Among children born extremely preterm, those with severe FGR appear to be at increased risk of multiple cognitive and behavioral deficits at age 10 years.
Abstract
OBJECTIVES:
We sought to evaluate the relationships between fetal growth restriction (FGR) (both severe and less severe) and assessments of cognitive, academic, and adaptive behavior brain function at age 10 years.
METHODS:
At age 10 years, the Extremely Low Gestational Age Newborns Cohort Study assessed the cognitive function, academic achievement, social-communicative function, psychiatric symptoms, and overall quality of life of 889 children born before 28 weeks’ gestation. A pediatric epileptologist also interviewed parents as part of a seizure evaluation. The 52 children whose birth weight z scores were <−2 were classified as having severe FGR, and the 113 whose birth weight z scores were between −2 and −1 were considered to have less severe FGR.
RESULTS:
The more severe the growth restriction in utero, the lower the level of function on multiple cognitive and academic achievement assessments performed at age 10 years. Growth-restricted children were also more likely than their extremely preterm peers to have social awareness impairments, autistic mannerisms, autism spectrum diagnoses, difficulty with semantics and speech coherence, and diminished social and psychosocial functioning. They also more frequently had phobias, obsessions, and compulsions (according to teacher, but not parent, report).
CONCLUSIONS:
Among children born extremely preterm, those with severe FGR appear to be at increased risk of multiple cognitive and behavioral dysfunctions at age 10 years, raising the possibility that whatever adversely affected their intrauterine growth also adversely affected multiple domains of cognitive and neurobehavioral development.
What’s Known on This Subject:
No cohort study of later school-aged children born extremely preterm has examined the relationship between fetal growth restriction and executive function, adaptive behaviors, or quality of life.
What This Study Adds:
Among children born extremely preterm, those born with fetal growth restriction appear to be at increased risk of multiple cognitive and behavioral dysfunctions at age 10 years.
Children born at term weighing much less than expected for their gestational age are at greater risk of developmental limitations than their peers with birth weights appropriate for gestational age (AGA),1–4 seemingly even into adulthood.5–9 Children born very preterm are also at increased risk of developmental disorders.10–13 The combination of severe growth restriction and extremely preterm birth might result in so-called double jeopardy,14 placing children with both characteristics at especially high risk of developmental problems.15–19
Researchers in follow-up studies of children born extremely preterm have used just a handful of instruments and questionnaires to assess motor, cognitive, speech and language, hearing, vision, academic, and some behavioral problems or other symptoms typically at ∼5 years of age.11,20,21 Whereas motor function appears stable by ∼5 years old,22–26 deficits in other domains involving higher-order cognitive processes do not.27,28 No researchers in cohort studies of later school-aged children born extremely preterm have examined the relationship between fetal growth restriction (FGR) and executive function, adaptive behaviors, or quality of life. The large Extremely Low Gestational Age Newborns (ELGAN) Study cohort of infants born before 28 weeks’ gestation provided us opportunities to fill this void and evaluate the relationships between FGR (both severe and less severe) and assessments of cognitive, academic, and behavioral functioning at age 10 years.
Methods
Participants
The ELGAN Study is a multicenter, prospective, observational study of the risk of structural and functional neurologic disorders in extremely preterm infants.29 All women delivering before 28 weeks’ gestation at 1 of 14 participating institutions were asked to enroll in the study during years 2002 to 2004. All the children they delivered who survived to have a cranial ultrasound scan were included. A total of 1506 infants born before 28 weeks’ gestation were enrolled, and 1200 survived to 2 years, when 1102 of them had a developmental assessment.30 At age 10 years, of the 966 children who were eligible to be recruited for follow-up (because of the availability of data on inflammation-related proteins in blood samples from their first postnatal month), 889 (92%) returned for an assessment of cognition, executive functioning, behaviors, and achievement. Children who survived but did not participate were more likely at the time of birth than participants to have indicators of social disadvantage (lower maternal education and receipt of public health insurance), but there were no differences on sex, gestational age, or birth weight z score. Enrollment and consent procedures for this follow-up study were approved by the institutional review boards of all participating institutions. Our previous publications provide additional information about the ELGAN Study design,29 pregnancy disorders,31 microbiologic and histologic characteristics of the placenta,32 systemic inflammation in children born with FGR,33 and the age 10 years assessments.34
Newborn Variables
The gestational age estimates were based on a hierarchy of the quality of available information. The most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week (62%). When these were not available, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks (29%), last menstrual period (LMP) without fetal ultrasound (7%), and gestational age recorded in the log of the NICU (1%).
The birth weight z score is the number of SDs an infant’s birth weight is above or below the mean weight of infants of the same gestational age in referent samples not delivered for preeclampsia or fetal indications.35,36 Three study groups were formed according to birth weight z score category: <−2, ≥−2 and <−1, and ≥−1.
Procedures
Families who were willing to participate were scheduled for 1 visit, during which all of the measures reported here were administered in 3 to 4 hours, including breaks. The assessments were selected to provide the most comprehensive information about cognitive and academic function in 1 testing session. While the child was being tested, the parent or caregiver completed questionnaires regarding the child’s medical and neurologic status, language, behavior, and quality of life.
Cognitive Measures
We selected cognitive measures that are well validated and provide recently normed standard scores, allowing for comparison with US population norms. Details about the assessments of cognition and executive function (the Differential Ability Scales–II [DAS-II]37), Developmental Neuropsychological Assessment-II [NEPSY-II]38), language (Oral and Written Language Scales [OWLS]39), social and communication function (Social Communication Questionnaire [SCQ]40), and autism spectrum disorder (ASD) diagnosis are provided in our previous publications.34,41,42 Each cognitive subtest is described elsewhere.
Academic Function
The Wechsler Individual Achievement Test-III (WIAT-III [C]) provides standard scores in word recognition and decoding, spelling, and numeric operations.43 We report the scores from the WIAT-III Numeric Operations, Word Reading, Pseudoword Decoding, and Spelling subtests.
Autism Assessment
Children determined to be at risk on the SCQ (see parent-completed questionnaires below) were assessed with the Autism Diagnostic Interview–Revised (ADI-R) and an in-depth parent interview.42,44 Children who met ADI-R modified criteria for ASD45 were administered the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2).46 All children who met standardized research criteria for ASD on both the ADI-R and ADOS-2 were classified as having ASD. In addition, 11 children were included who met ADOS-2 criteria but did not have an ADI-R assessment; of these children, 9 who had a previous clinical diagnosis of ASD or who the site psychologist thought were likely to meet diagnostic criteria for ASD were assessed with the ADOS-2, whereas the parents of the remaining 2 children did not complete the ADI-R interview.
Gross Motor Function
The children’s motor function was assessed with the Gross Motor Function Classification System.47 A child was classified as level 3 or higher if he or she needed mobility assistance (level 3, walks using a handheld mobility device; level 4, self-mobility with limitations, may use powered mobility; and level 5, transported in a manual wheelchair).
Manual Ability Classification System
The classification assigns a single level for the collaborative use of both hands when handling objects in daily life (level 1, handles objects easily and successfully; level 2, some reduction of quality and/or speed; level 3, handles objects with difficulty; level 4, significant limitations; and level 5, requires total assistance).48
Communication Function Classification System
The Communication Function Classification System allocates children to 1 of 5 levels of communication performance (level 1, effective with unfamiliar and familiar partners; level 2, effective but slower paced; level 3, effective with familiar partners but less so with unfamiliar partners; level 4, inconsistent with familiar partners; and level 5, seldomly effective with familiar partners).49 The system assesses speech, gestures, behaviors, eye gaze, facial expressions, and such augmentative and alternative communication systems as manual signs, pictures, communication boards, communication books, and speech-generating devices.
Parent-Completed Questionnaires
While the child was being tested, the parent or caregiver was asked to complete the following questionnaires regarding the child’s medical and neurologic status and behavior.
Child Symptom Inventory-4
While the child was being tested, the parent or caregiver completed questionnaires regarding the child’s medical and neurologic status and behavior, including the Child Symptom Inventory-4 (CSI-4) Parent Checklist.50 The child’s current teacher was also asked to complete the CSI-4 Teacher Checklist. Although the parent checklist has 20 more items than the teacher version (97 vs 77), both include the same 18 items specific to attention-deficit/hyperactivity disorder symptoms (9 for the inattentive domain and 9 for the hyperactive and/or impulsive domain) that are each rated on a scale from 0 (never) to 3 (often). Teachers and parents did not make any Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnosis. Rather, the CSI-4 program identified children as screening positive for these diagnoses on the basis of the parents’ or teachers’ acknowledgment of selected child characteristics.
Social Responsiveness Scale
The Social Responsiveness Scale (SRS) is a short, parent-completed questionnaire designed to evaluate a child’s social ability.51,52 This 65-item instrument was designed as a quantitative trait measure for ASD-related deficits that do not warrant a formal diagnosis in the general population.53,54 It provides a total score reflecting the severity of social deficits on the autism spectrum as well as 5 subscale scores: social awareness, social cognition, social communication, social motivation, and restricted interests and repetitive behavior.
SCQ
All children were screened for an autism disorder by the parent-completed SCQ.55 We used the current version, which is composed of 40 yes-or-no questions about the child’s behavior over the last 3 months.
Children’s Communication Checklist-2
The Children’s Communication Checklist-2 (CCC-2) has 70 items that are used to assess speech, vocabulary, sentence structure, and social language skills.56 The 10 subscales are discourse, syntax, semantics, coherence, inadequate initiation, stereotyped language, use of context, nonverbal communication, social relations, and interests. We calculated z scores using normative data.57
Data Analyses
We evaluated the null hypothesis that among children born before 28 weeks’ gestation, those who had severe and less severe intrauterine growth restriction do not differ from their peers who had higher weight for gestation on assessments of cognitive and executive function, behavior, language, and communication at age 10 years. We also described motor function, the frequencies of parent and teacher responses on CSI-4 items, the occurrence of seizures, and quality of life among children who were born growth restricted and children who were not.
Frequencies and proportions were calculated to describe the characteristics of each study group. For assessments that yield a continuous outcome, we used normative data described by the authors of the assessment37,58,59 to derive z scores. Associations with z scores <−2 or z scores ≥−2 and <−1 were evaluated for cognition and academic outcomes, as well as those measured by the SRS, SCQ, and CCC-2. We used logistic regression models to estimate odds ratios (ORs) with 95% confidence intervals (CIs) adjusting for potential confounders (sex and racial identity) that were selected a priori and were associated with the independent and dependent variables (see Supplemental Fig 5). ORs with 95% CIs that exclude 1.0 are statistically significant at P <.05.
Results
Correlates of Birth Weight Z Score Categories
The mothers of severely growth-restricted newborns were more likely than the mothers of other children to identify as neither white nor African American (Supplemental Fig 5). Most (69%) children who were delivered because of preeclampsia were growth restricted, as were half of those who were delivered for a fetal indication. Girls were more frequently growth restricted than boys.
Distributions of Age 10 Years Assessment Scores
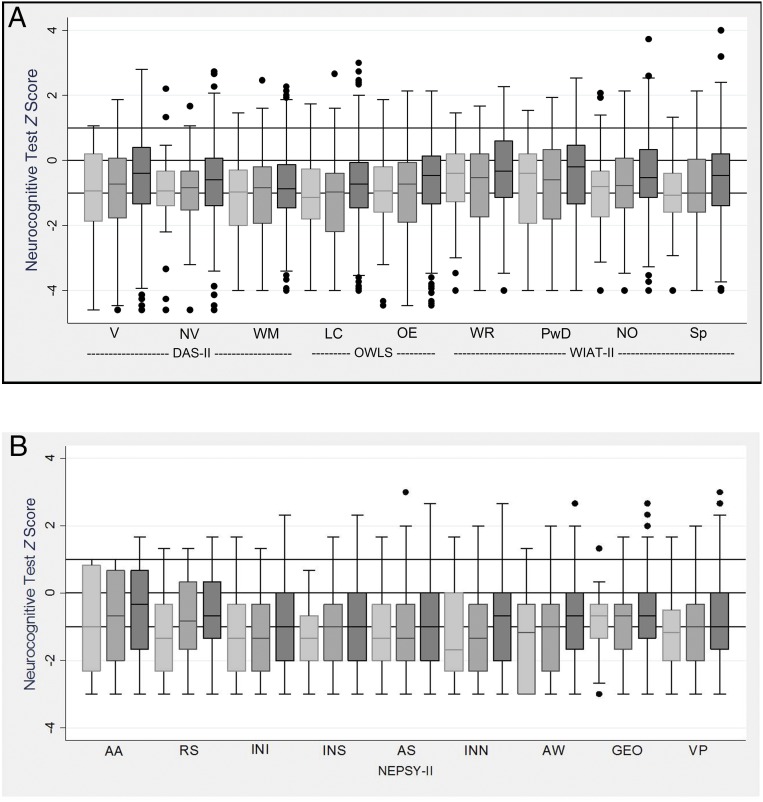
Figure 1 shows box plots for each measure; the 3 horizontal lines in the box plots correspond to the normative population 25th, 50th, and 75th percentile values for each measure. The distributions of scores on every assessment were lower than was expected on the basis of the distributions in the normative sample (ie, the medians lie below the horizontal line at 0).
FIGURE 1.
Box-and-whisker plots (A and B) of each cognitive subtest by birth weight z score category. All z scores are adjusted to population norms. Light gray is <−2; medium gray is ≥−2, <−1; and dark gray is ≥−1. The central line in the box indicates the median (50th percentile), whereas the top of the box indicates the 75th percentile, and the bottom of the box indicates the 25th percentile. If ELGAN had the expected normal distribution of term children, the middle of the box would be at z score = 0, and the upper and lower ends of the box would be at z score = 1 and z score = −1, respectively. AA, auditory attention; AS, animal sorting; AW, arrows; GEO, geometric puzzles; INI, inhibition inhibition; INN, inhibition naming; INS, inhibition switching; LC, listening comprehension; NO, numerical operations; NV, nonverbal reasoning; OE, oral expression; PwD, pseudoword decoding; RS, auditory response set; Sp, spelling; V, verbal; VP, visuomotor precision; WM, working memory; WR, word reading.
Compared with their peers who were not born with FGR, the most severely and the less severely growth-restricted newborns had relatively similar percentages of low scores on the DAS-II Verbal Reasoning, OWLS Listening Comprehension, NEPSY-II Visuomotor Precision, and WIAT-III Word Reading, Pseudoword Decoding, and Spelling subtests. In contrast, the more severe the growth restriction, the lower the scores for auditory attention, auditory response, inhibition inhibition, inhibition switching, inhibition naming, and arrows assessments. Differences in scores for the remaining assessments across the 3 study groups were minor, although median and 25th percentile scores generally tended to be higher among the AGA group than among their growth-restricted peers.
General Cognition and Achievement
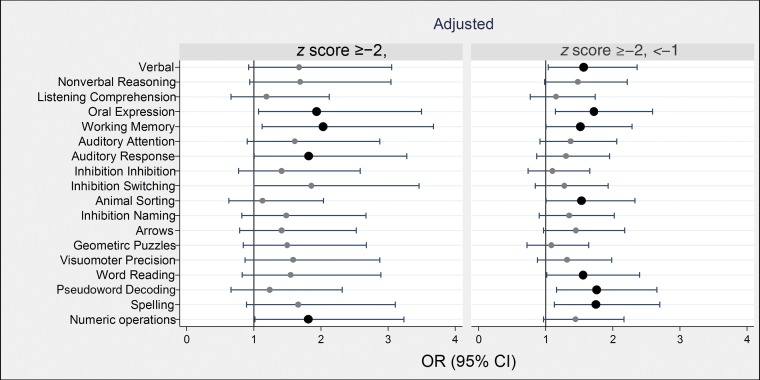
In analyses that were adjusted for race and sex, children born severely or less severely growth restricted were 1.5- to twofold more likely than their peers who were born with higher birth weight z scores to have low scores on the OWLS Oral Expression subtest, the DAS-II Working Memory subtest, the NEPSY-II Auditory Response subtest, and the WIAT-II Numeric Operations subtest (Fig 2, Supplemental Tables 1 and 2).
FIGURE 2.
Forest plots of ORs and 95% CIs of a z score ≤−1 on each DAS-II and NEPSY-II cognitive assessment at age 10 years associated with birth weight z score category <−2 (on left) and ≥−2, <−1 (on right). ORs are adjusted for racial identity and sex. BW, birth weight.
Children who were less severely growth restricted at birth were also at increased risk of low scores on the OWLS Oral Expression subtest and the DAS-II Working Memory subtest. These children also had higher risks of low scores on the DAS-II Verbal subtest, the NEPSY-II Animal Sorting subtest, and the WIAT-II Word Reading, Pseudoword Decoding, and Spelling subtest scores.
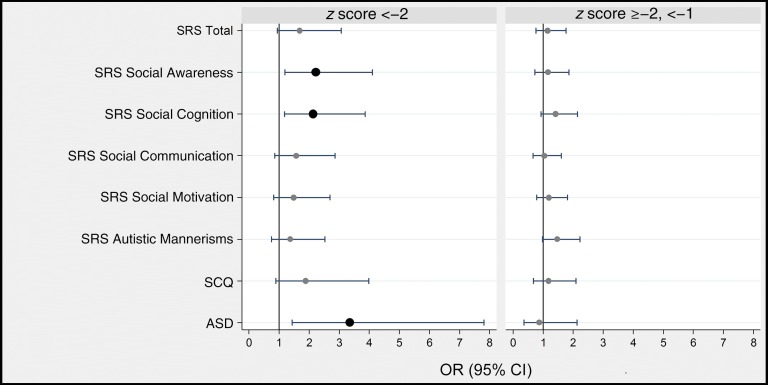
SRS
Clinically significant impairment (score of ≥60) at age 10 years on the social awareness and social cognition components of the SRS occurred more frequently among children who were severely growth restricted at birth than among children who were not growth restricted (Fig 3, Supplemental Table 3). The ORs of clinically significant impairment as defined by the total SRS score and the remaining SRS components (social cognition, social communication, social motivation, and autistic mannerisms) were not statistically different from 1.
FIGURE 3.
Forest plots of ORs and 95% CIs of a T score ≥60 on the SRS subtests, of a positive screening result on the SCQ, and of documented characteristics of ASD on the basis of the ADOS-2 at age 10 years associated with birth weight z score category <−2 (on left) and ≥−2, <−1 (on right). ORs are adjusted for racial identity and sex. BW, birth weight.
Children who were severely growth restricted at birth were also at increased risk of a rigorously defined ASD. Their increased risk of screening positive on the SCQ was not statistically significant, although they were considerably more likely than others to have been described as using odd phrases, socially inappropriate questions, and made-up words.
Children whose growth restriction at birth was less severe were not at increased risk of high scores on the SRS, screening positive on the SCQ, or positive ADOS-2.
CCC-2
Children with severe FGR were at increased risk of a z score ≤−1 on the CCC-2 subtests of coherence, context, nonverbal communication, and interests (Fig 4, Supplemental Tables 4 ). Children who were less severely growth restricted at birth were not at increased risk of a low score on any subtest of the CCC-2.
FIGURE 4.
Forest plots of ORs and 95% CIs of a z score ≤−1 on the CCC-2 subtests at age 10 years associated with birth weight z score category <−2 (on left) and ≥−2, <−1 (on right). ORs are adjusted for racial identity and sex. BW, birth weight.
CSI-4 Identified Behavioral Disorders
According to both parents and teachers, children who were born severely growth restricted screened positive for posttraumatic stress at age 10 years more frequently than their AGA peers (Supplemental Fig 6). Parents, but not teachers, also reported a higher frequency of vocal tics among children who were born severely growth restricted. In contrast, teachers, but not parents, reported higher frequencies of symptoms of specific phobia, obsessions, compulsions, and social phobia among the severely growth restricted than among those who were not growth restricted. The less severely growth restricted children were remarkably similar to their peers who had higher birth weight for gestation.
Other Dysfunctions
Inconsistent or seldom effective communication with familiar partners was more common among severely growth restricted than among children who were not born with FGR (Supplemental Fig 7). However, less severely growth-restricted children did not have such severe communication limitations. Children who were severely growth restricted at birth were also more likely than others to be strongly right-handed, but they were no more likely to have seizures or a limitation of manual ability or gross motor function.
The more severe the FGR, the higher the proportion of children who had limited quality of life in school functioning. Limited quality of life in social functioning and psychosocial functioning were also more common among children born severely growth restricted but not among those who were born less severely growth restricted.
Discussion
Our main finding is that by and large, the more severe the growth restriction in utero, the lower the scores on multiple neurodevelopmental assessments at age 10 years. Severely growth-restricted children were more likely than their extremely preterm peers to have social awareness impairments and autistic mannerisms (according to the SRS), a rigorously defined ASD, and difficulty with speech coherence, context, nonverbal communication, and interests (according to the CCC-2). These severely growth-restricted children also had diminished social and psychosocial function and quality of life (according to the Pediatric Quality of Life Inventory) compared with their peers who were not growth restricted. Children who were less severely growth restricted at birth were at increased risk of low scores on the OWLS Oral Expression subtest and the DAS-II Working Memory subtest.
Synthesis With Previous Studies
Some of the social and communication deficits we studied were particularly pronounced in children born extremely preterm who had severe FGR, as were some cognitive functioning deficits, but not all. We do not know if FGR at low gestational ages is associated with general deficits across most cognitive domains or with selective deficits in only some domains of brain function.11,20,21 Our search of PubMed identified no large study of associations between FGR (or being small for gestational age [SGA]) and cognitive and behavioral outcomes in children born before 28 weeks’ gestation. However, such associations have been assessed in 3 studies involving children who were born before 30 weeks’ gestation. In the first, excluding children who had cerebral palsy and/or sensory impairment, 6-year-old SGA children were more likely to have an IQ <75 than were their AGA peers (35%, 7 of 20 vs 14.6%, 12 of 82).60 The second included 8-year-olds who were born before 28 weeks’ gestation, but only 4 children were SGA. Nonetheless, birth weight z score was moderately correlated with IQ.61 In the third study, SGA children who also had absent or reversed end diastolic blood flow were compared with AGA controls and matched for sex, gestational age at birth, and year of birth. At 5 years to 8 years of age, a full-scale IQ <70 was more common (10 of 34 vs 2 of 34), and the mean verbal IQ was lower in the SGA group.62
Our findings are also generally consistent with those of 2 population-based cohorts of children born very preterm (ie, before the 32nd week). In a Dutch cohort of school-aged children who were born very preterm or very low birth weight (<1500 g), SGA children were more likely to have a speech and language abnormality and to receive special education.63 Similarly, in the Etude Epidémiologique sur les Petits Ages Gestationnels (EPIPAGE) cohort, 5- to 8-year-olds who were SGA were more likely to have minor cognitive difficulties, inattention-hyperactivity symptoms, and school difficulties (OR: 1.74; 95% CI 1.07–3).
The most likely explanation for the observation that girls are more likely than boys to be growth restricted at birth is based on the observation that preterm preeclampsia occurs more commonly among pregnancies with a female fetus than among pregnancies with a male fetus.64
FGR and the Brain
Some of the brain structure characteristics of growth-restricted children born preterm might account for some of the dysfunctions evident at age 10 years in children who were growth restricted at birth,65–70 although some morphologic correlates might be below current clinical MRI resolution.71 These brain structure abnormalities might, in turn, be a consequence of epigenetic phenomena that sensitize the brain,72–75 making it vulnerable to inflammatory phenomena that appear to increase the risk of brain damage in very preterm newborns.76 Indeed, the risk of brain damage in severely growth-restricted neonates born very preterm appears further heightened by their tendency to have more intense systemic inflammatory responses than their peers who were not growth restricted,33 perhaps acting in a 2-hit model77 (in which growth restriction is the first hit, and intermittent or sustained systemic inflammation is the second hit). Likewise, inflammation appears to account for some of the brain abnormalities in rats with FGR.78
Growth Restriction Might Be a First Hit Because of Impaired Placentation
In the ELGAN Study, almost two-thirds of all severely growth-restricted infants were born to women who had preeclampsia. Both of these disorders are characterized by impaired placentation79,80 and deficiencies of growth factors81,82 apparently involved in the regulation of intravillous or fetomaternal angiogenesis.83–86 Although the stimulus responsible for altered placental release of the molecules is not known,87–89 dysregulation of angiogenic-related factors is thought to affect pregnancy either by failing to promote growth90 or limiting the availability of nutrients.91 Both mechanisms have the potential to limit brain growth and maturation.15,92,93
A paucity of the enzyme heme oxygenase (HO) might also contribute to impaired fetal brain development.94,95 It helps regulate not only angiogenesis but also vascular tone, inflammation, apoptosis, and oxidation. Deficiencies of HO additionally appear to characterize preeclampsia,96–100 although not all researchers agree.101 The deletion of the gene HO-1 in mice leads to inadequate remodeling of spiral arteries and suboptimal placentation followed by intrauterine growth restriction.102 Consistent findings have been shown in rats.103
HO also modulates innate and adaptive immune responses,104–109 can contribute to the resolution of inflammation,110–112 and can also reduce oxidative stress.113,114 Moreover, an HO-1 inducer promotes preconditioning,115 perhaps thereby protecting the vulnerable brain.116–119 Consequently, the brains of very preterm children born to women who have severe preeclampsia might be more vulnerable than the brains of their peers who are delivered for spontaneous indications.120 Such vulnerability might explain the increased risk for cognitive impairment reported among children who were born to mothers affected by preeclampsia (and its correlates).121–124
Methodologic Issues
Defining FGR is not as simple as it might seem. This is reflected in the wide variation in terms and methods across studies.125–127 Not all infants whose weight is near the lower end of the spectrum have had disordered growth. Some will be small in part because of the tendency for children of his or her genetic predisposition to be small at birth.128 However, the contribution of such tendencies is thought to be small relative to the contributions of phenomena that lead to severe growth restriction.129 Consequently, customized percentiles based on maternal characteristics are not recommended.130,131
Some argue that growth restriction and SGA are not synonymous.127,132 We use the term FGR in light of the ongoing challenge to discern pathologically from constitutionally small newborns3,133,134 and because we prefer to avoid the impression that we used a cutoff of the lowest decile (which would define SGA). Indeed, our finding that some children who were relatively but not severely growth restricted at birth had cognitive limitation leads to this inference that growth restriction can be a continuum and not an either/or phenomenon.
Clinical Implications
The cognitive, social, and other behavioral impairments we and others have observed call for efforts to prevent and ameliorate these impairments among children with FGR born extremely preterm. Low-dose aspirin administered in early gestation has therapeutic benefits for some women who are at increased risk of preeclampsia (and its correlates [ie, FGR]135), and trials are underway to test additional strategies.135–140 Placental and other stem cells,141–148 proton-pump inhibitors,149 low-molecular-weight heparin150 and other molecules151–153 might also have therapeutic benefits. Indeed, compelling studies of rodents154–157 and nonhuman primates158 support the possibility of a therapeutic benefit from exogenous angiotrophins during gestation.
Interventions aiming to improve maternal diet and its correlates (eg, the mHealth coaching program159) would likely be more beneficial than a narrow focus on maternal weight gain.160–163 Postnatal care plans that were not specifically developed for children with FGR might nevertheless help minimize some of the limitations identified.164–175
Strengths and Limitations
The strengths of our study are the large number of infants, the enrollment of infants based on gestational age and not birth weight,176 the outcome assessments by individuals who did not know which study participants had a history of FGR, and the large number of instruments used to assess cognitive and other functions at age 10 years. To avoid the error of inappropriately drawing the inference that FGR has no influence, we did not adjust for multiple comparisons; it is possible that this increased type I error.177 However, we found 5 times as many statistically significant ORs than was expected by chance alone; this prompts us to infer that our findings are unlikely to reflect random phenomena. As with all observational studies, we are limited in our ability to infer causation from associations; ie, we cannot rule out the possibility that the observed association between FGR and increased risk of neurodevelopmental deficits was explained by alternative unmeasured or measured factors (eg, neonatal morbidities).
Conclusions
Among children born extremely preterm, those with severe FGR are at increased risk of a wide variety of neurodevelopmental dysfunctions and low achievement scores assessed at age 10 years.
Acknowledgments
We are grateful to the families who made this study possible as well as our colleagues at the following participating institutions: Boston Children’s Hospital, Boston, Massachusetts: Kathleen Lee, Anne McGovern, Jill Gambardella, Susan Ursprung, Ruth Blomquist, Kristen Ecklund, Haim Bassan, Samantha Butler, Adré Duplessis, Cecil Hahn, Catherine Limperopoulos, Omar Khwaja, and Janet S. Soul; Baystate Medical Center, Springfield, Massachusetts: Bhavesh Shah, Karen Christianson, Frederick Hampf, Herbert Gilmore, and Susan McQuiston; Beth Israel Deaconess Medical Center, Boston, Massachusetts: Camilia R. Martin, Colleen Hallisey, Caitlin Hurley, Miren Creixell, and Jane Share; Brigham and Women’s Hospital, Boston, Massachusetts: Linda J. Van Marter and Sara Durfee; Massachusetts General Hospital, Boston, Massachusetts: Robert M. Insoft, Jennifer G. Wilson, Maureen Pimental, Sjirk J. Westra, and Kalpathy Krishnamoorthy; Floating Hospital for Children at Tufts Medical Center, Boston, Massachusetts: Cynthia Cole, John M. Fiascone, Janet Madden, Ellen Nylen, Anne Furey, Roy McCauley, Paige T. Church, Cecelia Keller, and Karen J. Miller; University of Massachusetts Memorial Health Care, Worcester, Massachusetts: Francis Bednarek, Mary Naples, Beth Powers, Jacqueline Wellman, Robin Adair, Richard Bream, Alice Miller, Albert Scheiner, and Christy Stine; School of Medicine, Yale University, New Haven, Connecticut: Richard Ehrenkranz, Elaine Romano, Cindy Miller, Nancy Close, Elaine Romano, and Joanne Williams; Wake Forest University Baptist Medical Center and Forsyth Medical Center, Winston-Salem, North Carolina: T. Michael O’Shea, Debbie Gordon, Teresa Harold, Barbara Specter, Deborah Allred, Robert Dillard, Don Goldstein, Deborah Hiatt (deceased), Gail Hounshell, Ellen Waldrep, Lisa Washburn, and Cherrie D. Welch; University Health Systems of Eastern Carolina, Greenville, North Carolina: Stephen C. Engelke, Sherry Moseley, Linda Pare, Donna Smart, Joan Wilson, Ira Adler, Sharon Buckwald, Rebecca Helms, Kathyrn Kerkering, Scott S. MacGilvray, and Peter Resnik; North Carolina Children’s Hospital, Chapel Hill, North Carolina: Carl Bose, Gennie Bose, Lynn A. Fordham, Lisa Bostic, Diane Marshall, Kristi Milowic, and Janice Wereszczak; Helen DeVos Children’s Hospital, Grand Rapids, Michigan: Mariel Poortenga, Bradford W. Betz, Steven L. Bezinque, Joseph Junewick, Wendy Burdo-Hartman, Lynn Fagerman, Kim Lohr, Steve Pastyrnak, and Dinah Sutton; Sparrow Hospital, Lansing, Michigan: Carolyn Solomon, Ellen Cavenagh, Victoria J. Caine, Nicholas Olomu, and Joan Price; Michigan State University, East Lansing, Michigan: Nigel Paneth, Padmani Karna, and Madeleine Lenski; University of Chicago Medical Center, Chicago, Illinois: Michael D. Schreiber, Grace Yoon, Kate Feinstein, Leslie Caldarelli, Sunila E. O’Connor, Michael Msall, and Susan Plesha-Troyke; William Beaumont Hospital, Royal Oak, Michigan: Daniel Batton, Beth Kring, Karen Brooklier, Beth Kring, Melisa J. Oca, and Katherine M. Solomon.
Glossary
- ADI-R
Autism Diagnostic Interview–Revised
- ADOS-2
Autism Diagnostic Observation Schedule, Second Edition
- AGA
appropriate for gestational age
- ASD
autism spectrum disorder
- CCC-2
Children’s Communication Checklist-2
- CI
confidence interval
- CSI-4
Child Symptom Inventory-4
- DAS-II
Differential Ability Scales–II
- ELGAN
Extremely Low Gestational Age Newborns
- FGR
fetal growth restriction
- HO
heme oxygenase
- NEPSY-II
Developmental Neuropsychological Assessment-II
- OR
odds ratio
- OWLS
Oral and Written Language Scales
- SCQ
Social Communication Questionnaire
- SGA
small for gestational age
- SRS
Social Responsiveness Scale
- WIAT-III
Wechsler Individual Achievement Test-III
Footnotes
Dr Korzeniewski conceptualized and designed the analysis of the Extremely Low Gestational Age Newborns study data, drafted the initial manuscript, and coordinated revisions; Dr Allred conducted analyses and critically reviewed and revised manuscript drafts; Drs Joseph and Heeren critically reviewed and revised manuscript drafts; Drs Leviton, Kuban, and O’Shea conceptualized and designed the Extremely Low Gestational Age Newborns study and reviewed and revised manuscript drafts; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069-06A2), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5P30HD018655-28; L40 HD077654), the Office of the Director of the National Institutes of Health (1UG3OD023348-01), and the Wayne State University Perinatal Initiative. The funders did not take part in developing this article beyond funding the study. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Walker DM, Marlow N. Neurocognitive outcome following fetal growth restriction. Arch Dis Child Fetal Neonatal Ed. 2008;93(4):F322–F325 [DOI] [PubMed] [Google Scholar]
- 2.de Bie HM, Oostrom KJ, Delemarre-van de Waal HA. Brain development, intelligence and cognitive outcome in children born small for gestational age. Horm Res Paediatr. 2010;73(1):6–14 [DOI] [PubMed] [Google Scholar]
- 3.Savchev S, Sanz-Cortes M, Cruz-Martinez R, et al. Neurodevelopmental outcome of full-term small-for-gestational-age infants with normal placental function. Ultrasound Obstet Gynecol. 2013;42(2):201–206 [DOI] [PubMed] [Google Scholar]
- 4.Paz I, Gale R, Laor A, Danon YL, Stevenson DK, Seidman DS. The cognitive outcome of full-term small for gestational age infants at late adolescence. Obstet Gynecol. 1995;85(3):452–456 [DOI] [PubMed] [Google Scholar]
- 5.Pearce MS, Mann KD, Singh G, Sayers SM. Birth weight and cognitive function in early adulthood: the Australian aboriginal birth cohort study. J Dev Orig Health Dis. 2014;5(3):240–247 [DOI] [PubMed] [Google Scholar]
- 6.Løhaugen GC, Østgård HF, Andreassen S, et al. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J Pediatr. 2013;163(2):447–453 [DOI] [PubMed] [Google Scholar]
- 7.Berle JO, Mykletun A, Daltveit AK, Rasmussen S, Dahl AA. Outcomes in adulthood for children with fetal growth retardation. A linkage study from the Nord-Trøndelag Health Study (HUNT) and the Medical Birth Registry of Norway. Acta Psychiatr Scand. 2006;113(6):501–509 [DOI] [PubMed] [Google Scholar]
- 8.van Wassenaer A. Neurodevelopmental consequences of being born SGA. Pediatr Endocrinol Rev. 2005;2(3):372–377 [PubMed] [Google Scholar]
- 9.Strauss RS. Adult functional outcome of those born small for gestational age: 26-year follow-up of the 1970 British Birth Cohort. JAMA. 2000;283(5):625–632 [DOI] [PubMed] [Google Scholar]
- 10.Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med. 2014;19(2):90–96 [DOI] [PubMed] [Google Scholar]
- 11.Vohr BR. Neurodevelopmental outcomes of extremely preterm infants. Clin Perinatol. 2014;41(1):241–255 [DOI] [PubMed] [Google Scholar]
- 12.Jarjour IT. Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr Neurol. 2015;52(2):143–152 [DOI] [PubMed] [Google Scholar]
- 13.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269 [DOI] [PubMed] [Google Scholar]
- 14.Regev RH, Reichman B. Prematurity and intrauterine growth retardation–double jeopardy? Clin Perinatol. 2004;31(3):453–473 [DOI] [PubMed] [Google Scholar]
- 15.Rätsep MT, Hickman AF, Croy BA. The Elsevier trophoblast research award lecture: impacts of placental growth factor and preeclampsia on brain development, behaviour, and cognition. Placenta. 2016;48(suppl 1):S40–S46 [DOI] [PubMed] [Google Scholar]
- 16.Hutton JL, Pharoah PO, Cooke RW, Stevenson RC. Differential effects of preterm birth and small gestational age on cognitive and motor development. Arch Dis Child Fetal Neonatal Ed. 1997;76(2):F75–F81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guellec I, Lapillonne A, Renolleau S, et al. ; EPIPAGE Study Group . Neurologic outcomes at school age in very preterm infants born with severe or mild growth restriction. Pediatrics. 2011;127(4). Available at: www.pediatrics.org/cgi/content/full/127/4/e883 [DOI] [PubMed] [Google Scholar]
- 18.Kallankari H, Kaukola T, Olsén P, Ojaniemi M, Hallman M. Very preterm birth and fetal growth restriction are associated with specific cognitive deficits in children attending mainstream school. Acta Paediatr. 2015;104(1):84–90 [DOI] [PubMed] [Google Scholar]
- 19.Guellec I, Lapillonne A, Marret S, et al. Effect of intra- and extrauterine growth on long-term neurologic outcomes of very preterm infants. J Pediatr. 2016;175:93.e1–99.e1 [DOI] [PubMed] [Google Scholar]
- 20.Vohr B. Speech and language outcomes of very preterm infants. Semin Fetal Neonatal Med. 2014;19(2):78–83 [DOI] [PubMed] [Google Scholar]
- 21.Murray E, Fernandes M, Fazel M, Kennedy SH, Villar J, Stein A. Differential effect of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG. 2015;122(8):1062–1072 [DOI] [PubMed] [Google Scholar]
- 22.Korzeniewski SJ, Feldman JF, Lorenz JM, Pinto-Martin JA, Whitaker AH, Paneth N. Persistence of cerebral palsy diagnosis: assessment of a low-birth-weight cohort at ages 2, 6, and 9 years. J Child Neurol. 2016;31(4):461–467 [DOI] [PubMed] [Google Scholar]
- 23.McCormick A, Brien M, Plourde J, Wood E, Rosenbaum P, McLean J. Stability of the gross motor function classification system in adults with cerebral palsy. Dev Med Child Neurol. 2007;49(4):265–269 [DOI] [PubMed] [Google Scholar]
- 24.Nystrand M, Beckung E, Dickinson H, Colver A. Stability of motor function and associated impairments between childhood and adolescence in young people with cerebral palsy in Europe. Dev Med Child Neurol. 2014;56(9):833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48(6):424–428 [DOI] [PubMed] [Google Scholar]
- 26.Zarrinkalam R, Russo RN, Gibson CS, van Essen P, Peek AK, Haan EA. CP or not CP? A review of diagnoses in a cerebral palsy register. Pediatr Neurol. 2010;42(3):177–180 [DOI] [PubMed] [Google Scholar]
- 27.Lee K, Bull R, Ho RM. Developmental changes in executive functioning. Child Dev. 2013;84(6):1933–1953 [DOI] [PubMed] [Google Scholar]
- 28.Tucker-Drob EM. Differentiation of cognitive abilities across the life span. Dev Psychol. 2009;45(4):1097–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Shea TM, Allred EN, Dammann O, et al. ; ELGAN Study Investigators . The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helderman JB, O’Shea TM, Kuban KC, et al. ; ELGAN Study Investigators . Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics. 2012;129(3):494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McElrath TF, Hecht JL, Dammann O, et al. ; ELGAN Study Investigators . Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168(9):980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hecht JL, Allred EN, Kliman HJ, et al. ; Elgan Study Investigators . Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology. 2008;40(4):372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McElrath TF, Allred EN, Van Marter L, Fichorova RN, Leviton A; ELGAN Study Investigators . Perinatal systemic inflammatory responses of growth-restricted preterm newborns. Acta Paediatr. 2013;102(10):e439–e442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph RM, O’Shea TM, Allred EN, et al. ; ELGAN Study Investigators . Neurocognitive and academic outcomes at age 10 years of extremely preterm newborns. Pediatrics. 2016;137(4):e20154343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yudkin PL, Aboualfa M, Eyre JA, Redman CWG, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15(1):45–52 [DOI] [PubMed] [Google Scholar]
- 36.Leviton A, Paneth N, Reuss ML, et al. ; Developmental Epidemiology Network Investigators . Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Pediatr Res. 1999;46(5):566–575 [DOI] [PubMed] [Google Scholar]
- 37.Elliott CD. Differential Ability Scales. 2nd ed. San Antonio, TX: Pearson; 2007 [Google Scholar]
- 38.Korkman M, Kirk U, Kemp S. NEPSY-II: Clinical and Interpretive Manual. San Antonio, TX: Pearson Education; 2007 [Google Scholar]
- 39.Carrow-Woolfolk E. Oral and Written Language Scales (OWLS). San Antonio, TX: Pearson Education; 1995 [Google Scholar]
- 40.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 41.Kuban KC, Joseph RM, O’Shea TM, et al. ; Extremely Low Gestational Age Newborn (ELGAN) Study Investigators . Girls and boys born before 28 weeks gestation: risks of cognitive, behavioral, and neurologic outcomes at age 10 years. J Pediatr. 2016;173:69.e1–75.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joseph RM, O’Shea TM, Allred EN, et al. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism Res. 2017;10(2):224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wechsler D. The Wechsler Individual Achievement Test-III. Oxford, United Kingdom: Pearson Assessment; 2009 [Google Scholar]
- 44.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 45.Risi S, Lord C, Gotham K, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1094–1103 [DOI] [PubMed] [Google Scholar]
- 46.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule. 2nd ed. Torrance, CA: Western Psychological Services; 2012 [Google Scholar]
- 47.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised gross motor function classification system. Dev Med Child Neurol. 2008;50(10):744–750 [DOI] [PubMed] [Google Scholar]
- 48.Eliasson AC, Krumlinde-Sundholm L, Rösblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48(7):549–554 [DOI] [PubMed] [Google Scholar]
- 49.Hidecker MJ, Paneth N, Rosenbaum PL, et al. Developing and validating the communication function classification system for individuals with cerebral palsy. Dev Med Child Neurol. 2011;53(8):704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gadow KD, Sprafkin J. Child Symptom Inventory–4 Screening and Norms Manual. Stony Brook, NY: Checkmate Plus; 2002 [Google Scholar]
- 51.Constantino JN, Gruber CP. Social Responsiveness Scale, Second Edition (SRS-2). 2nd ed. Los Angeles, CA: Western Psychological Services; 2012 [Google Scholar]
- 52.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–433 [DOI] [PubMed] [Google Scholar]
- 53.Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr. 2000;21(1):2–11 [DOI] [PubMed] [Google Scholar]
- 54.Constantino JN, Hudziak JJ, Todd RD. Deficits in reciprocal social behavior in male twins: evidence for a genetically independent domain of psychopathology. J Am Acad Child Adolesc Psychiatry. 2003;42(4):458–467 [DOI] [PubMed] [Google Scholar]
- 55.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire - Manual. Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 56.Bishop DVM. Children’s Communication Checklist. 2nd ed. San Antonio, TX: Psychological Corporation; 2006 [Google Scholar]
- 57.Norbury CF, Nash M, Baird G, Bishop D. Using a parental checklist to identify diagnostic groups in children with communication impairment: a validation of the Children’s Communication Checklist–2. Int J Lang Commun Disord. 2004;39(3):345–364 [DOI] [PubMed] [Google Scholar]
- 58.Carrow-Woolfolk E. Oral and Written Language Scales: Written Expression Scale Manual. Circle Pines, MN: American Guidance Service; 1996 [Google Scholar]
- 59.Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. New York, NY: The Psychological Corporation; 1998 [Google Scholar]
- 60.Kono Y, Mishina J, Takamura T, et al. Impact of being small-for-gestational age on survival and long-term outcome of extremely premature infants born at 23-27 weeks’ gestation. J Perinat Med. 2007;35(5):447–454 [DOI] [PubMed] [Google Scholar]
- 61.Kan E, Roberts G, Anderson PJ, Doyle LW; Victorian Infant Collaborative Study Group . The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children. Early Hum Dev. 2008;84(6):409–416 [DOI] [PubMed] [Google Scholar]
- 62.Morsing E, Asard M, Ley D, Stjernqvist K, Marsál K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011;127(4). Available at: www.pediatrics.org/cgi/content/full/127/4/e874 [DOI] [PubMed] [Google Scholar]
- 63.Kok JH, den Ouden AL, Verloove-Vanhorick SP, Brand R. Outcome of very preterm small for gestational age infants: the first nine years of life. Br J Obstet Gynaecol. 1998;105(2):162–168 [DOI] [PubMed] [Google Scholar]
- 64.Schalekamp-Timmermans S, Arends LR, Alsaker E, et al. ; Global Pregnancy Collaboration . Fetal sex-specific differences in gestational age at delivery in pre-eclampsia: a meta-analysis. Int J Epidemiol. 2017;46(2):632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tolsa CB, Zimine S, Warfield SK, et al. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res. 2004;56(1):132–138 [DOI] [PubMed] [Google Scholar]
- 66.Lodygensky GA, Seghier ML, Warfield SK, et al. Intrauterine growth restriction affects the preterm infant’s hippocampus. Pediatr Res. 2008;63(4):438–443 [DOI] [PubMed] [Google Scholar]
- 67.Esteban FJ, Padilla N, Sanz-Cortés M, et al. Fractal-dimension analysis detects cerebral changes in preterm infants with and without intrauterine growth restriction. Neuroimage. 2010;53(4):1225–1232 [DOI] [PubMed] [Google Scholar]
- 68.Padilla N, Falcón C, Sanz-Cortés M, et al. Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: a magnetic resonance imaging study. Brain Res. 2011;1382:98–108 [DOI] [PubMed] [Google Scholar]
- 69.Padilla N, Junqué C, Figueras F, et al. Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res. 2014;1545:1–11 [DOI] [PubMed] [Google Scholar]
- 70.Tzarouchi LC, Drougia A, Zikou A, et al. Body growth and brain development in premature babies: an MRI study. Pediatr Radiol. 2014;44(3):297–304 [DOI] [PubMed] [Google Scholar]
- 71.Bisignano M, Rees S. The effects of intrauterine growth retardation on synaptogenesis and mitochondrial formation in the cerebral and cerebellar cortices of fetal sheep. Int J Dev Neurosci. 1988;6(5):453–460 [DOI] [PubMed] [Google Scholar]
- 72.Campbell LR, Pang Y, Ojeda NB, Zheng B, Rhodes PG, Alexander BT. Intracerebral lipopolysaccharide induces neuroinflammatory change and augmented brain injury in growth-restricted neonatal rats. Pediatr Res. 2012;71(6):645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu XF, Xu SS, Fu LC, Hu QY, Lv Y, Du LZ. Epigenetic changes in peripheral leucocytes as biomarkers in intrauterine growth retardation rat. Biomed Rep. 2016;5(5):548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kitsiou-Tzeli S, Tzetis M. Maternal epigenetics and fetal and neonatal growth. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):43–46 [DOI] [PubMed] [Google Scholar]
- 75.Hillman SL, Finer S, Smart MC, et al. Novel DNA methylation profiles associated with key gene regulation and transcription pathways in blood and placenta of growth-restricted neonates. Epigenetics. 2015;10(1):50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75(3):376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leviton A, Fichorova RN, O’Shea TM, et al. ; ELGAN Study Investigators . Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr Res. 2013;73(3):362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rideau Batista Novais A, Pham H, Van de Looij Y, et al. Transcriptomic regulations in oligodendroglial and microglial cells related to brain damage following fetal growth restriction. Glia. 2016;64(12):2306–2320 [DOI] [PubMed] [Google Scholar]
- 79.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213(suppl 4):S115–S122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Korzeniewski SJ, Romero R, Chaiworapongsa T, et al. Maternal plasma angiogenic index-1 (placental growth factor/soluble vascular endothelial growth factor receptor-1) is a biomarker for the burden of placental lesions consistent with uteroplacental underperfusion: a longitudinal case-cohort study. Am J Obstet Gynecol. 2016;214(5):629.e1–629.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alfaidy N, Hoffmann P, Boufettal H, et al. The multiple roles of EG-VEGF/PROK1 in normal and pathological placental angiogenesis. BioMed Res Int. 2014;2014:451906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grazul-Bilska AT, Johnson ML, Borowicz PP, et al. Placental development during early pregnancy in sheep: effects of embryo origin on vascularization. Reproduction. 2014;147(5):639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25(2–3):114–126 [DOI] [PubMed] [Google Scholar]
- 86.Fan X, Rai A, Kambham N, et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest. 2014;124(11):4941–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts JM. Pathophysiology of ischemic placental disease. Semin Perinatol. 2014;38(3):139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Myatt L, Roberts JM. Preeclampsia: syndrome or disease? Curr Hypertens Rep. 2015;17(11):83. [DOI] [PubMed] [Google Scholar]
- 89.Karumanchi SA. Angiogenic factors in preeclampsia: from diagnosis to therapy. Hypertension. 2016;67(6):1072–1079 [DOI] [PubMed] [Google Scholar]
- 90.Gourvas V, Dalpa E, Konstantinidou A, Vrachnis N, Spandidos DA, Sifakis S. Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction (review). Mol Med Rep. 2012;6(1):23–27 [DOI] [PubMed] [Google Scholar]
- 91.Dunlap KA, Brown JD, Keith AB, Satterfield MC. Factors controlling nutrient availability to the developing fetus in ruminants. J Anim Sci Biotechnol. 2015;6(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reuss ML, Paneth N, Susser M. Does the loss of placental hormones contribute to neurodevelopmental disabilities in preterm infants? Dev Med Child Neurol. 1994;36(8):743–747 [PubMed] [Google Scholar]
- 93.Dammann O, Leviton A. Perinatal brain damage causation. Dev Neurosci. 2007;29(4–5):280–288 [DOI] [PubMed] [Google Scholar]
- 94.Chen J. Heme oxygenase in neuroprotection: from mechanisms to therapeutic implications. Rev Neurosci. 2014;25(2):269–280 [DOI] [PubMed] [Google Scholar]
- 95.Schipper HM, Song W. A heme oxygenase-1 transducer model of degenerative and developmental brain disorders. Int J Mol Sci. 2015;16(3):5400–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.George EM, Warrington JP, Spradley FT, Palei AC, Granger JP. The heme oxygenases: important regulators of pregnancy and preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2014;307(7):R769–R777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venditti CC, Smith GN. Involvement of the heme oxygenase system in the development of preeclampsia and as a possible therapeutic target. Womens Health (Lond). 2014;10(6):623–643 [DOI] [PubMed] [Google Scholar]
- 98.Ramma W, Ahmed A. Therapeutic potential of statins and the induction of heme oxygenase-1 in preeclampsia. J Reprod Immunol. 2014;101–102:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levytska K, Kingdom J, Baczyk D, Drewlo S. Heme oxygenase-1 in placental development and pathology. Placenta. 2013;34(4):291–298 [DOI] [PubMed] [Google Scholar]
- 100.Wong RJ, Zhao H, Stevenson DK. A deficiency in haem oxygenase-1 induces fetal growth restriction by placental vasculature defects. Acta Paediatr. 2012;101(8):827–834 [DOI] [PubMed] [Google Scholar]
- 101.Tong S, Kaitu’u-Lino TJ, Onda K, et al. Heme oxygenase-1 is not decreased in preeclamptic placenta and does not negatively regulate placental soluble fms-like tyrosine kinase-1 or soluble endoglin secretion. Hypertension. 2015;66(5):1073–1081 [DOI] [PubMed] [Google Scholar]
- 102.Zenclussen ML, Linzke N, Schumacher A, et al. Heme oxygenase-1 is critically involved in placentation, spiral artery remodeling, and blood pressure regulation during murine pregnancy. Front Pharmacol. 2015;5:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang D, Wang L, Qiao C, Yu Y, Fu L, Shang T. The role of the reduction of spiral artery remodeling and heme oxygenase 1 in mediating AT1-AA-induced hypertension and intrauterine growth restriction in pregnant rats. Am J Perinatol. 2014;31(10):883–890 [DOI] [PubMed] [Google Scholar]
- 104.Schumacher A, Zenclussen AC. Effects of heme oxygenase-1 on innate and adaptive immune responses promoting pregnancy success and allograft tolerance. Front Pharmacol. 2015;5:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ozen M, Zhao H, Lewis DB, Wong RJ, Stevenson DK. Heme oxygenase and the immune system in normal and pathological pregnancies. Front Pharmacol. 2015;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang F, Xiao M, Lin XJ, Muhammad S, Piao XH, Liu L. Expression of heme oxygenase-1 and leukemia inhibitory factor in maternal plasma and placental tissue in a lipopolysaccharide-induced late pregnancy preterm birth mouse model. J Reprod Med. 2016;61(1–2):39–46 [PubMed] [Google Scholar]
- 107.Oh SY, Hwang JR, Lee Y, et al. Isolation of basal membrane proteins from BeWo cells and their expression in placentas from fetal growth-restricted pregnancies. Placenta. 2016;39:24–32 [DOI] [PubMed] [Google Scholar]
- 108.Solano ME, Kowal MK, O’Rourke GE, et al. Progesterone and HMOX-1 promote fetal growth by CD8+ T cell modulation. J Clin Invest. 2015;125(4):1726–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao H, Ozen M, Wong RJ, Stevenson DK. Heme oxygenase-1 in pregnancy and cancer: similarities in cellular invasion, cytoprotection, angiogenesis, and immunomodulation. Front Pharmacol. 2015;5:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.An L, Liu CT, Yu MJ, et al. Heme oxygenase-1 system, inflammation and ventilator-induced lung injury. Eur J Pharmacol. 2012;677(1–3):1–4 [DOI] [PubMed] [Google Scholar]
- 111.Wu ML, Ho YC, Lin CY, Yet SF. Heme oxygenase-1 in inflammation and cardiovascular disease. Am J Cardiovasc Dis. 2011;1(2):150–158 [PMC free article] [PubMed] [Google Scholar]
- 112.Guo X, Shin VY, Cho CH. Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci. 2001;69(25–26):3113–3119 [DOI] [PubMed] [Google Scholar]
- 113.Quincozes-Santos A, Bobermin LD, Latini A, et al. Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS One. 2013;8(5):e64372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haines DD, Lekli I, Teissier P, Bak I, Tosaki A. Role of heme oxygenase-1 in resolution of oxidative stress-related pathologies: focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol (Oxf). 2012;204(4):487–501 [DOI] [PubMed] [Google Scholar]
- 115.Le LL, Li XY, Meng D, et al. Heme oxygenase-1 mediated memorial and revivable protective effect of ischemic preconditioning on brain injury. CNS Neurosci Ther. 2013;19(12):963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ambegaokar SS, Kolson DL. Heme oxygenase-1 dysregulation in the brain: implications for HIV-associated neurocognitive disorders. Curr HIV Res. 2014;12(3):174–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Calabrese V, Scapagnini G, Colombrita C, et al. Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach. Amino Acids. 2003;25(3–4):437–444 [DOI] [PubMed] [Google Scholar]
- 118.Calabrese V, Butterfield DA, Stella AM. Nutritional antioxidants and the heme oxygenase pathway of stress tolerance: novel targets for neuroprotection in Alzheimer’s disease. Ital J Biochem. 2003;52(4):177–181 [PubMed] [Google Scholar]
- 119.Chang EF, Claus CP, Vreman HJ, Wong RJ, Noble-Haeusslein LJ. Heme regulation in traumatic brain injury: relevance to the adult and developing brain. J Cereb Blood Flow Metab. 2005;25(11):1401–1417 [DOI] [PubMed] [Google Scholar]
- 120.Mor O, Stavsky M, Yitshak-Sade M, et al. Early onset preeclampsia and cerebral palsy: a double hit model? Am J Obstet Gynecol. 2016;214(1):105.e1–105.e9 [DOI] [PubMed] [Google Scholar]
- 121.Tuovinen S, Eriksson JG, Kajantie E, et al. Maternal hypertensive disorders in pregnancy and self-reported cognitive impairment of the offspring 70 years later: the Helsinki Birth Cohort Study. Am J Obstet Gynecol. 2013;208(3):200.e1–200.e9 [DOI] [PubMed] [Google Scholar]
- 122.Rätsep MT, Hickman AF, Maser B, et al. Impact of preeclampsia on cognitive function in the offspring. Behav Brain Res. 2016;302:175–181 [DOI] [PubMed] [Google Scholar]
- 123.Morsing E, Maršál K. Pre-eclampsia- an additional risk factor for cognitive impairment at school age after intrauterine growth restriction and very preterm birth. Early Hum Dev. 2014;90(2):99–101 [DOI] [PubMed] [Google Scholar]
- 124.Ehrenstein V, Rothman KJ, Pedersen L, Hatch EE, Sørensen HT. Pregnancy-associated hypertensive disorders and adult cognitive function among Danish conscripts. Am J Epidemiol. 2009;170(8):1025–1031 [DOI] [PubMed] [Google Scholar]
- 125.American College of Obstetricians and Gynecologists ACOG practice bulletin no. 134: fetal growth restriction. Obstet Gynecol. 2013;121(5):1122–1133 [DOI] [PubMed] [Google Scholar]
- 126.Giuliani F, Ohuma E, Spada E, et al. Systematic review of the methodological quality of studies designed to create neonatal anthropometric charts. Acta Paediatr. 2015;104(10):987–996 [DOI] [PubMed] [Google Scholar]
- 127.Chauhan SP, Gupta LM, Hendrix NW, Berghella V; American College of Obstetricians and Gynecologists . Intrauterine growth restriction: comparison of American College of Obstetricians and Gynecologists practice bulletin with other national guidelines. Am J Obstet Gynecol. 2009;200(4):409.e1–409.e6 [DOI] [PubMed] [Google Scholar]
- 128.Kierans WJ, Joseph KS, Luo ZC, Platt R, Wilkins R, Kramer MS. Does one size fit all? The case for ethnic-specific standards of fetal growth. BMC Pregnancy Childbirth. 2008;8:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang X, Platt RW, Cnattingius S, Joseph KS, Kramer MS. The use of customised versus population-based birthweight standards in predicting perinatal mortality. BJOG. 2007;114(4):474–477 [DOI] [PubMed] [Google Scholar]
- 130.Hutcheon JA, Zhang X, Platt RW, Cnattingius S, Kramer MS. The case against customised birthweight standards. Paediatr Perinat Epidemiol. 2011;25(1):11–16 [DOI] [PubMed] [Google Scholar]
- 131.Chiossi G, Pedroza C, Costantine MM, Truong VTT, Gargano G, Saade GR. Customized vs population-based growth charts to identify neonates at risk of adverse outcome: systematic review and Bayesian meta-analysis of observational studies. Ultrasound Obstet Gynecol. 2017;50(2):156–166 [DOI] [PubMed] [Google Scholar]
- 132.Das UG, Sysyn GD. Abnormal fetal growth: intrauterine growth retardation, small for gestational age, large for gestational age. Pediatr Clin North Am. 2004;51(3):639–654, viii [DOI] [PubMed] [Google Scholar]
- 133.Chauhan SP, Beydoun H, Chang E, et al. Prenatal detection of fetal growth restriction in newborns classified as small for gestational age: correlates and risk of neonatal morbidity. Am J Perinatol. 2014;31(3):187–194 [DOI] [PubMed] [Google Scholar]
- 134.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta-analysis. BJOG. 2013;120(6):681–694 [DOI] [PubMed] [Google Scholar]
- 135.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110.e6–120.e6 [DOI] [PubMed] [Google Scholar]
- 136.O’Gorman N, Wright D, Poon LC, et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017;49(6):756–760 [DOI] [PubMed] [Google Scholar]
- 137.Mone F, Mulcahy C, McParland P, McAuliffe FM. Should we recommend universal aspirin for all pregnant women? Am J Obstet Gynecol. 2017;216(2):141.e1–141.e5 [DOI] [PubMed] [Google Scholar]
- 138.Adkins K, Allshouse AA, Metz TD, Heyborne KD. Impact of aspirin on fetal growth in diabetic pregnancies according to White classification [published online ahead of print June 30, 2017]. Am J Obstet Gynecol. doi: 10.1016/j.ajog.2017.05.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Gorman N, Wright D, Rolnik DL, Nicolaides KH, Poon LC. Study protocol for the randomised controlled trial: combined multimarker screening and randomised patient treatment with Aspirin for evidence-based preeclampsia prevention (ASPRE). BMJ Open. 2016;6(6):e011801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moore GS, Allshouse AA, Winn VD, Galan HL, Heyborne KD. Baseline placental growth factor levels for the prediction of benefit from early aspirin prophylaxis for preeclampsia prevention. Pregnancy Hypertens. 2015;5(4):280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Farmer D. Placental stem cells: the promise of curing diseases before birth [published online ahead of print April 26, 2017]. Placenta. doi: 10.1016/j.placenta.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 142.Vanover M, Wang A, Farmer D. Potential clinical applications of placental stem cells for use in fetal therapy of birth defects [published online ahead of print May 18, 2017]. Placenta. doi: 10.1016/j.placenta.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 143.Yang Y, Bolnick A, Shamir A, et al. Blastocyst-derived stem cell populations under stress: impact of nutrition and metabolism on stem cell potency loss and miscarriage. Stem Cell Rev. 2017;13(4):454–464 [DOI] [PubMed] [Google Scholar]
- 144.Paton MCB, McDonald CA, Allison BJ, Fahey MC, Jenkin G, Miller SL. Perinatal brain injury as a consequence of preterm birth and intrauterine inflammation: designing targeted stem cell therapies. Front Neurosci. 2017;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahn SY, Chang YS, Kim JH, Sung SI, Park WS. Two-year follow-up outcomes of premature infants enrolled in the phase I trial of mesenchymal stem cells transplantation for bronchopulmonary dysplasia. J Pediatr. 2017;185:49.e2–54.e2 [DOI] [PubMed] [Google Scholar]
- 146.Shroff G, Dhanda Titus J, Shroff R. A review of the emerging potential therapy for neurological disorders: human embryonic stem cell therapy. Am J Stem Cells. 2017;6(1):1–12 [PMC free article] [PubMed] [Google Scholar]
- 147.Perets N, Segal-Gavish H, Gothelf Y, et al. Long term beneficial effect of neurotrophic factors-secreting mesenchymal stem cells transplantation in the BTBR mouse model of autism. Behav Brain Res. 2017;331:254–260 [DOI] [PubMed] [Google Scholar]
- 148.Mohan KN. Stem cell models to investigate the role of DNA methylation machinery in development of neuropsychiatric disorders. Stem Cells Int. 2016;2016:4379425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Onda K, Tong S, Beard S, et al. Proton pump inhibitors decrease soluble fms-like tyrosine kinase-1 and soluble endoglin secretion, decrease hypertension, and rescue endothelial dysfunction. Hypertension. 2017;69(3):457–468 [DOI] [PubMed] [Google Scholar]
- 150.McLaughlin K, Baczyk D, Potts A, Hladunewich M, Parker JD, Kingdom JC. Low molecular weight heparin improves endothelial function in pregnant women at high risk of preeclampsia. Hypertension. 2017;69(1):180–188 [DOI] [PubMed] [Google Scholar]
- 151.Albrecht C, Caniggia I, Clifton V, et al. IFPA meeting 2015 workshop report III: nanomedicine applications and exosome biology, xenobiotics and endocrine disruptors and pregnancy, and lipid. Placenta. 2016;48(suppl 1):S12–S16 [DOI] [PubMed] [Google Scholar]
- 152.Ilekis JV, Tsilou E, Fisher S, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an executive workshop summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215(suppl 1):S1–S46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wixey JA, Chand KK, Colditz PB, Bjorkman ST. Review: neuroinflammation in intrauterine growth restriction. Placenta. 2017;54:117–124 [DOI] [PubMed] [Google Scholar]
- 154.Spradley FT, Tan AY, Joo WS, et al. Placental growth factor administration abolishes placental ischemia-induced hypertension. Hypertension. 2016;67(4):740–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Suzuki H, Ohkuchi A, Matsubara S, et al. Effect of recombinant placental growth factor 2 on hypertension induced by full-length mouse soluble fms-like tyrosine kinase 1 adenoviral vector in pregnant mice. Hypertension. 2009;54(5):1129–1135 [DOI] [PubMed] [Google Scholar]
- 156.Siddiqui AH, Irani RA, Zhang Y, et al. Recombinant vascular endothelial growth factor 121 attenuates autoantibody-induced features of pre-eclampsia in pregnant mice. Am J Hypertens. 2011;24(5):606–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhu L, Huang L, Wen Q, Wang T, Qiao L, Jiang L. Recombinant human erythropoietin offers neuroprotection through inducing endogenous erythropoietin receptor and neuroglobin in a neonatal rat model of periventricular white matter damage. Neurosci Lett. 2017;650:12–17 [DOI] [PubMed] [Google Scholar]
- 158.Makris A, Yeung KR, Lim SM, et al. Placental growth factor reduces blood pressure in a uteroplacental ischemia model of preeclampsia in nonhuman primates. Hypertension. 2016;67(6):1263–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Steegers-Theunissen RPM. Periconception mHealth platform for prevention of placental-related outcomes and non-communicable diseases [published online ahead of print April 23, 2017]. Placenta. doi: 10.1016/j.placenta.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 160.Susser M. Maternal weight gain, infant birth weight, and diet: causal sequences. Am J Clin Nutr. 1991;53(6):1384–1396 [DOI] [PubMed] [Google Scholar]
- 161.Cottrell E, Tropea T, Ormesher L, et al. Dietary interventions for fetal growth restriction - therapeutic potential of dietary nitrate supplementation in pregnancy. J Physiol. 2017;595(15):5095–5102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Johnson A, Gambrah-Sampaney C, Khurana E, et al. Risk factors for malnutrition among children with cerebral palsy in Botswana. Pediatr Neurol. 2017;70:50–55 [DOI] [PubMed] [Google Scholar]
- 163.Vohr BR, Poggi Davis E, Wanke CA, Krebs NF. Neurodevelopment: the impact of nutrition and inflammation during preconception and pregnancy in low-resource settings. Pediatrics. 2017;139(suppl 1):S38–S49 [DOI] [PubMed] [Google Scholar]
- 164.Moody C, Callahan TJ, Aldrich H, Gance-Cleveland B, Sables-Baus S. Early initiation of Newborn Individualized Developmental Care and Assessment Program (NIDCAP) reduces length of stay: a quality improvement project. J Pediatr Nurs. 2017;32:59–63 [DOI] [PubMed] [Google Scholar]
- 165.Ohlsson A, Jacobs SE. NIDCAP: a systematic review and meta-analyses of randomized controlled trials [published correction appears in Pediatrics. 2013;132(1):e881-e893]. Pediatrics. 2013;131(3). Available at: www.pediatrics.org/cgi/content/full/131/3/e881 [DOI] [PubMed] [Google Scholar]
- 166.Als H, Duffy FH, McAnulty G, et al. NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. J Perinatol. 2012;32(10):797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lagercrantz H. Are extremely preterm born children with autism the victims of too much isolation in the incubator? Acta Paediatr. 2017;106(8):1246–1247 [DOI] [PubMed] [Google Scholar]
- 168.Guivarch J, Murdymootoo V, Elissalde SN, et al. Impact of an implicit social skills training group in children with autism spectrum disorder without intellectual disability: a before-and-after study. PLoS One. 2017;12(7):e0181159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gates JA, Kang E, Lerner MD. Efficacy of group social skills interventions for youth with autism spectrum disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2017;52:164–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Choque Olsson N, Flygare O, Coco C, et al. Social skills training for children and adolescents with autism spectrum disorder: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2017;56(7):585–592 [DOI] [PubMed] [Google Scholar]
- 171.Parker KJ, Oztan O, Libove RA, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci USA. 2017;114(30):8119–8124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quintana DS, Westlye LT, Hope S, et al. Dose-dependent social-cognitive effects of intranasal oxytocin delivered with novel Breath Powered device in adults with autism spectrum disorder: a randomized placebo-controlled double-blind crossover trial. Transl Psychiatry. 2017;7(5):e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bou Khalil R. Is insulin growth factor-1 the future for treating autism spectrum disorder and/or schizophrenia? Med Hypotheses. 2017;99:23–25 [DOI] [PubMed] [Google Scholar]
- 174.Vahdatpour C, Dyer AH, Tropea D. Insulin-like growth factor 1 and related compounds in the treatment of childhood-onset neurodevelopmental disorders. Front Neurosci. 2016;10:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Costales J, Kolevzon A. The therapeutic potential of insulin-like growth factor-1 in central nervous system disorders. Neurosci Biobehav Rev. 2016;63:207–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134(6):604–613 [DOI] [PubMed] [Google Scholar]
- 177.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.