Abstract
Complicated pleural effusions and empyema with loculation and failed drainage are common clinical problems. In adults, intrapleural fibrinolytic therapy is commonly used with variable results and therapy remains empiric. Despite the intrapleural use of various plasminogen activators; fibrinolysins, for about sixty years, there is no clear consensus about which agent is most effective. Emerging evidence demonstrates that intrapleural administration of plasminogen activators is subject to rapid inhibition by plasminogen activator inhibitor-1 and that processing of fibrinolysins is importantly influenced by other factors including the levels and quality of pleural fluid DNA. Current therapy for loculation that accompanies pleural infections also includes surgery, which is invasive and for which patient selection can be problematic. Most of the clinical literature published to date has used flat dosing of intrapleural fibrinolytic therapy in all subjects but little is known about how that strategy influences the processing of the administered fibrinolysin or how this influences outcomes. We developed a new test of pleural fluids ex vivo, which is called the Fibrinolytic Potential or FP, in which a dose of a fibrinolysin is added to pleural fluids ex vivo after which the fibrinolytic activity is measured and normalized to baseline levels. Testing in preclinical and clinical empyema fluids reveals a wide range of responses, indicating that individual patients will likely respond differently to flat dosing of fibrinolysins. The test remains under development but is envisioned as a guide for dosing of these agents, representing a novel candidate approach to personalization of intrapleural fibrinolytic therapy.
Keywords: Intrapleural fibrinolytic therapy, plasminogen activators and personalized therapy
Introduction
With the passage of the 21st Century Cures Act1, a national focus on the development of personalized medicine was consolidated with the commitment of about $1.5 billion of new federal funding over the next ten years. This novel program is called the Precision Medicine Initiative or PMI and is designed to identify better ways of treating diseases that are refractory to therapy. The predicate of the PMI is that by better understanding individual genetic, environmental and lifestyle variability associated with these diseases, better therapy or preventative measures can be identified. In a similar vein, current diseases for which therapy is of variable efficacy may be improved.
Within the realm of pleural diseases, precision-guided medicine is in its infancy relative to other areas of medicine. In concept, the pharmacotherapy of loculation associated with pleural infection is one area where a precision guided approach could be of great advantage based upon several considerations related to current care. Complicated parapneumonic pleural effusions (CPE) and empyema are common clinical problems that are commonly referred to pulmonary specialists for consultation and management.
CPE and empyema with loculation have been reported to affect about 80,000 patients in the US and UK annually and are associated with mortality of up to 20 percent, considerable morbidity and costs of about half a billion dollars annually2–5. The incidence of pleural infections is said to be about 8-fold that of cystic fibrosis, five-fold that of idiopathic pulmonary fibrosis and mortality exceeds that of myocardial infarction or community acquired pneumonia3, 4, 6, 7. While it may be that the actual incidence of empyema/CPE may be lower in the United States; approaching 30,000 patients, per year (David Lapidus, proprietary review of the literature commissioned by Lung Therapeutics, Inc.), it is clear that these cases are commonly seen and of major impact in terms of resource utilization. These patients often require extended hospitalization during which time dedicated procedures may be required to expedite pleural drainage. In addition, the incidence of empyema is steadily increasing in both adult and pediatric populations world-wide, as recently reviewed3.
The treatment of choice in adult patients currently remains unsettled and a number of options are available to address failed drainage in the setting of empyema/CPE (Figure 1). Procedures including video-assisted thoracoscopy can be used but selection of patients most likely to benefit remains problematic. Sometimes, at least in the US, that decision to engage a surgical option hinges on what service the patient is admitted to. While surgical options are generally quite effective in facilitating drainage8–11, the radiographic picture of extensive loculation alone should not result in a surgical referral3. As reviewed, at least four clinical trials have failed to demonstrate clear benefits of surgical drainage for CPE/empyema patients3, although operable patients who remain septic clearly may benefit if other measures instituted to facilitate drainage have failed. In many centers in the US, local experience also guides whether patients are committed to surgery early after impaired drainage in CPE/empyema patients is detected11. That approach, however, is invasive and entails the risks of anesthesia and the development of chronic chest pain. The surgical approach is also relatively costly. It cannot be used in patients with exclusionary co-morbidities such as a bleeding diathesis or for patients otherwise deemed inoperable.
Figure 1.
No legend required.
Given these considerations, effective and well-tolerated and reliably effective pharmacotherapy is desirable. Unfortunately, dosing of intrapleural fibrinolytic therapy (IPFT) has since its inception remained empiric and off-label, which may underlie the variable responses to its application in adults. While a variety of approaches including the use of fibrinolysins alone or in combination with DNase have been advocated, the search for optimal IPFT and how best to deliver that in adults remains a high priority12. Along these lines, a personalized approach to the application of IPFT in adults remains unavailable. However, we infer that personalized therapy based on evaluation of the components of the fibrinolytic system in pleural fluids could be of clinical value. We specifically posit that this analysis, which we call the fibrinolytic potential assay (FPA) of pleural fluids could be useful for adult patients with pleural infection, loculation and failed drainage. We are now developing the FPA, which is an assessment of the effect of an ex vivo fibrinolysin or fibrinolysin plus adjunctive therapy on pleural fluid plasminogen activator (PA) activity. Our objective is to reliably improve outcomes in these patients.
The Origins and Evolution of Intrapleural Fibrinolytic Therapy
The concept that clearance of intrapleural fibrinous adhesions could expedite drainage of loculated pleural collections was based on the concept that intrapleural collections of loculated material were primarily fibrinous (Figure 2). Almost seventy years ago, Sherry and Tillett originally proposed the concept these collections could be lysed and drained by fibrinolysins like streptokinase or streptodornase13–15. IPFT has been part of the guideline-driven armamentarium for the treatment of CPE/empyema ever since that time8. Interestingly, two recent multicenter trials failed to show efficacy of fibrinolysins alone4, 16 and the results of one were challenged based on study design issues including inclusion of patients with and without pleural loculation17. More recently, a meta-analysis including both these studies concluded that IPFT provided benefits for adult patients with CPE/empyema, that it should, in particular, be considered for patients with loculation and that further investigation was required to identify best practices18. At some centers, IPFT is now the primary therapy for CPE/empyema, while other recommendations are more restrictive, calling for IPFT in inoperable patients with clear evidence of with pleural loculation19.
Figure 2.
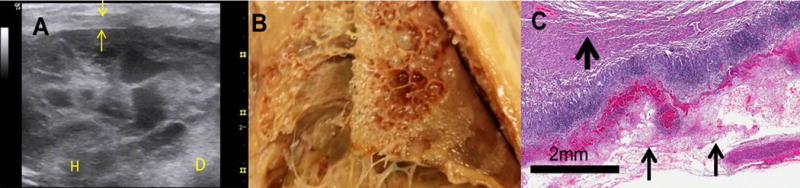
Representative images and histology from rabbits with S. pneumoniae empyema (EMP) in rabbits are illustrated. EMP and pleural injury were induced by intrapleural injection of S. pneumoniae (1×108 cfu). (A) Chest ultrasonography at 1 week after induction of the model. Yellow arrows indicate pleural thickening, adhesions seen as rounded whitish structures; H=heart, D=diaphragm. (B) Postmortem gross visual evaluation of multiloculated empyema following S. pneumoniae-induced empyema in rabbits. (C) The pleural surface displays extensive fibrin (arrows) at the visceral parietal pleural surface, inflammation and subpleural pneumonitis. The visceral pleural surface is oriented at the bottom left portion (thicker arrow indicates lung parenchyma). Representative images shown are shown.
The early reports of effectiveness resulted in an enduring approach to therapy that has been variably successful in adults but interestingly has been quite effective in children, as reviewed20. The reasons for the disparity are now unclear, but may be related to subtle genetic or epigenetic factors that remain to be elucidated. The disparities could relate to the ability of young and otherwise healthy pediatric patients to better handle infectious pleural episodes compared to older adults with comorbidities. The better outcomes could relate to frequently delayed presentation in adults, as patients with serious pleural infection in the MIST2 trial often presented weeks after the onset of symptoms4. It may be that children are more rapidly brought to medical evaluation by their parents or guardians, but that is speculation. Although a number of agents have been used, it is clear that IPFT is generally well-tolerated and effective in pediatric patients with pleural loculation associated with empyema or CPE20, 21. The safety profiles of current forms of IPFT are less certain in adults although the topic is an area of ongoing clinical investigation.
Variation in Innate Responses to Pleural Infection
A number of factors including the variable response of different patients to pleural infection, the efficacy of the local immune response, stage of disease at treatment onset, co-morbidities and variable elaboration of pleural fluid inhibitors, cross-linked transitional fibrin or nucleotides could influence outcomes of IPFT. The relative impact of each of these factors to independently or collectively influence the ability of IPFT to affect pleural drainage in a given patient with pleural loculation remains unclear.
The type of infection that a given subject experiences can clearly affect the severity of pleural loculation. We recently examined this issue in newly developed rabbit models of progressively organizing pleural empyema that develop as facsimiles of pleural loculation of the course of five days22. In this study, we compared the biochemical correlates and phenotypic changes associated with empyema induced by intrapleural administration of either P. multocida or S. pneumoniae. In the models, the animals responded to pleural infection with the development of an inflammatory pleural effusion that developed pleural collections reminiscent of loculated pleural effusions that persisted over the 5 day course of the experiments. The pleural effusions of P. multocida-injured animals had increased PAI-1 levels that correlated with increased PAI-1 activity and levels of each were comparable to those of animals with S.pneumoniae-induced empyema. Concentrations of PAI-1 in these fluids exceeded those of rabbits with tetracycline (TCN)-induced pleural injury that we previously studied23–25. IPFT with doses of tissue plasminogen activator (tPA) or single chain urokinase plasminogen activator (scuPA) that were effective in attenuating pleural collections in TCN-induced pleural injury were ineffective in P. multocida-induced empyema but scuPA demonstrated a trend towards improved outcomes in animals with S. pneumoniae-induced empyema. Overall, higher doses of IPFT were required in either form of empyema (2 mg/kg) than used in TCN-induced pleural injury, suggesting that PAI-1 and associated activity levels were responsible for the differential effects of the fibrinolysins on clearance of fibrinous, organizing pleural collections.
The role of PAI-1 in regulating the responses to IPFT in empyema in rabbits was comparable to the findings in which PAI-1 levels and activity were independently altered in the TCN model. In this study, adenovirus-mediated delivery of the human PAI-1 gene was delivered under a cytomegalovirus promoter to the rabbit pleura. In an independent study, PAI-1 levels were increased via transduction of the pleural mesothelium of rabbits after which TCN-induced pleural organizing injury was induced25. As levels of intrapleural PAI-1 and its activity increased in the model, the PA activity of pleural fluid fell in a linearly correlated manner in animals treated with either tPA or scuPA. These results suggest the possibility that PAI-1 is a legitimate target to improve IPFT. This is not to say that absolute deficiency of PAI-1 is desirable, as PAI-1 plays a regulatory role in normal healing and the control of fibroblast elaboration of collagen26. In a murine model of empyema that we recently developed, absolute PAI-1 deficiency was associated with attenuation of the extent of pleural thickening and lung restriction, likely attributable at least in part to increased mesomesenchymal transition of pleural mesothelial cells and cross talk with procoagulant pathways with increased tissue factor expression in the injured pleural tissues27.
In human pleural fluids from patients with empyema or complicated parapneumonic pleural effusions, PAI-1 levels are generally increased28 which has been demonstrated by a number of other groups, as reviewed20. Interestingly, we recently reported that levels of PAI-1 in pleural fluids of patients with empyema and other forms of pleural infection in the MIST2 clinical trial were similar to those detected in the pleural fluids of rabbits with either P. multocida or S. pneumoniae-induced empyema22. The levels of PAI-1 antigen and activity varied in individual patients, suggesting that dosing of fibrinolysins could be variably effective in patients depending on the levels of PAI-1 that were present within pleural fluids at the time that IPFT is initiated. PAI-1 levels were not assessed in the MIST2 trial at the time that patients were randomized to the treatment arms of either vehicle, tPA alone, DNase alone or tPA and DNase in combination. Whether the patients with high levels were evenly distributed within these groups is potentially important and could conceivably have affected the outcomes. The results of this and other trials in which PAI-1 expression was not interrogated in pleural fluids could similarly have been affected and may underlie the variability in the response to various forms of IPFT in adult patients.
To pursue the possibility that PAI-1-targeted therapy could enhance IPFT, we tested whether PAI-1-neutralizing monoclonal antibodies (mAbs) could be used as adjuncts to fibrinolysins and improve outcomes of IPFT23. Combinations of scuPA with mAbs affecting different aspects of the PAI-1 mechanism were intrapleurally administered to rabbits with TCN-induced pleural injury. We found that dosing with scuPA and anti-PAI-1 mAbs generated higher PF uPA amidolytic and PA activities, faster formation of αM/uPA complexes, and slower uPA inactivation. Targeting PAI-1 was well-tolerated in the animals and did not induce local or systemic bleeding. Interestingly, the combination rendered otherwise ineffective doses of scuPA to become effective in the model and cleared intrapleural collections effectively. PAI-1-neutralizing mAbs increased the durability of intrapleural PA activity which likely contributed to the salutary effect of the scuPA-PAI-1 monoclonal antibody adjuncts. The combination will soon be tested in our newly developed animal models of empyema. If this approach is likewise successful in the rabbit empyema models, the approach may be further developed for clinical application.
Overview of the Processing of Fibrinolysins in Pleural Fluids: Additional Considerations
The changes in the coagulation and fibrinolytic system that occur in pleural injury favor the formation of a fibrinous transitional neomatrix and extend beyond the increments of PAI-1 that characterize virtually all forms of pleural injury20. In evolving pleural injury, tissue factor is locally expressed by the mesothelium and likely in other resident cell types that are found in pleural tissues29–31. Although tissue factor pathway inhibitor is likewise expressed in organizing pleural injury and is expressed by human pleural mesothelial cells32, 33 and factor VIIa34 bound to tissue factor can be inhibited by PAI-1, the net effect of the inflammatory response on local fibrin turnover favors robust fibrin deposition that organizes29. Intrapleural coagulation may be enhanced as pleural mesothelial cells readily support the assembly of procoagulant complexes35. The interactions between the coagulation and fibrinolytic pathways are additionally more complex in the pleural compartment, as plasmin can stimulate expression of tissue factor activity at the surface of pleural mesothelial cells36. Like uPA, thrombin or factor Xa, plasmin can also induce mesenchymal transition of pleural mesothelial to myofibroblast-like cells27. While thrombin-antithrombin complexes have been proposed to be biomarkers of the severity of pleural inflammation37, thrombin can also decrease TFPI in pleural mesothelial cells and thereby predispose to accelerated local coagulation in a PI-3 kinase-NF-κB dependent manner38. Mesomesenchymal transition of pleural mesothelial cells is interestingly also related to activation of the same pathway39. Given that intrapleural coagulation is initiated by acute and chronic pleural injury, it is not surprising that fibrin deposition is a prominent feature of organizing pleural injuries both in the preclinical setting and in human pleural injury, as previously reviewed21, 29. The fibrinous neomatrix organizes and is remodeled in much the same way as occurs in wound healing generally40, 41.
Both uPA and tPA antigen are detectable in pleural fluids28 and both are expressed by pleural mesothelial cells and lung fibroblasts among other cell types represented in the injured pleural space30, 31. Elaboration of these plasminogen activators in the setting of pleural injury allows for clearance of excessive fibrin deposition or otherwise permits remodeling of the fibrin collections that occur in pleural loculation. Urokinase can bind its receptor at the surface of pleural mesothelial cells or lung fibroblasts42 and its expression is in part regulated at the posttranscriptional level42. Apart from their impact on remodeling of intrapleural fibrinous adhesions or collections, uPA is also capable of stimulating collagen expression of pleural mesothelial cells, which could contribute to maturation of the intrapleural neomatrix43.
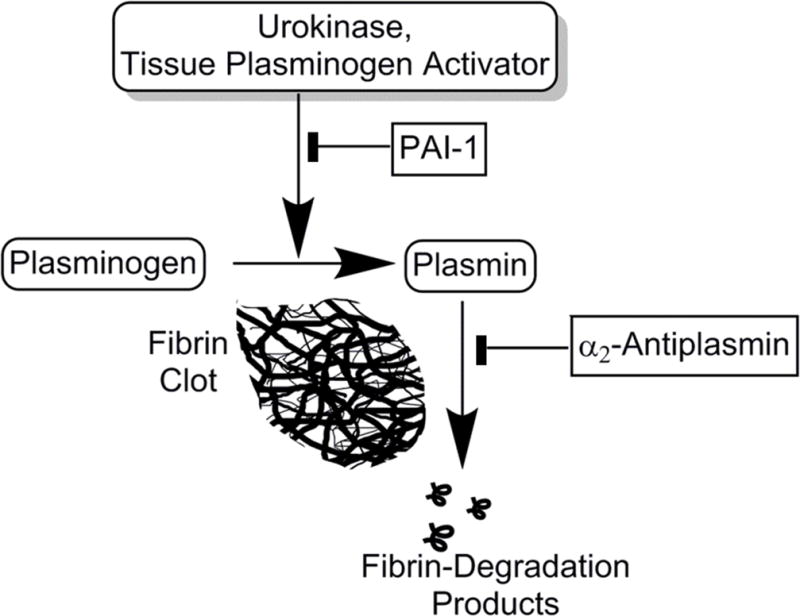
It is important to note that antiplasmins may also inhibit the activity of plasmin that is generated within the pleural compartment by the administration of IPFT20, 28. The designation of plasminogen activators such as tPA, various forms of uPA and streptokinase as fibrinolysins is somewhat of a misnomer, as these agents do not directly lyse fibrin, but rather cleave plasminogen to generate plasmin and thereby augment intrapleural fibrinolytic activity (Figure 3). Among the antiplasmins, α2-antiplasmin is elaborated and active in human pleural fluids28. On the other hand, α-2-macroglobulin, which functions in part as an antiplasmin, appears to play a unique role in the processing of intrapleurally administered scuPA, which is the proenzyme form of two chain urokinase44. While much of the scuPA IPFT is converted to two chain uPA and thereby subject to inactivation by PAI-1, a relatively small proportion of scuPA forms a “molecular cage complex with uPA derived from scuPA. These complexes resist inhibition by PAI-1 and lead to durable but low grade maintenance of plasminogen activator release that occurs over about 24h. This particular mode of processing offers potential advantages for scuPA IPFT, which has thus far been well-tolerated in preclinical testing22, 24, 25, 45–47 and GLP toxicology testing. scuPA is now undergoing safety testing in a Phase I dose escalation clinical trial being conducted in Australia and New Zealand under the sponsorship of Lung Therapeutics, Inc.
Figure 3.
PAI-1: plasminogen activator inhibitor-1. Solid tabs indicate inhibition, arrows indicate cleavage or activation. The fugue indicates the key interactions responsible for plasminogen activation and inhibition in the pleural compartment.
The FPA Methodology and Testing in Human Pleural Fluid Samples
While there is debate about the best approach to IPFT in adults, the postulate that a personalized guide to dosing might be of clinical advantage is less provocative. While the complexity of interactions and variability of expression of components of the fibrinolytic system represents a challenge, we inferred that assessment of the effects of freshly harvested pleural fluid constituents on fibrinolytic activity generated by a fibrinolysin could be helpful to decide on the dosing of IPFT. The FPA involves the ex vivo addition of a fibrinolysin to pleural fluids with subsequent analysis of the fibrinolytic activity, which reflects the aggregate effects of plasminogen activator inhibitors and antiplasmins in individual pleural fluids and the availability of plasminogen to support plasmin generation (Figure 4).
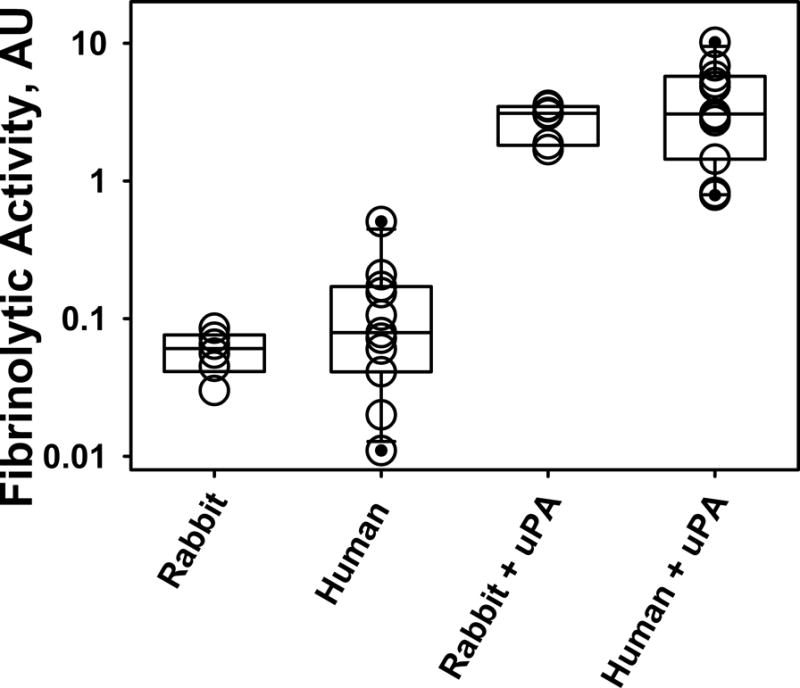
Figure 4. Similarity in the Fibrinolytic Potential of humans and rabbits with empyema.
A semi-logarithmic plot of changes in the fibrinolytic activity in pleural fluids of rabbits and humans with infectious pleural injury is illustrated. Low level baseline fibrinolytic activity (FA0) reflects inhibition of plasminogen activation that are characteristically observed in baseline empyema fluid samples. Supplementation of pleural fluid with exogenous plasminogen activator (4 nM uPA) inhibits PAI-1, activates accumulated plasminogen and results in a considerable (up to 3 orders of magnitude) increase in the level of the fibrinolytic activity (FAuPA). Notably, the Fibrinolytic Potential (FP= FAuPA – FAo; baseline) varies significantly among animals and among humans. While two chain uPA was used in this example, any fibrinolysin can be used in this assay.
To perform the FPA, baseline pleural fluid fibrinolytic activity is first determined, then a plasminogen activator is added to neutralize PAI-1 and activate the endogenous plasminogen. A FITC-fibrin film is formed (with or without incorporated DNA) at the bottom of a 96-well plate as we previously described48. In this assay format, fibrinolysis is monitored by increased fluorescence emission at 510 nm (excitation 490 nm) (Figure 5). While a number of parameters beyond active PAI-1 could contribute to the outcome of IPFT, including different levels of plasminogen, other proenzymes within pleural fluids, enzymes, serpins, or extracellular DNA; we believe that the FPA is a “snapshot” that accounts for the effects of all of these variables. It reflects the net fibrinolytic balance in a personalized way, which is unique for each subject at the time that the pleural fluid was obtained for analysis.
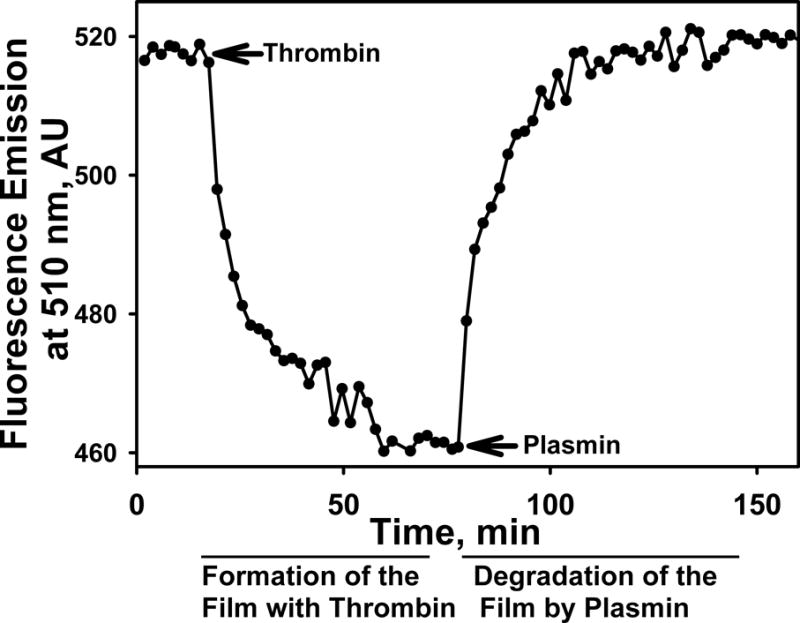
Figure 5. The plot shows changes in the fluorescence emission with time reflecting formation and degradation of the FITC-fibrin in a well of a 96-well plate.

An FITC-fibrin plate assay is described in detail elsewhere23,48. Briefly, thrombin, added at time indicated with arrow, induced polymerization of FITC-fibrinogen and a decrease in the fluorescence emission due to self-quenching of FITC groups in fibrin. Plasmin, added at the time point indicated by the arrow, digests FITC-fibrin and removes the quenching effect, resulting in an increase in the fluorescence emission to the original level. In the FPA, degradation of FITC-fibrin film at the bottom of wells of 96-well plates is used to detect fibrinolytic activity in samples of pleural fluids (Fig. 4) prior to (FA0) and after (FAuPA) supplementation with plasminogen activator.
The development of this assay is in its early stages, while intellectual property protection has been sought. Testing of the FPA in human pleural fluids suggests that the assay can detect a wide range of variable responses to the same dose of a fibrinolysin added to pleural fluids22, suggesting that it is worthy of further investigation. This data clearly demonstrate that a wide range of FPA responses are associated with patients with pleural infection, most of whom had empyema, as reported in the MIST2 trial4. The same variability occurred in pleural fluid from rabbits with P. multocida or S. pneumoniae-induced pleural injury. Baseline fibrinolytic activity in the pleural fluids from the MIST2 patients or rabbits with empyema was generally undetectable or very low, so that the increment of fibrinolytic activity after administration of the fibrinolysin (5nM two chain (tc) urokinase) was readily detectable in nearly all fluids that were tested. This data suggests that administration of tc uPA at this ex vivo dose or an alternative PA at a comparable dose would be sufficient to detect the FPA response in virtually all tested samples. The PAI-1 activity and total PAI-1 levels likewise varied in these pleural fluids and likely accounted for much of the suppression of pleural fluid fibrinolytic activity under baseline conditions.
The detection of pleural fluid fibrinolytic activity after addition of the supplemental tcuPA clearly indicates that the paucity of fibrinolytic activity was not due to a deficiency of pleural fluid plasminogen. Rather, the robust response to the addition of the tcuPA shortly after ex vivo administration indicates that plasminogen was readily available and present in a form amenable to cleavage by a plasminogen activator. These results are consistent with the prior demonstration that much of the plasminogen in human pleural fluids is in the glu-plasminogen form that can be converted to plasmin28. The variability of the responses further suggests that some patients demonstrating a low FPA value were less likely to respond to IPFT while other patients with a high level of response; high fibrinolytic activity after ex vivo dosing with the fibrinolysin, were more likely to respond to IPFT. Whether the PA generated by the 10 mg dose of tPA that was used in the MIST2 trial was sufficient to overcome this variability and whether the variability in the ex vivo responsiveness to supplemental PA administration remains unclear.
It is now unclear if the results of this or other trials were affected by this newly appreciated confounder, the issue remains as to how to select dosing for patients with loculation and failed drainage. At present, clinicians sometimes rely on the perceived viscosity or purulence to use an increased dose of fibrinolysin; for example 20 mg of tPA versus 10 mg. This strategy assumes that the viscosity and/or purulence of all of the pleural fluid in the pleural space of a loculated patient are the same, which may or not be the case in the individual patient. The characteristics of pleural fluid sequestered in a given loculus could differ from a proximate collection. The FPA is subject to the same challenge but offers some conceptual advantages. The technique can readily be performed by virtually any clinical laboratory and the results could be rapidly made available to clinicians at the bedside. The test itself could help stratify which patients would likely benefit from higher or lower dosing of a fibrinolysin or one in combination with DNase as well as to identify patients who would rather require multiple injections of the fibrinolysin. If pleural fluid with a low FPA value is obtained, theoretically a higher dose of fibrinolysin should be employed to help drain the most viscous portions of the loculated fluid. Whether the test will prove useful remains to be determined by clinical trial testing in patients with loculated pleural fluids and failed drainage who are otherwise deemed to be candidates for IPFT.
The Pathway for Development of the FPA and Testing of Its Ability to Guide Dosing of IPFT
The initial testing of the FPA was done in using pleural fluids from rabbits with TCN-induced pleural injury, which was followed by testing in the pleural fluids of rabbits with empyema and human pleural fluids. At this point, there is proof of concept data that the FPA can segregate pleural fluids based on their response to a fibrinolysin added ex vivo to samples collected from patients with pleural infection and otherwise deemed appropriate candidates for institution of IPFT. The next step will be to determine whether the FPA can discriminate between subjects who are likely to benefit from stratified dosing of the IPFT. Ongoing preclinical studies will be done to confirm that the baseline pleural fluid FPA (collected prior to IPFT) can be used to predict outcomes of pleural injury or dosing and dosing intervals. In studies now being planned and in parallel with further preclinical testing, the FPA will be done on baseline and pleural fluids collected at intervals from patients with pleural loculation undergoing IPFT to determine if the test is predictive in the clinical arena and if the test can be used to guide the dosing and dosing intervals in patients undergoing IPFT.
While there is little data of which we are aware to validate the proposition that higher dose IPFT could be beneficial in selected patients, the topic is now being explored in clinical trial testing. In the Phase I dose escalation safety trial that is ongoing in Australia and New Zealand, the concept is being tested. This is a safety trial and the primary objective is to determine the safety of escalating doses of scuPA IPFT in patients with COPD/empyema and loculated pleural fluid that is difficult to drain. The dosing range begins at 50,000 IU/unit dose given once daily for three days unless effective (complete) drainage is observed before the full course of therapy is given. At this dose, even if scuPA were to be fully converted to tcuPA, the dosage of IPFT would be lower than nearly all of the dosing reported in adults previously, where the typical range is 100,000–250,000 IU/unit dose49. Assuming that the 50,000 IU dose is well-tolerated, subsequent dose escalations then range from 100,000–200,000–400,000 to 800,000 IU doses, each given over three, days.
The trial is designed to identify a dose which is well-tolerated and at which pleural drainage seems to be expedited in each of the three patients that comprise the cohort for each dose escalation. If more than one dose is identified, then the lowest well-tolerated dose that appears to demonstrate an efficacy signal would be carried forward to phase II clinical trial testing. We are also setting up collaborations with other groups to obtain baseline and post-treatment pleural fluids and are harvesting baseline and post-treatment pleural fluids from our rabbit S. Pneumoniae empyema model which can be used to determine if the baseline FPA correlates with the response to IPFT. We will need to accrue a number of samples pre and post-treatment to understand whether a correlation exists between the FPA and outcome. Fortunately, there is a clear variability of the FPA values in the rabbits, so that we will likely be able to address this question in animal model using pleural fluid samples from the model even if clinical samples prove difficult to obtain. Using the model, we can also test the postulate that the initial FPA value in baseline pleural fluids can be used to determine whether a higher dose of fibrinolysin yields better outcomes in animals with a relatively low FPA.
Potential Advantages of the FPA, Potential Pitfalls and Their Remediation
While of conceptual appeal, the FPA remains to be validated as a clinical tool to predict outcomes of IPFT or stratify IPFT dosing, dosing intervals or the need for repeat dosing. Nonetheless, the test appears to be easy to perform, to yield reproducible results and uses technology that is amenable to adaptation in a clinical laboratory setting. In addition, the results can be rapidly generated. The preclinical pleural fluid samples that have been tested to date have been carefully collected, citrated and maintained at −80°C prior to testing. The clinical samples have been similarly processed and stored. Pleural fluid collection at the bedside may be delayed contingent on the engagement of the clinical team, availability of rapid transportation to the clinical laboratory and potential delays in processing at that point. All of these factors could potentially affect the reliability of the FPA measurements, but similar considerations apply to other pleural fluid tests, such as the pH.
Even if strong correlations are identified between outcomes in the S.pneumoniae model and the FPA from baseline pleural fluid samples, additional variability may emerge from clinical trial samples from patients with different severities of organization associated with empyema, different underlying infectious pathogens or mixtures of infecting organisms and different stages of the disease process. Many of the patients in the MIST2 trial were symptomatic for weeks prior to presentation and entry into the study4, suggesting that baseline pleural fluids may reflect very different stages of disease that might affect the FPA. Lastly, large numbers of patients will need to be recruited to appropriately assess the ability of the FPA to predict outcomes or guide the use of different dosages of IPFT, which would likely entail the continued engagement of a number of clinical sites from which patients can be recruited. These challenges are, in our view, formidable but not insurmountable.
Summary
The Fibrinolytic Potential Assay (FPA) is a candidate clinical assay that derives from a wealth of preclinical and clinical data about the role of the fibrinolytic system in the pathogenesis of pleural injury and the processing of different forms of IPFT. The FPA reflects the net balance between endogenous fibrinolytic enzymes, plasminogen and inhibitors for a particular subject and we believe that it can relate IPFT to drug dosing and ultimate outcome in a personalized manner. The FPA measures the difference between fibrinolytic activity in a baseline patient’s pleural fluid sample prior to and after activation of the endogenous plasminogen by exogenous plasminogen activator. The test is now early-on in development but is in our view promising enough to continue investigation into its potential to predict clinical outcomes or dosing of IPFT. Currently, IPFT dosing is generally empiric in adults and associated with variable results. We believe that the FPA will ultimately be able to introduce a more personalized approach to the dosing of IPFT, the selection of optimized therapy and to more reliable effectiveness of IPFT, particularly in adult patients with pleural loculation and failed drainage.
Acknowledgments
Supported by NIH 1 P50 HL107186 (CADET I, SI, AK) RO-1HL118401-01A1 (SI), R21ES025815-01A1 and R01HL133067-01 (SS), R01HL130133-01A1 (TT) and 1R01HL130402-01A1 (AK, GF, SI). Dr. S. Idell serves as an unpaid member of the Board of Directors, Founder and Chief Scientific Officer of Lung Therapeutics, Inc. (LTI) and has an equity position in the company, as does the University of Texas Horizon Fund and The University of Texas Health Science Center at Tyler. Dr. Shetty has an equity position in LTI. Drs. Shetty, Florova and Komissarov work on scientific projects related to the products being commercialized by LTI or involving molecules related to the company’s commercialization efforts.
Footnotes
Disclosures: All authors have conflict of interest plans acknowledging and managing these declared conflicts of interest through The University of Texas Health Science Center at Tyler. Dr. R. Idell has a declared Conflict of Interest given his relationship to Dr. S. Idell. The FPA; a test of pleural fluid that may predict outcomes of fibrinolytic therapy, guide dosing and dosing intervals. (GF, SI, AK) appl # 15/086,623, Notice of publication 10.2016. Dr. Rahman received an unrestricted educational grant from Roche UK in support of the MIST2 study. Kathy Koenig, Torry Tucker and Ali Azghani have no conflicts. All human and animal studies described in this manuscript were approved by the UTHSCT Human Subjects Institutional Review Board and Institutional Animal Care and Utilization Committees, respectively.
References
- 1.Hudson KL, Collins FS. The 21st Century Cures Act - A View from the NIH. N Engl J Med. 2017;376:111–3. doi: 10.1056/NEJMp1615745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grijalva CG, Zhu Y, Nuorti JP, Griffin MR. Emergence of parapneumonic empyema in the USA. Thorax. 2011;66:663–8. doi: 10.1136/thx.2010.156406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corcoran JP, Hallifax R, Rahman NM. New therapeutic approaches to pleural infection. Curr Opin Infect Dis. 2013;26:196–202. doi: 10.1097/QCO.0b013e32835d0b71. [DOI] [PubMed] [Google Scholar]
- 4.Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, Peckham D, Davies CW, Ali N, Kinnear W, Bentley A, Kahan BC, Wrightson JM, Davies HE, Hooper CE, Lee YC, Hedley EL, Crosthwaite N, Choo L, Helm EJ, Gleeson FV, Nunn AJ, Davies RJ. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011;365:518–26. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]
- 5.Sonnappa S, Cohen G, Owens CM, van Doorn C, Cairns J, Stanojevic S, Elliott MJ, Jaffe A. Comparison of Urokinase and Video-assisted Thoracoscopic Surgery for Treatment of Childhood Empyema. Am J Respir Crit Care Med. 2006;174:221–7. doi: 10.1164/rccm.200601-027OC. [DOI] [PubMed] [Google Scholar]
- 6.Neill AM, Martin IR, Weir R, Anderson R, Chereshsky A, Epton MJ, Jackson R, Schousboe M, Frampton C, Hutton S, Chambers ST, Town GI. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax. 1996;51:1010–6. doi: 10.1136/thx.51.10.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baigent C, Collins R, Appleby P, Parish S, Sleight P, Peto R. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ. 1998;316:1337–43. doi: 10.1136/bmj.316.7141.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colice GL, Curtis A, Deslauriers J, Heffner J, Light R, Littenberg B, Sahn S, Weinstein RA, Yusen RD. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest. 2000;118:1158–71. doi: 10.1378/chest.118.4.1158. [DOI] [PubMed] [Google Scholar]
- 9.Colice GL, Idell S. Response. Chest. 2014;146:e104–e5. doi: 10.1378/chest.14-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colice L, Idell S. Rebuttal from drs colice and idell. Chest. 2014;145:21–3. doi: 10.1378/chest.13-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colice L, Idell S. Counterpoint: should fibrinolytics be routinely administered intrapleurally for management of a complicated parapneumonic effusion? No. Chest. 2014;145:17–20. doi: 10.1378/chest.13-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YC, Idell S, Stathopoulos GT. Translational Research in Pleural Infection and Beyond. Chest. 2016 doi: 10.1016/j.chest.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Tillett WS, SHERRY S. The Effect In Patients Of Streptococcal Fibrinolysin (Streptokinase) And Streptococcal Desoxyribonuclease On Fibrinous, Purulent, And Sanguinous Pleural Exudations. J Clin Invest. 1949;28:173–90. doi: 10.1172/JCI102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tillett WS, SHERRY S, READ CT. The use of streptokinase-streptodornase in the treatment of postneumonic empyema. J Thorac Surg. 1951;21:275–97. [PubMed] [Google Scholar]
- 15.Zhang JC, Sakthivel R, Kniss D, Graham CH, Strickland DK, McCrae KR. The low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor regulates cell surface plasminogen activator activity on human trophoblast cells. J Biol Chem. 1998;273:32273–80. doi: 10.1074/jbc.273.48.32273. [DOI] [PubMed] [Google Scholar]
- 16.Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, Gabe R, Rees GL, Peto TE, Woodhead MA, Lane DJ, Darbyshire JH, Davies RJ. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352:865–74. doi: 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 17.Huggins JT, Doelken P, Sahn SA. Intrapleural therapy. Respirology. 2011;16:891–9. doi: 10.1111/j.1440-1843.2011.02011.x. [DOI] [PubMed] [Google Scholar]
- 18.Janda S, Swiston J. Intrapleural fibrinolytic therapy for treatment of adult parapneumonic effusions and empyemas: a systematic review and meta-analysis. Chest. 2012;142:401–11. doi: 10.1378/chest.11-3071. [DOI] [PubMed] [Google Scholar]
- 19.Davies HE, Davies RJ, Davies CW. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii41–ii53. doi: 10.1136/thx.2010.137000. [DOI] [PubMed] [Google Scholar]
- 20.Tucker T, Idell S. Plasminogen-plasmin system in the pathogenesis and treatment of lung and pleural injury. Semin Thromb Hemost. 2013;39:373–81. doi: 10.1055/s-0033-1334486. [DOI] [PubMed] [Google Scholar]
- 21.Idell S. The pathogenesis of pleural space loculation and fibrosis. Curr Opin Pulm Med. 2008;14:310–5. doi: 10.1097/MCP.0b013e3282fd0d9b. [DOI] [PubMed] [Google Scholar]
- 22.Komissarov AA, Florova G, Azghani AO, Buchanan A, Boren J, Allen T, Rahman NM, Koenig K, Chamiso M, Karandashova S, Henry J, Idell S. Dose dependency of outcomes of intrapleural fibrinolytic therapy in new rabbit empyema models. Am J Physiol Lung Cell Mol Physiol. 2016;311:L389–99. doi: 10.1152/ajplung.00171.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Florova G, Azghani A, Karandashova S, Schaefer C, Koenig K, Stewart-Evans K, Declerck PJ, Idell S, Komissarov AA. Targeting of Plasminogen Activator Inhibitor 1 Improves Fibrinolytic Therapy for Tetracycline Induced Pleural Injury in Rabbits. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2014-0168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komissarov AA, Florova G, Azghani A, Karandashova S, Kurdowska AK, Idell S. Active alpha-macroglobulin is a reservoir for urokinase after fibrinolytic therapy in rabbits with tetracycline-induced pleural injury and in human pleural fluids. Am J Physiol Lung Cell Mol Physiol. 2013;305:L682–L92. doi: 10.1152/ajplung.00102.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karandashova S, Florova G, Azghani AO, Komissarov AA, Koenig K, Tucker TA, Allen TC, Stewart K, Tvinnereim A, Idell S. Intrapleural adenoviral delivery of human plasminogen activator inhibitor-1 exacerbates tetracycline-induced pleural injury in rabbits. Am J Respir Cell Mol Biol. 2013;48:44–52. doi: 10.1165/rcmb.2012-0183OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marudamuthu AS, Shetty SK, Bhandary YP, Karandashova S, Thompson M, Sathish V, Florova G, Hogan TB, Pabelick CM, Prakash YS, Tsukasaki Y, Fu J, Ikebe M, Idell S, Shetty S. Plasminogen activator inhibitor-1 suppresses profibrotic responses in fibroblasts from fibrotic lungs. J Biol Chem. 2015;290:9428–41. doi: 10.1074/jbc.M114.601815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucker TA, Jeffers A, Alvarez A, Owens S, Koenig K, Quaid B, Komissarov AA, Florova G, Kothari H, Pendurthi U, Rao LV, Idell S. Plasminogen Activator Inhibitor-1 Deficiency Augments Visceral Mesothelial Organization, Intrapleural Coagulation and Lung Restriction in Mice with Carbon Black/Bleomycin-Induced Pleural Injury. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2013-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idell S, Girard W, Koenig KB, McLarty J, Fair DS. Abnormalities of pathways of fibrin turnover in the human pleural space. American Review of Respiratory Disease. 1991;144:187–94. doi: 10.1164/ajrccm/144.1.187. [DOI] [PubMed] [Google Scholar]
- 29.Mutsaers SE, Prele CM, Brody AR, Idell S. Pathogenesis of pleural fibrosis. Respirology. 2004;9:428–40. doi: 10.1111/j.1440-1843.2004.00633.x. [DOI] [PubMed] [Google Scholar]
- 30.Idell S, Zwieb C, Kumar A, Koenig KB, Johnson AR. Pathways of fibrin turnover of human pleural mesothelial cells in vitro. American Journal of Respiratory Cell & Molecular Biology. 1992;7:414–26. doi: 10.1165/ajrcmb/7.4.414. [DOI] [PubMed] [Google Scholar]
- 31.Idell S, Zwieb C, Boggaram J, Holiday D, Johnson AR, Raghu G. Mechanisms of fibrin formation and lysis by human lung fibroblasts: influence of TGF-beta and TNF-alpha. American Journal of Physiology. 1992;263:L487–L94. doi: 10.1152/ajplung.1992.263.4.L487. [DOI] [PubMed] [Google Scholar]
- 32.Idell S, Pendurthi U, Pueblitz S, Koenig K, Williams T, Rao LV. Tissue factor pathway inhibitor in tetracycline-induced pleuritis in rabbits. Thrombosis & Haemostasis. 1998;79:649–55. [PubMed] [Google Scholar]
- 33.Bajaj MS, Pendurthi U, Koenig K, Pueblitz S, Idell S. Tissue factor pathway inhibitor expression by human pleural mesothelial and mesothelioma cells. Eur Respir J. 2000;15:1069–78. doi: 10.1034/j.1399-3003.2000.01515.x. [DOI] [PubMed] [Google Scholar]
- 34.Sen P, Komissarov AA, Florova G, Idell S, Pendurthi UR, Vijaya Mohan RL. Plasminogen activator inhibitor-1 inhibits factor VIIa bound to tissue factor. J Thromb Haemost. 2011;9:531–9. doi: 10.1111/j.1538-7836.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A, Koenig KB, Johnson AR, Idell S. Expression and assembly of procoagulant complexes by human pleural mesothelial cells. Thrombosis & Haemostasis. 1994;71:587–92. [PubMed] [Google Scholar]
- 36.Kothari H, Kaur G, Sahoo S, Idell S, Rao LV, Pendurthi U. Plasmin enhances cell surface tissue factor activity in mesothelial and endothelial cells. J Thromb Haemost. 2008 doi: 10.1111/j.1538-7836.2008.03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MH, Nahm CH, Choi JW. Thrombin-antithrombin III complex, proinflammatory cytokines, and fibrinolytic indices for assessing the severity of inflammation in pleural effusions. Ann Clin Lab Sci. 2010;40:342–7. [PubMed] [Google Scholar]
- 38.Jeffers A, Owens S, Koenig K, Quaid B, Pendurthi UR, Rao VM, Idell S, Tucker TA. Thrombin down-regulates tissue factor pathway inhibitor expression in a PI3K/nuclear factor-kappaB-dependent manner in human pleural mesothelial cells. Am J Respir Cell Mol Biol. 2015;52:674–82. doi: 10.1165/rcmb.2014-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens S, Jeffers A, Boren J, Tsukasaki Y, Koenig K, Ikebe M, Idell S, Tucker TA. Mesomesenchymal transition of pleural mesothelial cells is PI3K and NF-kappaB dependent. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1265–L73. doi: 10.1152/ajplung.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. [Review] [73 refs] New England Journal of Medicine. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 41.LF B, AM D, HF D. Leaky vessels, fibrin deposition, and fibrosis: A sequence of events common to solid tumrs and many other types of disease. Am Rev Respir Dis. 1989;140:1104–7. doi: 10.1164/ajrccm/140.4.1104. [DOI] [PubMed] [Google Scholar]
- 42.Shetty S, Idell S. A urokinase receptor mRNA binding protein from rabbit lung fibroblasts and mesothelial cells. Am J Physiol. 1998;274:L871–L82. doi: 10.1152/ajplung.1998.274.6.L871. [DOI] [PubMed] [Google Scholar]
- 43.Tucker TA, Williams L, Koenig K, Kothari H, Komissarov AA, Florova G, Mazar AP, Allen TC, Bdeir K, Mohan Rao LV, Idell S. Lipoprotein receptor-related protein 1 regulates collagen 1 expression, proteolysis, and migration in human pleural mesothelial cells. Am J Respir Cell Mol Biol. 2012;46:196–206. doi: 10.1165/rcmb.2011-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komissarov AA, Mazar AP, Koenig K, Kurdowska AK, Idell S. Regulation of intrapleural fibrinolysis by urokinase-alpha-macroglobulin complexes in tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol. 2009;297:L568–L77. doi: 10.1152/ajplung.00066.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Idell S, Allen T, Chen S, Koenig K, Mazar A, Azghani A. Intrapleural activation, processing, efficacy, and duration of protection of single-chain urokinase in evolving tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol. 2007;292:L25–L32. doi: 10.1152/ajplung.00118.2006. [DOI] [PubMed] [Google Scholar]
- 46.Idell S, Azghani A, Chen S, Koenig K, Mazar A, Kodandapani L, Bdeir K, Cines D, Kulikovskaya I, Allen T. Intrapleural low-molecular-weight urokinase or tissue plasminogen activator versus single-chain urokinase in tetracycline-induced pleural loculation in rabbits. Exp Lung Res. 2007;33:419–40. doi: 10.1080/01902140701703333. [DOI] [PubMed] [Google Scholar]
- 47.Idell S, Jun NM, Liao H, Gazar AE, Drake W, Lane KB, Koenig K, Komissarov A, Tucker T, Light RW. Single-chain urokinase in empyema induced by Pasturella multocida. Exp Lung Res. 2009;35:665–81. doi: 10.3109/01902140902833277. [DOI] [PubMed] [Google Scholar]
- 48.Komissarov AA, Florova G, Idell S. Effects of extracellular DNA on plasminogen activation and fibrinolysis. J Biol Chem. 2011;286:41949–62. doi: 10.1074/jbc.M111.301218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Idell S. Update on the Use of Fibrinolysins in Pleural Disease. Clinical Pulmonary Medicine. 2005;12:184–90. [Google Scholar]


