Abstract
Streptococcus pneumoniae (pneumococcus) is responsible for serious pediatric respiratory infections, and kills close to one million children under the age of five each year. Unfortunately, the Prevnar-13 vaccine (PCV-13) that is used to protect children from the serious consequences of pneumococcus infections is not always successful. Given that vitamin A deficiency (VAD) is known to affect children in both developed and developing countries, we asked if VAD could be responsible, at least in part, for PCV-13 vaccine failures. In a mouse model for VAD, we found that PCV-13 failed to elicit binding and neutralizing antibody activities. Unlike vaccinated, vitamin-replete animals, vaccinated VAD animals were not protected from lethal pneumococcus infections. Results suggest that children with VAD may be susceptible to serious pneumococcal infections even after having received the PCV-13 vaccine.
Keywords: pneumococcus, vitamin A deficiency, antibody neutralization, protection, challenge
Introduction
Streptococcus pneumoniae (pneumococcus) is frequently found in the human respiratory tract and can cause a wide range of diseases, from mild otitis media to fatal pneumonia or meningitis. It is estimated that worldwide pneumococcus kills close to one million children under the age of five each year [1, 2].
Prevnar-13 (PCV-13) is typically administered to young children as part of the routine immunization schedule to induce antibody responses toward pneumococcal capsules and protect against invasive disease [3–5]. PCV-13 is also used in adults to a lesser extent. The vaccine is multi-valent, representing 13 different serotypes of the pneumococcus pathogen, including serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. Each component consists of the serotype-specific polysaccharide capsular antigen individually linked to a diphtheria CRM197 protein, a non-toxic variant of diphtheria toxin. These conjugates induce robust, T-dependent antibody responses in young children. Routine immunizations with the pneumococcal conjugate vaccine have resulted in highly significant reductions in pneumococcus bacteremia among young children [4, 5].
Vitamin A deficiencies (VAD) and insufficiencies are present in both developed and developing countries [6–9] and have been associated with poor immune responses toward respiratory tract pathogens [10, 11], as well as an increased incidence of and morbidity from respiratory disease in school age children. Knowing that prevention of infection is critical in these highly susceptible populations, we employed a murine model of VAD to determine if PCV-13 was efficacious in the context of VAD.
Materials and Methods
Animals and Vitamin A Deficiency
C57BL/6 (H2-b) mice were purchased from Jackson Laboratories (Bar harbor, ME). Day 4–5 estrus pregnant females were placed on either a control or VAD diet (Test Diets) upon their arrival in the animal facility at St. Jude Children’s Research Hospital (St. Jude). VAD (cat. no. 5WA2) and control (cat. no. 5W9M) diets differed only in vitamin A content, containing either 0 or 15 IU/g vitamin A palmitate, respectively. Mice remained on their assigned diets throughout their pregnancies and while nursing. Weaned pups were continued on the appropriate diets throughout experimentation. Both male and female offspring were used in experiments. All experiments were repeated to ensure reproducibility. Mice were sacrificed by anesthetization with tribromoethanol (avertin) followed by exsanguination or by carbon dioxide asphyxiation followed by cervical dislocation, as approved by the Institutional Animal Care and Use Committee (IACUC). St. Jude follows the standards established by the Animal Welfare Act and by the document entitled “Principles for the Use of Animals and Guide for the Care and Use of Laboratory Animals” and maintains continuous AAALAC approvals.
Vaccinations, Infections and Blood Collections
Mice were vaccinated and boosted with Prevnar-13 (Wyeth Pharm. Inc.) using a three week interval between prime and boost. The vaccine was diluted 1:40 in PBS and 100 μL were administered intraperitoneally to each mouse. At 3 weeks post-prime and 9–10 days post-boost, animals were anesthetized with isoflurane and blood was collected via the retro-orbital route for enzyme-linked immunosorbent assays (ELISAs). Two weeks post-boost, vaccinated and unvaccinated mice were challenged intranasally with 106 colony forming units (CFU) of the pneumococcus Strain TIGR4 in 35 μL PBS. One day post-infection, blood (5 μL) was collected from tails of infected mice into 45 μL of PBS with Heparin (16.5 IU/mL) for measurement of bacterial titers by colony forming unit enumeration. Animals were euthanized when there were signs of meningitis or when animals became moribund.
ELISAs
For IgM and IgG ELISAs, 96-well plates (cat. no. 9018; Corning) were coated with 5 μg/mL T4 (cat. no. 173-X; ATCC) or 19F (cat. no. 99-X; ATCC) polysaccharides in PBS (50 μL/well) overnight (O/N) at 4°C. Plates were then washed 3X with phosphate buffered saline (PBS) and blocked for ~5 hours at room temperature (RT) with 1% BSA in PBS (200 μL/well). Sera were serially diluted 1:200, 1:1000 and 1:5000 in dilution buffer (1% BSA + 0.05% Tween in PBS). Blocking buffer was removed and sera were added to plates (50 μL/well) and incubated O/N at 4°C. Plates were then washed 3X with PBS + 0.05% Tween. Secondary antibodies (anti-IgM-AP and anti-IgG-AP, cat. no. 1020-04 and cat. no. 1030-04, respectively; Southern Biotechnologies) were diluted 1:1000 in dilution buffer, added to plates (100 μL/well) and incubated at RT for 1 hour. Plates were washed 3X with PBS + 0.05% Tween. Substrate was added to plates (1 mg/mL p-nitrophenyl phosphate (NPP [cat. no. N2640; Sigma]) in 5% diethanolamine buffer (cat. no. D8885; Sigma); 100 μL/well) and plates were developed for 10–20 minutes before being read at 405 nM on a Molecular Devices Precision Microplate Reader. Antibody titers were determined as the reciprocal of the serum dilution that would produce an OD reading of 0.1.
Bacterial Titers
For the measurement of bacterial titers, blood was serially diluted 1:10 and 15 uL of each dilution was plated on a blood agar plate. Plates were incubated at 37°C overnight. Colonies were counted and excel software was used to calculate titers based on results from dilutions resulting in 10–100 colonies. All statistical analyses, as described in figure legends, were performed using GraphPad Prism software.
Results
Prevnar-13 fails to elicit antibody responses in VAD animals
VAD and control mice were vaccinated with the pneumococcal vaccine Prevnar-13 in a prime-boost regimen using a three week interval. Mice were bled approximately three weeks following the first vaccination and 10 days after the boost (~4.5 weeks post-prime). Sera were then evaluated for responses to two polysaccharides represented in the vaccine, serotype 4 (T4) and serotype 19F.
Following the first vaccination, antibody responses were difficult to detect in VAD and control animals (data not shown). Ten days following the booster vaccine, robust IgM and IgG antibody responses to both T4 and 19F polysaccharides were detectable in vitamin A-replete mice (Figure 1A–D). However, responses in vaccinated VAD animals were significantly reduced. Additionally, when IgG1, IgG2b and IgG3 were evaluated independently, all were weak in VAD animals compared to controls (Figure 2).
Figure 1. Weak antibody responses toward PCV-13 in the context of VAD.
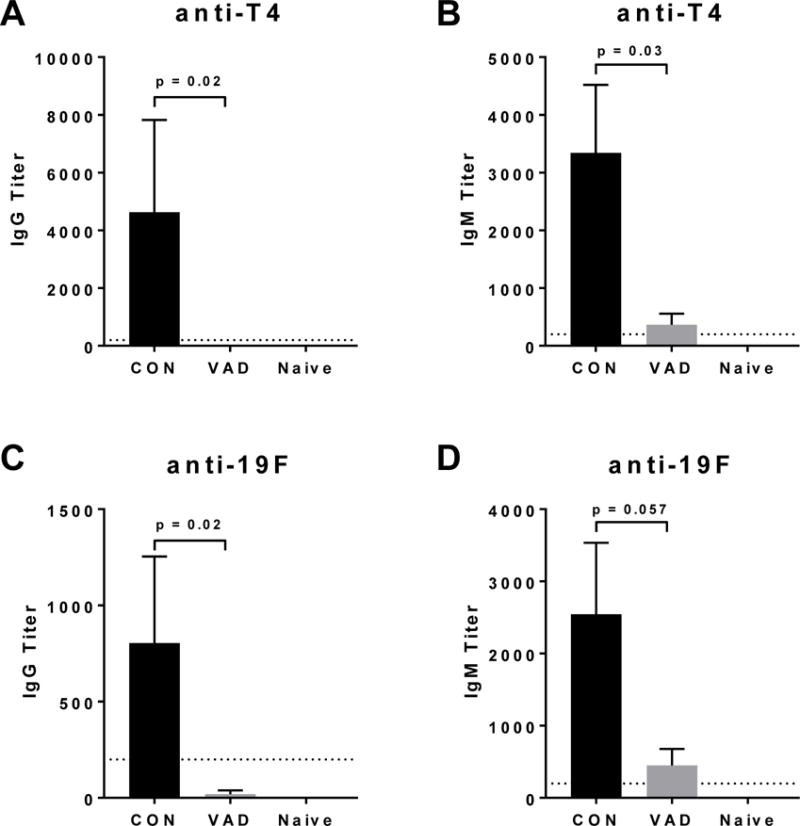
Antibodies were evaluated by ELISA 10 days after a booster vaccine with PCV-13 in VAD and control mice (N = 7 per group, N = 2 for naive). IgG and IgM responses are shown against serotypes 4 (T4) (A-B) and 19F (C-D) of pneumococcus. Statistical significance in antibody titers between VAD and control mice was determined using a Mann-Whitney U test.
Figure 2. Poor IgG subtype responses toward PCV-13 in the context of VAD.
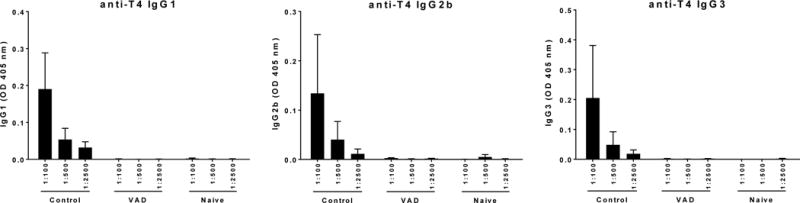
Serotype 4 (T4)-specific antibodies were evaluated by ELISA 10 days after a booster vaccine with PCV-13. OD readings for (A) IgG1, (B) IgG2b and (C) IgG3 are shown for VAD and control animals (N = 10 per group, N = 2 for naïve).
Vaccinated VAD animals are poorly protected from pneumococcus infection
Two weeks after the second administration of vaccine, both VAD and control mice were challenged with 106 colony forming units (CFU) of pneumococcus type 4, an invasive strain represented in the PCV-13 vaccine. As demonstrated in Figure 3, the protection conferred by the vaccine was significantly reduced in VAD animals compared to controls. Vaccination failed to prevent the development of bacteremia in the VAD animals whereas robust protection was observed in control animals. No bacteria were detected in the blood of control animals vaccinated with PCV-13 at 24 hours post-infection (Figure 3A). Similarly, the percent survival of vaccinated and unvaccinated VAD animals was not significantly different, whereas vaccinated control animals were well protected from the otherwise lethal challenge, unlike their unvaccinated counterparts (Figure 3B).
Figure 3. Failed PCV-13 vaccine efficacy in the context of VAD.
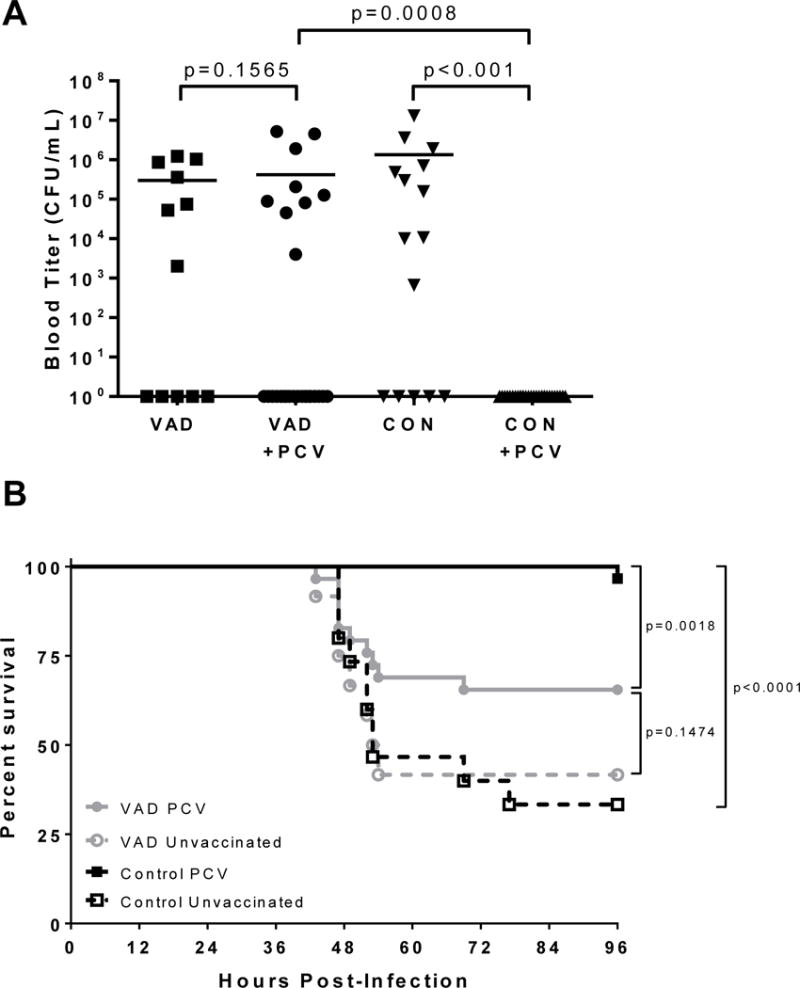
(A) Bacterial titers and (B) survival are shown for PCV-13 vaccinated (N = 29–30 per group) and unvaccinated (N = 12–15 per group) VAD and control animals following challenge with pathogenic pneumococcus. All mice were infected with 106 CFU of serotype T4. Results are shown for two experiments combined. CON=control animals. Statistical significance was determined using a Mann-Whitney U test for comparisons of bacterial titers. Significant differences were observed between vaccinated and unvaccinated mice and between VAD and control mice. Significant differences in survival were evaluated using a Mantel-Cox test.
Discussion
Combined, these data demonstrate a clear failure of PCV-13 to induce a protective response in the context of VAD. Our IgG results are reminiscent of a previous study, in which VAD rats were shown to generate poor IgG responses following immunization with pneumococcal capsules [12]. In this study, we additionally found that IgM and multiple subtypes of IgG (including IgG1, IgG2b and IgG3) responses were significantly reduced in VAD mice compared to controls. We further found that vaccinated VAD mice were incompletely protected from invasive bacterial challenge, whereas the PCV-13 vaccine protected control mice. The poor response to PCV-13 vaccination observed in VAD mice complements previous studies of respiratory virus vaccines in the context of VAD whereby mucosal antibody responses were poor toward a parainfluenza virus (Sendai virus), a human influenza virus vaccine (FluMist), and a cold-adapted, PR8-derived influenza virus vaccine [10, 13]. Molrine et. al. further demonstrated that when VAD SCID mice were reconstituted with human peripheral blood lymphocytes from tetanus toxoid-positive donors, the mice exhibited impaired tetanus toxoid-specific antibody responses compared to vitamin-replete control animals. The antibody defect was corrected by supplementation of test mice with vitamin A prior to reconstitution [14].
Vitamin A supplementation programs are supported by the World Health Organization in the developing world. These programs have been associated with large reductions in childhood morbidity and mortality, encouraging the continuation of vitamin supplementation programs in low and middle income countries. A systematic meta-analysis of 17 trials including almost 200,000 participants between the ages of 6 months and 5 years revealed a 24% reduction in all-cause mortality among vitamin-supplemented individuals. Several studies also revealed associations between vitamin A supplementation and a reduced incidence of measles, diarrhea, night blindness, and xerophthalmia [9].
Previously, we have found that in Memphis, Tennessee, low serum vitamin A levels (scored by measuring the surrogate retinol binding protein) are quite common. Unfortunately, nutritional deficits among individuals in inner cities of the United States are not fully recognized. The situation may be worsening, as low income families gain better access to ‘fast’ foods rather than unprocessed and fortified foods. Our study in Memphis showed that low vitamin A levels associated with low serum IgA as well as poor influenza virus-specific neutralizing antibodies [6]. A placebo controlled, randomized, blinded, clinical study is now in progress to determine if vitamin supplements can be partnered with the administration of influenza virus vaccines to increase vaccine-induced immune responses in vitamin-deficient or vitamin-insufficient children in Memphis (Clinicaltrials.gov, CTG ID# NCT02649192, FLUVIT).
There are several cases of PCV-7 and PCV-13 clinical vaccine failures described in the literature [15, 16]. Data described in this report support the hypothesis that vitamin deficiencies may contribute to sub-optimal vaccine responses in the clinic. Possibly, vitamin supplementation will prove beneficial for improving vaccine efficacy. Programs that measure vitamin levels in children receiving PCV-13 are now encouraged. If poor responses correlate with low vitamin levels, data may drive future efforts to partner vitamin supplementation with PCV-13 vaccination, to prevent the serious consequences of pneumococcus infections in children.
HIGHLIGHTS.
In a mouse model for VAD, PCV-13 failed to elicit binding antibody activities.
PCV-13 failed to elicit neutralizing antibody activities in mice with VAD.
PCV-13 failed to protect mice with VAD from lethal pneumococcus infections.
Possibly, PCV-13 is also failing in children with VAD.
Acknowledgments
We thank Beth Mann who conducted ELISAs upon initiation of this project.
Funding: This work was supported by the National Institutes of health [NIAID R01 AI088729 and NIH NCI P30 CA21765], and ALSAC.
Abbreviations
- PCV-13
Prevnar 13
- VAD
Vitamin A deficiency
- CFU
Colony forming units
- SCID
Severe combined immunodeficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iroh Tam PY, Thielen BK, Obaro SK, Brearley AM, Kaizer AM, Chu H, et al. Childhood pneumococcal disease in Africa - A systematic review and meta-analysis of incidence, serotype distribution, and antimicrobial susceptibility. Vaccine. 2017;35:1817–27. doi: 10.1016/j.vaccine.2017.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 4.Boelsen LK, Dunne EM, Lamb KE, Bright K, Cheung YB, Tikoduadua L, et al. Long-term impact of pneumococcal polysaccharide vaccination on nasopharyngeal carriage in children previously vaccinated with various pneumococcal conjugate vaccine regimes. Vaccine. 2015;33:5708–14. doi: 10.1016/j.vaccine.2015.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenhow TL, Hung YY, Herz A. Bacteremia in Children 3 to 36 Months Old After Introduction of Conjugated Pneumococcal Vaccines. Pediatrics. 2017 doi: 10.1542/peds.2016-2098. [DOI] [PubMed] [Google Scholar]
- 6.Surman SL, Penkert RR, Jones BG, Sealy RE, Hurwitz JL. Vitamin supplementation at the time of immunization with cold-adapted influenza virus vaccine corrects poor antibody responses in mice deficient for vitamins A and D. Clin Vaccine Immunol. 2016;23:219–27. doi: 10.1128/CVI.00739-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer A. Vitamin A, infectious disease, and childhood mortality: a 2 solution? J Infect Dis. 1993;167:1003–7. doi: 10.1093/infdis/167.5.1003. [DOI] [PubMed] [Google Scholar]
- 8.Sommer A, Katz J, Tarwotjo I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am J Clin Nutr. 1984;40:1090–5. doi: 10.1093/ajcn/40.5.1090. [DOI] [PubMed] [Google Scholar]
- 9.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011;343:d5094. doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surman SL, Jones BG, Sealy RE, Rudraraju R, Hurwitz JL. Oral retinyl palmitate or retinoic acid corrects mucosal IgA responses toward an intranasal influenza virus vaccine in vitamin A deficient mice. Vaccine. 2014;32:2521–4. doi: 10.1016/j.vaccine.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton KA, Mora-Plazas M, Marin C, Villamor E. Vitamin A deficiency is associated with gastrointestinal and respiratory morbidity in school-age children. The Journal of nutrition. 2014;144:496–503. doi: 10.3945/jn.113.185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasatiempo AM, Kinoshita M, Taylor CE, Ross AC. Antibody production in vitamin A-depleted rats is impaired after immunization with bacterial polysaccharide or protein antigens. FASEB J. 1990;4:2518–27. doi: 10.1096/fasebj.4.8.2110538. [DOI] [PubMed] [Google Scholar]
- 13.Surman SL, Jones BG, Rudraraju R, Sealy RE, Hurwitz JL. Intranasal administration of retinyl palmitate with a respiratory virus vaccine corrects impaired mucosal IgA response in the vitamin A-deficient host. Clin Vaccine Immunol. 2014;21:598–601. doi: 10.1128/CVI.00757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molrine DC, Polk DB, Ciamarra A, Phillips N, Ambrosino DM. Impaired human responses to tetanus toxoid in vitamin A-deficient SCID mice reconstituted with human peripheral blood lymphocytes. Infect Immun. 1995;63:2867–72. doi: 10.1128/iai.63.8.2867-2872.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harboe ZB, Valentiner-Branth P, Ingels H, Rasmussen JN, Andersen PH, Bjerre CC, et al. Pediatric invasive pneumococcal disease caused by vaccine serotypes following the introduction of conjugate vaccination in Denmark. PLoS One. 2013;8:e51460. doi: 10.1371/journal.pone.0051460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moraga-Llop F, Garcia-Garcia JJ, Diaz-Conradi A, Ciruela P, Martinez-Osorio J, Gonzalez-Peris S, et al. Vaccine Failures in Patients Properly Vaccinated with 13-Valent Pneumococcal Conjugate Vaccine in Catalonia, a Region with Low Vaccination Coverage. Pediatr Infect Dis J. 2016;35:460–3. doi: 10.1097/INF.0000000000001041. [DOI] [PubMed] [Google Scholar]


