Abstract
With novel genetic technologies available, there is a paradigm shift in the way that risk assessments, diagnoses and therapies for genetic susceptibility syndromes are addressed. Hereditary pancreatitis is among these conditions, for which genetic counseling and next generation sequencing, help families better understand, cope with and live healthier lives. Identifying a genetic etiology to a condition formally believed to be solely environmental induced can alter the path for treatment for many patients. This finding introduces the concept of gene-environment interactions in human disease and the relationship between genetic predisposition and exposure risk in disease development. The genetic counseling process is complex with medical explanations, psychosocial issues relating to coping with diagnosis, potential future health problems, recurrence risks and family planning. These sometimes difficult conversations can be facilitated by a genetic counselor as a member of the multidisciplinary team. This chapter addresses the intricate medical and psychosocial issues that can arise in the setting of treating patients with hereditary pancreatitis.
Keywords: Hereditary pancreatitis, acute pancreatitis, chronic pancreatitis, genetic counseling, genetic counselor, psychosocial, genetic testing, risk assessment, family history, PRSS1, SPINK1, CFTR, CTRC, CASR, personalized medicine, next generation sequencing, whole genome/exome sequencing, gene-environment interactions
Introduction
Acute pancreatitis (AP) and chronic pancreatitis (CP) are syndromes that describe the pathologic appearance of pancreatic tissue with inflammatory cells and evidence of severe short term or long term complications of the inflammatory process (1–3). Because the inflammatory response does not depend on the etiology or mechanism (4), these two related syndromes are classified by the diagnostic codes ICD9 577.0 and 577.1. This view of pancreatitis from a histological description, with the associated abdominal imaging appearance, is useful for diagnosing a syndrome, but not for predicting clinical course, directing therapy, or otherwise managing the patient.
Discovery that genetic variants, as well as different environmental factors can be strong risk factors for susceptibility and progression of AP, RAP and CP. This has provided leverage for unlocking the mystery of inflammatory diseases of the pancreas. A new paradigm has emerged that views tissue pathology as a biomarker of a variety of complex gene-environment interactions that cause injury and lead to inflammation and its consequences. Thus, the pathologic diagnosis does not define the true disorder. This new paradigm is emerging as careful evaluation and comparison is made between thousands of patients enrolled in major translational studies in the United States, Europe and other locations.
The North American Pancreatitis Study 2 (NAPS2) (5) and other prospective cross sectional cohort and population studies demonstrate that alcohol plays a smaller role than previously thought, that smoking is an equally important, and synergistic risk factor for CP, and that genetic susceptibility factors are common in patients with RAP and CP, but they are often in complex combinations. The recognition of the variety of syndromes related to different genetic factors in RAP and CP requires more personalized assessment and care.
Genetic factors in pancreatitis
Genetic variants in five genes have been identified as being associated with susceptibility to CP and replicated in multiple cohorts (6–8). There are clearly additional genetic variants being evaluated and replicated which will likely be added to this list in the future. Currently, all of the major genetic susceptibility factors center on the control of trypsin activity within the pancreas (7), in part because they were identified as candidate genes linked to intra-pancreatic trypsin control. The five replicated genetic factors including the cationic trypsinogene gene (PRSS1) the pancreatic secretory trypsin inhibitor gene (SPINK1), the cystic fibrosis transmembrane conductance regulator gene (CFTR), the chymotrypsinogene gene (CTRC) and the calcium sensing receptor (CASR). In this review we focus on hereditary pancreatitis and the clinical implications of identifying genetic variants related to hereditary pancreatitis. The complexities of such results bring the importance of genetic counseling related to testing, the psychological aspects of genetic testing, and the role of genetic counselors as health care extenders for multidisciplinary teams.
Genetic Counseling and Genetic Testing
Genetic counselors are graduate level healthcare professionals who work within a multidisciplinary team to offer risk assessment and genetic testing, discussion of genetic test results as well as counseling services to patients and their families. Genetic counseling is the process of helping people understand and adapt to the medical, psychological and familial implications of genetic contributions to disease. The goal of the genetic counselor begins with integration of family and medical histories and to help assess the incidence of variable phenotypic expression of disease features within a patient and the patient’s family. Secondly, they help educate the patient about inheritance patterns, environmental factors, genetic testing, prevention, resources and research as well as counseling to promote informed choices and adaptation to the risk or condition. In addition they play a major role as a resource to the physician for the evaluation of genetic test results and provide resources for complex decision-making.
Hereditary pancreatitis
Hereditary pancreatitis is an unusual form of RAP and CP that begins in childhood (median age of onset, 10 years), chronic pancreatitis in young adulthood, and high risk of pancreatitis cancer beginning in the fifth decade of life (9–11). The disease gene is typically cationic trypsinogen (PRSS1) with a limited number of gain-of-function mutations. The most common are p.A16V, p.N29I, and p.R122H, although a couple dozen sequence have been reported (11). In addition, copy number variants (CNV) are clearly associated with hereditary pancreatitis (12). The disorder has been well described, and serves as an important disorder for discussing many of the critical issues related to genetic counseling in pancreatic diseases (13, 14).
The complexity of genotypes and familial, medical, social, ethical and legal issues related to genetic testing of patients with pancreatic disease justifies incorporation of a genetic counselor into multidisciplinary teams dealing with pancreatitis and pancreatic cancer patients. Furthermore, they can serve as valuable health care extenders.
A genetic counseling session for hereditary pancreatitis includes interviewing the patient about personal medical history and obtaining at least a three generation pedigree. The genetic counselor obtains information about vital status, current age or age of death for all relatives. Health information in the family includes history of pancreatitis, age of onset and diagnosis ages (if multiple attacks). Medical records and laboratory data of lipase and amylase values are obtained when possible to confirm/document pancreatitis attacks in the family. Any history of pancreatic cancer in a relative along with age of diagnosis is documented. It is also important to collect information about diabetes, exocrine insufficiency, male infertility, chronic sinusitis/nasal polyps and presence of cystic fibrosis in the family as well as smoking and alcohol abuse histories.
Risk assessment is based on the information provided in the pedigree and the observed pattern of disease in the generations. Discussions ensue at this point during the genetic counseling session, including education defining genes, chromosome, inheritance patterns and the differential diagnoses.
In our practice, genetic testing is offered when specific disease features or symptom combinations (i.e syndromes) are already present. Genetic testing for hereditary or familial pancreatitis is recommended for patients with recurrent acute or chronic pancreatitis and a family history of pancreatitis, patients with early onset, unexplained pancreatitis often before the age of 25 and asymptomatic family members of a known mutation carrier.
Issues surrounding genetic testing differ quite substantially from standard laboratory testing. Genetic test results have implications beyond the patient’s current diagnosis. There may be risks for asymptomatic relatives, for future generations and even for the future health of the patient. Many of these issues require intensive discussions and counseling due to the psycho-social nature of the significance of genetic test results. However, with assurances against insurance discrimination and wider acceptance of genetic testing the anxiety and reluctance of patients is now much less than what was seen in the past.
Psychosocial Aspects to Genetic Testing
Issues surrounding consent for genetic testing in adults differs from that of children because there is greater perspective, experience and ability to make better, informed decisions. Decisions in younger patients require more care and consideration. Practice guidelines are established for the consideration of psychosocial implications in testing children and adolescents for genetic conditions. The psychosocial impact includes timing of testing as it relates to disease onset, anxiety, depression and self image for the child or adolescent and the consideration of balancing benefits and harms prior to testing. It is also important to consider the family’s involvement in the decision making process. Genetic counselors should educate and counsel the parents and child about the medical and psychosocial issues. Another important consideration is to discuss obtaining assent from the child, when appropriate (15).
Families report feeling misunderstood, misdiagnosed or even denied care from healthcare providers unaware of medical issues relating to rare syndromes such as hereditary pancreatitis. For this reason, it is critical to educate patients about documenting the diagnosis and obtaining medical records for proof of diagnosis.
Recurrence risks/family planning
Recurrence risk describes the chance that a genetic syndrome will occur in the offspring of the affected individual or carrier parents. Recurrence risk is dependent on the genotype in the family along with environmental exposures which can exacerbate symptoms. PRSS1 mutations are inherited in an autosomal dominant pattern, while mutations in CFTR, SPINK1 and CTRC can be inherited in multiple modalities and are dependent on co-transmission of mutations. This information can be complex, emotional and overwhelming to families coping with the initial diagnosis for themselves, when thinking about future family planning. Genetic counseling is strongly suggested during these conversations. Prenatal diagnosis and pre-implantation genetic diagnosis and in vitro fertilization for hereditary pancreatitis has been offered through specific laboratories and fertility centers across the US, however thorough pre-test genetic counseling and medical evaluations are strongly encouraged, including the facts that there are incomplete penetrance, variable expression and improving treatment. In addition, testing leads to important moral issues surrounding human life that must be considered and respected.
Direct to consumer testing
Some genetic laboratories market genetic tests directly to the patient population. Patients undergoing their own genetic testing may provide such results to the clinic with expectation of clinical utility. It is important to be aware of such testing and to educate patients about the risks and benefits of pursuing such tests on their own, without genetic counseling prior to initiating testing. Furthermore, the quality and reliability of these test is not always known. Therefore, if there is any doubt or concern, a repeated test linked to appropriate counseling may be needed.
Future Opportunities for Families
Personalized medicine technologies including whole exome sequence analysis are coming to the forefront of healthcare. Complete genotyping offers clinicians an opportunity to establish connections between low penetrant genes and complex diseases such as pancreatitis. Further, the data from next generation sequencing technologies may provide options for personalized treatment and even prevention of disease altogether.
Next Generation Sequencing
Traditional DNA sequencing was typically done using the Sanger method with each exon or DNA strand being amplified by specific PCR primers, and read by an expert technician. Next Generation Sequencing (NGS) is an emerging technique that takes advantage of massive parallel sequencing linked with a series of computational steps that lead to high-quality DNA sequence variant calls. Several commercial approaches to whole exome sequencing and whole genome sequencing have produced innovative approaches and rapidly dropping costs. NGS provides a time- and cost-effect method for obtaining the DNA sequence of large genes and enables the assessment of many exons in many genes for the same cost as one gene. This approach allows investigators to rapidly screen all the genes that contribute to a pathologic pathway or disease process. It is expected that NGS will be used to evaluate all of the exons in the five replicated pancreatitis susceptibility genes, and new genes that are yet to be defined.
There are several disadvantages of NGS (16). The first is that there are occasional errors in the DNA sequence reading. To overcome this limitation, the entire exome, or genome, is sequenced many times over (e.g., 50×) to more accurately determine the presence of true sequence variants, which consistently lowers the error rate to less than 5%. The second problem is how to evaluate an entire genome. This can be overcome by “masking” the majority of data, and focusing on specific disease-associated genes, stratifying the observed variants according to potential importance, and verify the variants using standard Sanger sequencing. The third problem is the discovery of variants of unknown significance. In many cases these may be benign polymorphisms. Unless the variant has been shown to have functional significance, or is associated with pathology in larger cohorts (cases and controls) then it is difficult to determine whether or not a variant is associated with the pancreatitis phenotype. The answer to this final question is complex, because there will likely be a variety of ways to provide some level of confidence that a variant is, or is not part of the disease process. Over time, new data may suggest the variant as a true mutation or as a polymorphism and it is uncertain whether the testing laboratory or the clinician will bear the responsibility to report such updates to the patient. Policies for such follow-up reporting are not in placed at this time.
Complex genotypes - splitting
Hereditary pancreatitis was chosen as a well-defined syndrome with well-defined genetic risk factors. A much larger fraction of patients with pancreatitis have mutations in the other susceptibility genes, especially CFTR and SPINK1. Table 1 illustrates the complex genotypes and the simple or complex phenotypes (syndromes) that are linked with combinations of these genes.
Table 1.
Examples of genotype-phenotype correlation and multi-organ syndromes.
| Genotype (variants) | Phenotype (syndromes) | Comment |
|---|---|---|
| PRSS1 | hereditary pancreatitis | Genetic counseling recommended |
| CFTRsev/CFTRsev | cystic fibrosis (CF) | Manage with a CF center |
| CFTRsev/CFTRm-v | atypical CF | Manage with a CF center |
| SPINK1/SPINK1 | familial pancreatitis | Usually progresses to severe CP |
| CFTRbicarb/CFTRany | Pancreas/Sinus/CBAVD | Newly defined syndrome |
| CFTRany/SPINK1 | RAP / CP | Pancreas only |
| CTRC/SPINK1 | RAP / CP | Pancreas only – not well studied |
| CASR / SPINK1 | RAP/CP | Pancreas only – not well studied |
CFTR: sev=severe mutations (typically functional class I–III), m-v = mild-variable mutations, (typically CFTR functional class IV), bicarb = bicarbonate conductance disrupting variant (e.g. R75Q), any= either severe, mild-variable or bicarbonate disrupting variants.
The latest finding is that patients with common CFTR variants that were thought to be benign because they did not change sweat chloride concentrations or lead to lung disease are strongly associated with chronic pancreatitis. The prototype is CFTR R75Q, which acts by specifically disrupting bicarbonate, but not chloride conductance (17). This pancreas-targeting variant may also lead to chronic sinusitis and congenital bilateral absence of the vas deferens (CBAVD) – since all three of these organs utilize CFTR for bicarbonate secretion.
Mutations in SPINK1 are also complex. SPINK1 codes for the pancreatic secretory trypsin inhibitor, which is an acute phase protein that is a specific trypsin inhibitor that is expressed in the pancreatic acinar cells following the development of inflammation (18). Thus, it is not a susceptibility factor for acute pancreatitis, but rather a modifier of the disease process linking trypsin activating susceptibility factors to recurrent injury and chronic pancreatitis (19–22). This biology is important, because it amplifies minimal risk factors such as heterozygous mutations in CFTR (17, 23), CTRC and CASR (8, 24), to the point that they become disease associated. While observed genotypes are listed in Table 1, the implications of these genotypes has not been fully sorted out.
Complex genotypes - lumping
Figure 1 is a diagram that summarize the location at which trypsin is at greatest risk of becoming activated depending on the underlying problem. Within the acinar cell most of the risk is associated with calcium dysregulation, and can include both environment factors as well as trypsin related mutations (25). On the other hand risk of trypsin activation can also be associated with alterations in the pancreatic duct with the most important being CFTR. While it is unclear where the calcium sensing receptor variance are increasing the risk of pancreatitis it is interesting to note that the loss of function mutations become pathologic in the presence of SPINK mutations whereas the gain of function mutations are associated with alcoholic pancreatitis, suggesting that alcohol associated pancreatitis has a different mechanism for development of pancreatitis (8). Note also that SPINK mutations appear to enhance RAP and CP regardless of an origin in the duct cell or the acinar cell. The reason that this model is important is that it provides a conceptual paradigm from which to think about potential therapies. Within the acinar cells dysregulation of calcium leading to intracellular hypercalcemia might be targeted by strategies to reduce the magnitude of calcium fluctuations. On the other hand the problems within the duct cell are more hydrodynamic since the purpose of the duct is to quickly flush enzymes out of the pancreas. Therefore either proximal failure to initiate sufficient fluid volume and hydrostatic pressure, or more distal problems of high resistance to flow can work independently or synergistically to diminish the rate and effectiveness of flushing the digested enzymes out of the pancreas. In addition there appears to be protective mechanisms associated with duct function, including sensors to calcium concentration, sensors to trypsin activation, pancreas activated receptors (PAR 2 and PAR 4) (26) and alterations of pH (25, 27).
Figure 1.
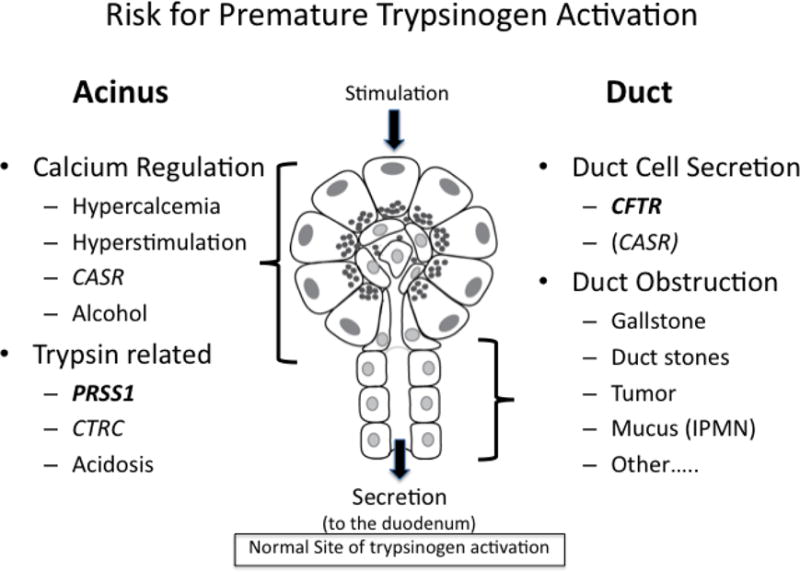
Risk factors for premature trypsinogen activation during the stimulation-secretion process classified by hypothesized site of their effect within the acinar cells (acinus) or pancreatic duct. Classification of risk according to mechanism and location may allow for rational therapeutic approaches to groups of risk factors.
It is becoming more and more apparent that proper management of complex disorders such as chronic pancreatitis requires expert physicians who can tease out the nuances of the disease and work at a multidisciplinary fashion to not only provide effect insights into pancreatic treatment. There is also an important role for physician extenders with expertise in both genetics and counseling to provide appropriate education and counsel to the family about the importance of genetics from the standpoint of Mendelian and non-Mendelian (complex) traits.
Summary.
The recognition of the role of genetic factors in pancreatic disease and the modifying effects of environmental factors has changed the focus of our understanding of pancreatitis from making a histological diagnosis to understanding and intervening in the underlying mechanism. Relating this new information to the patients through the use of genetic counselors as part of a multidisciplinary approach is an important aspect of optimal patient care. In the future, as more sophisticated testing and risk calculators are developed, patients will have even better opportunity for early detection of underlying pathology and effective treatment that is personalized to and target the mechanism in an individual patient.
Acknowledgments
Dr Whitcomb has been supported by the Wayne Fusaro Pancreatic Cancer Research Fund, The Frieda G. and Saul F. Shapira BRCA Cancer Research Program and the National Institutes of Health (DK061451, DK075803, DK054709).
References
- 1.Etemad B, Whitcomb DC. Chronic pancreatitis: Diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 2.Pandol SJ, Gukovsky I, Satoh A, Lugea A, Gukovskaya AS. Emerging concepts for the mechanism of alcoholic pancreatitis from experimental models. J Gastroenterol. 2003;38(7):623–8. doi: 10.1007/s00535-003-1134-7. [DOI] [PubMed] [Google Scholar]
- 3.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007 Apr;132(4):1557–73. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Shanbhogue AK, Fasih N, Surabhi VR, Doherty GP, Shanbhogue DK, Sethi SK. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics. 2009 Jul-Aug;29(4):1003–26. doi: 10.1148/rg.294085748. [DOI] [PubMed] [Google Scholar]
- 5.Whitcomb DC, Yadav D, Adam S, Hawes RH, Brand RE, Anderson MA, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2) Pancreatology. 2008;8(4–5):520–31. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JM, Ferec C. Chronic pancreatitis: genetics and pathogenesis. Annu Rev Genomics Hum Genet. 2009;10:63–87. doi: 10.1146/annurev-genom-082908-150009. [DOI] [PubMed] [Google Scholar]
- 7.Whitcomb DC. Genetic aspects of pancreatitis. Annu Rev Med. 2010;61:413–24. doi: 10.1146/annurev.med.041608.121416. [DOI] [PubMed] [Google Scholar]
- 8.Larusch J, Whitcomb DC. Genetics of pancreatitis. Curr Opin Gastroenterol. 2011 Sep;27(5):467–74. doi: 10.1097/MOG.0b013e328349e2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nature Genetics. 1996;14(2):141–5. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 10.Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2(3):252–61. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 11.Rebours V, Levy P, Ruszniewski P. An overview of hereditary pancreatitis. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2011 Sep 8; doi: 10.1016/j.dld.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Masson E, Le Marechal C, Delcenserie R, Chen JM, Ferec C. Hereditary pancreatitis caused by a double gain-of-function trypsinogen mutation. Hum Genet. 2008 Jun;123(5):521–9. doi: 10.1007/s00439-008-0508-6. [DOI] [PubMed] [Google Scholar]
- 13.Applebaum SE, O’Connell JA, Aston CE, Whitcomb DC. Motivations and concerns of patients with access to genetic testing for hereditary pancreatitis. American Journal of Gastroenterology. 2001;96(5):1610–7. doi: 10.1111/j.1572-0241.2001.03787.x. [DOI] [PubMed] [Google Scholar]
- 14.Ellis I, Lerch MM, Whitcomb DC, Committee C Genetic Testing for Hereditary Pancreatitis: Guidelines for indications, counseling, consent and privacy issues. Pancreatology. 2001;1(5):401–11. doi: 10.1159/000055840. [DOI] [PubMed] [Google Scholar]
- 15.Wilfond BS. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. American Society of Human Genetics Board of Directors, American College of Medical Genetics Board of Directors. American journal of human genetics. 1995 Nov;57(5):1233–41. [PMC free article] [PubMed] [Google Scholar]
- 16.Depristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011 May;43(5):491–8. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider A, Larusch J, Sun X, Aloe A, Lamb J, Hawes R, et al. Combined Bicarbonate Conductance-Impairing Variants in CFTR and SPINK1 Variants Are Associated With Chronic Pancreatitis in Patients Without Cystic Fibrosis. Gastroenterology. 2011 Jan;140(1):162–71. doi: 10.1053/j.gastro.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalid A, Finkelstein S, Thompson B, Kelly L, Hanck C, Godfrey TE, et al. A 93 year old man with the PRSS1 R122H mutation, low SPINK1 expression, and no pancreatitis: insights into phenotypic non-penetrance. Gut. 2006 May;55(5):728–31. doi: 10.1136/gut.2005.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfutzer RH, Barmada MM, Brunskill AP, Finch R, Hart PS, Neoptolemos J, et al. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000 Sep;119(3):615–23. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 20.Threadgold J, Greenhalf W, Ellis I, Howes N, Lerch MM, Simon P, et al. The N34S mutation of SPINK1 (PSTI) is associated with a familial pattern of idiopathic chronic pancreatitis but does not cause the disease. Gut. 2002;50(5):675–81. doi: 10.1136/gut.50.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoun E, Chang CC, Greer JB, Papachristou GI, Barmada MM, Whitcomb DC. Pathways to injury in chronic pancreatitis: decoding the role of the high-risk SPINK1 N34S haplotype using meta-analysis. PLoS ONE. 2008;3(4):e2003. doi: 10.1371/journal.pone.0002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoun E, Muddana V, Papachristou GI, Whitcomb DC. SPINK1 N34S is strongly associated with recurrent acute pancreatitis but is not a risk factor for the first or sentinel acute pancreatitis event. Am J Gastroenterol. 2010 Feb;105(2):446–51. doi: 10.1038/ajg.2009.630. [DOI] [PubMed] [Google Scholar]
- 23.Noone PG, Zhou Z, Silverman LM, Jowell PS, Knowles MR, Cohn JA. Cystic fibrosis gene mutations and pancreatitis risk: relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology. 2001;121(6):1310–9. doi: 10.1053/gast.2001.29673. [DOI] [PubMed] [Google Scholar]
- 24.Felderbauer P, Klein W, Bulut K, Ansorge N, Dekomien G, Werner I, et al. Mutations in the calcium-sensing receptor: a new genetic risk factor for chronic pancreatitis? Scand J Gastroenterol. 2006 Mar;41(3):343–8. doi: 10.1080/00365520510024214. [DOI] [PubMed] [Google Scholar]
- 25.Thrower EC, Gorelick FS, Husain SZ. Molecular and cellular mechanisms of pancreatic injury. Curr Opin Gastroenterol. 2010 Sep;26(5):484–9. doi: 10.1097/MOG.0b013e32833d119e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namkung W, Han W, Luo X, Muallem S, Cho KH, Kim KH, et al. Protease-activated receptor 2 exerts local protection and mediates some systemic complications in acute pancreatitis. Gastroenterology. 2004;126(7):1844–59. doi: 10.1053/j.gastro.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Bhoomagoud M, Jung T, Atladottir J, Kolodecik TR, Shugrue C, Chaudhuri A, et al. Reducing Extracellular pH Sensitizes the Acinar Cell to Secretagogue-Induced Pancreatitis Responses in Rats. Gastroenterology. 2009 May 18; doi: 10.1053/j.gastro.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]


