Abstract
Background: Chronic myelogenous leukemia (CML) is a hematological malignancy that originates in an abnormal pluripotent bone marrow stem cell. CML is consistently associated with the expression of the BCR-ABL1 fusion gene, generated by the t(9;22) chromosomal translocation, thus creating the Philadelphia chromosome, a target for protein tyrosine kinase inhibitor (TKI) therapy. There is no published data, demonstrating increased risk for development of second cancers, associated with the exposure to TKIs.
Case report: We report a case of a primary extranodal marginal zone B-cell lymphoma (EMZBCL), diagnosed in a 53-year-old woman with CML receiving treatment with TKI the preceding seven years. Both diagnoses were confirmed by cytological/histological examination. Regarding CML, complete hematological, cytogenetic, and molecular remission was achieved by imatinib therapy. Regarding EMZBCL complete remission with an anthracycline-based polychemotherapy was achieved, while the patient was on continuous treatment with imatinib. Currently, the patient is in major molecular response for CML (151 months) and also in complete remission for EMZBCL (60 months).
Conclusion: We report this case as we consider that any secondary neoplasia developing in patients treated with TKIs should be reported for assessment and further detailed analyzes. Hippokratia 2016, 20(3): 241-243
Keywords: Extranodal marginal zone B-cell lymphoma, chronic myeloid leukemia, tyrosine kinase inhibitor
Introduction
Isaacson and Wright were the first who described certain extranodal lymphomas related to mucosa-associated lymphoid tissue (MALT), as a new separate entity rather than that of peripheral lymph nodes in 19831. These observations were extended later to include other extranodal low-grade B-cell lymphomas, especially those of salivary gland, lung, and thyroid2. At present, they were recently included both in the WHO-classification3 in the Mature (peripheral) B-cell neoplasms with the general term of marginal zone lymphoma (MZL), that includes the three closely related lymphoma subtypes: i) nodal, ii) primary splenic, and iii) extranodal lymphomas of MALT type.
Completely different is the chronic myelogenous leukemia (CML) which belongs to the myeloproliferative neoplasms (MPN) that originates in an abnormal pluripotent bone marrow (BM) stem cell. CML is consistently associated with the BCR-ABL1 fusion gene located in the Philadelphia (Ph) chromosome4. The BCR-ABL fusion protein mediates the development and maintenance of CML through interaction with multiple downstream signaling partners which result in altered cellular adhesion, activation of mitogenic signaling, and inhibition of apoptosis that lead to the transformation of hematopoietic stem cells. BCR-ABL-mediated signaling is also associated with defective DNA repair, resulting in additional chromosomal alterations and mutations, which may partly explain the aggressive nature of advanced CML5. Thereby, BCR-ABL1 fusion gene provides a target for tyrosine kinase inhibitor (TKI) therapy6. Imatinib mesylate, a TKI, is now standard therapy for patients with CML, and more than 80 % of patients accomplish a complete cytogenetic response (CCyR) and ≤ 70 % may attain a major molecular response (MMR) by five years of therapy6. Newer therapies with other TKIs (e.g. bosutinib, dasatinib, nilotinib) are effective after the failure of imatinib mesylate. More recently these TKIs as frontline therapy have shown superiority compared with imatinib mesylate7-9. However, with the success of TKIs in CML treatment and prolonged survival, questions arise regarding the possibility of late effects of TKI treatment, and especially about the possibility of developing other malignancies. Some data from case-reported studies show incidence of secondary malignancies during TKIs therapy where most of the patients had solid cancer and others had lymphoma10-12. Verma et al, reported a study of 1,445 patients with CML/MPN13. They found 5 % cumulative incidence of non-Hodgkin lymphoma (NHL), i.e. chronic lymphoid leukemia, diffuse large B-cell lymphoma and myeloproliferative lymphoma, but the standardized incidence ratios (SIRs) did not reach statistical significance13. Additionally epidemiological analysis of Novartis suggested that exposure to TKIs increases the risk of developing second NHL14. The etiology of secondary neoplasias in patients treated with imatinib is unknown. In vitro studies have shown that imatinib inhibits CD4+ or CD8+ T-cell proliferation and activation, as well as it downregulates the antigen-presenting function of dendric cells (DC)15. In contrast, other studies have shown that imatinib enhances the antigen-presenting function of DC and fosters DC-natural killer (NK) cells reciprocal activation, thus promoting the antitumoral function of NK cells16. Therefore, it is still uncertain whether the subsequent tumor results from imatinib therapy, previous antitumor treatment or from other independent risk factors (e.g. age). For this reasons we consider that any secondary neoplasia developing in patients treated with TKIs should be reported for assessment and further detailed analyses. We report the case of myeloproliferative and lymphoproliferative neoplastic disorders, which occurred in the same patient.
Case presentation
A 53-year-old woman presented with a clinical history of swelling of the left parotid gland, no B-symptoms, with CML proven eight years ago, under treatment with imatinib 400 mg daily for the preceding seven years. The physical evaluation revealed a painless two cm mass in the left parotid gland, which was not adherent to the surrounding tissue. No lymphadenopathy was recorded. The laboratory findings showed normochromic anemia [hemoglobin: 11.9 g/dL, normal range (NR): 12.0-14.0 g/dL], no leukocytosis [white blood cells (WBC): 3.9 x 103/µl, NR: 3.5-10.5 x103/µl], and no thrombocytopenia (platelets: 229 x103/µl, NR: 150-500 x103/µl). Also, blood tests performed did not detect hepatitis virus (HCV, HBV) or HIV infection. All biochemical parameters checked were normal. The control examination of CML status showed complete hematological, cytogenetic and molecular response.
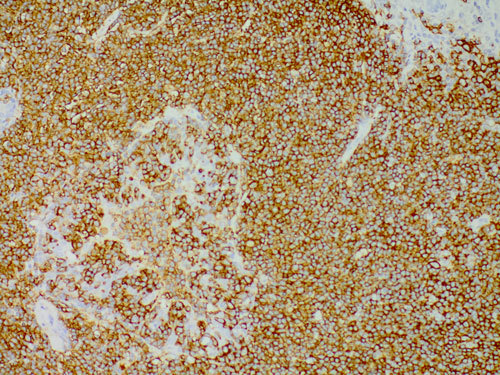
The histological findings, from the left parotid biopsy, were typically indicative for extranodal MALT lymphoma (Figure 1). The immunohistochemical study demonstrated positivity of lymphoid cells for the B-cell lineage marker CD20; no positivity was found for CD5, CD10, CD23, CyD1 (Figure 2). After complete staging for lymphoma with whole body computed tomography scan and trephine bone marrow biopsy, a final diagnosis of primary extranodal marginal zone B-cell lymphoma (EMZBCL) MALT-type was made arising in a salivary gland, Ann Arbor stage IЕ. Anthracyclin-based polychemotherapy (six cycles of CHOP regimen) was carried out simultaneously with Imatinib. Lymphoma complete response (CR) was achieved after four months.
Figure 1. Morphological diagnosis of extranodal marginal zone lymphoma (EMZL): nodular and diffuse infiltrates obscure the normal glandular structure, most neoplastic lymphocytes being small to medium size with moderate amount of clear cytoplasm - so called monocytiod appearance. Further, clear evidence of aggressive behavior is seen in the well-formed lympho-epithelial lesions (hematoxylin-eosin, original magnification ×40).
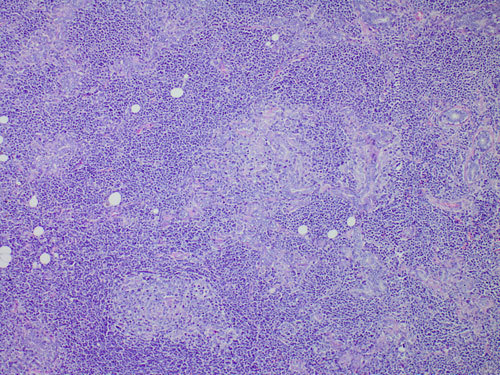
Figure 2. Lymphomatous cells show a strong positivity for CD20 immunostaining (CD20 immunostain, original magnification x20).
The first malignancy, CML Ph positive, the chronic phase was diagnosed by bone-marrow aspirate (hypercellular bone marrow aspirate smears) with a maturation pattern similar to that observed in the peripheral blood. There was a prominent increase of neutrophil myelocytes (“myelocyte bulge”) and segmented neutrophils. Dyserythropoiesis was absent or minimal. The number of megakaryocytes varied considerably. Moderate to marked megakaryocytic proliferation was observed. Blood film showed leucocytosis (WBC: 22 x103/µl) and this increase in the WBC count was largely due to neutrophils in different stages of maturation, with peaks in the percentages of myelocytes, and segmented neutrophils. The neutrophils were normal and there was no significant dysplasia. Blasts accounted for less than 2 % of WBCs. Absolute basophilia was observed. Red cells showed mild anisocytosis, platelets revealed no significant abnormalities, except for a single giant form. Cytogenetic examination conformed t(9;22) (M-BCR-ABL-b3a2 transcripts). Therapy with TKI (imatinib) was started in a standard dose of 400 mg daily. A complete hematological response was achieved one year after initiation of the therapy. On the eighteenth month CCyR and MMR were reported. Since the CML diagnosis was established imatinib has been given permanently.
Currently, the patient is disease free survival both for CML (151 months) and EMZBCL MALT-type (60 months).
Discussion
EMZBCL account for approximately 9 % of all NHL with a frequency of primary origin in the salivary gland 26 %2. Like with other malignancies, etiopathogenesis remains unclear. Some findings suggest that MALT lymphoma is an antigen driven neoplasm associated with chronic antigenic stimulation by autoantigens17 and/or microbial pathogens18, inducing an accumulation of lymphoid tissue in the typical sites of involvement for each lymphoma entity. Less is known about the gene translocations which result to protein dysregulation, playing a role in the pathogenesis of MALT lymphomas. However, the mechanism of action of the protein products for the most frequent chromosome translocation in MALT lymphoma t(11;18)(q21;q21) and t(1;14)(p22;q32), was established. The fusion proteins (API2-MALT1 and over-expressed Bcl10), by synergism, lead to a block of the inhibitory IκB proteins which activate the NF-κB resulting in transcription of a number of different genes involved in inflammation, cell viability, immune response, and unregulated cell proliferation19. There is no evidence that imatinib used in the treatment of CML could promote certain steps in the described pathological pathways. Moreover, imatinib inhibits specifically the kinase activity of proteins that contain ABL, ABL-related gene (ARG) protein, or platelet-derived growth factor receptor, or KIT receptor20 for which there are no data to participate in NHL lymphoma pathogenesis or chronic autoimmune inflammation. Additionally, Verma et al13, after following-up 1,445 patients (CML/MPN or other hematologic malignancies) treated with TKIs for up to two years, concluded that exposure to TKIs did not increase the risk of developing secondary malignancies. This observation was in accordance with another independent study on 9,500 patients treated with TKIs14.
In conclusion, the described clinical case assumes with a high probability that the two hematological neoplasms could occur independently of each other.
Conflict of interest
The authors report no conflict of interest
Acknowledgement
We acknowledge the contribution of Petrov Y who helped in manuscript preparation.
References
- 1.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer. 1984;53:2515–2524. doi: 10.1002/1097-0142(19840601)53:11<2515::aid-cncr2820531125>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edition, IARC Press, Lyon. 2008:10–12. [Google Scholar]
- 4.Melo JV, Barnes DJ. Chronic myeloid leukemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7:441–453. doi: 10.1038/nrc2147. [DOI] [PubMed] [Google Scholar]
- 5.Sawyers CL. Signal transduction pathways involved in BCR-ABL transformation. Baillieres Clin Haematol. 1997;10:223–231. doi: 10.1016/s0950-3536(97)80004-2. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Schiffer C, Jones D, Cortes J. Monitoring the response and course of chronic myeloid leukemia in the modern era of BCR-ABL tyrosine kinase inhibitors: practical advice on the use and interpretation of monitoring methods. Blood. 2008;111:1774–1780. doi: 10.1182/blood-2007-09-110189. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117:1141–1145. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 9.Paydaş S, Bozkurt Duman B, Ergin M. Non-Hodgkin’s lymphoma a chronic myelocytic leukemia patient treated with imatinib. Turk J Hematol. 2011;28:232–234. doi: 10.5152/tjh.2011.60. [DOI] [PubMed] [Google Scholar]
- 10.Duman BB, Paydas S, Disel U, Besen A, Gurkan E. Secondary malignancy after imatinib therapy: eight cases and review of the literature. Leuk Lymphoma. 2012;53:1706–1708. doi: 10.3109/10428194.2012.666545. [DOI] [PubMed] [Google Scholar]
- 11.Roy L, Guilhot J, Martineau G, Larchée R, Guilhot F. Unexpected occurrence of second malignancies in patients treated with interferon followed by imatinib mesylate for chronic myelogenous leukemia. Leukemia. 2005;19:1689–1692. doi: 10.1038/sj.leu.2403874. [DOI] [PubMed] [Google Scholar]
- 12.Piccin A, Conneally E, Lynch M, McMenamin ME, Langabeer S, McCann S. Adenomatoid tumor of the testis in a patient on imatinib therapy for chronic myeloid leukemia. Leuk Lymphoma. 2006;47:1394–1396. doi: 10.1080/10428190600556098. [DOI] [PubMed] [Google Scholar]
- 13.Verma D, Kantarjian H, Strom SS, Rios MB, Jabbour E, Quintas-Cardama A, et al. Malignancies occurring during therapy with tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML) and other hematologic malignancies. Blood. 2011;118:4353–4358. doi: 10.1182/blood-2011-06-362889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilot PR, Sablinska K, Owen S, Hatfield A. Epidemiological analysis of second primary malignancies in more than 9500 patients treated with imatinib. Leukemia. 2006;20:148. doi: 10.1038/sj.leu.2404025. author reply 149. [DOI] [PubMed] [Google Scholar]
- 15.Appel S, Rupf A, Weck MM, Schoor O, Brümmendorf TH, Weinschenk T, et al. Effects of imatinib on monocyte-derived dendritic cells are mediated by inhibition of nuclear factor-kappaB and Akt signaling pathways. Clin Cancer Res. 2005;11:1928–1940. doi: 10.1158/1078-0432.CCR-04-1713. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Cheng F, Cuenca A, Horna P, Zheng Z, Bhalla K, et al. Imatinib mesylate (STI-571) enhances antigen-presenting cell function and overcomes tumor-induced CD4+ T-cell tolerance. Blood. 2005;105:1135–1143. doi: 10.1182/blood-2004-01-0027. [DOI] [PubMed] [Google Scholar]
- 17.Hyjek E, Isaacson PG. Primary B cell lymphoma of the thyroid and its relationship to Hashimoto’s thyroiditis. Hum Pathol. 1988;19:1315–1326. doi: 10.1016/s0046-8177(88)80287-9. [DOI] [PubMed] [Google Scholar]
- 18.Zucca E, Bertoni F, Roggero E, Bosshard G, Cazzaniga G, Pedrinis E, et al. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid-tissue lymphoma of the stomach. N Engl J Med. 1998;338:804–810. doi: 10.1056/NEJM199803193381205. [DOI] [PubMed] [Google Scholar]
- 19.Lucas PC, Yonezumi M, Inohara N, McAllister-Lucas L, Abazeed ME, Chen FF, et al. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-kappa B signaling pathway. J Biol Chem. 2001;276:19012–19019. doi: 10.1074/jbc.M009984200. [DOI] [PubMed] [Google Scholar]
- 20.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]


