Summary
The power of foodomics as a discipline that is now broadly used for quality assurance of food products and adulteration identification, as well as for determining the safety of food, is presented. Concerning sample preparation and application, maintenance of highly sophisticated instruments for both high-performance and high-throughput techniques, and analysis and data interpretation, special attention has to be paid to the development of skilled analysts. The obtained data shall be integrated under a strong bioinformatics environment. Modern mass spectrometry is an extremely powerful analytical tool since it can provide direct qualitative and quantitative information about a molecule of interest from only a minute amount of sample. Quality of this information is influenced by the sample preparation procedure, the type of mass spectrometer used and the analyst’s skills. Technical advances are bringing new instruments of increased sensitivity, resolution and speed to the market. Other methods presented here give additional information and can be used as complementary tools to mass spectrometry or for validation of obtained results. Genomics and transcriptomics, as well as affinity-based methods, still have a broad use in food analysis. Serious drawbacks of some of them, especially the affinity-based methods, are the cross-reactivity between similar molecules and the influence of complex food matrices. However, these techniques can be used for pre-screening in order to reduce the large number of samples. Great progress has been made in the application of bioinformatics in foodomics. These developments enabled processing of large amounts of generated data for both identification and quantification, and for corresponding modeling.
Key words: foodomics, food safety, foodborne pathogens, sample preparation, analytical technologies
Introduction
Traditional food analysis as a discipline of food science was developed together with other basic sectors of analytical chemistry (1). The first and most important aim of food analysis has always been to ensure food safety and quality, and to protect consumers against adulteration (1, 2). New developments in food technology, production of fast and ready-to-eat food, globalization of the market, and modern nutritional trends lead to new, or increasingly actual problems such as unhealthy diets that can cause obesity or food allergies. Large-scale industrial food production brings new aspects of microbiological safety and safety of genetically engineered food as additional challenges (1–5).
Food safety is a matter of global importance, and the prevention of health problems caused by false nutrition or contaminated food is a worldwide topic of extraordinary social, economic and public health importance (3, 6). Outbreaks of diseases caused by foodborne pathogens such as fungi, bacteria and protozoa are still a problem in developing countries, but also in the industrialized, highly developed Western World. Increasing globalization of the food market and new nutritional trends, such as consumption of fresh and raw food, ready-to-eat meals, dry products and exotic ingredients, cause additional outbreaks of food poisoning, but also allergies. Firstly, of unknown origin, the fatal outbreak of food poisoning caused by Shiga toxin-producing pathogen bacterium Escherichia coli O104:H4 during the year 2011 in both Germany and France originated from food imported from a developing country (7, 8). Changes in world climate and increasing environmental pollution in some countries can also cause generation of new toxic agents, and as a consequence, new toxic effects will be identified. Contamination with mycotoxins, bacterial toxins and toxins coming from other organisms via soil, water and air, as well as via livestock that was fed with contaminated food, have global implications (9).
In Croatia and neighboring Southeastern European countries, endemic nephropathy is a chronic disease that is caused by an unknown agent. There are many hypotheses, but the two most actual ones are focused on food contamination either by aristolochic acid, possibly originating in flour (10) or by ochratoxin A, a mycotoxin (11). These environmental agents are defined as main risk factors for this disease that can end in kidney failure and are associated with urothelial cancer (10–13). New regulations in the European Union, such as regulation EC 258/97 (14) or ISO 9000/EN 29000 (15) and the subsequent ones, including the Nutrition Labeling and Education Act (16, 17) that has been a Federal law in the United States since 1990, as well as similar regulations worldwide, had a crucial influence on further development of food analysis. Consequently, food analysis is becoming one of the most important areas in applied analytical chemistry, and the newest and most sophisticated up-to-date techniques are used in this field. According to these regulations, and to the law that is accepted worldwide, food safety is a responsibility equally shared by all participants in food production, from the handling of raw materials and food processing to food distribution. Regulatory and control agencies are finally responsible for compliance with regulatory rules regarding food quality and consumer protection (3, 6, 9).
The main tasks of food safety regulations are the elimination of bacterial, fungal and other pathogens and their toxins that cause foodborne diseases, the reduction of allergens and the elimination of other agents that can contaminate food and have harmful teratogenic, immunotoxic, nephrotoxic, estrogenic or similar effects. The increasing standards for food safety and new developments in analytical methods, especially in high-throughput mode and fast data analyses, result in the application of new, reliable and rapid test methods. Genomic, proteomic, peptidomic, metabolomic and similar high-throughput and high-resolution techniques that are applied for food analysis are newly summarized under the highly actual term ‘foodomics’ (3, 6). According to Herrero et al. (18) ‘Foodomics has been defined as a new discipline that studies the food and nutrition domains through the application of advanced ‘omic’ technologies to improve consumer’s well-being, health and confidence. As a global discipline, foodomics includes the working areas in which food, nutrition, corresponding advanced analytical techniques and bioinformatics are brought together’. These techniques are being increasingly applied in food analysis, as well as in food monitoring during harvest, processing, storage and transportation, until final consumption. Moreover, they can be used for identifying possible agents of food poisoning or food allergies (2, 4–6, 18–20). In both contemporary food science and nutrition, new terms such as allergomics, nutrigenomics, nutrigenetics, nutriproteomics, nutrimetabolomics and similar are frequently used. Unfortunately, they are connected with poor definitions and systematics that causes problems regarding their understanding, so the logical consequence is their relatively low acceptance (18).
In this Journal, we have already published two reviews about the application of proteomics and peptidomics in food technology and biotechnology with a focus on process development, quality control and product safety (2, 4). In the last few years several excellent studies and reviews dedicated to microbial safety and microbiota dynamics have been published (21, 22). Investigation of allergies and detection of allergens in food is also a focus of foodomic investigations (19, 23). This paper gives an overview of recent developments in foodomics as a new discipline that summarizes all high-throughput ‘omic’ techniques applied in food analysis (5, 18, 19), and their use in early, rapid, safe and reliable identification of possible hazards, such as foodborne pathogens, toxins and other harmful components that can be present in food, or can occur as contaminants during food processing and distribution.
Foodomics and the Application of ‘Omic’ Methods in Food Analysis
In a recent review, Gallo and Ferranti (1) give an excellent overview of the historical development of the application of instrumental methods in food analysis that leads to up-to-date foodomic techniques. As mentioned above, the term ‘foodomics’ summarizes genomics, transcriptomics, proteomics, peptidomics, metabolomics, lipidomics and similar high-throughput and high-resolution techniques that offer considerable opportunities to assess quality and safety of food of both plant and animal origin. Starting with the health assessment of plants and animals as food producers (3), followed by the production and monitoring of both food quality and safety by the producer, the process finally ends in control of food quality and authenticity, and protection against adulteration by authorities in corresponding reference laboratories (2–4, 17).
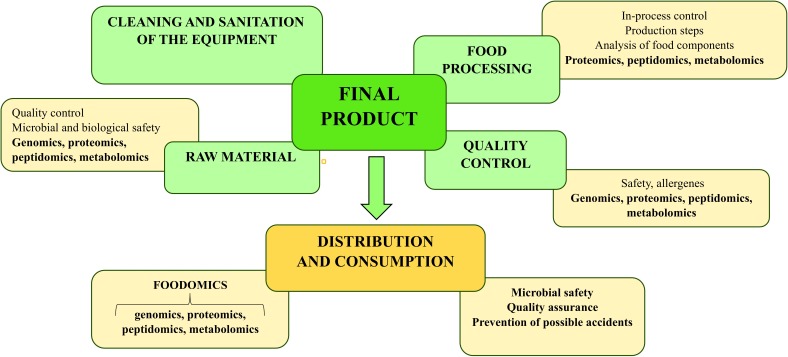
Foodomic analyses of food during the production until consumation (see Fig. 1) and the presence or the development of allergens and/or foodborne pathogens and their toxins provide decisive information and help to prevent the outbreak of foodborne infections and development of food allergies. In the case of disease and side-effect outbreaks, foodomics will lead to the identification of causative agents and support healing (7–9). Identification of proteins or other agents that are relevant for the outbreaks leads to the discovery of biomarkers for this kind of foodborne disease (or side effects) (6, 24). In this case, biomarkers (or only ‘markers’) are polynucleotides, proteins, peptides and metabolites that are relevant for tracing microbial infections and identification of toxins and allergens, as well as for the identification of the adultery of food products. Consequently, in vivo foodomic analyses can further efficiently support effective food quality control and quality assurance (3, 20, 25, 26) see also Fig. 1).
Fig. 1.
Foodomic analyses of food from the production to the consumption
Analytical tools that concerned parties have at their disposal in the struggle for food safety are standardized reference methods. Different immunological, spectroscopic and chromatographic methods with selected detection techniques are still most frequently used for analysis and identification of harmful agents in food (6). Most of the current standardized reference methods for the detection of bacteria in food are based on traditional culture methods (U.S. Food and Drug Administration Bacteriological Analytical Manual (27)). Despite reliability and robustness, traditional culture methods may display different limitations that should be overcome in order to make food safety management systems more efficient and eligible to purpose. These methods can be laborious and time consuming, they lack quantitative information and provide limited identification of post-translational modifications of proteins and peptides, they are unable to detect viable but non-culturable bacteria and are far from optimal for the detection of some harmful agents that are present in low concentrations (20, 28, 29). Development of new analytical methods for the detection of bacterial contamination in food is a challenging task, which requires a multidisciplinary approach. A special concern about newly developed methods is that they have to be validated against standard reference methods. It is important to note that a gold standard method is not necessarily perfect. These methods can also produce false-positive or false-negative results. By definition, a gold standard method provides correct results and hence a false-positive (negative) result obtained with the gold standard method would be interpreted as a false-negative (positive) result with the new method being evaluated (30). Thus, standard reference methods should be reviewed from sample preparation procedure through subsequent steps in order to perform comparison and validation of a new method (31). Increased sensitivity of new methods can cause confusion regarding legal issues since ‘safe levels’ of bacteria in a food sample are hard to define. Thus, most countries demand ‘absence’ for most pathogens. But, ‘absence’ (‘zero tolerance’) usually means ‘below detection limit’ by use of a particular method, and confusion arises if a new method is of higher sensitivity (31). Here we provide a short overview on what contemporary proteomic/peptidomic tools are providing in the detection and quantification of bacteria in food samples.
Advances in the development of up-to-date instrumental methods, especially in high-performance mass spectrometers (32), but also the introduction of label-free quantitative analyses of proteins and other biological molecules by use of new bioinformatic tools (33, 34) substantially helped in overcoming these limitations (35). The importance of proper sample preparation and the introduction of laboratory robotics for high-throughput analyses have also found necessary recognition (1, 9, 18, 20, 36–40). These developments will improve fast detection of foodborne pathogens and their toxins, allergens and other harmful components in food, and also help producers, food control authorities and consumers to detect adulterations and protect safe and original food (39–41). Ensuring food safety and identification, monitoring and assessing of both food authenticity and foodborne hazards will require further development of already existing mass spectrometry (MS)-based methods (34, 38–42). In proteomics and peptidomics, MS represents a very powerful, high-performance method and is an excellent tool for protein and peptide identification and quantitative analysis. High-resolution MS is frequently hyphenated with the ultra-high performance chromatography or capillary electrophoresis and does not require a prior knowledge of the proteins and peptides that are to be identified and quantified (18, 43).
Additionally, the introduction and application of new or already known but seldom used techniques, such as imaging mass spectrometry, nuclear magnetic resonance (NMR) and differential scanning calorimetry (DSC), can further contribute to faster and more efficient foodomic analyses (44–46). Further optimization and parallel use of the already established reference methods will support the control and validation of results acquired by the application of these newly developed techniques.
Sample Preparation
Representative sampling and adequate sample preparation are two starting and crucial steps of food analysis and correct data interpretation. However, these two steps are frequently neglected or carried out incorrectly. Consequently, both wrong sampling and sample treatment in the sample preparation stage of the analysis cannot be corrected any more, and they can cause very dangerous systematic errors. For correct sampling, deep knowledge of both the food matrix and the analytical protocol are necessary. Sample preparation itself can be a significant bias of a foodomic method since the experimental data accuracy, reproducibility and confidence essentially depend on the accuracy and quality of the cleanup technique. This becomes even more complex if very low-abundance molecules such as mycotoxins, bacterial toxins or biomarkers of microbial food contamination are targets of analysis (6). Recent review by Gallo and Ferranti (1) gives an extensive overview of sample preparation techniques in food analysis and can be highly recommended.
It is difficult to give a short overview of sample preparation for foodomic analyses. Firstly, the food itself is a very complex matrix, and the molecules of interest are very different. In genomics, DNA, RNA and their degradation products are the topics of investigation. For proteomic and peptidomic analyses, proteins and peptides have to be concentrated during sample preparation. However, the most complex task is sample preparation for metabolomic analysis of small molecules, products of microbial metabolism. The first rule is that the choice of the sample preparation method in metabolomics depends on the type of the molecule(s) of interest.
The simplest sample preparation method mostly used for the detection of small molecules such as mycotoxins, antibiotics or pesticides is the ‘dilute and shoot’ approach. It is a direct injection of diluted (or extracted) sample into the system for liquid chromatography (LC) without a complex cleanup procedure. This approach, when followed by LC-MS/MS or less frequently capillary electrophoresis (CE)-MS/MS, provides a robust method for detection and quantification (41–43). Conventional sample preparation methods in food analysis that are used especially for the detection of small molecules are liquid- -liquid extraction, solid-liquid extraction and supercritical fluid extraction (1). However, these techniques are time and/or labor intensive. The introduction of solid-phase extraction (SPE) and corresponding pre-packed commercially available columns enabled the application of high- -throughput methods and robotics for fast and reproducible sample preparation, even when multiple steps must be introduced (47, 48). Campone et al. (49) introduced pressurized liquid extraction and online SPE coupled with ultra HPLC-MS/MS. By use of this method, small molecules like mycotoxins or pesticides can be detected and quantified. Recovery and quantification limits of target substances have to be carefully checked and validated (50, 51). However, the applied method is time consuming, requires large amounts of solvents, and systematic errors caused by the absorption loss as well as the interaction between the sample and the solid phase were also observed. Next optimization step was the introduction of solid phase microextraction, where the amounts of solvent were minimized to a few milligrams (52, 53). Use of iron oxide-based nanoparticles as materials for so-called ‘magnetic solid-phase extraction’ is a further step towards the introduction of a simple, rapid, selective, sensitive and highly efficient method for high-throughput enrichment of molecules of interest (54). However, further development of very sensitive analytical methods, as well as software systems capable of handling very high volumes of required data, especially mass spectrometry with a very low detection limit for targeted substances, was necessary to enable application of this sample preparation method (38, 39, 41, 42, 55–58). As mentioned above, there are commercially available columns and ready-to-use cartridges packed with different chromatographic stationary phases for selective enrichment of small molecules (1). Commercially available solid phase extraction kit (so- -called QuEChErs kit by Agilent, Santa Clara, CA, USA) can be used as a fast alternative to traditional liquid-liquid and solid-phase extraction in analysis of small molecules. The sample preparation process involves two steps, namely the extraction with organic and salt solutions followed by a clean-up with dispersive solid-phase extraction resins (1, 59).
Sampling and sample preparation are also key steps in proteomic and peptidomic analyses (60). Wiśniewski et al. (61) recommend a filter-aided sample preparation by a complete solubilization of the proteome in sodium dodecyl sulfate which is subsequently exchanged by urea on a standard filtration device (filter-aided sample preparation). According to the authors, ‘peptides eluted after digestion on the filter were pure, allowing a single-run analyses of organelles and unprecedented depth of proteome coverage’. This method summarizes previous experiences in sample preparation for proteomic analyses that was reviewed by several authors (62, 63). However, if cell and tissue samples are prepared using this method, some very hydrophobic proteins and proteins with posttranslational modifications (PTMs) such as glycosylation are frequently not solubilized. In food analysis, the extracellular matrix is frequently only partially (or not at all) destroyed. These proteins are therefore lost for subsequent MS analysis. For analysis of matrix proteins that are important for meat tenderness, and for overall food consistency and quality, as well as tracing of possible adulteration and manipulation (such as repeated freezing and thawing), D’Alessandro and Zolla (64) recommend a stepwise solubilization method, which makes use of detergents, chaotropic agents and reagents for chemical digestion such as cyanogen bromide. This method was developed by Hill et al. (65), for the analysis of proteins that are important for tissue engineering. There are also difficulties with proteolytic (most frequently tryptic) digestion of these proteins. Consequently, they are only partially detected or not detected at all, and additional methods for their solubilization and proteolytic digestion must be developed (66). Blonder et al. (67) recommend extraction of hydrophobic proteins by organic solvents, mostly methanol, followed by tryptic digestion. This method enables detection of different, highly hydrophobic proteins that were previously not covered by other approaches. Furthermore, Lu and Zhu (68) developed the so-called ‘tube- -gel digestion’ of proteins for more complete proteomic analysis and detection of very hydrophobic proteins and proteins with posttranslational modifications (PTMs). Use of this method enabled identification of different hydrophobic membrane proteins that had previously not been detected (66). Application of MALDI mass spectrometry, requests for miniaturization, and introduction of on-chip sample preparation (69), as well as reduction of sample amount used for proteomic analyses (70), were the main driving forces for the introduction of high-throughput methods that enable analyses of hundreds or even thousands of samples. Development of these methods, use of laboratory robotics and introduction of special chromatographic supports like monoliths further enhanced the development of high-throughput methods for sample preparation, especially in genomics, proteomics and metabolomics (48, 71).
Genomic analysis that is followed by transcriptomics gives the first information about the changes in the transcription rate of genes in a genome in a particular cell type or tissue during development, but also in different disease states (72). Preparation of genomic DNA is now a routine that is described in several protocols for use of commercial kits for sample preparation and preparation of DNA microarrays (73). DNA microarrays are genome- -wide hybridization devices that are used to get information about changes at the transcriptional levels. This technology is one of the most successful and most effective methodologies of high-throughput genomic analyses (74). Flow-through DNA microarray assay can be successfully used for rapid detection and quantification of food pathogens (75).
However, analyses of samples have their own rules, especially if detection of foodborne bacteria and their toxins, as well as contamination by fungi and mycotoxins, is requested. We demonstrated that for the detection of Gram-positive bacteria, their thorough decomposition by use of different mechanical methods, followed by extraction with urea and different detergents is necessary (37). As a rule, sample preparation for detection of proteins and peptides in foodomics has to be carefully elaborated, but already published recommendations (37, 61–63) can be used as basic information and a first step in the development of a sample preparation protocol. After solubilization of proteins/peptides of interest, they can be separated by gel electrophoresis, i.e. gel-based proteomics (76) or by different chromatographic or capillary electrophoretic techniques, i.e. gel-free methods (6, 39, 77, 78). In proteomic analyses of food, gel-based methods are still frequently used. However, when a quantitative analysis of detected proteins or polypeptides is necessary, special staining and quantification programs have to be used (76).
Analytical Techniques
The strategy of the integration of different analytical techniques and approaches is the main power of foodomics. This kind of strategy demonstrates the flexibility and knowledge in order to follow and incorporate fast technological developments in all fields of instrumental techniques and shall be open for new ideas and approaches. The foodomics expert shall integrate the sample preparation knowledge with the knowledge of main fields of advanced analytical technology, strategically integrated under a strong bioinformatic environment. The opinion of the authors of this review is that a comprehensive knowledge of the methods that are actually applied for food analysis and quality assurance should be combined with recently developed high-throughput methods that will be presented here.
Affinity-based methods
Affinity recognition is usually an antibody- and lectin-based approach that benefits from high selectivity. This method is based on specific affinity recognition of antibodies and lectins with the epitopes on the cell surface of microbial species of interest, or with the corresponding parts of the molecule of secreted products of their metabolism. Affinity recognition can also be based on other highly specific molecular interactions such as affinity recognition of components of a bacteriophage with bacterial cell wall components. Different technical solutions have been developed to exploit affinity recognition: immuno-diffusion discs, ELISA, lateral flow assays, biosensors and fast affinity chromatography on monolithic supports (78–81). A serious drawback of all affinity-based methods is the cross reactivity between similar molecules. Antibodies developed against surface antigens may cross--react with microbial cells within the same genera. In this case, cross reactivity would lead to false positive identification. However, this method can be used for pre-screening to reduce the large number of samples to a low number of positive ones, which will subsequently be analyzed by a more stringent technique. Antibody and lectin microarrays incorporating several molecules directed against the same target substance have been designed to overcome the problem of cross reactivity. However, complex food matrices may contain substances (e.g. polyphenols, tannins), which interact or covalently modify target molecules. These substances can significantly interfere by inducing or reducing specific bindings. These phenomena can lead to over- or underestimation (79–82).
Rapid response of foodborne pathogens to their environment typically involves altered gene expression in order to favor both their survival and subsequent growth under extreme conditions. These changes in the physiological state that frequently result in damages of cellular surface (e.g. high/low pH, pressure, temperature, availability of oxygen, exposure to radiation and UV light, or use of specific disinfectants) are typical for food processing and can interfere with the affinity-based detection system (83).
Nuclear magnetic resonance spectroscopy
The discussion about nuclear magnetic resonance spectroscopy (NMR) as a very promising method with great potential to improve both food safety and taste started years ago, before the time of broad use of mass spectrometry-based methods started. First applications of NMR were performed for measurement of water movement during processing in order to study heat and mass transfer, composition and structure of food (84). The main goal was to follow the killing and inactivation of food pathogens and their toxins (85–89). Different promising discussions started, but almost nothing happened for a long time. An intensive literature study shows that some applications gave very interesting results, but follow-up work either failed or was never done (86). More than twenty years ago, alterations in food during packaging were followed by this technique (87). Later, there were some very promising applications in the field of metabolomics, mostly for studying the interaction between different food components, and in the field of food lipidomics, for quantitative determination of olive oil quality (88, 89). Albeit its high potential, NMR was still rarely used for food analysis, even less frequently for detection of food pathogens, their toxins and other metabolites (90).
More than ten years ago, NMR came back in focus as a very effective, high-resolution, quality control method in food industry (91, 92). Moreau and Guichard (88) demonstrated that this method can be used for the determination of interactions between flavor compounds and high molecular, non-volatile food components, as well as the influence of these reactions on flavor perception of processed foods.
In the last ten years, the number of quantitative NMR applications in foodomics has significantly increased (93, 94), especially toward detection and quantitative determination of specific molecules (95–97). NMR can be used for determining food origin (98–100), quality and purity (97, 98, 101). The most important application of NMR in the field of safety is the use of this method for the determination of changes in food during storage, with particular emphasis on degradation processes and possible contamination with food pathogens and their toxins (93, 94). Changes during freezing and storing at different temperatures in dependence of time were determined in fish (102, 103), meat (104) and vegetables (105). NMR was also used for detecting metabolic profiles of transgenic grapes and their comparison with non-modified fruits (106). Although there are only a few NMR applications for the determination of food pathogens, they seem to be very promising. Ercolini et al. (104) used this technique for monitoring the microbial metabolites and bacterial diversity in beef during storage under different packaging conditions. Picone et al. (107) followed the effect of the natural terpenoid carvacrol on the metabolome of a model Escherichia coli strain by use of proton nuclear magnetic resonance (1H-NMR) spectroscopy.
In general, 1H NMR spectroscopy is the most frequently used method (95–99, 108), but NMR spectroscopy can also detect other isotopes such as 13C, 15N, 17O and 31P (79, 89, 91, 92, 108). The advantages of NMR spectroscopy are that minimal sample preparation is necessary, and practically every kind of biological fluids or food extracts can be analyzed using this method (91, 92, 108). However, sensitivity is still a problem, and less abundant substances cannot be detected. This fact reduces the utilization of this method, so that mass spectrometry is still the method of choice, when low-abundance food components are the targeted substances (108).
Other techniques
There are several methods for visualizing changes in food during processing such as X-ray diffraction (109), a combination of high-resolution imaging techniques like confocal scanning microscopy with specific staining of food components, and a combination of several physicochemical methods (110–112) that are followed by computer simulation techniques (113). These strategies are still either time consuming, or they yield only very specific information, so as a consequence, the use of these methods is still limited. However, further development in the direction of simplification and high-throughput can open new perspectives for the utilization of these techniques.
Use of differential scanning calorimetry for the determination of melting and caramelization of sugars as an important component in food was also discussed, but the correct application of this technique is still a topic of controversial debate (45, 114, 115).
Techniques based on genomic methods
Different PCR/qPCR-based methods (116), second and third generation sequencing techniques (117) and other nucleic acid-based methods (e.g. microarrays) are powerful tools for the detection of foodborne pathogens and their metabolites. Simultaneous detection and quantification of several microorganisms (118) and their serotyping (119), performed in a high-throughput fashion, can be combined with a microfluidic technique. However, application of polymerase-based techniques also has some limitations, and until now, most gene expression studies have been done on monobacterial cultures, mixtures and on artificially contaminated food samples. As a complex matrix, food may contain substances that inhibit polymerase reaction (118, 119). Additionally, some compounds may be introduced during sample preparation. Specific design of internal positive control can help to overcome these shortcomings (30, 120). Gene expression microarrays are successfully used for analyses of bacterial transcriptome, and RNA sequencing has also been used as a method for investigating the physiological state of foodborne pathogens at the molecular level (121, 122). The quantification of expression of specific genes and the determination of both stress resistance and cell viability biomarkers can be performed by the use of reverse transcription qPCR (123). Together with the results of targeted proteomic analyses, such as detection of endo- and exotoxins, this kind of transcriptomic data provide further understanding of the physiology of foodborne pathogens (124). That is why proteomic and metabolomic tools are compatible with DNA- (and RNA)-based approaches in the analysis of foodborne pathogens and can be used for validation of these assays. Rosselló-Móra and Amann (125) used a strategy that includes comparative analysis of bacterial proteomes in search for clone-specific proteins whose sequences are used for the design of PCR probes for their quantitative determination. Such improved understanding of behavior and metabolic activity of microorganisms during production is crucial for preserving and improving food quality, safety and nutritional value (126).
All DNA-based microarray assays are plausible high--throughput techniques. The automation and introduction of laboratory robots resulted in an improved capacity and enabled the analysis of a large number of samples. Donhauser et al. (127) developed a DNA-based, sensitive chemiluminiscence flow-through microarray assay for the rapid and very sensitive determination and quantification of food pathogens Escherichia coli, Salmonella enterica and Campylobacter jejuni in water samples. This method was further developed and applied for the detection of other foodborne pathogenic microorganisms and viruses (128, 129). However, the use of such microarray chips for analyses of food samples requires further development of high--throughput sample preparation methods (6, 39, 130).
Mass Spectrometry
Mass spectrometry (MS) is one of the most powerful analytical tools because it can provide direct qualitative and quantitative information about the molecule of interest from a minute amount of sample. In the last 10–15 years, the increase in the performance and adaptability of instrumentation in mass spectrometry has enormously contributed to the development of new strategies in food analysis, rendering MS as one of its main tools (131). The quality of the information gained by MS is influenced by sample preparation procedure, type of mass spectrometer and analyst’s skills. As a consequence, foodomic approaches need to be adapted to each analytical problem and the special characteristics of each matrix have to be taken into consideration. Technical advances are bringing to the market new mass spectrometers of increased sensitivity and resolution, with a lower sample and solvent consumption, that offer rapid analysis, and even in-field analysis. However, all presented advantages still cannot be incorporated into one analytical device, thus comprehensive knowledge of the current technology status is a prerequisite for the successful design of foodomic approaches (3, 6, 18).
Mass analyzers can achieve low accuracy levels of 100–10 ppm (e.g. quadrupole, ion trap, linear time-of- -flight (TOF) or can be of medium accuracy with levels down to 1 ppm (e.g. reflectron TOF). This level of accuracy is not enough to unambiguously identify a molecule of interest. Thus, mass analyzers are often combined, together and with a collision cell (fragmentation cell), into tandem mass spectrometers (MS/MS) which can provide enough information to unambiguously identify large numbers of compounds of interest in food analysis. Improvement of existing and development of new mass analyzers is necessary to achieve higher accuracy, resolution, sensitivity, dynamic range and speed. Mass analyzer with the highest accuracy and resolving power is Fourier transform ion cyclotron resonance (FT-ICR). The platform of FT-ICR MS dates from 1974 (132). However, the acquisition speed of FT-ICR of 1 Hz is still not fast enough for comprehensive analysis of highly complex mixtures which need to be fractioned by liquid chromatography (LC) prior to mass analysis. In the year 2000, a new mass analyzer named Orbitrap was described by Makarov (133). In the years that followed, this mass analyzer has quickly become a very important mass analyser for LC-MS/MS analysis of high complex mixtures. With improvements described in 2009 (134), Orbitrap, now as part of a hybrid mass spectrometer, provides resolving power of 500 000 (at m/z=200) and accuracy in low-sub ppm range. The advantage of Orbitrap over FT-ICR is in its speed of up to 20 Hz, which is much more suitable for coupling with LC in analysis of samples of high complexity. However, depending on the particular application, higher resolving power is sometimes more beneficial (135–137).
This chapter gives a short overview of principles of high-resolution mass spectrometric methods that are most frequently applied in food analysis with a focus on food safety. A more general overview of these methods was published by Herrero et al. (18) and recently by Ibáñez et al. (138).
MALDI-TOF mass spectrometry
Matrix-assisted laser desorption/ionisation time-of- -flight mass spectrometry (MALDI-TOF MS) is a frequently used method that is complementary to other soft ionisation techniques, such as electrospray ionization (ESI). The advantage of MALDI-TOF instruments is their higher tolerance toward contaminants arising from the food matrix. This technique can be applied for the analysis of large molecules such as proteins, but it is also applicable to analysis of intermediate and small size molecules. However, the signal suppression due to matrix background ions presents a limitation when small molecules are analyzed (139). MALDI-TOF MS is an effective and simple method for high-throughput analysis, and the main use of this technique in food authentication and safety analyses is the identification of foodborne pathogens (140, 141). Phenotypic tests, such as colony characteristics, growth on selective agar plates, biochemical pattern characterization and Gram staining, are methods that are currently used for the identification of bacteria. However, they are time consuming and less practical if a fast analysis is needed. Known for its sensitivity, accuracy and reproducibility, MALDI-TOF MS is a method most commonly used for the identification of bacteria and their toxins (139, 141). This approach is very rapid since it does not involve a sample preparation step, but rather relies upon the introduction of a bacterial colony onto a MALDI plate. The result is a unique intact or tryptically digested ribosomal or intracellular protein and peptide profile of whole bacterial cells, so called bacterial ‘fingerprint’, which allows for an accurate identification of bacterial contamination. Acquired bacterial MALDI-TOF MS fingerprints are matched against spectral libraries previously collected under identical MALDI conditions without further identification. Consequently, identification success remains highly dependent on the number of well characterized food pathogen biomarker sequences available in reference databases that are in the majority of cases still not publicly available (142–144). It has been demonstrated that sample preparation is also a critical point that includes destruction of bacterial cells and reproducible digestion of liberated proteins (145–147).
MALDI-TOF MS can be successfully used for the discrimination between bacterial subtypes and the detection of the foodborne pathogen Mycobacterium avium subspecies paratuberculosis (MAP) (147). MAP is a pathogenic bacterium that affects cattle and causes paratuberculosis. This microorganism is heat resistant, making its contamination of milk a major cause of MAP transmission to humans (148). The most common diagnostic method used to detect MAP in cattle is ELISA, using specific monoclonal antibodies. However, the use of this technique is limited because of its relatively low sensitivity and possible cross-reaction of antibodies with environmental mycobacteria. Moreover, because of the above-mentioned relatively low sensitivity, ELISA cannot be used for specific detection of early infections. Thus, more sensitive proteomic approaches have been developed for MAP detection, such as 2D gel electrophoresis coupled with MS (148). Lin et al. (149) have effectively shown that MALDI-TOF MS is applicable for rapid discrimination of M. avium from other Mycobacterium species. These results also demonstrate a serious potential of this method for clinical application, as well as for early detection of these bacteria. MALDI-TOF MS as a tool for classification and identification of bacteria has been systematically used for about ten years (150), and its use as an alternative to conventional methods for identification of food pathogens was described by Schumann and Maier (151). It was also shown that this method can be used as a very efficient tool for the identification of high-risk toxin-producing E. coli strains (152). The major bottleneck for further implementation of MALDI-TOF MS in food monitoring systems remains sample preparation. It covers the isolation of microorganisms, culturing by conventional methods and/or enrichment of anaerobic, demanding or slow-growing bacterial strains (153). For that reason, sample preparation protocols for viable but non-culturable (VBNC) or difficult to culture food poisoning bacterial strains Vibrio cholerae, enterohemorrhagic E. coli, Shigella flexneri and Salmonella enterica should still be developed (154). It is important to note that not all food pathogens pose a health threat themselves, but proteins and other metabolites that they secrete can be severely toxic to humans. The significant disadvantage of MALDI--TOF MS is that this technique can only provide information on the occurrence or absence of food-contaminating food pathogens, and it does not give any data about the expression of toxin-encoding genes or levels of toxins and other microbial metabolites or other secreted substances in food. For this sake, other methods, again mostly based on mass spectrometry, have to be employed for their detection (6, 9).
Imaging mass spectrometry
Imaging mass spectroscopy is a combination between MALDI-TOF MS and histochemistry. This technique gives information about both the molecular size of the substance and its localization in tissue samples (44, 155). However, this potentially powerful strategy was seldom used in foodomics (44, 156–158). Hong et al. (156) used MALDI imaging mass spectrometry for enhanced visualization of small peptides in rat intestine, presenting only the optimization of the method. An example of the application of imaging mass spectrometry in the field of food safety is the detection of procymidone, a frequently used fungicide in cucumber samples by nanoparticle-assisted laser desorption-ionization mass spectrometry imaging (157). Recently, Yoshimura et al. (158) have given an extensive overview about the state-of-the art and future use of imaging mass spectrometry in food analysis.
Similar strategies based on both transmission electron microscopy and single particle inductively coupled plasma-mass spectrometry were used for the analysis of the release of potentially harmful silver nanoparticles that are used as pastry decorations (159).
Together with MALDI-TOF, additional mass spectrometric methods were used for the determination of structural elements and large molecules that are cellular components of foodborne pathogens as well as their metabolites. These substances are used as biomarkers for indirect detection of pathogens in food (160).
Fourier transform ion cyclotron resonance mass spectrometry
Mass analyzer with highest accuracy and resolving power is Fourier transform ion cyclotron resonance (FT- -ICR). As already mentioned above, the platform of FT- -ICR MS dates already from 1974 (132). Development of stronger magnets, improvement of the ICR cell, ion optics, data acquisition systems and signal processing are only some of the possible ways for improvements in FT- -ICR performance (161, 162). Nowadays, commercial FT- -ICR instruments with a resolving power of 10 000 000 (at m/z=400) and with accuracy in low-sub ppm or ppb range are available. Acquisition speed of FT-ICR of 1 Hz is not fast enough for a comprehensive analysis of highly complex mixtures, which is needed for the fractionation by LC prior to mass analysis, but the improved instrument can be used for the analysis of complex mixtures containing large molecules such as proteins (135, 136). The large potential of FT-ICR MS for metabolite analysis was discovered relatively early (163), and a big advantage of this method is the possibility of direct infusion (164). The hyphenation with a liquid chromatograph is also practicable, but there are some limitations caused by relatively slow analysis by this instrument (165–167). For analysis of small molecules, Oliveira et al. (168) demonstrated the power of ultra-high resolution of FT-ICR operating in negative ESI mode with a direct infusion of complex extract of mango fruit for characterization of primary and secondary metabolites in order to achieve higher nutraceutical value of this fruit. The application of FT-ICR MS in lipidomics (169, 170) is increasingly pushed back by faster and easier-to-use Orbitrap and other hybrid mass spectrometers (171–173). According to Schwudke et al. (173), ‘Despite its recognized potential, the scope of FT MS-driven lipidomics remined rather limited. Sampling of abundant ions of chemical background could exceed the storage capacity of an ion cyclotron resonance (ICR) cell and impair the accuracy of isotopic profiles, thus compromising the species quantification’. However, although published in a very high quality peer-reviewed journal, this pretty critical evaluation is coming from two competitors, and careful analysis of the instrument (174) yielded in significant improvements, so that this method is again gaining in popularity for lipidomic and other analyses of biological samples (175). For example, He et al. (176) used this method for characterization of polar lipids by online LC hyphenated with hybrid linear quadrupole ion trap FT-ICR MS. Furthermore, Le et al. (177) applied MALDI FT-ICR MS for tissue imaging of lipids. In a metabolomic analysis, Milev et al. (178) used this MS approach for the analysis of fermented raw cocoa beans and the determination of their origin by direct infusion of purified raw extract. Together with other high-performance MS and LC methods, Chen et al. (179) used FT-ICR mass spectrometry for the screening of antioxidants of longan seed extract. There are further applications of FT-ICR for determining protein glycosylation, glycoscreening and sequencing (180–182). In food safety, these analyses are important for the determination of allergenicity of proteins and peptides (183, 184). Recently, Ghaste et al. (185) gave a comprehensive overview about the use of FT-ICR MS in metabolomics and lipidomics. However, a clear differentiation between the use of this kind of instrument and Orbitrap-based metabolomics is not given. In conclusion, the use of this powerful method in foodomics, especially in both proteomics and metabolomics, is still not fully exploited, and the use of the newly developed instruments with increased throughput will contribute to a more frequent use of FT-ICR mass spectrometry in food analysis (186). A recent impressive investigation of the E. coli proteome by LC FT-ICR MS (187) and a metabolomic study by Witting et al. (188) of both worm Caenorhabditis elegans and bacterium Pseudomonas aeruginosa opened the way for use of this method for high-throughput analysis of food pathogen metabolomes.
Hybrid mass spectrometers – Orbitrap and other instruments
In the year 2000 a new mass analyzer, Orbitrap, was described (133). In the years that followed, the interfacing of the Orbitrap mass analyzer with an electrospray ion source was realized (189), and this MS instrument has quickly become a very important tool for LC-MS/MS analysis of highly complex mixtures. Further optimization of the instrument was described in 2009 (134) and the development of corresponding data acquisition has been constantly improved since (190). Orbitrap, now as part of a hybrid mass spectrometer, provides resolving power of 500 000 (at m/z=200) and accuracy in the low-sub ppm range (134, 191). The advantage of Orbitrap over FT-ICR is in its speed of up to 20 Hz, which is much more suitable for coupling with LC when analyzing samples of high complexity. Hybrid Orbitrap mass spectrometers can perform combined fragmentations, and this property of the instrument is currently the most important fragmentation scheme for the analysis of posttranslational modifications of proteins (134, 183, 184, 192). In food safety, these investigations have been shown to be crucial for the studying of allergenicity of proteins (183, 184). It has to be stressed that the ongoing optimization of speed, performance, practicability, collaboration with leading scientist in this field from both academia and industry (33, 34, 134, 173, 183, 184), its user friendliness and excellent marketing, make the Orbitrap a leading instrument for mass spectrometry analyses in foodomics (33, 133-135, 183, 186, 193), as well as one of the most expensive ones.
As mentioned above, Orbitrap is both a very popular and frequently used instrument for high-resolution mass spectrometric experiments in foodomics. The detection of marker molecules is highly important for food safety, and there is no surprise that most references regarding the application of this method point to works that were performed by this instrument (18, 33–36, 55, 57, 138, 141, 173). A further interesting application of this method for control of food safety and authenticity is the determination of the triacylglycerol profile of Iberian dry-cured ham (194). The results were further combined with chemometrics to prove the authenticity of this product (195). The use of the ESI-Orbitrap method for profiling of epicuticular wax from olive fruits shortened the analysis, and a detailed compositional overview of a wide range of chemical species was obtained. Otherwise, this profile could be obtained by applying a combination of multiple analytical techniques (196). Proteomic investigation of the interaction of foodborne pathogen Salmonella enterica with macrophages gave important information about the pathogenicity of this bacterium (197). Orbitrap mass spectrometers are also frequently used for the determination of food contaminants and metabolites (55, 56). This kind of information is also very important for the determination of metabolites in genetically modified food (138, 198). However, with corresponding sample preparations and in- -front separation by liquid chromatography or capillary electrophoresis, FT-ICR MS (199), MALDI-TOF MS (200) and other mass spectrometry techniques (201) have been proven to be equally efficient analytical tools for these kinds of tests.
The use of other hybrid instruments has recently been reviewed by Ibañez et al. (138), and the conclusion can be made that there are different alternatives, especially if corresponding sample preparation and separation of the component of interest were done by LC and capillary electrophoresis. These strategies are applied for both the identification of bacterial proteins (202) and food authentication (187, 203, 204), as well as for the identification of peptides that reflect changes in food products during processing and storage (205). While 2D electrophoresis as a sample preparation method is mostly coupled with MALDI-TOF, LC-MS/MS can be used as well. The comparison of isotope dilution MALDI-TOF MS and LC ESI- -MS/MS (with a hybrid instrument, different than Orbitrap) used for quantification of anthrax lethal toxin in plasma and serum by Kuklenyik et al. (206) gave similar, very precise quantitative results. Detection of foodborne pathogen bacteria and their toxins can be performed by isotope dilution MALDI-TOF MS, but other, more targeted investigations and detections of food pathogens such as E. coli, Salmonella sp., Clostridium sp., Mycobacteria sp. and their toxins, as well as possible antibiotic resistance, were performed by high-performance LC (less frequently CE)–MS/MS and by using different instruments (138, 141, 207, 208). At the same time, hybrid type mass spectrometers were used for investigation of proteins in milk as putative biomarkers of milk contamination (208) and antibiotic resistance of potential foodborne pathogens (209, 210). Picariello et al. (211) and Andjelkovic et al. (19) reviewed the use of mass spectrometry-based techniques for the determination of allergens in food. A comprehensive overview of applied techniques and instruments that can also be used for the determination of other quality and toxicity markers in food was given (like the above-mentioned isotope dilution MALDI-TOF MS). Use of different methods and MS–based techniques in allergenomics, frequently combined with corresponding sample preparation, was recently published, among others, for the determination of barley contamination (212), peptidomic analysis of the possibly immunogenic potential of polypeptides in beer (213), whey-based supplements (214), glycosylation of soybean proteins (215), as well as new food allergens from hazelnut (216). Peptidomics are also a useful tool for quality control and control of curing time of processed meat products such as Spanish dry-cured ham. Gallego et al. (217) identified two signature peptides that can be used for the determination of the curing process. In a practically parallel investigation, Bayés-García et al. (195) used a relatively complicated analytical approach based on the analysis of the lipid fraction extracted from Iberian dry- -cured ham by ultrahigh resolution mass spectrometry (UHRMS) using an Orbitrap–mass spectrometer combined with scanning calorimetry, X-ray diffraction and thermo- -optical polarized microscopy analysis, in combination with chemometrics. The resulting lipidomic profile obtained by UHRMS and the fingerprint obtained by other methods can be used for analytical discrimination of this high-value nutritional product. However, the question of practicability of this very complicated and time-consuming method remains open. The final goal should always be to develop a validated rapid, simple and highly reliable method for the detection of safety risks and the identification of foodborne pathogens. The use of specific validated markers for undesirable changes and contamination of food enables fast and reliable recognition of safety risks (9).
Data Processing and Bioinformatics
The acquisition of large amounts of data during genomic, proteomic, peptidomic and especially metabolomic analyses requires highly sophisticated software for further data processing (9, 58, 171, 218, 219). There are published databases and specific applications in genomics (34), peptidomics (220), proteomics (34, 221), metabolomics (58) and lipidomics (171, 172). In the last ten years (so-called the ‘years of bioinformatics’) of their application in foodomics, including automated data processing, interactome analyses and modeling, great progress has been made (173, 222). Large amounts of data are generated during each non-targeted MS analysis of food samples for both identification and quantification of components of interest. We have recently presented a comprehensive overview of some omic data repositories (9) that are in a process of constant improvement and completion (171), and bioinformatic research is without doubt a crucial tool in the whole field of foodomics (4, 9, 18). It is difficult, sometimes practically impossible, to implant them in a laboratory for routine foodomic analyses. During the last twenty years, the development of high-speed computer technology and sophisticated hardware capable of processing a large number of results has growth enabled a fast growth of chemometrics. The most common definition of chemometrics is that it is ‘a chemical discipline that uses mathematical, statistical and other methods based on formal logic for the construction or selection of optimum measurement methods and experimental designs and also the extraction of the most important information in the analysis of experimental data’ (92, 222). In a chemometric analysis, projecting tens of thousands of initial multidimensional data onto only a few axes is possible. When placed in such new coordinates, interpretation of presented data is relatively easy. Consequently, it is not necessary to know the mathematical fundaments of a method in detail for the effective practical application of chemometrics – it is sufficient to understand the main ideas of this approach (92). Bayés-García et al. (195) used chemometrics for authentication of Iberian dry-cured ham. Different foodomic methods based on ultrahigh resolution mass spectrometry, differential scanning calorimetry, X-ray diffraction and microscopy analysis were combined with chemometrics and were used for analytical discrimination of this highly valuable product. Köbler et al. (223) presented a combination of NMR spectroscopy, sensory analysis and chemometrics for the identification of pine nuts that cause taste disturbance.
Conclusions
The power of foodomics, as omics in general, is in the integration of different techniques and approaches which all show an increased trend of development. However, the educational system should demonstrate more flexibility to follow and incorporate fast technological developments that are necessary to reach the full power of foodomics. Use and maintenance of highly sophisticated mass spectrometers, data analysis and data interpretation require a special attention to be paid to the development of skilled analysts. A new curriculum for analysts in the food sector should integrate basic biochemistry, molecular biology, biophysics, food technology and sample preparation knowledge, together with the knowledge of advanced analytical technologies, some of which are mentioned in this review. All this should be integrated under a strong bioinformatic environment which would enable integrative data analysis and meaningful interpretation of data obtained from the analyzed material.
According to our experience, targeted and careful sample preparation in combination with a sophisticated up-to-date mass spectrometer is an essential part of foodomic analysis that guarantees reproducibility of quantitative analyses and enables the detection of low-abundance components. Additional methods presented here, such as NMR, give further information and can be used as complementary tools or for the validation of obtained results, especially in the case of glycomic investigations.
Genomic and transcriptomic techniques, as well as affinity-based methods, still have a broad use in food analysis. A serious drawback of all affinity-based methods is the cross reactivity between similar molecules that can lead to a false-positive identification. Additionally, complex food matrices may contain substances (e.g. polyphenols, tannins), which interact or covalently modify target molecules. These substances can significantly interfere by inducing or reducing specific bindings. These phenomena can lead to over- or underestimation. However, these techniques can be used for the prescreening to reduce the large number of samples to a low number of positive ones. The subsequent final validation will be achieved by use of a more stringent technique such as high-resolution mass spectrometry.
During the last ten years, great progress has been made in the application of bioinformatics in foodomics. These progressive developments enabled the processing of large amounts of data that are generated during the analysis of food samples for both identification and quantification of components of interest, and for the corresponding modeling and generation of interactome.
Acknowledgements
This paper was supported by a bilateral scientific project financed by the Ministries of Sciences of Croatia and Serbia. U.A. acknowledges support from the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant no. 172048).
References
- 1.Gallo M, Ferranti P. The evolution of analytical chemistry methods in foodomics. J Chromatogr A. 2016;1428:3–15. 10.1016/j.chroma.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 2.Gašo-Sokač D, Kovač S, Josić Dj. Application of proteomics in food technology and food biotechnology: process development, quality control and product safety. Food Technol Biotechnol. 2010;48:284–95. [Google Scholar]
- 3.García-Cañas V, Simó C, Herrero M, Ibáñez E, Cifuentes A. Present and future challenges in food analysis: foodomics. Anal Chem. 2012;84:10150–9. 10.1021/ac301680q [DOI] [PubMed] [Google Scholar]
- 4.Gašo-Sokač D, Kovač S, Josić Dj. Use of proteomic methodology in optimization and processing and quality control of food of animal origin. Food Technol Biotechnol. 2011;49:397–412. [Google Scholar]
- 5.Rešetar D, Kraljević Pavelić S. Josić Dj. Foodomics in investigations of food toxins. Curr Opin Food Sci. 2015;4:86–91. 10.1016/j.cofs.2015.05.004 [DOI] [Google Scholar]
- 6.Martinović T, Andjelković U, Šrajer Gajdošik M, Rešetar D. Josić Dj. Food borne pathogens and their toxins. J Proteomics. 2016;147:226–35. 10.1016/j.jprot.2016.04.029 [DOI] [PubMed] [Google Scholar]
- 7.King LA, Nogareda F, Weill FX, Mariani-Kurkdjian P, Loukiadis E, Gault G, et al. Outbreak of Shiga toxin-producing Escherichia coli O104:H4 associated with organic fenugreek sprouts, France, June 2011. Clin Infect Dis. 2012;54:1588–94. 10.1093/cid/cis255 [DOI] [PubMed] [Google Scholar]
- 8.Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–80. 10.1056/NEJMoa1106483 [DOI] [PubMed] [Google Scholar]
- 9.Giacometti J, Josić Dj. Foodomics in microbial safety. Trends Analyt Chem. 2013;52:16–22. 10.1016/j.trac.2013.09.003 [DOI] [Google Scholar]
- 10.Grollman AP, Jelaković B. Role of environmental toxins in endemic (Balkan) nephropaty. J Am Soc Nephrol. 2007;18:2817–23. 10.1681/ASN.2007050537 [DOI] [PubMed] [Google Scholar]
- 11.Pešić I, Stefanović V, Müller GA, Müller CA, Čukuranović R, Jahn O, et al. Identification and validation of six proteins as markers for endemic nephropathy. J Proteomics. 2011;74:1994–2007. 10.1016/j.jprot.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 12.Arlt VM, Stiborová M, vom Brocke J, Simões ML, Lord GM, Nortier JL, et al. Aristolochic acid mutagenesis: molecular clues to the aetiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogenesis. 2007;28:2253–61. 10.1093/carcin/bgm082 [DOI] [PubMed] [Google Scholar]
- 13.Huang F, Clifton J, Yang X, Rosenquist T, Hixson D, Kovač S, et al. SELDI-TOF as a method for biomarker discovery in the urine of aristolochic-acid-treated mice. Electrophoresis. 2009;30:1168–74. 10.1002/elps.200800548 [DOI] [PubMed] [Google Scholar]
- 14.Regulation EC 258/97 of the European Parliament and of the Council concerning novel foods and novel food ingredients. Of J Eur Commun. 1997;L43:1–6. [Google Scholar]
- 15.Searstone K. Total quality management: BS 5750 (ISO 9000/ EN 29000). Total Qual Manage Bus Excell. 2006;2:249–54. 10.1080/09544129100000029 [DOI] [Google Scholar]
- 16.Ingredients, packaging and labeling. Silver Spring, MD, USA: US Food and Drug Administration. Available from: www.da.gov/food/ingedientspackaginglabeling.
- 17.Cowburn G, Stockley L. Consumer understanding and use of nutrition labelling: a systematic review. Public Health Nutr. 2005;8:21–8. 10.1079/PHN2005666 [DOI] [PubMed] [Google Scholar]
- 18.Herrero M, Simó C, García-Cañas V, Ibáñez E, Cifuentes A. Foodomics: MS-based strategies in modern food science and nutrition. Mass Spectrom Rev. 2012;31:49–69. 10.1002/mas.20335 [DOI] [PubMed] [Google Scholar]
- 19.Andjelković U, Martinović T, Josić Dj. Foodomic investigations of food allergies. Curr Opin Food Sci. 2015;4:92–8. 10.1016/j.cofs.2015.06.003 [DOI] [Google Scholar]
- 20.Giacometti J, Buretić Tomljenović A. Josić Dj. Application of proteomics and metabolomics for investigation of food toxins. Food Res Int. 2013;54:1042–51. 10.1016/j.foodres.2012.10.019 [DOI] [Google Scholar]
- 21.Soggiu A, Piras C, Levi Mortera S, Alloggio I, Urbani A, Bonizzi L, et al. Unraveling the effect of clostridia spores and lysozyme on microbiota dynamics in Grana Padano cheese: A metaproteomics approach. J Proteomics. 2016;147:21–7. 10.1016/j.jprot.2016.03.035 [DOI] [PubMed] [Google Scholar]
- 22.Piras C, Roncada P, Rodrigues PM, Bonizzi L, Soggiu A. Proteomics in food: quality, safety, microbes, and allergens. Proteomics. 2016;16:799–815. 10.1002/pmic.201500369 [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann-Sommergruber K. Proteomics and its impact on food allergy diagnosis. EuPA Open Proteom. 2016;12:10–2. 10.1016/j.euprot.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO initiative to estimate the global burden of foodborne diseases report. Geneva, Switzerland: World Health Organization (WHO); 2013. Available from: http://apps.who.int/iris/bitstream/10665/159847/1/9789241507967_eng.pdf. [DOI] [PubMed]
- 25.Havelaar AH, Brul S, de Jong A, de Jonge R, Zwietering MH, ter Kuile BH. Future challenges to microbial food safety. Int J Food Microbiol. 2010;139:S79–94. 10.1016/j.ijfoodmicro.2009.10.015 [DOI] [PubMed] [Google Scholar]
- 26.Hartung T, Koeter H. Food for thought…on food safety testing. ALTEX. 2008;25:259–64. 10.14573/altex.2008.4.259 [DOI] [PubMed] [Google Scholar]
- 27.Bacteriological analytical manual. Silver Spring, MD, USA: US Food and Drug Administration. Available from: https://www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm2006949.htm
- 28.Josefsen MH, Bhunia AK, Olsson Engvall E, Fachmann MSR, Hoorfar J. Monitoring Campylobacter in the poultry production chain – from culture to genes and beyond. J Microbiol Methods. 2015;112:118–25. 10.1016/j.mimet.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 29.Senoh M, Ghosh-Banerjee J, Ramamurthy T, Colwell RR, Miyoshi S, Nair GB, et al. Conversion of viable but nonculturable enteric bacteria to culturable by co-culture with eucaryotic cells. Microbiol Immunol. 2012;56:342–5. 10.1111/j.1348-0421.2012.00440.x [DOI] [PubMed] [Google Scholar]
- 30.Wiedmann M, Wang S, Post L, Nightingale K. Assessment criteria and approaches for rapid detection methods to be used in the food industry. J Food Prot. 2014;77:670–90. 10.4315/0362-028X.JFP-13-138 [DOI] [PubMed] [Google Scholar]
- 31.López-Campos G, Martinez-Suarez JV, Aguado-Urda M, Lopez Alonso V. Detection, identification, and analysis of foodborne pathogens. In: Hartel RW, Clark JP, Rodriguez-Lazaro D, Topping D, editors. SpringerBriefs in food, health, and nutrition: Microarray detection and characterization of bacterial foodborne pathogens. New York, NY, USA: Springer Science + Business Media, LLC; 2012. pp.13–32. https://doi.org/ 10.1007/978-1-4614-3250-0_2 [DOI] [Google Scholar]
- 32.Löfström C, Schelin J, Norling B, Vigre H, Hoorfar J, Rådström P. Culture-independent quantification of Salmonella enterica in carcass gauze swabs by flotation prior to real-time PCR. Int J Food Microbiol. 2011;145:S103–9. 10.1016/j.ijfoodmicro.2010.03.042 [DOI] [PubMed] [Google Scholar]
- 33.Michalski A, Damoc E, Lange O, Denisov E, Nolting D, Müller M, et al. Ultra high resolution linear ion Orbitrap mass spectrometer (Orbitrap Elite) facilitates top down LC MS/MS and versatile peptide fragmentation modes. Mol Cell Proteomics. 2012;11:013698–11. 10.1074/mcp.O111.013698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selevsek N, Chang CY, Gillet LC, Navarro P, Bernhardt OM, Reiter L, et al. Reproducible and consistent quantification of the Saccharomyces cerevisiae proteome by SWATH-mass spectrometry. Mol Cell Proteomics. 2015;14:739–49. 10.1074/mcp.M113.035550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao X, Li R, Song W, Miao WJ, Liu J, Chen HB, et al. A targeted strategy to analyze untargeted mass spectral data: rapid chemical profiling of Scutelaria baicelensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J Chromatogr A. 2016;1441:83–95. 10.1016/j.chroma.2016.02.079 [DOI] [PubMed] [Google Scholar]
- 36.Lesur A, Gallien S, Domon B. Hyphenation of fast liquid chromatography with high-resolution mass spectrometry for quantitative proteomics analyses. Trends Analyt Chem. 2016;84:144–50. https://doi.org/101016/j.trac2016.01.023 10.1016/j.trac.2016.01.023 [DOI] [Google Scholar]
- 37.Šrajer Gajdošik M, Gašo-Sokač D, Pavlović H, Clifton J, Breen L, Cao L, et al. Sample preparation and further proteomic investigation of the inhibitory activity of pyridinium oximes fo Gram-positive and Gram-negative food pathogens. Food Res Int. 2013;51:46–52. 10.1016/j.foodres.2012.11.018 [DOI] [Google Scholar]
- 38.Zachariasova M, Cajka T, Godula M, Malachova A, Veprikova Z, Hajslova J. Analysis of multiple mycotoxins in beer employing (ultra)-high-resolution mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:3357–67. 10.1002/rcm.4746 [DOI] [PubMed] [Google Scholar]
- 39.Rubert J, Dzuman Z, Vaclavikova M, Zachariasova M, Soler C, Hajslova J. Analysis of mycotoxins in barley using ultra high liquid chromatography high resolution mass spectrometry: comparison of efficiency and efficacy of different extraction procedures. Talanta. 2012;99:712–9. 10.1016/j.talanta.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 40.Dzuman Z, Zachariasova M, Lacina O, Veprikova Z, Slavikova P, Hajslova J. A rugged high-throughput analytical approach for the determination and quantification of multiple mycotoxins in complex feed matrices. Talanta. 2014;121:263–72. 10.1016/j.talanta.2013.12.064 [DOI] [PubMed] [Google Scholar]
- 41.Nathanail AV, Syvähuoko J, Malachová A, Jestoi M, Varga E, Michlmayr H, et al. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal Bioanal Chem. 2015;407:4745–55. 10.1007/s00216-015-8676-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hickert S, Gerding J, Ncube E, Hübner F, Flett B, Cramer B, et al. A new approach using micro HPLC-MS/MS for multi-mycotoxin analysis in maize samples. Mycotoxin Res. 2015;31:109–15. 10.1007/s12550-015-0221-y [DOI] [PubMed] [Google Scholar]
- 43.Kašička V. Recent developments in capillary and microchip electroseparations of peptides (2013–middle 2015). Electrophoresis. 2016;37:162–88. 10.1002/elps.201500329 [DOI] [PubMed] [Google Scholar]
- 44.Canela N, Rodríguez MÁ, Baiges I, Nadal P, Arola L. Foodomics imaging by mass spectrometry and magnetic resonance. Electrophoresis. 2016;37:1748–67. 10.1002/elps.201500494 [DOI] [PubMed] [Google Scholar]
- 45.Lee JW, Thomas LC, Schmidt SJ. Effects of heating conditions on the glass transition parameters of amorphous sucrose produced by melt-quenching. J Agric Food Chem. 2011;59:3311–9. 10.1021/jf104853s [DOI] [PubMed] [Google Scholar]
- 46.Schmidt SJ. Response to comment on the melting and decomposition of sugars. J Agric Food Chem. 2012;60:10363–71. 10.1021/jf3011855 [DOI] [PubMed] [Google Scholar]
- 47.Wan Ibrahim WA, Abd Ali LI, Sulaiman A, Sanagi MM, Aboul-Enein HY. Application of solid-phase extraction for trace elements in environmental and biological samples: a review. Crit Rev Anal Chem. 2014;44:233–54. 10.1080/10408347.2013.855607 [DOI] [PubMed] [Google Scholar]
- 48.Breen L, Cao L, Eom K, Šrajer Gajdošik M, Camara L, Giacometti J, et al. High-throughput fractionation of human plasma for fast enrchment of low- and high-abundance proteins. Blood Transfus. 2012;10 Suppl 2:s89–100. 10.2450/2012.013S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campone L, Piccinelli AL, Alberti L, Rastrelli L. Application of pressurized liquid extraction in the analysis of aflatoxins B1, B2, G1, and G2, in nuts. J Sep Sci. 2009;32:3837–44. 10.1002/jssc.200900329 [DOI] [PubMed] [Google Scholar]
- 50.Li W, Wang Z, Chen L, Zhang J, Han L, Hou J, et al. Pressurized liquid extraction followed by LC-ESI/MS for analysis of four chromones in Radix Saposhnikoviae. J Sep Sci. 2010;33:2881–7. 10.1002/jssc.201000336 [DOI] [PubMed] [Google Scholar]
- 51.Erdogan S, Ates B, Durmaz G, Yilmaz I, Seckin T. Pressurized liquid extraction of phenolic compounds from Anatolia propolis and their radical scavenging capacities. Food Chem Toxicol. 2011;49:1592–7. 10.1016/j.fct.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 52.Yan H, Wang H. Recent development and applications of dispersive liquid-liquid microextraction. J Chromatogr A. 2013;1295:1–15. 10.1016/j.chroma.2013.04.053 [DOI] [PubMed] [Google Scholar]
- 53.Al-Hadithi N, Kössler P, Karlovsky P. Determination of ochratoxin A in wheat and maize by solid bar microextraction with liquid chromatography and fluorescence detection. Toxins (Basel). 2015;7:3000–11. 10.3390/toxins7083000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan Ibrahim WA, Nodeh HR, Aboul-Enain HY, Sangi MM. Magnetic solid-phase extraction based on modified ferum oxides for enrichment, preconcentration, and isolation of pesticides and selected pollutants. Crit Rev Anal Chem. 2015;45:270–87. 10.1080/10408347.2014.938148 [DOI] [PubMed] [Google Scholar]
- 55.Skrabákova Z, O’Halloran J, van Pelt FNAM, James KJ. Food contaminant analysis at ultra-high mass resolution: application of hybrid linear ion trap – orbitrap mass spectrometry for the determination of the polyether toxins, azaspiracids, in shellfish. Rapid Commun Mass Spectrom. 2010;24:2966–74. 10.1002/rcm.4724 [DOI] [PubMed] [Google Scholar]
- 56.Yang M, Zhou Z, Guo D. A strategy for fast screening and identification of sulfur derivatives in medicinal Pueraria species based on the fine isotopic pattern filtering method using ultra-high-resolution mass spectrometry. Anal Chim Acta. 2015;894:44–53. 10.1016/j.aca.2015.07.050 [DOI] [PubMed] [Google Scholar]
- 57.Marínez-Domínguez G, Romero-González R, Arrebola FJ, Garrido Frenich A. Multi-class determination of pesticides and mycotoxins in isoflavones supplements obtained from soy by liquid chromatography coupled to Orbitrap high resolution mass spectrometry. Food Control. 2016;59:218–24. 10.1016/j.foodcont.2015.05.033 [DOI] [PubMed] [Google Scholar]
- 58.Carreer WJ, Flight RM, Moseley HNB. A computational framework for high-throughput isotopic natural abundance correction of omics-level ultra-high resolution FT-MS datasets. Metabolites. 2013;3:853–66. 10.3390/metabo3040853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anastassiades M, Lehotay SJ, Štajnbaher D, Schenk FJ. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and ‘dispersive solid-phase extraction’ for the determination of pesticide residues in produce. J AOAC Int. 2003;86:412–31. [PubMed] [Google Scholar]
- 60.Josić Dj. Strategies for complete proteomic analysis of hydrophobic proteins in complex biological samples – hide-and- -seek. J Data Mining Genomics Proteomics. 2014;5:e111 10.4172/2153-0602.1000e111 [DOI] [Google Scholar]
- 61.Wiśnievski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation for proteome analysis. Nat Methods. 2009;6:359–62. 10.1038/nmeth.1322 [DOI] [PubMed] [Google Scholar]
- 62.Bodzon-Kulakowska A, Bierczynska-Krzysik A, Dylag T, Drabik A, Suder P, Noga M, et al. Methods for sample preparation in proteomic research. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;849:1–31. 10.1016/j.jchromb.2006.10.040 [DOI] [PubMed] [Google Scholar]
- 63.Josić Dj, Clifton J. Mammalian plasma membrane proteomics. Proteomics. 2007;7:3010–29. 10.1002/pmic.200700139 [DOI] [PubMed] [Google Scholar]
- 64.D’Alessandro A, Zolla L. Foodomics to investigate meat tenderness. Trends Analyt Chem. 2013;52:47–53. 10.1016/j.trac.2013.05.017 [DOI] [Google Scholar]
- 65.Hill RC, Calle EA, Dzieciatkowska M, Niklason LE, Hansen KC. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol Cell Proteomics. 2015;14:961–73. 10.1074/mcp.M114.045260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao L, Clifton JG, Reutter W, Josić Dj. Mass spectrometry-based analysis of rat liver and hepatocellular carcinoma Morris hepatoma 7777 plasma membrane proteome. Anal Chem. 2013;85:8112–20. 10.1021/ac400774g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blonder J, Hale ML, Chang KC, Yu LR, Lucas DA, Conrads TP, et al. Quantitative profiling of detergent-resistant membrane proteome of iota-B toxin induced vero cells. J Proteome Res. 2005;4:523–31. 10.1021/pr049790s [DOI] [PubMed] [Google Scholar]
- 68.Lu X, Zhu H. Tube-gel digestion: a novel proteomic approach for high throughput analysis of membrane proteins. Mol Cell Proteomics. 2005;4:1948–58. 10.1074/mcp.M500138-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moon H, Wheeler AR, Garell RL, Loo JA, Kim CJ. An integrated digital microfluidic chip for multiplexed proteomic sample preparation and analysis by MALDI-MS. Lab Chip. 2006;6:1213–9. 10.1039/b601954d [DOI] [PubMed] [Google Scholar]
- 70.Feist P, Hummon AB. Proteomic challenges: sample preparation techniques for microgram-quantity protein analysis from biological samples. Int J Mol Sci. 2015;16:3537–63. 10.3390/ijms16023537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pučić M, Knežević A, Vidič J, Adamczyk B, Novokmet M, Polašek O, et al. High throughput isolation and glycosylation analysis of IgG-variability and heteritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics. 2011;10:010090. 10.1074/mcp.M111.010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stoughton RB. Application of DNA microarrays in biology. Annu Rev Biochem. 2005;74:53–82. 10.1146/annurev.biochem.74.082803.133212 [DOI] [PubMed] [Google Scholar]
- 73.Preparing samples for sequencing genomic DNA. San Diego, CA, USA: Illumina, Inc; 2008. Available from: http://mmjggl.caltech.edu/sequencing/Genomic_DNA_Sample_Prep_1003 806 _RevB.pdf.
- 74.Trevino V, Falaciani F, Barrera-Saldaña HA. DNA microarrays: a powerful genomic tool for biomedical and clinical research. Mol Med. 2007;13:527–41. [Trevino] 10.2119/2006-00107.Trevino [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schulze H, Giraud G, Crain J, Bachmann TT. Multiple optical pathogen detection with lab-on-a-chip devices. J Biophotonics. 2009;2:199–211. 10.1002/jbio.200910009 [DOI] [PubMed] [Google Scholar]
- 76.Piras C, Soggiu A, Bonizzi L, Gaviraghi A, Deriu F, De Martino L, et al. Comparative proteomics to evaluate drug resistance in Escherichia coli. Mol Biosyst. 2012;8:1060–7. 10.1039/C1MB05385J [DOI] [PubMed] [Google Scholar]
- 77.Acunha T, Ibáñez C, García-Cañas V, Simó C, Cifuentes A. Recent advances in the application of capillary electromigration methods for food analysis and foodomics. Electrophoresis. 2016;37:111–41. 10.1002/elps.201500291 [DOI] [PubMed] [Google Scholar]
- 78.Vallverdú-Queralt A, Lamuela-Raventós RM. Foodomics: a new tool to differentiate between organic and conventional foods. Electrophoresis. 2016;37:1784–94. 10.1002/elps.201500348 [DOI] [PubMed] [Google Scholar]
- 79.Wu S, Duan N, Shi Z, Fang CC, Wang Z. Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolour upconversion nanoparticles labels. Anal Chem. 2014;86:3100–7. 10.1021/ac404205c [DOI] [PubMed] [Google Scholar]
- 80.Dong ZM, Zhao GC. Label-free detection of pathogenic bacteria via immobilized antimicrobial peptides. Talanta. 2015;137:55–61. 10.1016/j.talanta.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 81.Pfaunmiller EL, Paulemond ML, Dupper CM, Hage DS. Affinity monolith chromatography: a review of principles and recent analytical applications. Anal Bioanal Chem. 2013;405:2133–45. 10.1007/s00216-012-6568-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoorfar J. Rapid detection, characterization, and enumeration of foodborne pathogens. APMIS Suppl. 2011;119:1–24. 10.1111/j.1600-0463.2011.02767.x [DOI] [PubMed] [Google Scholar]
- 83.Denes T, Wiedmann M. Environmental responses and phage susceptibility in foodborne pathogens: implications for improving applications in food safety. Curr Opin Biotechnol. 2014;26:45–9. 10.1016/j.copbio.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 84.Richardson Schmidt SJ. Characterization of water in foods by NMR. In: Finley JW, Schmidt SJ, Serianni AS, editors. NMR application in biopolymers. New York, NY, USA: Plenum Press; 1990. pp. 415–59. [Google Scholar]
- 85.Hills BP, Takacs SF, Belton PS. A new interpretation of proton NMR relaxation time measurements of water in food. Food Chem. 1990;37:95–111. 10.1016/0308-8146(90)90084-H [DOI] [Google Scholar]
- 86.Komoroski RA. Nonmedical applications of NMR imaging. Anal Chem. 1993;65:1068A–77A. 10.1021/ac00072a714 [DOI] [Google Scholar]
- 87.Wright JJ, Hall LD. NMR imaging of packed foods. In: Mathlouthi M, editor. Food packing and preservation. Dordrecht, The Netherlands: Springer Science + Business Media; 1994. pp. 197–208. [Google Scholar]
- 88.Moreau C, Guichard E. Flavor-food compond interactions by NMR spectroscopy. In: Webb GA, editor. Modern magnetic resonance. Dordrecht, The Netherlands: Springer; 2006. pp. 1611–5. https://doi.org/ 10.1007/1-4020-391-7_179 [DOI] [Google Scholar]
- 89.Spyros A, Dais P. Application of 31P NMR spectroscopy in food analysis. 1. Quantitative determination of the mono- and diglyceride composition of olive oils. J Agric Food Chem. 2000;48:802–5. 10.1021/jf9910990 [DOI] [PubMed] [Google Scholar]
- 90.Xu YJ, Wu X. Foodomics in microbiological investigations. Curr Opin Food Sci. 2015;4:51–5. 10.1016/j.cofs.2015.05.001 [DOI] [Google Scholar]
- 91.Guthausen G, Todt H, Burk W, Schmalbein D, Guthausen A, Kamlowski A. Single-sided NMR in foods. In: Webb GA, editor. Modern magnetic resonance. Dordrecht, The Netherlands: Springer; 2006. pp. 1895–7. https://doi.org/ 10.1007/1-4020-3910-7_216 [DOI] [Google Scholar]
- 92.Todt H, Guthausen G, Burk W, Schmalbein D, Kamlowski A. Time-domain NMR in quality control: standard applications. In: Webb GA, editor. Modern magnetic resonance. Dordrecht, The Netherlands: Springer; 2006. pp. 1739–43. https://doi.org/ 10.1007/1-4020-391-7_196 [DOI] [Google Scholar]
- 93.Trimigno A, Marincola FC, Dellarosa N, Picone G, Laghi L. Definiton of food quality by NMR-based foodomics. Curr Opin Food Sci. 2015;4:99–104. 10.1016/j.cofs.2015.06.008 [DOI] [Google Scholar]
- 94.Monakhova YB, Kuballa T, Lachenmeir DW. Chemometric methods in NMR spectrometric analysis of food products. J Anal Chem. 2013;68:755–66. 10.1134/S1061934813090098 [DOI] [Google Scholar]
- 95.Li ZY, Walbeck E, Wang RF, Liu Q, Yang YB, Chou GX, et al. A universal quantitative 1H nuclear magnetic resonanace (qNMR) method for assessing the purity of damarane-type ginsenosides. Phytochem Anal. 2015;26:8–14. 10.1002/pca.2527 [DOI] [PubMed] [Google Scholar]
- 96.Cao R, Nonaka A, Komura F, Matsui T. Application of diffusion ordered- 1H nuclear magnetic resonanace spectroscopy to quantify sucrose in beverages. Food Chem. 2015;171:8–12. 10.1016/j.foodchem.2014.08.105 [DOI] [PubMed] [Google Scholar]
- 97.Hohmann M, Koospal V, Bauer-Christoph C, Christoph N, Wachter H, Diel B, et al. Quantitative 1H NMR analysis of egg yolk, alcohol and total sugar content in egg liqueurs. J Agric Food Chem. 2015;63:4112–9. 10.1021/acs.jafc.5b00940 [DOI] [PubMed] [Google Scholar]
- 98.Longobardi F, Ventrella A, Napoli C, Humpfer E, Schütz B, Schäfer H, et al. Classification of olive oils according to geographical origin by using 1H NMR fingerprinting with multivariate analysis. Food Chem. 2012;130:177–83. 10.1016/j.foodchem.2011.06.045 [DOI] [Google Scholar]
- 99.Godelmann R, Fang F, Humpfer E, Schütz B, Bansbach M, Schäfer H, et al. Targeted and nontargeted wine analysis by 1H NMR spectroscopy combined with multivariate statistical analysis. Differentiation of important parameters: grape variety, geographical origin, year of vintage. J Agric Food Chem. 2013;61:5610–9. 10.1021/jf400800d [DOI] [PubMed] [Google Scholar]
- 100.Cagliani LR, Culeddu N, Chessa M, Consonni R. NMR investigations for a quality assessment of Italian PDO saffron (Crocus sativus L). Food Control. 2015;50:342–8. 10.1016/j.foodcont.2014.09.017 [DOI] [Google Scholar]
- 101.Gallo V, Mastrorilli P, Cafagna I, Nitti GI, Latronico M, Longobardi F, et al. Effects of agronomical practices on chemical composition of table grapes evaluated by NMR spectroscopy. J Food Compos Anal. 2014;35:44–52. 10.1016/j.jfca.2014.04.004 [DOI] [Google Scholar]
- 102.Piras C, Scano P, Locci E, Sanna R, Cesare Marincola F. Analysing the effects of frozen storage and processing on the metabolome profile of raw mullet roes using 1H NMR spectroscopy. Food Chem. 2014;159:71–9. 10.1016/j.foodchem.2014.02.160 [DOI] [PubMed] [Google Scholar]
- 103.Shumilina E, Ciampa A, Capozzi F, Rustad T, Dikiy A. NMR approach for monitoring post-mortem changes in Atlantic salmon fillets stored at 0 and 4oC. Food Chem. 2015;184:12–22. 10.1016/j.foodchem.2015.03.037 [DOI] [PubMed] [Google Scholar]
- 104.Ercolini D, Ferrocino I, Nasi A, Ndagijimana M, Vernocchi P, La Storia A, et al. Monitoring of microbial metabolites and bacterial diversity in beef stored under different packaging conditions. Appl Environ Microbiol. 2011;77:7372–81. 10.1128/AEM.05521-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kortesniemi M, Vuorinen AL, Sinkkonen J, Yang B, Rajala A, Kallio H. NMR metabolomics of ripened and developing oilseed rape (Brassica napus) and turnip rape (Brassica rapa). Food Chem. 2015;172:63–70. 10.1016/j.foodchem.2014.09.040 [DOI] [PubMed] [Google Scholar]
- 106.Picone G, Mezzetti B, Babini E, Capocasa F, Placucci G, Capozzi F. Unsupervised principal component analysis of NMR metabolic profiles for the assessment of substancial equivalence of transgenic grapes (Vitis vinifera). J Agric Food Chem. 2011;59:9271–9. 10.1021/jf2020717 [DOI] [PubMed] [Google Scholar]
- 107.Picone G, Langhi L, Gardini F, Lanciotti R, Siroli L, Capozzi F. Evaluation of the effect of carvacrol on the Escherichia coli 555 metabolome by using 1H NMR spectroscopy. Food Chem. 2013;141:4367–74. 10.1016/j.foodchem.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 108.Canela N, Rodríguez MÁ, Baiges I, Nadal P, Arola L. Foodomics imaging by mass spectrometry and magnetic resonance. Electrophoresis. 2016;37:1748–67. 10.1002/elps.201500494 [DOI] [PubMed] [Google Scholar]
- 109.Nielsen MS, Bøgelund Munk M, Diaz A, Pedersen EBL, Holler M, Bruns S, et al. Ptychographic X-ray computed tomography of extended colloidal networks in food emulsions. Food Structure. 2016;7:21–8. 10.1016/j.foostr.2016.01.001 [DOI] [Google Scholar]
- 110.Oechsle AM, Akgün D, Krause F, Maier C, Gibis M, Kohlus R, et al. Microstructure and physical-chemical properties of chicken collagen. Food Structure. 2016;7:29–37. 10.1016/j.foostr.2016.02.001 [DOI] [Google Scholar]
- 111.Hagsten C, Altskär A, Gustafsson S, Lorén N, Hamberg L, Innings F, et al. Composition and structure of high temperature dairy fouling. Food Structure. 2016;7:13–20. 10.1016/j.foostr.2015.12.002 [DOI] [Google Scholar]
- 112.Keenan DF, Auty MAE, Doran L, Kerry JP, Hamill RM. Investigating the influence of inulin as a fat substitute in comminuted products using rheology, calorimetric and microscopy techniques. Food Structure. 2014;2:1–13. 10.1016/j.foostr.2014.06.001 [DOI] [Google Scholar]
- 113.Pink DA, Razul MSG. Computer simulation techniques for food science and engineering: simulating atomic scale and coarse-grained models. Food Structure. 2014;1:71–90. 10.1016/j.foostr.2013.11.005 [DOI] [Google Scholar]
- 114.Schmidt SJ, Thomas LC, Lee JW. Response to comment on the melting and decomposition of sugars. J Agric Food Chem. 2012;60:10363–71. 10.1021/jf3011855 [DOI] [PubMed] [Google Scholar]
- 115.Roos YH, Karel M, Labuza TP, Levine H, Mathlouthi M, Reid D, et al. Melting and crystallization of sugars in high-solids systems. J Agric Food Chem. 2013;61:3167–78. 10.1021/jf305419y [DOI] [PubMed] [Google Scholar]
- 116.Law JWF, Ab Mutalib NS, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principals, applications, advantages and limitations. Front Microbiol. 2015;5:770. 10.3389/fmicb.2014.00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Diaz-Sanchez S, Hanning I, Pendleton S, D’Souza D. Next- -generation sequencing: the future of molecular genetics in poultry production and food safety. Poult Sci. 2013;92:562–72. 10.3382/ps.2012-02741 [DOI] [PubMed] [Google Scholar]
- 118.Ishii S, Segava T, Okabe S. Simultaneous quantification of multiple food- and waterborne pathogens by use of microfluidic quantitative PCR. Appl Environ Microbiol. 2013;79:2891–8. 10.1128/AEM.00205-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salazar JK, Wang Y, Yu S, Wang H, Zhang W. Polymerase chain reaction-based serotyping of pathogenic bacteria in food. J Microbiol Methods. 2015;110:18–26. 10.1016/j.mimet.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 120.Murphy NM, McLauchlin J, Ohai C, Grant KA. Construction and evaluation of a microbiological positive process internal control of PCR-based examination of food samples for Listeria monocytogenes and Salmonella enterica. Int J Food Microbiol. 2007;120:110–9. 10.1016/j.ijfoodmicro.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 121.Conway T, Schoolnik GK. Microarray expression profiling: capturing a genome-wide portrait of transcriptome. Mol Microbiol. 2003;47:879–89. 10.1046/j.1365-2958.2003.03338.x [DOI] [PubMed] [Google Scholar]
- 122.Croucher NJ, Thompson NR. Studying bacterial transcriptomes using RNA-seq. Curr Opin Microbiol. 2010;13:619–24. 10.1016/j.mib.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Allen KJ, Lepp D, McKellar RC, Griffiths MW. Examination of stress and virulence gene expression in Escherichia coli O157:H7 using targeted microarray analysis. Foodborne Pathog Dis. 2008;5:437–47. 10.1089/fpd.2008.0100 [DOI] [PubMed] [Google Scholar]
- 124.Mataragas M, Rovetto F, Bellio A, Alessandria V, Rantsiou K, Decastelli L, et al. Differential gene expression profiling in Listeria monocytogenes in Cacciatore and Fellino salami to reveal potential stress resistance biomarkers. Food Microbiol. 2015;46:408–17. 10.1016/j.fm.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 125.Rosselló-Móra R, Amann R. Past and future species definitions for Bacteria and Archea. Syst Appl Microbiol. 2015;38:209–16. 10.1016/j.syapm.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 126.Cocolin L, Dolci P, Rantsiou K. Biodiversity and dynamics of meat fermentations: the contribution of molecular methods for a better comprehension of a complex ecosystem. Meat Sci. 2011;89:296–302. 10.1016/j.meatsci.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 127.Donhauser SC, Niessner R, Seidel M. Sensitive quantification of Escherichia coli O157:H7, Salmonella enterica and Campylobacter jejuni by combining stopped polymerase chain reaction with chemiluminscence flow-through DNA microarray analysis. Anal Chem. 2011;83:3153–60. 10.1021/ac2002214 [DOI] [PubMed] [Google Scholar]
- 128.Wunderlich A, Torggler C, Elsässer D, Lück C, Niessner R, Seidel M. Rapid quantification method for Legionella pneumophila in surface water. Anal Bioanal Chem. 2016;408:2203–13. 10.1007/s00216-016-9362-x [DOI] [PubMed] [Google Scholar]
- 129.Kunze A, Dilcher M, Abd El Wahed A, Hufert F, Niessner R, Seidel M. On-chip isothermal nucleic acid amplification on flow-based chemiluminescence microarray analysis platform for the detection of viruses and bacteria. Anal Chem. 2016;88:898–905. 10.1021/acs.analchem.5b03540 [DOI] [PubMed] [Google Scholar]
- 130.Seidel M, Niessner R. Chemiluminescence microarrays: a critical review. Anal Bioanal Chem. 2014;406:5589–612. 10.1007/s00216-014-7968-4 [DOI] [PubMed] [Google Scholar]
- 131.Cunsolo V, Muccilli V, Saletti R, Foti S. Mass spectrometry in food proteomics: a tutorial. J Mass Spectrom. 2014;49:768–84. 10.1002/jms.3374 [DOI] [PubMed] [Google Scholar]
- 132.Comisarow M, Marshall AG. Fourier transform ion cyclotron resonance spectroscopy. Chem Phys Lett. 1974;25:282–3. 10.1016/0009-2614(74)89137-2 [DOI] [Google Scholar]
- 133.Makarov A. Electrostatic axially harmonic orbital trapping: a high-performance technique for mass analysis. Anal Chem. 2000;72:1156–62. 10.1021/ac991131p [DOI] [PubMed] [Google Scholar]
- 134.Makarov A, Denisov E, Lange O. Performance evaluation of a high-field Orbitrap mass analyzer. J Am Soc Mass Spectrom. 2009;20:1391–6. 10.1016/j.jasms.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 135.Tolmachev AV, Robinson EW, Wu S, Paša-Tolić Lj, Smith RDFT-ICR. MS optimization for the analysis of intact proteins. Int J Mass Spectrom. 2009;281:32–8. 10.1016/j.ijms.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weisbrod CR, Hoopmann MR, Senko MW, Bruce JE. Performance evaluation of a dual linear ion trap-Fourier transform ion cyclotron resonance mass spectrometer for proteomic research. J Proteomics. 2013;88:109–19. 10.1016/j.jprot.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marshall AG, Hendrickson CL, Jackson GS. Fourier transform ion cyclotron resonance mass spectrometry. Mass Spectrom Rev. 1998;17:1–35. [DOI] [PubMed] [Google Scholar]
- 138.Ibáñez C, Simó C, García-Cañas V, Acunha T, Cifuentes A. The role of direct high-resolution mass spectrometry in foodomics. Anal Bioanal Chem. 2015;407:6275–87. 10.1007/s00216-015-8812-1 [DOI] [PubMed] [Google Scholar]
- 139.Tevell Åberg A, Björnstad K, Hedeland M. Mass spectrometric detection of protein-based toxins. Biosecur Bioterror. 2013;11 Suppl 1:S215–26. 10.1089/bsp.2012.0072 [DOI] [PubMed] [Google Scholar]
- 140.Siciliano RA, d’Esposito D, Mazzeo MF. Food authentication by MALDI MS: MALDI-TOF MS analysis of fish species. In: Cramer R, editor. Advances in MALDI and laser-induced soft ionization mass spectrometry. Cham, Switzerland: Springer International Publishing, 2016. pp. 263–77. https://doi.org/ 10.1007/978-3-319-04819-2_14 [DOI] [Google Scholar]
- 141.Kalb SR, Boyer AE, Barr JR. Mass spectrometric detection of bacterial protein toxins and their enzymatic activity. Toxins (Basel). 2015;7:3497–511. 10.3390/toxins7093497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Böhme K, Fernández-No IC, Barros-Velázquez J, Gallardo JM, Calo-Mata P, Cañas B. Species differentiation of seafood spoilage and pathogenic Gram-negative bacteria by MALDI--TOF mass fingerprinting. J Proteome Res. 2010;9:3169–83. 10.1021/pr100047q [DOI] [PubMed] [Google Scholar]
- 143.Böhme K, Fernández-No IC, Barros-Velázquez J, Gallardo JM, Cañas B, Calo-Mata P. Rapid species identification of seafood spoilage and pathogenic Gram-pozitive bacteria by MALDI-TOF mass fingerprinting. Electrophoresis. 2011;32:2951–65. 10.1002/elps.201100217 [DOI] [PubMed] [Google Scholar]
- 144.Oros D, Andjelković U, Kraljević Pavelić S. Josić Dj, Čeprnja M, Melvan E, et al. Identification of pathogens from urine samples by MALDI-TOF/TOF. In: Küster B, Lemeer S, Marcus K, Urlaub H, editors. EMBO Workshop, 10th European Summer School: Advanced Proteomics; 2016 July 31–August 6; Bressanone, Italy; 2016. p. 60. [Google Scholar]
- 145.Giebel R, Worden C, Rust SM, Kleinheinz GT, Robins M, Sandrin TR. Microbial fingerprinting using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) applications and challenges. In: Gadd GM, Sariaslani S, editors. Advances in applied microbiology, vol. 71. San Diego, CA, USA: Academic Press; 2010. pp. 149–84. http://doi.org/ 10.1016/S0065-2164(10)71006-6 [DOI] [PubMed] [Google Scholar]
- 146.Böhme K, Fernández-No IC, Pazos M, Gallardo JM, Barros-Velázquez J, Cañas B, et al. Identification and classification of seafood-borne pathogenic and spoilage bacteria: 16S rRNA sequencing versus MALDI-TOF MS fingerprinting. Electrophoresis. 2013;34:877–87. 10.1002/elps.201200532 [DOI] [PubMed] [Google Scholar]
- 147.Hettick JM, Kashon ML, Slaven JE, Ma Y, Simpson JP, Siegel PD, et al. Discrimination of intact mycobacteria at a strain level, a combined MALDI-TOF MS and biostatistical analysis. Proteomics. 2006;6:6416–25. 10.1002/pmic.200600335 [DOI] [PubMed] [Google Scholar]
- 148.Piras C, Soggiu A, Bonizzi L, Greco V, Ricchi M, Arrigoni N, et al. Identification of immunoreactive proteins of Mycobacterium avium subsp. paratuberculosis. Proteomics. 2015;15:813–23. 10.1002/pmic.201400276 [DOI] [PubMed] [Google Scholar]
- 149.Lin CS, Su CC, Hsieh SC, Lu CC, Wu TL, Jia JH, et al. Rapid identification of Mycobacterium avium clinical isolates by matrix-assisted laser desorption/ionization time-of-flifgt mass spectrometry. J Microbiol Immunol Infect. 2015;48:205–12. 10.1016/j.jmii.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 150.Sauer S, Kliem M. Mass spectrometry tools for the classification and identification of bacteria. Nat Rev Microbiol. 2010;8:74–82. 10.1038/nrmicro2243 [DOI] [PubMed] [Google Scholar]
- 151.Schumann P, Maier T. MALDI-TOF mass spectrometry applied to classification and identification of bacteria. Methods Microbiol. 2014;41:275–306. 10.1016/bs.mim.2014.06.002 [DOI] [Google Scholar]
- 152.Clark CG, Kruczkiewicz P, Guan C, McCorrister SJ, Chong P, Wyle J, et al. Evaluation of MALDI-TOF mass spectroscopy methods for determination of Escherichia coli pathotypes. J Microbiol Methods. 2013;94:180–91. 10.1016/j.mimet.2013.06.020 [DOI] [PubMed] [Google Scholar]
- 153.Biswas S, Rolain JM. Use of MALDI-TOF mass spectrometry for identification of bacteria that are difficult to culture. J Microbiol Methods. 2013;92:14–24. 10.1016/j.mimet.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 154.Senoh M, Ghosh-Banerjee J, Ramamurthy T, Colwell RR, Miyoshi SI, Balakrish Nair G, et al. Conversion of viable but nonculturable enteric bacteria to culturable by co-culture with eukaryotic cells. Microbiol Immunol. 2012;56:342–5. 10.1111/j.1348-0421.2012.00440.x [DOI] [PubMed] [Google Scholar]
- 155.Josić Dj, Kovač S. Molecular imaging mass spectrometry. Kem Ind. 2009;58:207–13. [Google Scholar]
- 156.Hong SM, Tanaka M, Yoshii S, Mine Y, Matsui T. Enhanced visualization of small peptides absorbed in rat small intestine by phytic-acid-aided matrix-assisted laser desorption/ionization-imaging mass spectrometry. Anal Chem. 2013;85:10033–9. 10.1021/ac402252j [DOI] [PubMed] [Google Scholar]
- 157.Taira S, Tokai M, Kaneko D, Katano H, Kawamura-Konishi Y. Mass spectrometry imaging analysis of location of procymidone in cucumber samples. J Agric Food Chem. 2015;63:6109–12. 10.1021/acs.jafc.5b00957 [DOI] [PubMed] [Google Scholar]
- 158.Yoshimura Y, Goto-Inoue Y, Moriyama T, Zaima N. Significant advancement of mass spectrometry imaging for food chemistry. Food Chem. 2016;210:200–11. 10.1016/j.foodchem.2016.04.096 [DOI] [PubMed] [Google Scholar]
- 159.Verleysen E, Van Doren E, Waegeneers N, De Temmerman PJ, Francisco MAD, Mast J. TEM and SP-ICP-MS analysis of the release of silver nanoparticles from decoration of pastry. J Agric Food Chem. 2015;63:3570–8. 10.1021/acs.jafc.5b00578 [DOI] [PubMed] [Google Scholar]
- 160.Ojima-Kato T, Yamamoto N, Suzuki M, Fukunaga T, Tamura H. Discrimination of Escherichia coli O157, O26 and O111 from other serovars by MALDI-TOF MS based on S10- -GERMS method. PLoS One. 2014;9:e113458. 10.1371/journal.pone.0113458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marshall AG, Guan S. Advantages of high magnetic field for Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun Mass Spectrom. 1996;10:1819–23. [DOI] [PubMed] [Google Scholar]
- 162.Guan S, Marshall AG. Ion traps for Fourier transform ion cyclotron resonance mass spectrometry: principles and design of geometric and electric configuration. Int J Mass Spectrom Ion Process. 1995;146–147:261–96. 10.1016/0168-1176(95)04190-V [DOI] [Google Scholar]
- 163.Aharoni A, Ric de Vos CH, Verhoeven HA, Maliepaard CA, Kruppa G, Bino R, et al. Nontargeted metabolome analysis by use of Fourier transform ion cyclotron mass spectrometry. OMICS. 2002;6:217–34. 10.1089/15362310260256882 [DOI] [PubMed] [Google Scholar]
- 164.Raternik RJ, van der Kloet FM, Li J, Wattel NA, Schaaf MJM, Spaink HP, et al. Rapid metabolic screening of early zebrafish embryogenesis based on direct infusion-nano-ESI-FTMS. Metabolomics. 2013;9:864–73. 10.1007/s11306-012-0493-6 [DOI] [Google Scholar]
- 165.Brown SC, Kruppa G, Dasseux JL. Metabolomics applications of FT-ICR mass spectrometry. Mass Spectrom Rev. 2005;24:223–31. 10.1002/mas.20011 [DOI] [PubMed] [Google Scholar]
- 166.Junot C, Madalanski G, Tabet JC, Ezan E. Fourier transform mass spectrometry for metabolome analysis. Analyst. 2010;135:2203–19. 10.1039/c0an00021c [DOI] [PubMed] [Google Scholar]
- 167.Lei Z, Huhman DV, Sumner LW. Mass spectrometry strategies in metabolomics. J Biol Chem. 2011;286:25435–42. 10.1074/jbc.R111.238691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oliveira BG, Costa HB, Ventura JA, Kondratyuk TP, Barroso MES, Correia RM, et al. Chemical profile of mango (Mangifera indica L.) using electrospray ionisation mass spectrometry (ESI-MS). Food Chem. 2016;204:37–45. 10.1016/j.foodchem.2016.02.117 [DOI] [PubMed] [Google Scholar]
- 169.Jones JJ, Borgmann S, Wilkins CL, O’Brien RM. Characterizing the phospolipid profiles in mammalian tissues by MALDI FTMS. Anal Chem. 2006;78:3062–71. 10.1021/ac0600858 [DOI] [PubMed] [Google Scholar]
- 170.Leavell MD, Leary JA. Fatty acid analysis tool (FAAT): an FT--ICR MS lipid analysis algorithm. Anal Chem. 2006;78:5497–503. 10.1021/ac0604179 [DOI] [PubMed] [Google Scholar]
- 171.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitaion of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. 10.1002/mas.20023 [DOI] [PubMed] [Google Scholar]
- 172.Han X, Yang K, Gross RW. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom Rev. 2012;31:134–78. 10.1002/mas.20342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schwudke D, Hannich JT, Surendranath V, Grimard V, Moehring T, Burton L, et al. Top-down lipidomic screens by multivariate analysis of high-resolution survey mass spectra. Anal Chem. 2007;79:4083–93. 10.1021/ac062455y [DOI] [PubMed] [Google Scholar]
- 174.Jones JJ, Mariccor S, Batoy AB, Wilkins CL. A comprehensive and comparative analysis for MALDI FTMS lipid and phospholipid profiles from biological samples. Comput Biol Chem. 2005;29:294–302. 10.1016/j.compbiolchem.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 175.Nicolardi S, Bogdanov B, Deelder AM, Palmblad M, van der Burgt YEM. Developments in FTICR-MS and its potential for body fluid signatures. Int J Mol Sci. 2015;16:27133–44. 10.3390/ijms161126012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.He H, Rodgers RP, Marshall AG, Hsu CS. Algae polar lipids by online liquid chromatography coupled with hybrid linear quadrupole ion trap/Fourier transform cyclotron resonance mass spectrometry. Energy Fuels. 2011;25:4770–5. 10.1021/ef201061j [DOI] [Google Scholar]
- 177.Le CH, Han J, Borchers CH. Dithranol as a MALDI matrix for tissue imaging of lipids by Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem. 2012;84:8391–8. 10.1021/ac301901s [DOI] [PubMed] [Google Scholar]
- 178.Milev BP, Patras MA, Dittmar T, Vrancken G, Kuhnert N. Fourier transform ion cyclotron resonance mass spectrometrical analysis of raw fermented cocoa beans of Cameroon and Ivory Coast origin. Food Res Int. 2014;64:958–61. 10.1016/j.foodres.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 179.Chen J, Ge ZZ, Zhu W, Xu Z, Li CM. Screening of key antioxidant compounds of longan (Dimocarpus longan Lour.) seed extract by combining online fishing/knockout, activity evaluation, Fourier transform ion cyclotron resonance mass spectrometry, and high-performance liquid chromatography electrospray ionization mass spectrometry methods. J Agric Food Chem. 2014;62:9744–50. 10.1021/jf502995z [DOI] [PubMed] [Google Scholar]
- 180.Froesch M, Bindila LM, Baykut G, Allen M, Peter-Katalinić J, Zamfir AD. Coupling of fully automated chip electrospray to Fourier transform ion cyclotron resonance mass spectrometry for high-performance glycoscreening and sequencing. Rapid Commun Mass Spectrom. 2004;18:3084–92. 10.1002/rcm.1733 [DOI] [PubMed] [Google Scholar]
- 181.Seipert RR, Barboza M, Niñouevo MR, LoCascio RG, Mills DA, Freeman SL, et al. Analysis and quantitation of fructooligosaccharides using matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem. 2008;80:159–65. 10.1021/ac7017298 [DOI] [PubMed] [Google Scholar]
- 182.Clowers BH, Dodds ED, Seipert RR, Lebrilla CB. Site determination of protein glycosylation based on digestion with immobilized nonspecific proteases and Fourier transform ion cyclotron resonance mass spectrometry. J Proteome Res. 2007;6:4032–40. 10.1021/pr070317z [DOI] [PubMed] [Google Scholar]
- 183.Halim A, Carlsson MC, Madsen CB, Brand S, Møller SR, Olsen CE, et al. Glycoproteomic analysis of seven major allergenic proteins reveals novel post-translational modifications. Mol Cell Proteomics. 2015;14:191–204. 10.1074/mcp.M114.042614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Koeberl M, Clarke D, Lopata AL. Next generation of food allergen quantification using mass spectrometric systems. J Proteome Res. 2014;13:3499–509. 10.1021/pr500247r [DOI] [PubMed] [Google Scholar]
- 185.Ghaste M, Mistrik R, Shulaev V. Application of Fourier transform ion cyclotron resonance (FT-ICR) and Orbitrap based high resolution mass spectrometry in metabolomics and lipidomics. Int J Mol Sci. 2016;17:816. 10.3390/ijms17060816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cifuentes A. Foodomics: advanced mass spectrometry in modern food science and nutrition. Hoboken, NJ, USA: John Willey & Sons, Inc; 2013. [Google Scholar]
- 187.Schmidt A, Kochanowski K, Vedelaar S, Ahrné E, Volkmer B, Callipo L, et al. The quantitative and condition-dependent Escherichia coli proteome. Nat Biotechnol. 2016;34:104–10. 10.1038/nbt.3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Witting M, Lucio M, Tziotis D, Wägele B, Suhre K, Voulhoux R, et al. DI-ICR-FT-MS-based high-throughput metabotyping: a case study of the Caenorhabditis elegans-Pseudomonas aeruginosa infection model. Anal Bioanal Chem. 2015;407:1059–73. 10.1007/s00216-014-8331-5 [DOI] [PubMed] [Google Scholar]
- 189.Hardman M, Makarov AA. Interfacing the Orbitrap mass analyzer to an electrospray ion source. Anal Chem. 2003;75:1699–705. 10.1021/ac0258047 [DOI] [PubMed] [Google Scholar]
- 190.Kalli A, Smith GT, Sweredoski MJ, Hess S. Evaluation and optimization of mass spectrometric settings during data-dependent acquisition mode: focus on LTQ-Orbitrap mass analyzers. J Proteome Res. 2013;12:3071–86. 10.1021/pr3011588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Makarov A, Denisov E, Kholomelov A, Balschun W, Lange O, Strupat K, et al. Performance evaluation of hybrid linear ion trap/Orbitrap mass spectrometer. Anal Chem. 2006;78:2113–20. 10.1021/ac0518811 [DOI] [PubMed] [Google Scholar]
- 192.Eliuk S, Makarov A. Evolution of Orbitrap mass spectrometry instrumentation. Annu Rev Anal Chem (Palo Alto Calif). 2015;8:61–80. 10.1146/annurev-anchem-071114-040325 [DOI] [PubMed] [Google Scholar]
- 193.Kelstrup CD, Young C, Lavallee R, Nielsen ML, Olsen JV. Optimized fast and sensitive acquisition methods for shotgun proteomics on quadrupole Orbitrap mass spectrometer. J Proteome Res. 2012;11:3487–97. 10.1021/pr3000249 [DOI] [PubMed] [Google Scholar]
- 194.Motilva MJ, Toldrá F, Aristoy MC, Flores J. Subcutaneous adipose tissue lipolysis in the processing of dry-cured ham. J Food Biochem. 1992;16:323–35. 10.1111/j.1745-4514.1992.tb00455.x [DOI] [Google Scholar]
- 195.Bayés-García L, Tres A, Vichi S, Calvet T, Cuevas-Diarte MA, Codony R, et al. Authentication of Iberian dry-cured ham: new approaches by polymorphic fingerprint and ultrahigh resolution mass spectrometry. Food Control. 2016;60:370–7. 10.1016/j.foodcont.2015.07.047 [DOI] [Google Scholar]
- 196.Vichi S, Cortés-Francisco N, Romero A, Caixach J. Direct chemical profiling of olive (Olea europaea) fruit epicuticular waxes by direct electrospray-ultrahigh resolution mass spectrometry. J Mass Spectrom. 2015;50:558–66. 10.1002/jms.3562 [DOI] [PubMed] [Google Scholar]
- 197.Shi L, Chowdhury SM, Smallwood HS, Yoon H, Mottaz-Brewer HM, Norbeck AD, et al. Proteomic investigation of the time course responses of RAW 264.7 macrophages to infection with Salmonella enterica. Infect Immun. 2009;77:3227–33. 10.1128/IAI.00063-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.García-Cañas V, Simó C, León C, Ibáñez E, Cifuentes A. MS-based analytical methodologies to characterize genetically modified crops. Mass Spectrom Rev. 2011;30:396–416. 10.1002/mas.20286 [DOI] [PubMed] [Google Scholar]
- 199.León C, Rodríguez-Meizoso I, Lucio M, Garcia-Cañas V, Schmitt-Kopplin P, Cifuentes A. Metabolomics of transgenic maize combining Fourier transform cyclotron resonance-mass spectrometry, capillary electrophoresis-mass spectrometry and pressurized liquid extraction. J Chromatogr A. 2009;1216:7314–23. 10.1016/j.chroma.2009.04.092 [DOI] [PubMed] [Google Scholar]
- 200.Levandi T, Leon C, Kaljurand M, Garcia-Cañas V, Cifuentes A. Capillary electrophoresis time-of-flight mass spectrometry for comparative metabolomics of transgenic versus conventional maize. Anal Chem. 2008;80:6329–35. 10.1021/ac8006329 [DOI] [PubMed] [Google Scholar]
- 201.Rocco M, Corrado G, Arena S, D’Ambrosio C, Tortiglione C, Sellaroli S, et al. The expression of tomato prosystemin gene in tobacco plants highly affects host proteomic repertoire. J Proteomics. 2008;71:176–85. 10.1016/j.jprot.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 202.Ruiz L, Hidalgo C, Blanco-Míguez A, Lourenço A, Sánchez B, Margolles A. Tracing probiotic and gut microbiota through proteomics. J Proteomics. 2016;147:28–39. 10.1016/j.jprot.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 203.Ortea I, O’Conor G, Maquet A. Review on proteomics for food authentication. J Proteomics. 2016;147:212–25. 10.1016/j.jprot.2016.06.033 [DOI] [PubMed] [Google Scholar]
- 204.Mazzeo MF, Siciliano RA. Proteomics for the authentication of fish species. J Proteomics. 2016;147:119–24. 10.1016/j.jprot.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 205.Ebner J, Baum F, Pischetsrieder M. Identification of sixteen peptides reflecting heat and/or storage induced process by profiling of commercial milk samples. J Proteomics. 2016;147:66–75. 10.1016/j.jprot.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 206.Kuklenyik Z, Boyer AE, Lins R, Quinn CP, Gallegos-Candela M, Woolfitt A, et al. Comparison of MALDI-TOF-MS and HPLC-ESI-MS/MS for endopeptidase activity-based quantification of antrax lethal factor in serum. Anal Chem. 2011;83:1760–5. 10.1021/ac1030144 [DOI] [PubMed] [Google Scholar]
- 207.Cheng K, Chui H, Domisch L, Hernandez D, Wang G. Recent development of mass spectrometry and proteomics applications in identification and typing of bacteria. Proteomics Clin Appl. 2016;10:346–57. 10.1002/prca.201500086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chiaradia E, Valiani A, Tartaglia M, Scoppetta F, Renzone G, Arena S, et al. Ovine subclinical mastitis: proteomic analysis of whey and milk fat globules unveils putative diagnostic biomarkers in milk. J Proteomics. 2013;83:144–59. 10.1016/j.jprot.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 209.Charretier Y, Schrenzel J. Mass spectrometry methods for predicting antibiotic resistance. Proteomics Clin Appl. 2016;10:964–81. 10.1002/prca.201600041 [DOI] [PubMed] [Google Scholar]
- 210.Piras C, Soggiu A, Greco V, Martino PA, Del Chierco F, Putignani L, et al. Mechanisms of antibiotic resistance in uropathogenic Escherichia coli in dog. J Proteomics. 2015;127:365–76. 10.1016/j.jprot.2015.05.040 [DOI] [PubMed] [Google Scholar]
- 211.Picariello G, Mamone G, Addeo F, Ferranti P. The frontiers of mass spectrometry-based techniques in food allergenomics. J Chromatogr A. 2011;1218:7386–98. 10.1016/j.chroma.2011.06.033 [DOI] [PubMed] [Google Scholar]
- 212.Colgrave ML, Byrne K, Brundell M, Howitt CA. Identification of barley-specific peptide markers that persist in processed food and are capable of detecting barley contamination by LC-MS/MS. J Proteomics. 2016;147:169–76. 10.1016/j.jprot.2016.03.045 [DOI] [PubMed] [Google Scholar]
- 213.Picariello G, Mamone G, Nitride C, Addeo F, Camarca A, Vocca I, et al. Shotgun proteome analysis in beer and the immunogenic potential of beer polypeptides. J Proteomics. 2012;75:5872–82. 10.1016/j.jprot.2012.07.038 [DOI] [PubMed] [Google Scholar]
- 214.Garrido BC, Souza GHMF, Laurenço DC, Fasciotti M. Proteomics in quality control: whey protein-based supplements. J Proteomics. 2016;147:48–55. 10.1016/j.jprot.2016.03.044 [DOI] [PubMed] [Google Scholar]
- 215.Picariello G, Amigo-Benavent M, del Castillo MD, Ferranti P. Structural characterization of the N-glycosylation of individual soybean β-conglycinin subunits. J Chromatogr A. 2013;1313:96–102. 10.1016/j.chroma.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 216.Nitride C, Mamone G, Picariello G, Mills C, Nocerino R, Canani RB, et al. Proteomic and immunological characterization of a new food allergen from hazelnut (Corylus avellana). J Proteomics. 2013;86:16–26. 10.1016/j.jprot.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 217.Gallego M, Mora L, Toldrá F. Peptidomics as a tool for quality control in dry-cured ham processing. J Proteomics. 2016;147:98–107. 10.1016/j.jprot.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 218.Monakhova YB, Kuballa T, Lachenmeier DW. Chemometric methods in NMR spectrometric analysis of food products. J Anal Chem. 2013;68:755–66. 10.1134/S1061934813090098 [DOI] [Google Scholar]
- 219.Kim S, Kim J, Yun EJ, Kim KH. Food metabolomics: from farm to human. Curr Opin Biotechnol. 2016;37:16–23. 10.1016/j.copbio.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 220.Pinu FR. Metabolomics – the new frontier in food safety and quality research. Food Res Int. 2015;72:80–1. 10.1016/j.foodres.2015.03.028 [DOI] [Google Scholar]
- 221.Cheng J, Gao R, Li H, Wu S, Fang J, Ma K, et al. Evaluating potential markers of spoilage foods using a metabolomic profiling approach. Food Anal Methods. 2015;8:1141–9. 10.1007/s12161-014-9999-z [DOI] [Google Scholar]
- 222.Deming SN, Michotte Y, Massart DL, Kaufman L, Vandeginste BGM. Chemometrics: a textbook. New York, NY, USA: Elsevier; 1988. [Google Scholar]
- 223.Köbler H, Monakhova YB, Kuballa T, Tschiersch C, Vancutsem J, Thielert G, et al. Nuclear magnetic resonance spectroscopy and chemometrics to identify pine nuts that cause taste disturbance. J Agric Food Chem. 2011;59:6877–81. 10.1021/jf2014827 [DOI] [PubMed] [Google Scholar]