) |
6.1 ± 2.1 (0, 7) |
6.6 ± 1.3 (0, 7) |
0.2620 |
|
| ||||
| Diabetes knowledge (SKILLD) (KR-20=.73) | 3.5 ± 2.4 (0, 9) |
2.9 ± 2.5 (0, 9) |
4.2 ± 2.2 (0, 9) |
0.0113 |
|
| ||||
| SF-12v2 Physical Health (αc=.82) | 48.3 ± 8.6 (23.9, 62.2) |
48.9 ± 9.2 (23.9, 62.2) |
47.5 ± 7.7 (26.9, 57.6) |
0.3080 |
|
| ||||
| SF-12v2 Mental Health (αc =.86) |
47.8 ± 11.5 (26.8, 68.4) |
45.9 ± 10.2 (29.1, 64.6) |
50.2 ± 12.7 (26.8, 68.4) |
0.0779 |
Patient Medication Use
Overall, about half of patients were on lipid lowering (49%) and hypertensive (52%) medications, with slightly fewer in the intervention group (I 43% vs. C 56% for lipid lowering; I 45% vs. C 61% for hypertensive). About four-fifths of the patients were on antiglycemic agents, oral or noninsulin injectables (I 80% versus C 83%). Twenty percent of intervention patients were using insulin compared to 39% of control patients. Eight percent in the intervention group were on antiplatelets compared to 15% in the control group. Five patients were on antidepressants or antianxiety medications (I 6% versus C 5%).
Change Over Time for Patients
Table 2 provides group comparisons from longitudinal analysis of change over time for patients. There were significant changes over time (i.e., group by time interaction effects) in SKILLD diabetes knowledge scores (p < 0.001), DSE scores (p = 0.007), and CIRS scores (p = 0.028), adjusting for propensity score weighting and repeated measures. However, significant improvements were not observed in CIRS scores. Post hoc comparisons revealed that mean diabetes knowledge SKILLD scores were 8.6 (out of a possible 10) for the intervention group compared to 6.3 for controls at post-intervention (p < 0.001), and remained significantly higher at the 1-month post-intervention follow-up (I M = 7.7 vs. C M = 6.5, p = 0.016). Finally, mean DSE scores were higher at immediate post-intervention for the intervention group than for controls (M = 8.5 vs. 7.3, p = 0.004), after accounting for baseline differences.
Table 2.
Impact of diabetes intervention on change in patient outcomes over time
| Time-Point1 | Overall2 P |
Pairwise comparison P for I vs. C3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention (1) | Post-Intervention (2) | 1-Month Follow-up (3) |
6-Month Follow-up (4) |
|||||||||
| I | C | I | C | I | C | I | C | 2 vs. 1 | 3 vs. 1 | 4 vs. 1 | ||
| SKILLD diabetes knowledge (KR-20=.73) |
3.0 (2.3, 3.8) |
4.2 (3.4, 4.9) |
8.6 (8.0, 9.1) |
6.3 (5.5, 7.2) |
7.7 (7.0, 8.4) |
6.5 (5.8, 7.1) |
7.3 (6.8, 7.7) |
6.6 (6.0, 7.3) |
<0.001 | <0.001 | 0.016 | 0.146 |
| DSE diabetes self-efficacy (α=.85) |
6.4 (5.9, 6.9) |
6.8 (6.0, 7.6) |
8.5 (8.1, 8.9) |
7.3 (6.6, 7.9) |
8.5 (8.2, 8.8) |
8.3 (7.7, 8.9) |
8.0 (7.7, 8.4) |
7.8 (7.3, 8.2) |
0.007 | 0.004 | 0.516 | 0.366 |
| CIRS family support (α=.63) |
1.4 (1.3, 1.5) |
1.7 (1.6, 1.8) |
1.7 (1.5, 1.9) |
1.8 (1.7, 2.0) |
1.6 (1.4, 1.7) |
2.0 (1.8, 2.1) |
1.7 (1.5, 1.8) |
1.7 (1.6, 1.9) |
0.028 | 0.115 | <0.001 | 0.194 |
| IPAQ MET-min/wk. |
5174.9 (3353.7, 6996.2) |
3902.3 (2038.1, 5766.5) |
5527.0 (3777.1, 7276.9) |
4692.1 (3185.1, 6199.0) |
5666.1 (3903.8, 7428.5) |
5004.2 (3471.4, 6537.0) |
6350.6 (4226.9, 8474.2) |
6539.5 (3836.0, 9243.0) |
0.096 | 0.066 | 0.048 | 0.019 |
| BRFSS fruits & veggies consumption |
2.7 (2.5, 2.8) |
2.6 (2.5, 2.8) |
2.8 (2.7, 2.9) |
2.8 (2.7, 2.9) |
2.8 (2.7, 2.9) |
2.8 (2.7, 2.9) |
2.7 (2.6, 2.8) |
2.7 (2.6, 2.9) |
0.934 | 0.834 | 0.885 | 0.847 |
| SDSCA Meds # days in past 7 |
6.4 (5.9, 6.9) |
6.7 (6.3, 7.1) |
6.3 (5.9, 6.8) |
6.6 (6.2, 7.0) |
6.3 (5.8, 6.8) |
6.6 (6.2, 7.0) |
6.2 (5.6, 6.8) |
6.5 (6.0, 7.0) |
0.946 | 0.276 | 0.241 | 0.165 |
| SDSCA blood sugar testing (α=.84) |
2.2 (1.5, 2.8) |
2.1 (1.4, 2.8) |
2.2 (1.6, 2.8) |
2.1 (1.4, 2.7) |
2.2 (1.6, 2.8) |
2.1 (1.4, 2.7) |
2.3 (1.6, 3.0) |
2.0 (1.2, 2.8) |
0.2676 | 0.8646 | 0.8129 | 0.6091 |
| SDSCA foot (α=.58) |
2.6 (1.9, 3.3) |
4.2 (3.3, 5.0) |
5.9 (5.1, 6.8) |
5.5 (4.5, 6.4) |
5.2 (4.5, 6.0) |
6.3 (5.7, 6.8) |
5.5 (4.9, 6.0) |
6.2 (5.7, 6.6) |
0.083 | 0.596 | 0.067 | 0.035 |
| SDSCA general diet (α=.87) |
3.1 (2.5, 3.7) |
3.5 (2.7, 4.2) |
4.7 (4.0, 5.5) |
3.9 (3.1, 4.7) |
4.4 (3.7, 5.1) |
3.5 (2.8, 4.3) |
3.7 (3.2, 4.2) |
3.5 (3.0, 4.0) |
0.061 | 0.116 | 0.086 | 0.517 |
| A1C (%) | 8.5 (7.9, 9.1) |
9.4 (8.8, 10.0) |
7.7 (7.1, 8.2) |
8.7 (8.1, 9.3) |
7.7 (7.1, 8.3) |
9.0 (8.3, 9.6) |
8.4 (7.8, 9.0) |
8.2 (7.7, 8.8) |
<0.001 | 0.020 | 0.005 | 0.674 |
| A1C (mmol/mol) |
65 (63, 76) |
79 (73, 86) |
61 (54, 66) |
72 (65, 78) |
61 (54, 67) |
75 (67, 81) |
68 (62, 75) |
66 (61, 73) |
<0.001 | 0.020 | 0.005 | 0.674 |
| SF-12v2 PCS physical HRQOL (αc=.82) |
49.5 (47.2, 51.8) |
49.1 (46.9, 51.3) |
50.6 (48.8, 52.4) |
49.5 (47.4, 51.5) |
51.1 (49.4, 52.8) |
49.6 (47.5, 51.7) |
53.3 (51.4, 55.1) |
50.3 (47.2, 53.5) |
0.678 | 0.150 | 0.079 | 0.006 |
| SF-12v2 MCS mental HRQOL (αc=.86) |
47.2 (44.7, 49.8) |
50.0 (46.8, 53.3) |
47.7 (45.3, 50.1) |
51.7 (49.1, 54.4) |
47.9 (45.5, 50.4) |
52.4 (49.8, 55.0) |
48.9 (45.9, 52.0) |
55.7 (52.0, 59.4) |
0.154 | 0.264 | 0.309 | 0.626 |
Note. Results are based on mixed-effect models for outcome with IPW propensity score adjustment (except ranks of IPAQ tested).
Numbers presented are model-based least-squares means using median time from pre-intervention, (95% CI for means). Median time from pre-intervention to post-intervention, 1-month follow-up, and 6-month follow-up were 2.5 months, 3.5 months, and 8.8 months, respectively;
P = Overall p-value is for omnibus test (group by time interaction) for any differences in change over time between groups;
P-value from testing difference in least-square mean estimates in post-hoc pairwise comparisons with baseline/pre-intervention.
There were no significant changes over time in behavioral outcomes, including IPAQ MET-min/week (p = 0.096), BRFSS fruit and vegetable consumption (p = 0.934), SDSCA adherence to recommended diabetes medications (p = 0.946), blood sugar testing (p = 0.268), foot care (p = 0.083), and general diet (p = 0.061).
There were significant changes over time in A1C (p < 0.001) after adjusting for repeated measures and propensity score weighting. Mean A1C in the intervention group was 7.7% (61 mmol/mol) at post-intervention and 8.7% (72 mmol/mol) in the attention control group (p = 0.020), after adjusting for baseline differences. Similarly, a significant difference between groups at 1-month post-intervention follow-up was found (I M = 7.7% [61 mmol/mol] vs. C M = 9.0% [75 mmol/mol], p = 0.005).
There were no significant differences between groups in change over time for either physical health-related quality of life using the PCS (p = 0.678), or mental health-related quality of life using the MCS (p = 0.154).
Change Over Time for Family Members
Table 3 provides findings for family members. There were significant differences in change over time between groups in SKILLD diabetes knowledge scores (p < 0.001), A1C (p = 0.002) and physical health-related quality of life using the PCS (p = 0.001). Specifically, mean diabetes knowledge SKILLD scores were 7.4 for the intervention group and 5.4 for controls at post-intervention (p = 0.001), and they remained significantly higher at 1-month post-intervention follow-up (I M = 6.5 vs. C M = 5.3, p = 0.027). Despite a significant omnibus test for change of time, A1C did not significantly differ between groups at post-intervention (p = 0.805), 1-month follow-up (p = 0.218), or at 6-month follow-up (p = 0.153). For physical health-related quality of life using the PCS, mean scores were significantly higher (p = 0.005) at 6-month follow-up for intervention family members (M = 54.5) than controls (M = 50.1).
Table 3.
Impact of family-based diabetes intervention on change in family member outcomes over time
| Time-Point1 | Overall2 P |
Pairwise comparison P for I vs. C3 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention (1) | Post-Intervention (2) | 1-Month Follow-up (3) |
6-Month Follow-up (4) |
||||||||||
| I | C | I | C | I | C | I | C | 2 vs. 1 | 3 vs. 1 | 4 vs. 1 | |||
| SKILLD diabetes knowledge (KR-20=.73) |
2.0 (1.5, 2.6) |
3.0 (2.4, 3.6) |
7.4 (6.6, 8.2) |
5.4 (4.6, 6.2) |
6.5 (5.8, 7.2) |
5.3 (4.5, 6.0) |
6.1 (5.4, 6.7) |
5.5 (4.7, 6.3) |
<0.001 | <0.001 | 0.027 | 0.283 | |
| IPAQ MET-min/wk. |
5213.8 (3442.5, 6985.1) |
5696.4 (3715.4, 7677.5) |
5501.1 (3785.1, 7217.0) |
6103.3 (4215.6, 7991.0) |
5615.0 (3811.1, 7418.9) |
6264.6 (4275.8, 8253.4) |
6185.9 (3355.7, 9016.1) |
7073.1 (3848.2, 10298.0) |
0.809 | 0.377 | 0.462 | 0.981 | |
| BRFSS fruits & veggies consumption | 2.6 (2.5, 2.7) |
2.6 (2.5, 2.8) |
2.8 (2.7, 2.9) |
2.7 (2.6, 2.9) |
2.8 (2.7, 2.9) |
2.7 (2.6, 2.9) |
2.8 (2.6, 2.9) |
2.8 (2.6, 2.9) |
0.709 | 0.463 | 0.489 | 0.921 | |
| A1C (%) | 5.9 (5.5, 6.3) |
6.0 (5.6, 6.5) |
5.9 (5.6, 6.1) |
5.9 (5.6, 6.2) |
5.7 (5.5, 6.0) |
6.0 (5.7, 6.3) |
6.0 (5.7, 6.3) |
5.7 (5.3, 6.0) |
0.002 | 0.805 | 0.218 | 0.153 | |
| A1C (mmol/mol) |
41 (37, 45) |
42 (38, 48) |
41 (38, 43) |
41 (38, 44) |
39 (37, 42) |
42 (39, 45) |
42 (39, 45) |
39 (34, 42) |
0.002 | 0.805 | 0.218 | 0.153 | |
| SF-12v2 PCS physical HRQOL (αc=.82) |
51.9 (50.6, 53.3) |
52.8 (51.2, 54.3) |
52.7 (51.3, 54.1) |
52.0 (50.4, 53.5) |
53.0 (51.5, 54.5) |
51.6 (50.0, 53.3) |
54.5 (52.4, 56.6) |
50.1 (47.6, 52.5) |
0.001 | 0.273 | 0.144 | 0.005 | |
Note. Results are based on mixed-effect models for outcome with IPW propensity score adjustment (except ranks of IPAQ tested).
Numbers presented are model-based least-squares means using median time from pre-intervention, (95% CI for means). Median time from pre-intervention to post-intervention, 1-month follow-up, and 6-month follow-up were 2.5 months, 3.5 months, and 8.8 months, respectively;
P = Overall p-value is for omnibus test (group by time interaction) for any differences in change over time between groups;
P-value from testing difference in least-square mean estimates in post-hoc pairwise comparisons with baseline/pre-intervention.
Discussion
The 8-session, 9 month-long culturally tailored, family-based intervention produced significant improvements in A1C and diabetes knowledge from baseline to post-intervention and 1-month follow-up; and significantly greater improvements in diabetes self-efficacy at post-intervention among the intervention group than the control group although the improvements were not maintained at 6-month follow-up. While there were no significant patient differences in diabetes self-management, physical activity, or health-related quality of life, positive trends were noted in the intervention group in physical activity, diabetes self-management (general diet and self-checking of feet), family support and the physical component of health-related quality of life. Family support was significantly higher in the attention control group at 1-month follow-up, than in the intervention group on average, but it was significantly higher at baseline, before sessions were conducted. A1C level decreased from baseline to 6-month follow-up in the control group although there was no significant difference between the groups at this time.
The intervention group family members improved their knowledge about diabetes at post intervention and 1-month follow-up relative to pre-intervention significantly more than the control groups; and their mean scores on the physical component of health-related quality of life at 6-month follow-up were also significantly higher relative to pre-intervention than family members in the control group.
The effects of the family-based intervention for diabetes self-management were noteworthy. In 2010–2014, 33.2% to 44.9% of Hispanics were without health insurance, and unable or delayed in receiving needed medical care or medications.36 Our study showed short-term clinical improvements in glycemic control with a decrease in A1C of −0.8% (−8.7 mmol/mol), which is clinically significant for behavioral programs.37 The control group also had a decrease in A1C, by −1.2% (−13.1 mmol/mol) at 6 months, but started out significantly higher at baseline. According to the United Kingdom Prospective Diabetes Study, every 1% of decrease in A1C is associated with a 35% reduction in diabetes-related complications.38
The findings of our study are consistent with those of other community-based intervention studies of Hispanics, which have shown a reduction in A1C by −0.69 to −0.85%.12 Many factors may have contributed to the lack of sustained improvement in A1C in our study. One factor might have been the improvement in glycemic control in the attention control group, which is consistent with previous study finding that participants in control groups demonstrated improvements in A1C in clinical trials.7 Strategies for sustaining glycemic control need to be developed in future intervention studies, including possible reinforcement over time.
Participants in our study were characterized by low-income, low education, lack of health insurance, and uncontrolled diabetes status; and they showed poor knowledge of diabetes at baseline. Using bilingual/bicultural interventionists and teaching strategies targeted at low literacy participants with diabetes experienced a 187% increase in average diabetes knowledge score from baseline to post-intervention and a 157% increase from baseline to 1-month follow-up. For their family members, average knowledge scores increased 270% from baseline to post-intervention and 225% at 1-month follow-up. Both participants and family members sustained higher knowledge scores but differences from the control group at 6 month follow-up were not significant. It should be noted, however, that the attention control group started with a significantly higher average score on knowledge of diabetes for both participants (p = 0.011) and their family members (p = 0.020) and reported receiving information on diabetes prior to beginning the study. The findings of our study suggest that providing educational programs at a low literacy level and in Spanish to Hispanics can enhance their understanding of the importance of glycemic control, lifestyle modification and recognition of diabetes related symptoms and complications in diabetes.
Our family-focused intervention incorporated cultural values (familismo) in facilitating stress management, problem solving, confidence in diabetes self-management and support in the context of family enhanced diabetes self-efficacy and family support. Self-efficacy was significantly increased by 33% at post-intervention in the intervention group on average. This is consistent with literature that social support, including family support, leads to improved self-efficacy.9 Although the attention control group had significantly higher scores on family support than the intervention group at 1-month follow-up, family support in the intervention group showed positive trends in improvements over time, despite having significantly lower scores at baseline. For example, differences between groups at post-intervention were not significant and also were comparable at 6 month follow-up relative to the attention control group. The fact that more participants in the attention control group in the current study were recruited in church-based sites, where support has already been provided, for example, spiritual support, information on health, preventive screenings, lectures on health topics39, might help to explain the difference between groups in family support. Strategies to sustain self-confidence in managing diet, exercise, controlling over diabetes and facilitate family support are needed.
Diabetes self-management activities including physical activities, diet and medications, were not improved in the current study. However, there were positive trends in physical activity measured as MET-min/wk; activity had increased 9.5% on average at 1-month follow-up and 23% at 6-month follow-up. General diet showed a 42% change at 1-month follow-up on average and average self-checking of feet scores showed a 112% change at 6-month follow-up in the intervention group. Our intervention used pedometers, daily pictorial logs for behavioral checklists40, and ethnic food models and pictures of food to facilitate change, physical activities and diet. Despite our strategies the changes were not statistically significant. Possible explanation for the non-significant finding in physical activity may be related to the high levels of overweight and obesity found among the sample as well as the sedentary activity level at baseline. Additionally, the cost of fresh vegetables and fruits may not have been economically feasible for this sample comprised mostly of low-income Hispanics. Challenges in improving and sustaining physical activity and diet in Hispanic populations with diabetes have also been reported in previous studies.7,9 Environmental factors, for example, family, ethnic food preference16, home, neighborhood, jobs and the social environment may prevent Hispanics with diabetes from participating in physical activity and following a healthy diet.9 Thus an ecological approach to support lifestyle changes may be required, using efforts at community, organizational and policy levels. Further research is needed to gain understanding of intervention strategies that will result in sustained healthy lifestyle changes among Hispanics.
Participants in the study had lower average scores on both the physical and mental health of the health-related quality of life at baseline than the general national norm scores.41 Mean scores of perceived physical component of the health-related quality of life had a 7.7% change in the intervention group at 6 months follow-up for participants and a 5% change for their family members. Hispanics consistently have perceived poorer health-related quality of life than Whites. Further, diabetes-related complications can negatively influence health-related quality of life.42 Our intervention, which emphasized family support and promotion of physical activity and diet may have had positive effects on health-related quality of life. The lack of significant changes in the patients may have been associated with severity of disease and complications of diabetes compounded by socioeconomic status.42
The study used a quasi-experimental design since randomization was not feasible. Such a design is not without limitations, but this study suggests efficacy of the intervention over 9 months of follow-up.
Implications
This intervention utilized multiple strategies to culturally tailor diabetes education and make it accessible to a highly vulnerable and at risk population. As has been noted, language translation of content is only one method to provide cultural tailoring. The use of simple language, demonstrations and easy to use tools like pedometers and food logs contribute to improved outcomes.43
In this study, the use of bilingual and bicultural team members provided direct linkages for recruitment, retention and trust building. Our 9-month intervention had high participation and low attrition rates. Inclusion of family members also played an important role as they benefited from the intervention as well. In fact, even participants and family members in the attention control group improved outcomes, perhaps attenuating the result of the intervention for particular outcomes. This findings suggest the importance of ensuring that health education, information, and outreach screening programs and activities are provided throughout the community. Partnerships with churches, providers, social and cultural organizations, have also shown positive outcomes39, and may provide sustainable opportunities for intervention that are less costly than formal health system service provision. More importantly, multilevel interventions similar to this one, which included both the individual and family have a better chance of achieving positive outcomes both in the short term and long term.
Future efforts in both research and practice to prevent diabetes and improve the health of those suffering from diabetes will require intimate understanding and immersion in the community of interest. Further, inclusion of multiple levels of intervention and appropriate methods will be important to delay the consequences of diabetes among the growing Hispanic population. The next steps are to develop sustainability avenues within systems and communities and to evaluate the cost-benefit of the intervention.
Contributor Information
Jie Hu, The Ohio State University, Columbus, OH.
Karen A. Amirehsani, The University of North Carolina at Greensboro, School of Nursing.
Debra C. Wallace, The University of North Carolina at Greensboro, School of Nursing.
Thomas P. McCoy, The University of North Carolina at Greensboro, School of Nursing.
Zulema Silva, The University of North Carolina at Greensboro, School of Nursing.
References
- 1.World Health Organization. Prevalence of diabetes. 2011 Available from http://www.who.int/diabetes/actionnow/en/mapdiabprev.pdf. Accessed October 30, 2015.
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 3.Dominguez K, Penman-Aguilar A, Chang M-H, Moonesinghe R, Castellanos T, Rodriguez-Lainz A, et al. Vital signs: Leading causes of death, prevalence of diseases and risk factors, and use of health services among Hispanics in the United States—2009–2013. MMWR Morbidity and Mortality Weekly Report. 2015;64:469–78. [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Foundations of care: education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Sec. 4. In Standards of Medical Care in Diabetes 2015. Diabetes Care. 2015;38:S20–S30. doi: 10.2337/dc15-S007. [DOI] [PubMed] [Google Scholar]
- 5.Agency for Healthcare Research and Quality [AHRQ] 2010 National Healthcare Disparities Report. Rockville, MD: U.S. Department of Health and Human Services; 2011. (AHRQ Publication No. 11-0005). [Google Scholar]
- 6.Mier N, Smith ML, Carrillo-Zuniga G, Wang X, Garza N, Ory MG. Personal and cultural influences on diabetes self-care behaviors among older Hispanics born in the U.S. and Mexico. J Immigr Minor Health. 2012;14:1052–62. doi: 10.1007/s10903-012-9639-x. [DOI] [PubMed] [Google Scholar]
- 7.Rosal MC, Ockene IS, Restrepo A, White MJ, Borg A, Olendzki B, et al. Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management Intervention for Low-Income Latinos: Latinos en Control. Diabetes Care. 2011;34:838–844. doi: 10.2337/dc10-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long JM, Sowell R, Bairan A, Holtz C, Curtis AB, Fogarty KJ. Exploration of commonalities and variations in health related beliefs across four Latino subgroups using focus group methodology: implications in care for Latinos with type 2 diabetes. J Cult Div. 2012;4:133–142. [PubMed] [Google Scholar]
- 9.Toobert DJ, Strycker LA, King DK, Barrera M, Osuna D, Glasgow RE. Long-term outcomes from a multiple-risk-factor diabetes trial for Latinas: ¡Viva Bien! ransl Behav Med. 2011;1:416–426. doi: 10.1007/s13142-010-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King DK, Glasgow RE, Toobert DJ, Strycker LA, Estabrooks PA, Osuna D, et al. Self-efficacy, problem solving, and social-environmental support are associated with diabetes self-management behaviors. Diabetes Care. 2010;33:751–753. doi: 10.2337/dc09-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prezio EA, Cheng D, Balasubramanian BA, Shuval K, Kendzor DE, Culica D. Community Diabetes Education (CoDE) for uninsured Mexican Americans: A randomized controlled trial of a culturally tailored diabetes education and management program led by a community health worker. Diabetes Res Clin Pract. 2013;100:19–28. doi: 10.1016/j.diabres.2013.01.027. r. [DOI] [PubMed] [Google Scholar]
- 12.Spencer MS, Rosland A-M, Kieffer EC, Sinco BR, Valerio M, Palmisano G, et al. Effectiveness of a community health worker intervention among African American and Latino adults with Type 2 diabetes: A Randomized controlled trial. Am J Public Health. 2011;101:2253–2260. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valen MS, Narayan S, Wedeking L. An innovative approach to diabetes education for a Hispanic population utilizing community health workers. J Cult Divers. 2012;19:10–17. [PubMed] [Google Scholar]
- 14.Rosland AM, Heisler M, Choi HJ, Silveira MJ, Piette JD. Family influences on self-management among functionally independent adults with diabetes or heart failure: do family members hinder as much as they help? Chronic Illn. 2010;6:22–33. doi: 10.1177/1742395309354608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesla CA, Fisher L, Skaff MM, Mullan JT, Kanter R. Family predictors of disease management over on year in Latino and European American patients with type 2 diabetes. Family Process. 2003;42:375–390. doi: 10.1111/j.1545-5300.2003.00375.x. [DOI] [PubMed] [Google Scholar]
- 16.Denham SA, Ware LJ, Raffle H, Leach K. Family inclusion in diabetes education: A nationwide survey of diabetes educators. Diabetes Edu. 2011;37:528–535. doi: 10.1177/0145721711411312. [DOI] [PubMed] [Google Scholar]
- 17.Denham SA. Family Health: A Framework for Nursing. Philadelphia, PA: FA Davis; 2003. [Google Scholar]
- 18.Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans the Starr county border health initiative. Diabetes care. 2002;25:259–268. doi: 10.2337/diacare.25.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuart EA, Rubin DB. Best practices in quasi-experimental designs: Matching methods for causal inference. In: Osborne J, editor. Best Practices in Quantitative Methods. Thousand Oaks, CA: Sage; 2007. pp. 155–176. [Google Scholar]
- 20.Hu J, Wallace DC, McCoy TP, Amirehsani KA. A family-based diabetes intervention for Hispanic adults and their family members. Diabetes Educ. 2014;40:48–59. doi: 10.1177/0145721713512682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bode BW, Irvin BR, Pierce JA, Allen M, Clark AL. Advances in hemoglobin A1C point of care technology. J Diabetes Sci Technol. 2007;1:405–411. doi: 10.1177/193229680700100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman RL. The Spoken Knowledge in Low Literacy in Diabetes Scale: A diabetes knowledge scale for vulnerable patients. Diabetes Educ. 2005;31:215–224. doi: 10.1177/0145721705275002. [DOI] [PubMed] [Google Scholar]
- 23.Eakin EG, Reeves MM, Bull SS, Floyd S, Riley KM, Glasgow RE. Validation of the Spanish-language version of the chronic illness resources survey. Int J Behav Med. 2007;14:76–85. doi: 10.1007/BF03004172. [DOI] [PubMed] [Google Scholar]
- 24.Stanford Patient Education Research Center. Self-efficacy for diabetes tool. Palo Alto CA: 2008. http://patienteducation.stanford.edu/research/sediabetes.html. [Google Scholar]
- 25.Vincent D, McEwen MM, Pasvogel A. The validity and reliability of a Spanish version of the summary of diabetes self-care activities questionnaire. Nurs Res. 2008;57:101–106. doi: 10.1097/01.NNR.0000313484.18670.ab. [DOI] [PubMed] [Google Scholar]
- 26.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Oja P. International Physical Activity Questionnaire (IPAQ): 12-country reliability and validity. Med Sci Sports Exercs. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) Behavioral risk factor surveillance system survey data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 28.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Berglund P, Heeringa S. Multiple imputation of missing data using SAS. Cary, N.C: SAS Institute; 2014. [Google Scholar]
- 30.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015 doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margolis DJ, Gupta J, Hoffstad O, Papdopoulos M, Glick HA, Thom SR, Mitra N. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care. 2013;36:1961–1966. doi: 10.2337/dc12-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brookhart MA. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen HQ, Ackermann RT, Berke EM, Cheadle A, Williams B, Lin E, LoGerfo JP. Impact of a managed-medicare physical activity benefit on health care utilization and costs in older adults with diabetes. Diabetes Care. 2007;30:43–48. doi: 10.2337/dc06-1013. [DOI] [PubMed] [Google Scholar]
- 34.Joffe MM, Ten Have TR, Feldman HI, Kimmel SE. Model selection, confounder control, and marginal structural models: review and new applications. Am Stat. 2004;58:272–279. [Google Scholar]
- 35.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd. Hoboken, New Jersy, NJ: Wiley; 2011. [Google Scholar]
- 36.Agency for Healthcare Research and Quality [AHRQ] 2014 National Healthcare Quality and Disparities Report chartbook on access to health care. 0007. Rockville, MD: 2015. pp. 1–EF. [Google Scholar]
- 37.Pillay J, Chordiya P, Dhakal S, Vandermeer B, Hartling L, Armstrong MJ, Butalia S, Donovan LE, Sigal RJ, Featherstone R, Nuspl M, Dryden DM. Behavioral programs for diabetes mellitus: Evidence report/technology assessment no. 221. E003. Rockville, MD: Agency for Healthcare Research and Quality; 2015. p. EF. [Google Scholar]
- 38.Srimanunthiphol J, Beddow R, Arakaki R. A review of the United Kingdom Prospective Diabetes Study (UKPDS) and a discussion of the implications for patient care. Hawaii Med J. 2000;59:295–298. [PubMed] [Google Scholar]
- 39.Baig AA, Locklin CA, Wilkes AE, Oborski DD, Acevedo JC, Gorawara-Bhat R, et al. Integrating diabetes self-management interventions for Mexican-Americans into the catholic church setting. J Relig Health. 2014;53:105–18. doi: 10.1007/s10943-012-9601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia AA, Brown SA, Wincheil M, Hanis CL. Using the behavioral checklist to document diabetes self-management behaviors in the Starr county diabetes education study. Diabetes Educ. 2003;29:758–766. doi: 10.1177/014572170302900508. [DOI] [PubMed] [Google Scholar]
- 41.Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12® Health Survey (With a Supplement Documenting Version 1) Lincoln, RI: Quality Metric, Inc.; 2004. [Google Scholar]
- 42.Graham JE, Stoebner-May DG, Ostir GV, Al Snih S, Peek MK, Markides K, et al. Health related quality of life in older Mexican Americans with diabetes: A cross-sectional study. Health Qual Life Outcomes. 2007;5:39. doi: 10.1186/1477-7525-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coffman MJ, Ferguson BL, Steinman L, Talbot LA, Dunbar-Jacob J. A health education pilot for Latina women with diabetes. Clin Nurs Res. 2012:70–81. doi: 10.1177/1054773812451746. [DOI] [PubMed] [Google Scholar]