Summary
Highly active antiretroviral therapy including HIV protease inhibitors has led to a marked reduction of clinically relevant mucosal candidiasis. We have previously shown that HIV protease inhibitors directly inhibit adhesion of Candida albicans to epithelial cells at concentrations that are reached in vivo during antiretroviral therapy. The aim of this study was to establish whether HIV protease inhibitors also inhibit adhesion of Candida to endothelial cells, which play a major role in systemic fungal disease. Three C. albicans strains were incubated with human umbilical vein endothelial cells or an endothelial cell line in the presence of either Ritonavir, Saquinavir or Indinavir. Subsequently, adherence was determined by counting colony-forming units. The results were comparable and revealed that Ritonavir and Saquinavir significantly inhibited adherence to endothelial cells at only very high concentrations which are likely not reached in vivo, and Indinavir did not even inhibit then. Inhibition of adhesion of C. albicans to human cells by HIV protease inhibitors is not a general feature, but strongly cell type-dependent, and clearly not observed for endothelial cells in vitro, which are a main target of systemic candidiasis in vivo.
Keywords: Candida albicans, human immunodeficiency virus, protease inhibitors, endothelial cells, adherence
Introduction
Members of the genus Candida are part of healthy human flora but can also cause mucocutaneous as well as systemic infections, especially in immunocompromised subjects.1 Patients infected with HIV often suffer from a mucocutaneous form of candidiasis, with oropharyngeal candidiasis being one of the first and most commonly reported opportunistic infections of untreated AIDS patients. However, a systemic disseminated candidiasis is rarely reported in these subjects.2 Systemic candidiasis will usually develop only when an additional defect in the phagocytic system occurs, either inherited or acquired, such as chemotherapy in cancer or immunosuppression in transplantation.1 Among the yeast, the relative contribution of Candida albicans is decreasing, but it is still the most frequent species isolated.2,3
With the introduction of the new highly active antiretroviral therapy (HAART), involving HIV protease inhibitors, this mucocutaneous infection is nowadays observed only rarely in treated patients.4,5 We recently investigated whether HIV protease inhibitors have a direct attenuating effect on C. albicans secreted aspartic proteases (Saps), an important virulence factor of the yeast.6 This investigation was prompted by the fact that both Saps and the HIV protease belong to the same superfamily of aspartic proteases and furthermore share a particular similarity, and by the observation that oropharyngeal candidiasis in HAART-treated patients sometimes even resolves in the absence of an immunological improvement of the host.7,8
Indeed, we first – but concurrently with Cassone et al. – discovered that the HIV protease inhibitors were weak but specific inhibitors of Saps,7,9,10 which were corroborated by us and others,8,11,12 summarised by Munro & Hube [13].
As Saps play a key role in adhesion, colonisation and penetration of host tissues,14 we also tested the influence of HIV protease inhibitors on adhesion of Candida to a monkey15 or human epithelial cell layer.16 Adhesion was significantly inhibited, clearly at concentrations which were reached systemically during HAART, which might, at least in part, explain the resolution of oropharyngeal candidiasis in HIV-positive subjects, where epithelial cells represent the target.
It was proposed that not necessarily HIV protease-specific, but rather putatively more efficient Sap-specific protease inhibitors might form an alternative in the treatment of Sap-producing yeast, in addition to, or even instead of, the currently available antimycotics.7,16 In this respect, it would be important to know whether adhesion itself or only adhesion to epithelial cells can be inhibited by protease inhibitors.
The aim of the present study was to evaluate the influence of HIV protease inhibitors on C. albicans adherence to endothelial cells in vitro, the important target for systemic spread of yeast infections.
Materials and methods
C. albicans cultivation
Candida albicans CBS 5982 (Central Bureau voor Schimmelcultures, Baarn, the Netherlands), C. albicans ATCC 90028 [American Type Culture Collection (ATCC), Rockville, MD, USA] and C. albicans SC5314 (a kind gift of R. Eck, Jena, Germany) were initially grown on Sabouraud dextrose agar (SDA; Oxoid, Basingstoke, UK) plates for 24 h and then transferred into RPMI 1640 medium (Hyclone, Cramlington, UK) without any supplements. This cell suspension was used as stock solution and was kept for 1 week at 4 °C. All experiments were performed under sterile conditions.
HIV protease inhibitors
Three HIV protease inhibitors, namely Ritonavir (Abbott, Chicago, IL, USA), Indinavir (Merck, Rahway, NJ, USA) and Saquinavir (Roche, Welwyn Garden City, UK) were used for this study. They were prepared as follows: Ritonavir was dissolved in methanol at a concentration of 40 mmol l−1. Indinavir and Saquinavir were dissolved in Aqua bidest at concentrations of 20 mmol l−1 and 2 mmol l−1, respectively. These solutions were used as stock solutions and were kept at −70 °C.
Endothelial cells
The immortalised human endothelial cell line EAhy 926, kindly provided by Dr Edgell (Chapel Hill, NC, USA), was used as one of the source of endothelial cells. This cell line has been conclusively shown to represent a legitimate model for human endothelial cells, as reviewed elsewhere.17
It was cultivated in RPMI medium (Hyclone) containing 10% fetal calf serum (FCS; Boehringer, Ingelheim, Germany) and l-glutamine (Hyclone) in cell culture flasks (Falcon, 75 cm3; Costar, Cambridge, UK). The cells were incubated at 37 °C (5% CO2, 95% humidity). For the adherence assay the cells were prepared as follows: the medium was poured off and the cells were washed once with phosphate-buffered saline (PBS). For the detachment of the cells, they were incubated at 37 °C (5% CO2, 95% humidity) for 7 min in RPMI/ethylenediaminetetraacetic acid (EDTA) containing 50% RPMI, 5% FCS, 50% PBS and 5 mmol l−1 EDTA. Later, the cells were rigorously shaken causing cell detachment. The EAhy 926 cells were poured into a 50 ml Falcon tube (Costar) containing 10 ml PBS and centrifuged at 300 g for 5 min. The supernatant was poured off and the cells were diluted in RPMI (containing 10% FCS and l-glutamine) at a concentration of 5 × 105 cells ml−1. The wells of a microtitre plate were filled with 100 μl EAhy 926 cell suspension each. Prior to adherence assay, the endothelial cells were incubated for 24 h at 37 °C (5% CO2, 95% humidity) in this microtitre plate. The concentration used enabled the generation of a continuous cell lawn for the adherence assay described below.
In order to account for changes introduced during culture, both a frequently thawn and frozen aliquot of the cell line, and one which had been stored for a decade in liquid nitrogen, have been used. Although shown to be representative for endothelial cells in many models investigated, we have also compared the data with those obtained from freshly obtained human umbilical vein endothelial cells (HUVECs).
Human umbilical vein endothelial cells were obtained from umbilical cords by digestion with collagenase II (Sigma, St Louis, MO, USA) and plated into culture flasks (Falcon) precoated with 0.2% gelatine (Sigma). Subsequent cultivation was performed in endothelial growth medium (EGM; BioWhittaker, Rockland, ME, USA) at 37 °C, 5% CO2, as described previously.18,19 Cells were identified by their typical cobblestone appearance in monolayer and confirmed by tumour necrosis factor-α induced expression of E-selectin. At passage 2, cells were plated into 96-well plates (Falcon) at 100 000 cells ml−1, allowed to grow for 48 h and further used for the adhesion assay, described below.
Adherence assay
Candida albicans, diluted in RPMI 1640 medium without any supplements, was prepared in RPMI (containing 10% FCS and l-glutamine) at a concentration of 2 × 104 cells ml−1, where they presented as >99% oval yeast cell forms. HIV protease inhibitors, Ritonavir, Indinavir and Saquinavir, diluted as described above, were also prepared in RPMI (containing 10% FCS and l-glutamine) at different concentrations. Candida albicans (final concentration 104 cell ml−1) and HIV protease inhibitors (final concentrations 500, 100, 20, 4 and 0.8 μmol l−1) were mixed at equal volumes. Candida albicans without HIV protease inhibitors in the absence or the presence of 5% methanol served as control. All preparations were incubated at 37 °C (without CO2) for 1 h, after which slight signs of germination were seen in most yeast cells. The wells of the microtitre plate containing the adherent endothelial cells (EAhy 926 or HUVEC) were washed once with PBS and then incubated with 100 μl of C. albicans/HIV protease inhibitor solution. Subsequently, the plate was incubated at 37 °C (with 5% CO2) for 30 min. Thereafter, all wells of the microtitre plate were washed twice with PBS to remove non-adherent yeast cells. Liquid SDA (100 μl, 6.5%, 40 °C) was added to each well. Finally, the microtitre plate was incubated at 30 °C for 16 h. This incubation was performed both to enable an easy quantification of adherent colonies the next day (under microscopic control at a magnification of ×40) and to restrict the number of adherent organisms to those that were able to replicate. Incubation at 37 °C was comparable but the colonies were larger and confluent and thus more difficult to count. The colony-forming units (CFU) derived from samples lacking HIV protease inhibitors were set to 100%.
Statistics
Statistical analyses were performed using SPSS for Windows version 11.0 (SPSS Inc., Birmingham, UK). Statistical significance was determined by using Student’s t-test analysis. All comparisons were two-sided and a P-value of <0.05 was considered significant.
Results
Effect of HIV protease inhibitors on C. albicans adherence to endothelial cells
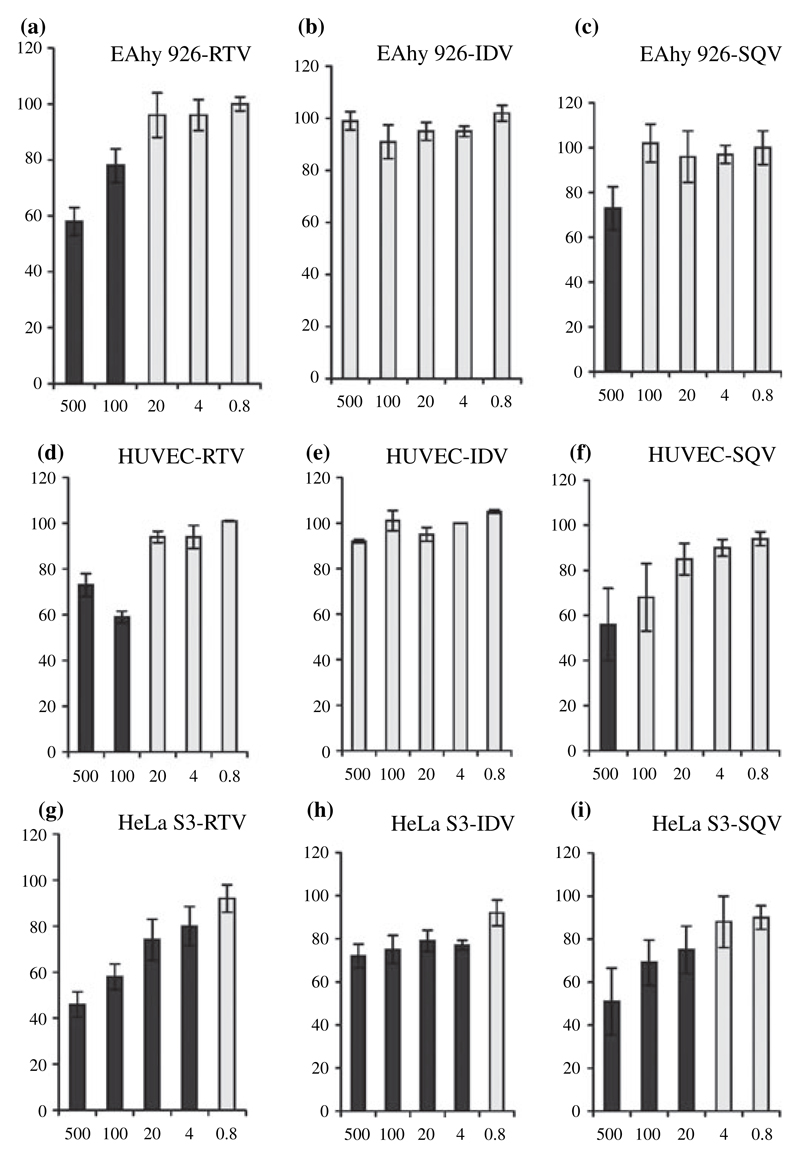
The influence of HIV protease inhibitors (Ritonavir, Indinavir and Saquinavir) on the adherence of C. albicans CBS 5982 to the HUVECs or the endothelial cell line EAhy 926 was tested using RPMI as adherence medium. After washing off the non-adherent yeast cells, the remaining organisms were cultured in SDA and their number (CFU) assessed under microscopic control the next day. The CFU derived from samples lacking HIV protease inhibitors were set to 100%. Ritonavir was found to be the most effective inhibitor of C. albicans adherence. Significant inhibition of fungal adhesion on EAhy 926 was observed at 100 and 500 μmol l−1 for all three C. albicans isolates – for CBS 5982 the inhibition was about 20% and 40%, respectively (Fig. 1a). In contrast, Indinavir had no influence on fungal adherence to this cell line – no inhibition was seen even at the highest concentration used for all three isolates, as depicted for CBS 5982 (Fig. 1b). Virtually identical data were obtained using HUVECs instead of the cell line (Fig. 1d,e). On both EAhy 926 and HUVECs Saquinavir inhibited only at 500 μmol l−1 using all three strains, as depicted for CBS 5982 (Fig. 1c,f), but this inhibition was over-shadowed by a cytotoxic effect for the cell line (colonies were present, but the cell lawn was destroyed), whereas HUVECs or epithelial cells were able to withstand this HIV protease inhibitor concentration.
Figure 1.
Adherence of Candida albicans CBS 5982 to EAhy 926 cells (a–c) or human umbilical vein endothelial cells (d–f) in the presence of Ritonavir (RTV, left), Indinavir (IDV, centre) or Saquinavir (SQV, right). The bottom boxes show the results for the epithelial cell line HeLa S3, as previously published,16 for comparison (g–i). The HIV protease inhibitors were used at final concentrations of 500, 100, 20, 4 and 0.8 μmol l−1 (x-axis). The number of C. albicans colony-forming units (CFUs) was assessed under microscopic control. The CFUs derived from samples lacking HIV protease inhibitor were set to 100% (control). Specific adherence values (CFU) are shown as a percentage of the control (y-axis). Standard deviations varied between 1% and 14%. Significant (P < 0.05) results are indicated by black columns.
The data obtained for an epithelial cell line (HeLa S3) using the same experimental set up16 are shown for comparison (Fig. 1g–i).
Discussion
The critical step in the pathogenesis of disseminated candidiasis is the adherence of Candida to the vascular structures such as endothelial cells and subendothelial basement membrane.20 Systemic fungal disease, however, is not common among HIV-positive subjects as such, but may occur in later states of AIDS, when the innate immune defence system is also affected.2 Because of the relatively low number, there are, however, no specific reports on the resolvement of systemic candidiasis in HAART-treated AIDS patients who do not show an improvement of their cellular immunity, in contrast to reports concerning mucosal disease.21,22 A previous report, corroborating that HIV protease inhibitors have a direct effect in vivo by showing that HAART exerts an early immune reconstitution-independent effect directly on the Saps23 did also focus on mucosal disease only.
In the present study, we have evaluated the effect of HIV protease inhibitors on endothelial cell adherence of C. albicans in the presence of HIV protease inhibitors, as these cells form the target for systemic disease.
While all three HIV protease inhibitors tested could inhibit adhesion to epithelial, but not to endothelial cells at concentrations which are reached in vivo,15,16 we conclude that while a direct influence on C. albicans adherence to epithelial cells appears possible, HIV protease inhibitors are not likely to play a role in endothelial adherence in vivo and thus in modulating systemic fungal disease.
Although HIV protease inhibitors clearly inhibit Saps, it is conceivable, but not unequivocal, that the inhibition of adhesion is due to an inhibition of Saps and not due to an independent mechanism. Saps, as important virulence factors of Candida, degrade host barriers, evade the host’s immune system by destroying immunoglobulins and complement, impair phagocytosis, and, importantly, facilitate adhesion.24 In order to fulfil these tasks, a battery of similar isoenzymes is selectively and differentially expressed adapting to the microenvironment of the yeast and the stage of the infection.14 Ten genes encoding Saps from C. albicans have been cloned to date:14 Saps 1–3 as well as Saps 4–6 form two subgroups, each containing closely related enzymes, whereas Saps 7–10 do not form specific groups. Inhibition studies and the use of mutants with targeted gene disruptions showed that Saps 1–3 were important during infections of skin and mucosa, while Saps 4–6 were less important,25 but most relevant for systemic infections.26 In line with these results, Saps 1–3 do not appear to be involved in adhesion to endothelial cells,27 whereas Saps 4–6 are required for the invasion of parenchymal organs.28 Interestingly, only Saps 1–3, but not Saps 4–6, are strongly affected by HIV protease inhibitors15 that may explain the absence of inhibition of endothelial adhesion using these drugs in the present study.
When HIV protease inhibitors were previously compared, it was found that Ritonavir was the most potent; however, also showing the highest cross-reactivity to pepsin.8 With respect to their fungicidal properties, Saquinavir was found to be the most potent inhibitor of yeast mitochondrial activity and fungal viability at a concentration of 1 mmol l−1,8 which implies that at high concentrations, Saquinavir may be toxic for some cells, such as the endothelial cell line, but not for HUVECs or the epithelial cell line. However, as these concentrations are approximately 100 times higher than the drug concentrations measured in vivo, it appears unlikely that such an inhibition has a clinical relevance.
In conclusion, inhibition of C. albicans adhesion by HIV protease inhibitors is clearly epithelial cell-specific. Although the design of this study does not allow such a conclusion, we believe, from the data available, that HIV protease inhibitors may not prevent attachment of C. albicans to endothelial cells in vivo. A quite recent study shows that even in the oral mucosa HIV protease inhibitors were not able to eliminate C. albicans.29 While we have previously proposed HIV protease inhibitors as prototype of novel antifungal drugs, this now appears to be restricted to mucosal disease, and likely only as preventive measure, before significant adhesion of Candida itself has taken place.
Acknowledgments
The authors would like to thank Univ-Prof. Dr. DDr.h.c. Bernd-Michael Rode for a personal grant covering accommodation (to P.I.) to perform this research work in Innsbruck, Austria. The study was further supported by a grant from the Austrian 'Fonds zur Förderung der wissenschaftlichen Forschung' (P17043-B13) and the Network of Excellence EuroPathoGenomics (LSHB-CT-200-512061). The authors express their appreciation to Merck (Rahway, NJ, USA), Roche (Welwyn Garden City, UK) and Abbott (Chicago, IL, USA) for supplying HIV-1 proteinase inhibitors.
References
- 1.Odds FC, editor. Candida and candidosis. London: Balliere Tindall; 1988. [Google Scholar]
- 2.Fidel PL. Immunity to Candida. Oral Dis. 2002;8:69–75. doi: 10.1034/j.1601-0825.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 3.Korting HC, Ollert M, Georgii A, Frosch M. In vitro susceptibility and biotypes of Candida albicans isolates from the oral cavities of patients infected with human immunodeficiency virus. J Clin Microbiol. 1998;26:2626–31. doi: 10.1128/jcm.26.12.2626-2631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zingman BS. Resolution of refractory AIDS-related mucosal candidiasis after initiation of didanosine plus saquinavir. N Engl J Med. 1996;334:1674–5. doi: 10.1056/NEJM199606203342516. [DOI] [PubMed] [Google Scholar]
- 5.Sepkowitz KA. Effect of HAART on natural history of AIDS-related opportunistic disorders. Lancet. 1998;351:228–30. doi: 10.1016/S0140-6736(05)78279-9. [DOI] [PubMed] [Google Scholar]
- 6.White TC, Agabian M. Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type; and levels are determined by environmental factors. J Bacteriol. 1995;177:5215–21. doi: 10.1128/jb.177.18.5215-5221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruber A, Speth C, Lukasser-Vogl E, et al. Human immunodeficiency virus type 1 protease inhibitor attenuates Candida albicans virulence properties in vitro. Immunopharmacology. 1999;41:227–34. doi: 10.1016/s0162-3109(99)00035-1. [DOI] [PubMed] [Google Scholar]
- 8.Gruber A, Berlit J, Speth C, et al. Dissimilar attenuation of Candida albicans virulence properties by human immunodeficiency virus type 1 protease inhibitors. Immunobiology. 1999;201:133–44. doi: 10.1016/S0171-2985(99)80052-7. [DOI] [PubMed] [Google Scholar]
- 9.Gruber A, Lukasser-Vogl E, Zangerle R, Borg-von Zepelin M, Dierich MP, Würzner R. Candida albicans: attenuation of fungal virulence properties in vitro by an HIV-1 protease inhibitor. Immunobiology. 1998;199:647. [Google Scholar]
- 10.Cassone A, De Bernardis F, Torosantucci A, Tacconelli E, Tumbarello M, Cauda R. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. J Infect Dis. 1999;180:448–53. doi: 10.1086/314871. [DOI] [PubMed] [Google Scholar]
- 11.Korting HC, Schaller M, Eder G, Hamm G, Böhme U, Hube B. Effects of the human immunodeficiency virus (HIV) proteinase inhibitors Saquinavir and Indinavir on in vitro activities of secreted aspartyl proteinases of Candida albicans isolates from HIV-infected patients. Antimicrob Agents Chemother. 1999;43:2038–42. doi: 10.1128/aac.43.8.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monod M, Borg-von Zepelin M, Telenti A, Sanglard D. The inhibition of Candida albicans-secreted aspartic proteases by three different HIV protease inhibitors. Dermatology. 1999;198:412–4. [PubMed] [Google Scholar]
- 13.Munro CA, Hube B. Anti-fungal therapy at the HAART of viral therapy. Trends Microbiol. 2002;10:173–7. doi: 10.1016/s0966-842x(02)02330-2. [DOI] [PubMed] [Google Scholar]
- 14.Hube B, Naglik J. Candida albicans proteinases: resolving the mystery of a gene family. Microbiology. 2001;147:1997–2005. doi: 10.1099/00221287-147-8-1997. [DOI] [PubMed] [Google Scholar]
- 15.Borg-von Zepelin M, Meyer I, Thomssen R, et al. HIV-protease inhibitors reduce cell adherence of Candida albicans strains by inhibition of yeast secreted aspartic proteases. J Invest Dermatol. 1999;113:747–51. doi: 10.1046/j.1523-1747.1999.00747.x. [DOI] [PubMed] [Google Scholar]
- 16.Bektic J, Lell CP, Fuchs A, et al. HIV protease inhibitors attenuate adherence of Candida albicans to epithelial cells in vitro. FEMS Immunol Med Microbiol. 2001;31:65–71. doi: 10.1111/j.1574-695X.2001.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 17.Würzner R, Langgartner M, Spötl L, et al. Temperature-dependent surface expression of the β2-integrin analogue of Candida albicans and its role in adhesion to the human endothelium. Exp Clin Immunogenet. 1996;13:162–73. [PubMed] [Google Scholar]
- 18.Holland JA, Pritchard KA, Rogers NJ, Stemerman MB. Perturbation of cultured human endothelial cells by atherogenic levels of low density lipoprotein. Am J Pathol. 1988;132:474–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–35. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 21.Hoegl L, Thomagreber E, Rocken M, Korting HC. HIV protease inhibitors influence the prevalence of oral candidosis in HIV-infected patients: a 2-year study. Mycoses. 1998;41:321–5. doi: 10.1111/j.1439-0507.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 22.Cauda R, Tacconelli E, Tumbarello M, et al. Role of protease inhibitors in preventing recurrent oral candidosis in patients with HIV infection: a prospective case-control study. J Acquir Immune Defic Syndr. 1999;21:20–5. doi: 10.1097/00126334-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Cassone A, Tacconelli E, De Bernardis F, et al. Antiretroviral therapy with protease inhibitors has an early, immune reconstitution-independent beneficial effect on Candida virulence and oral candidiasis in human immunodeficiency virus-infected subjects. J Infect Dis. 2002;185:188–95. doi: 10.1086/338445. [DOI] [PubMed] [Google Scholar]
- 24.De Bernardis F, Sullivan PA, Cassone A. Aspartyl proteinases of Candida albicans and their role in pathogenicity. Med Mycol. 2001;39:303–13. doi: 10.1080/mmy.39.4.303.313. [DOI] [PubMed] [Google Scholar]
- 25.Schaller M, Korting HC, Schafer W, Bastert J, Chen W, Hube B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol. 1999;34:169–80. doi: 10.1046/j.1365-2958.1999.01590.x. [DOI] [PubMed] [Google Scholar]
- 26.Sanglard D, Hube B, Monod M, Odds FC, Gow GA. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–46. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim AS, Filler SG, Sanglard D, Edwards JE, Jr, Hube B. Secreted aspartyl proteinases and interactions of Candida albicans with human endothelial cells. Infect Immun. 1998;66:3003–5. doi: 10.1128/iai.66.6.3003-3005.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felk A, Kretschmar M, Albrecht A, et al. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect Immun. 2002;70:3689–700. doi: 10.1128/IAI.70.7.3689-3700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Bernardis F, Tacconelli E, Mondello F, et al. Anti-retroviral therapy with protease inhibitors decreases virulence enzyme expression in vivo by Candida albicans without selection of avirulent fungus strains or decreasing their anti-mycotic susceptibility. FEMS Immunol Med Microbiol. 2004;41:27–34. doi: 10.1016/j.femsim.2003.12.006. [DOI] [PubMed] [Google Scholar]