Abstract
Background
The complement system is tightly controlled by several regulators. Two of these, factor H (FH) and C4b-binding protein (C4BP), can be acquired by pathogens conveying resistance to complement attack. The aim of the study was to characterize the FH binding molecule of Candida albicans, a potentially life-threatening yeast.
Methods
The gene coding for this molecule was identified by probing an expression library and homozygous deletion mutants of the respective gene were constructed. Binding and functional assays were undertaken to compare wild-type and knockout strains.
Results
The high-affinity glucose transporter 1 (CaHgt1p) was identified as an FH-binding molecule. Homozygous hgt1Δ/Δ deletion mutants, but not the restored strain in which HGT1 was reintegrated, showed a decreased binding of FH and even of C4BP, demonstrating its function as an FH- and C4BP-binding protein. This led to an enhanced terminal complement complex deposition after incubation with human serum; CaHgt1p thus functions as complement inhibitor. hgt1Δ/Δ mutants failed to form rosettes with complement-coated sheep erythrocytes, and show reduced binding to HIV-gp160, implying that a complement receptor 3 (CR3) moiety, known as fungal HIV binding molecule is lacking.
Conclusions
CaHgt1p is a multifunctional evasion molecule, as complement inhibitor, CR3 analogue and HIV receptor.
Candida albicans is a normal colonizer of mucous membranes and is predominantly known as a physiological inhabitant of the human gastrointestinal tract. In recent years, Candida has emerged as a leading pathogen in the opportunistic fungal infection of locally or systemically immunocompromised hosts [1]. Pathogenicity of C. albicans is mediated by various virulence factors, including surface-expressed proteins, such as adhesins, which allow adherence to epithelial and endothelial cells, or secreted proteins, such as aspartyl proteases and phospholipases. These important factors enable C. albicans dissemination in the host, enhancing its ability to colonize organs [2]. Additionally, the reversible transition between unicellular yeast cells and filamentous growth forms is considered essential to the infection process. Increased adhesion to host cells also facilitates tissue penetration after the expression of specific surface antigens during hyphal growth [1]. Early diagnosis of invasive fungal infections is difficult and treatment problematic because of the limited number of clinically available drugs [3]. A detailed knowledge regarding interactions between fungi and host immunity is, therefore, important for recognition and treatment of infections.
The innate immunity plays a crucial role in antifungal defense, via immediate reactions and recognition of a broad spectrum of microbial pathogens. The complement system is a key machinery of the innate immunity, recognizing and killing pathogens such as bacteria, virus-infected cells, parasites, and fungi [4]. Complement components are activated within seconds or minutes after microorganisms enter the host. The cascade of activation steps of complement factors leads to the opsonisation, chemotaxis, and the formation of the terminal complement complex, termed membrane attack complex when formed on a membrane, destroying membranes of pathogens and causing osmotic lysis. In the case of fungal pathogens, opsonisation may play an important role in phagocytosis [4].
The complement cascade is tightly regulated—also to preserve normal “self” cells—by several protein components, which are present in soluble form in different body fluids or bound to cell membranes. Factor H (FH), a soluble plasma protein, is the main fluid phase regulator of the complement alternative pathway [5]. It is employed by various pathogens for their protection against complement destruction when circulating in the host [6]. Further, C4b-binding protein (C4BP), the main fluid-phase inhibitor of the classical and lectin complement pathways, can also decorate the surface of various human pathogens, conferring resistance to complement destruction and thus promoting fungal pathogenicity [4]. Candida species [7, 8], similar to molds such as Aspergillus species [9], can bind FH as well as C4BP. FH and C4BP binding to Aspergillus species appears to be even stronger than to Candida species [9], a FH-binding molecule has not yet been characterized for Aspergillus species The aim of this study was to identify the FH-binding protein of C. albicans and to evaluate its relevance for complement binding and evasion.
Methods
Strains and Growth Conditions
Candida albicans SN152 [10], which was used both as wild-type and as parental strains for the knockout procedure described below, was grown on yeast–peptone–dextrose (YPD, Becton Dickinson) agar and then transferred to YPD or Roswell Park Memorial Institute (RPMI) medium (GIBCO-Invitrogen) for 16 hours at 30°C or 37°C. All results of SN152 were confirmed by using the C. albicans SC5314 strain [7] (data not shown).
Plasma, Antibodies, and Proteins
Plasma obtained from 5 healthy human donors was pooled. We obtained FH from Calbiochem, polyclonal goat anti-FH immunoglobulin G (IgG) from Quidel, alkaline phosphatase conjugated antigoat IgG and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) substrate from Sigma, FITC-conjugated antigoat, antirabbit, and antimouse IgG from Dako, and rabbit anti-Candida IgG from Biotrend. WU 13–15, a neoepitope-specific antineo C9 for the quantitation of the terminal complement complex (TCC) antibody [11] was generated in-house. C4BP and polyclonal rabbit anti-C4BP was kindly provided by Anna Blom, Sweden, and rabbit, goat, and mouse IgG by the Institute of Immunology, University of Göttingen.
Identification of the C. albicans FH–Binding Protein
To isolate and sequence the DNA corresponding to the fungal protein(s) that react with FH, a C. albicans SC5314 expression library was generated using the lysogene gt11 virus and the ZAP-cDNA Gigapack II Cloning Kit, both from Stratagene. The SC5314 strain had been grown in RPMI medium overnight at 37°C prior to its use for the expression library. For the identification of FH-binding clones, the membranes containing the colonies were probed with 50 μg/mL FH, followed by incubation with goat anti-FH at a 1:50 dilution, followed by secondary alkaline phosphate conjugated antigoat immunoglobulin G (IgG, 1:500 dilution) and detection using BCIP substrate. Positive colonies were identified using the chloroform technique [12] and reused at appropriate dilutions for a second screen to gain single plaques for DNA isolation and sequencing [13].
Sequences were identified using the Basic Local Alignment Search Tool (BLAST) nucleotide sequence similarity search; alignment was performed via blastN (comparing nucleotide sequences and blastX [translated query vs protein database]). We performed a further check for homologies with a translated query in the SWISSPROT database. Results were regarded as significant when P ≤ .05.
Construction of C. albicans hgt1Δ/Δ Deletion Mutants
We performed fusion polymerase chain reaction (PCR) [10] to create a gene disruption cassette for C. albicans HGT1 coding sequences using appropriate primers. HGT1 gene disruption fragments were created, employing SN152 as parental strain, using a LEU2 marker in the first round, and a HIS1 marker in the second. Disruption of both alleles was confirmed by colony PCR and Southern blot analyses (data not shown).
We used the SAT1 marker cassette of the plasmid pSFS2A and the fusion PCR strategy [10] to generate the gene complementation construct for HGT1. We inserted the wild-type allele of HGT1 into the genome of hgt1Δ/Δ mutants at the locus of the LEU2 marker. We performed transformation via electroporation and verified genomic integration events by PCR analysis.
Cellular Morphology of C. albicans Strains
C. albicans SN152 wild type and parental, hgt1Δ/Δ deletion mutant and hgt1Δ/Δ::HGT1 revertant strains were incubated in RPMI or YPD medium for 24 hours at 30°C or 37°C, in 24-well plates. We used microscopy to evaluate cellular morphology.
Indirect Immunofluorescence
C. albicans strains, after overnight incubation in YPD medium at 30°C, were placed at 1 x 106/mL (final concentration) on glass slides (precoated with 50 μl of poly-l-lysine [Sigma] for 30 minutes) for 1 hour at room temperature and fixed with acetone–methanol (1:1). Then they were incubated with rabbit anti-Candida IgG or rabbit IgG (both at 50 μg/ml) or human ethylenediaminetetraacetic acid (EDTA)–plasma (50%) or FH (50 μg/mL) for 4 hours at 4°C. After fixation and blocking at room temperature, phosphate-buffered saline (PBS) or goat anti-FH IgG or goat IgG (the latter two at 50 μg/mL) were added. Detection was performed via secondary fluorescein isothiocyanate (FITC)–labeled antigoat or antirabbit IgG at 1:50 dilutions.
Flow Cytometry
C. albicans strains, after overnight incubation in YPD medium at 30°C, were incubated at 2 x 106/mL (final concentration) with human EDTA-plasma (50%), FH (50 μg/mL) or C4BP (50 μg/mL) for 2 hours at 4°C. After blocking with 1% PBS-skim milk for 30 minutes, the primary antibodies (goat anti-FH (50 μg/mL); rabbit anti-C4BP (50 μg/mL); mouse anti-TCC (WU 13–15, 50 μg/mL) or control IgG (50 μg/mL)) were added for 1 hour at 4°C. Secondary FITC-labelled antigoat, antirabbit or antimouse IgG were used at dilutions of 1:50 for 30 minutes. Cells were fixed in PBS supplemented with 1% formalin and 0.1% azide. We examined labeled cells by a fluorescent-activated cell sorter (FACS, Becton Dickinson) with forward and sideward scatters, and 10 000 events were routinely counted.
Rosetting Test With Complement-Coated Sheep Erythrocytes
We tested C. albicans strains for their ability to form rosettes with sheep erythrocytes that were preincubated with complement (hemolytic system [HS], Virion/Serion) as described in detail elsewhere [14]. C. albicans cells were grown overnight in RPMI medium at 30°C, incubated (1:1) with HS for 1, 12, or 24 hours at 37°C, and evaluated by light microscopy.
Binding of hgt1Δ/Δ Mutants to gp160 HIV Envelope Protein
We tested C. albicans strains for their ability to bind gp160 by enzyme-linked immunosorbent assay (ELISA). Overnight Candida cell cultures (in RPMI medium at 30°C) were washed twice with PBS and diluted in PBS to 2.5 × 107 cells/mL; 40 μL of the cell suspension were distributed to each well of an ELISA plate and incubated for 1 hour at 30°C. After 2 careful washing steps with PBS, the recombinant HIV gp160 antigen (a generous gift from F. Dorner (Immuno AG)) was added at a concentration of 5 μg/well and incubated for 2 hours at 4°C. Human HIV-antibody positive serum (dilution 1:500, pooled from 5 patients) and antihuman–alkaline phosphatase (AP) immunoglobulin (IgG) (Sigma) served as detecting reagents. We verified microscopically the presence of still-coated hyphae after each washing step. We used a microdilution plate reader for recording the absorbance at 450 nm with a reference wavelength of 630 nm.
Results
Identification of CaHgt1p as FH Binding Molecule and Generation of C. albicans hgt1Δ/Δ Mutants
We identified CaHgt1p as an FH-binding molecule by probing an expression library with serum followed by anti-FH antibodies. We initially screened about 100 000 colonies. We probed positive clones with purified FH, followed by anti-FH antibodies. The sequence corresponding to HGT1 was most frequently found (11 times); sequences of different hypothetical, DNA-processing, metabolic, or ribosomal genes were found fewer than 3 times each. Although the sequence of HGT2 is to 93% homologous to HGT1 [15], it was always truncated (646–921 base pairs sequenced) HGT1 sequence (HGT gene size: 1638 bp [15]) found, and never the sequence of HGT2 or of other members of that family. To evaluate the role and importance of HGT1, we constructed a homozygous C. albicans hgt1Δ/Δ deletion mutant using a disruption marker cassette and a modified fusion PCR protocol that permits a rapid and highly efficient generation of homozygous knockout mutants [10]. The resulting deletion strains, as well as the restored strain hgt1Δ/Δ::HGT1 in which HGT1 was reintegrated, were analyzed for growth and morphology phenotypes.
Reduced Ability of C. albicans hgt1Δ/Δ to Form Hyphae in RPMI Medium at 30°C and 37°C
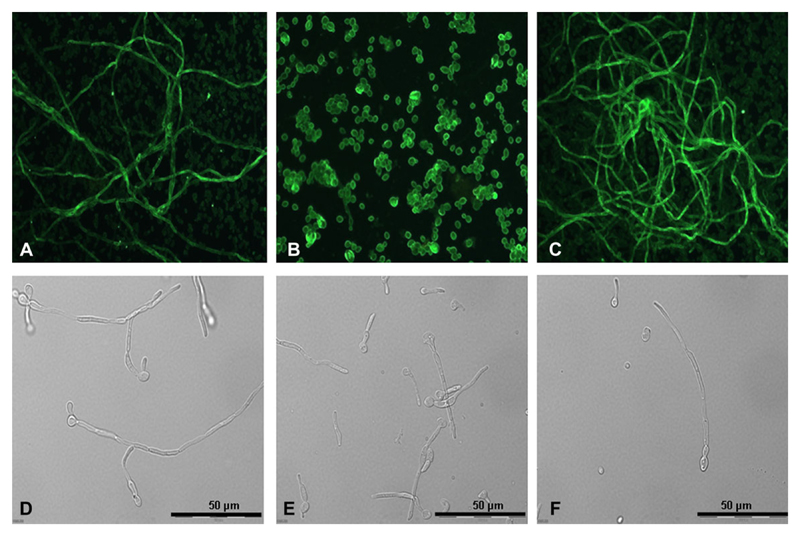
Fluorescent microscopic analyses using rabbit anti-Candida IgG and then antirabbit-FITC labelled IgG revealed that hgt1Δ/Δ mutants (Figure 1B), in contrast to parental (Figure 1A) or restored strains (Figure 1C), have impaired ability to form hyphae in RPMI medium at 30°C. After incubation at 37°C, hgt1Δ/Δ mutants were able to form hyphae; however, these were much and visibly shorter (Figure 1E) than those generated by parental (Figure 1D) or restored strains (Figure 1F).
Figure 1.
Morphology of Candida albicans high-affinity glucose transporter 1 (hgt1)Δ/Δ. Microscopic analyses of cellular morphology, in part via immunofluorescence using anti-Candida immunoglobulin G (IgG) (A–C), of C. albicans hgt1Δ/Δ (B, E) compared with SN152 parental (A, D) and hgt1Δ/Δ::HGT1 restored (C, F) strains after 24 hours incubation in RPMI at 30°C (A–C) or at 37°C (D–F). Representative examples of quadruplicate experiments are shown.
Reduced Binding of FH to C. albicans hgt1Δ/Δ
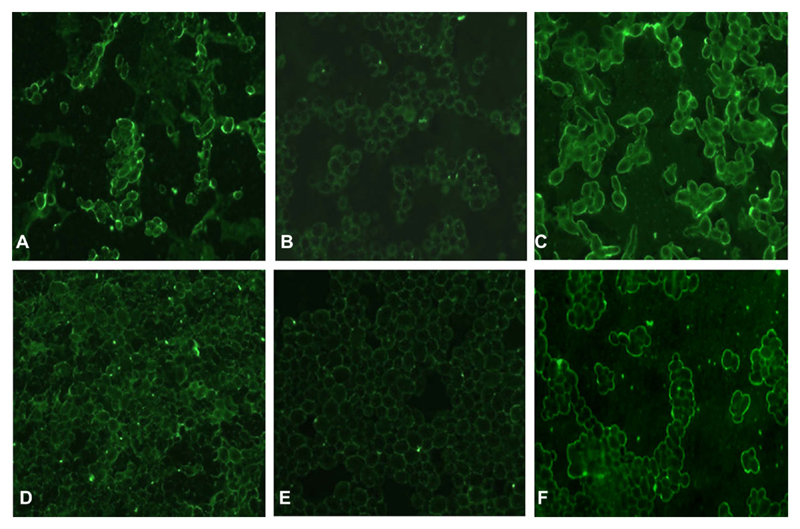
Binding of FH, following incubation in EDTA–plasma or with purified FH, to C. albicans parental, hgt1Δ/Δ, and restored strains was tested by immunofluorescence assay. Fluorescent microscopy results showed that the binding of FH—in its purified form as well as from human EDTA plasma—to C. albicans hgt1Δ/Δ mutants (Figures 2B and 2E, respectively) was reduced when compared with parental (Figure 2A and 2D) or restored strains (Figure 2C and 2F).
Figure 2.
Binding of factor H (FH) by Candida albicans high-affinity glucose transporter 1 (hgt1)Δ/Δ-qualitative assessment. Immunofluorescence analyses of binding of FH from ethylenediaminetetraacetic acid (EDTA)–plasma (A–C) or in its purified form (D–F) by hgt1Δ/Δ (B, E) compared with SN152 parental (A, D) and hgt1Δ/Δ::HGT1 restored (C, F) strains via goat anti-FH IgG. Representative examples of quadruplicates experiments are shown.
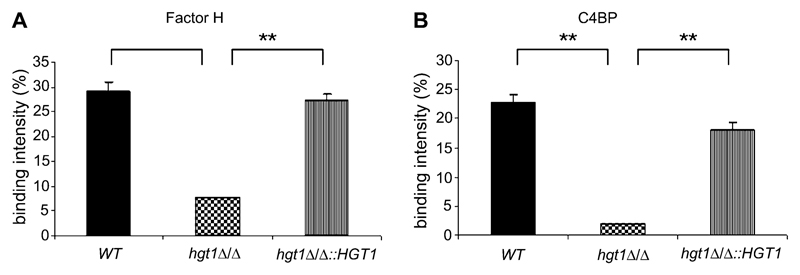
Reduced binding of FH to C. albicans hgt1Δ/Δ mutants, compared with binding to parental or restored strains, was also confirmed by flow cytometry using homogeneous cell populations obtained by filtration of the respective yeast cell suspensions. C. albicans hgt1Δ/Δ mutants had a decreased ability to bind FH in its purified form (Figure 3A) when compared with parental or restored strains (Figure 3A).
Figure 3.
Binding of factor H (FH) and C4b- binding protein (C4BP) by Candida albicans high-affinity glucose transporter 1 (hgt1)Δ/Δ–quantitative assessment. Binding of FH (A) and C4BP (B) to SN152 parental (or wild type [WT], black columns), hgt1Δ/Δ (chequered columns), and hgt1Δ/Δ::HGT1 restored (striped columns) strains was assessed by determining the percentage of binding cells within the entire population. Results from quadruplicate experiments performed on 4 different days are shown; **, P <.01.
Reduced Binding of C4b Binding Protein to C. albicans hgt1Δ/Δ
We tested binding of C4BP by flow cytometry using homogeneous cell populations obtained by filtration of cell suspensions of parental, hgt1Δ/Δ, and restored strains. Following incubation with purified C4BP, C. albicans hgt1Δ/Δ mutants had a reduced ability to bind C4BP when compared with parental or restored strains (Figure 3B).
Increased Deposition of Terminal Complement Complex on C. albicans hgt1Δ/Δ as a Measure of Lower Inhibition of Complement Activation on the Yeast Cell Surface
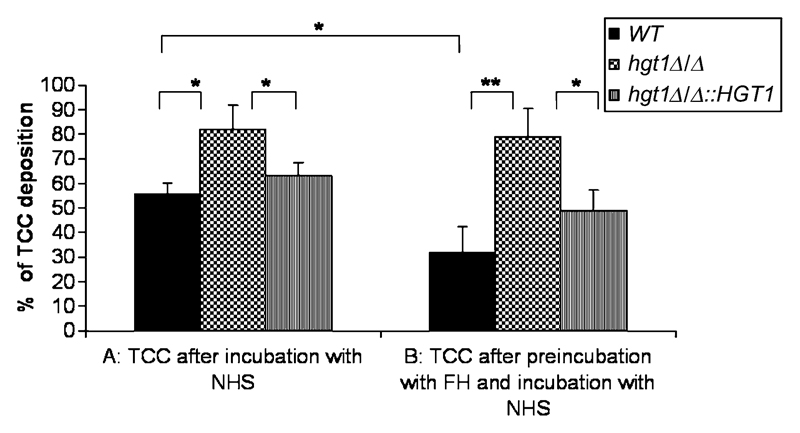
We tested C. albicans hgt1Δ/Δ mutants for the deposition of terminal complement complexes (TCC) following complement activation. Yeast cells were preincubated with FH (50 μg/mL) followed by incubation with normal human serum (NHS) and compared with yeast cells incubated with NHS only. Flow cytometry analyses revealed that TCC deposition on the yeast cell surface (after incubation of yeast cells with NHS for 2 hours at 37°C) was much more pronounced for the hgt1Δ/Δ mutant than for the parental and restored strains (Figure 4A), which still have the binding site for the complement inhibitor FH. We preincubated the strains with FH to corroborate these findings. Whereas there was no difference in TCC deposition for the hgt1Δ/Δ mutants after preincubation with FH (50 μg/mL, for 2 hours at 4°C followed by incubation with NHS for 2 hours at 37°C; Figure 4B), as expected from the absence of the major FH-binding molecule, the parental strain showed an even lower deposition of TCC on preincubation with FH (Figure 4B).
Figure 4.
Deposition of terminal complement complex (TCC) on Candida albicans high-affinity glucose transporter 1 (hgt1)Δ/Δ as a measure of complement activation on the yeast cell surface. Deposited TCC was detected by flow cytometry using mouse antineoC9 antibodies followed by fluorescein isothiocyanate (FITC)–labelled antimouse antibodies on SN152 parental (black columns), hgt1Δ/Δ (checkered columns), and hgt1Δ/Δ::HGT1 restored (striped columns) strains after incubation with normal human serum (NHS) (A) or after preincubation with factor H (50 μg/mL) followed by incubation with NHS (B). Percentages of deposited TCC on the yeast cell surface are shown. Cells incubated in buffer only were used as 100% control. Results from quadruplicate experiments performed on 4 different days are shown; *, P <.05, **, P <.01.
Reduced Rosette Formation on C. albicans hgt1Δ/Δ as a Measure of Low or Absent CR3 Analogue Expression on the Yeast Cell Surface
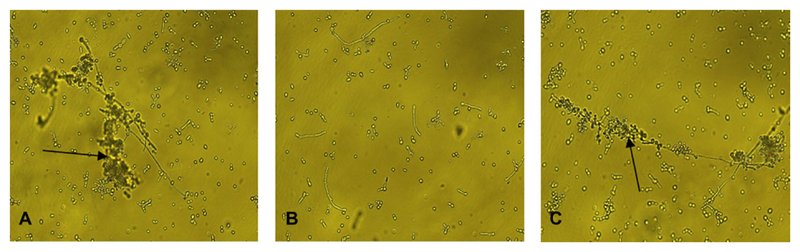
Whereas formation of rosettes with complement-coated erythrocytes was observed for parental (Figure 5A) and restored (Figure 5C) strains after 1 hour, the hgt1Δ/Δ mutant failed to form rosettes both at 1 hour (not shown) and after prolonged incubations of 12 hours (Figure 5B) or 24 hours (data not shown), suggesting that hgt1Δ/Δ mutants have a markedly reduced expression of a CR3-like protein.
Figure 5.
Rosette formation on Candida albicans hgt1Δ/Δ as a measure of CR3 analogue expression on the yeast cell surface. Rosettes (indicated by arrows) of C. albicans SN152 parental and high-affinity glucose transporter 1 (hgt1)Δ/Δ::HGT1 restored (C) strains after incubation with complement-coated sheep erythrocytes for 1 hour. No rosettes were observed for hgt1Δ/Δ at 1 hour (not shown) and not even after extended periods of 12 hours (B) and 24 hours. Results from quadruplicate experiments performed on 4 different days are shown.
Reduced Binding of Recombinant HIV gp160 to C. albicans hgt1Δ/Δ
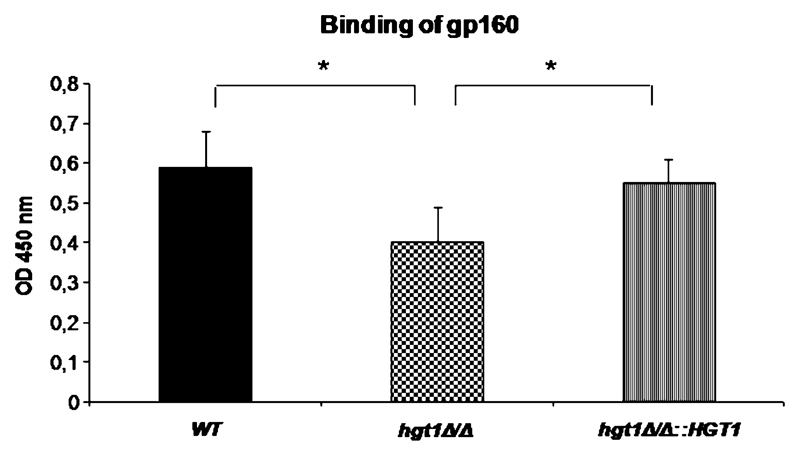
Binding of recombinant HIV gp160 to C. albicans strains was determined by Candida cell ELISA. Human HIV-antibody positive serum followed antihuman–alkaline phosphatase (AP) IgG revealed a reduced binding of recombinant HIV protein to C. albicans hgt1Δ/Δ mutants when compared with parental or restored strains (Figure 6).
Figure 6.
Binding of recombinant human immunodeficiency virus (HIV) gp160 to Candida albicans. Binding of recombinant HIV gp160 to C. albicans SN152 parental (black column), high-affinity glucose transporter 1 (hgt1)Δ/Δ (chequered column) and hgt1Δ/Δ::HGT1 restored (striped column) strains was determined by yeast cell enzyme-linked immunosorbent assay (ELISA). Human HIV antibody-positive serum (1:500) and antihuman-AP IgG served as detecting reagents. Results from quadruplicate experiments performed on 4 different d are shown; * P <.05.
Discussion
Human microbial pathogens, including facultative pathogenic yeasts, are able to counteract the destructive action of complements by acquisition of soluble complement inhibitors FH and C4BP from the host [4, 6–8]. We identified a putative FH-binding molecule of C. albicans by sequencing DNA isolated from clones of a C. albicans cDNA expression library, screened with purified FH. The most frequently identified gene was the C. albicans HGT1 (CaHGT1) encoding a putative hexose transporter [15].
To evaluate the role and importance of Hgt1p, a homozygous C. albicans hgt1Δ/Δ deletion mutant was constructed using a disruption marker cassette and a modified fusion PCR protocol [10]. Although this has not been directly tested, we expect that Hgt2p and other members of this transporter family are able to compensate for the loss of Hgt1 concerning the vital sugar transport, as the deletion mutants are viable, but are likely not FH-binding molecules, as deduced from the marked phenotype of the deletion mutant.
Reduced binding of FH to C. albicans hgt1Δ/Δ mutants confirmed that CaHgt1p may indeed be implicated in direct binding of FH. The marked reduction in , but not complete lack of, binding may be explained by the presence of other FH-binding proteins in C. albicans. Zipfel and coworkers have recently described 2 FH-binding molecules, the phosphoglycerate mutase (CaGpm1p) [16] and the pH-regulated antigen (Pra1p) [17]. However, Cagmp1 knockout mutants behaved similarly to wild-type cells regarding FH binding [16]. Pra1p was not even knocked out because the authors concluded, first, from the finding that only 17% of the FH binding could be inhibited by a Pra1p-specific antiserum, and, second, from the existence of different FH-binding proteins on the surface of the yeast cells, that a Pra1 knockout mutant will not differ substantially in FH binding [17]. In contrast, the hgt1Δ/Δ mutants used in this study clearly show a significant decrease in binding (Figure 3A), suggesting that CaHgt1p may play a dominant role in the acquisition of FH by the C. albicans cell surface.
Microorganisms that cause systemic disease employ a variety of complement evasion mechanisms, and acquisition of complement regulators is a frequently adopted principle [6]. Interestingly, the same molecule is often employed for binding both the alternative pathway regulator FH and the classical pathway regulator C4BP [6]. We have therefore investigated binding of C4BP and observed that CaHgt1p appears to be not only an FH-binding but also a C4BP-binding molecule.
FH ensures that the complement system does not damage endogenous host tissue. However, bound to pathogens, FH may protect them from destruction [6], leading to evasion of host defense by downregulating complement activation, detected via a reduced formation of the potentially lytic terminal complex (TCC) [6]. Flow cytometry analyses revealed that TCC deposition on the yeast cell surface was much more pronounced when CaHgt1p was absent. Furthermore, in the mutant it was not reduced by preincubation with FH. This confirms that CaHgt1p appears to be a major FH binding molecule and strongly implies that CaHgt1p plays a functional biological role in downregulating complement activation. This may also be important considering the finding that TCC on the fungal surface represents a trigger for the release of C6 and C7 from polymorphonuclear leukocytes (PMNs) [18], indicating that the reduced TCC on the wild type does not trigger PMN functions locally at the site of inflammation. In contrast, it has been very recently shown that FH bound to the surface of C. albicans enhances PMN antimicrobial activity [19], so carrying FH may protect from complement, but likewise enforce neutrophilic attack.
The protein on the PMNs that binds to FH is the αMβ2 integrin CD11b/CD18 or CR3 [19, 20]. As CR3-like molecules have been described for C. albicans, reviewed elsewhere [4], it was assessed whether the FH-binding CaHgt1p is such a CR3-like analogue, which are detected via their ability to induce the formation of rosettes, ie, hyphae surrounded by bound complement-coated erythrocytes (EAC, [21]). CR3-like molecules have been shown to be important for fungal virulence and complement evasion in general [22, 23], and in particular to be involved in adhesion and filamentous growth [23].
Candida albicans hgt1Δ/Δ mutants fail to form rosettes with complement-coated erythrocytes, which suggests that hgt1Δ/Δ mutants have a markedly reduced expression of a CR3-like protein. Hence, CaHgt1p likely represents a dominant—as no rosettes at all are visible for the deletion mutant—CR3-like protein.
A CR3 analogue has previously been identified as a HIV gp160/gp41 binding moiety on C. albicans [24]. On HIV glycoprotein–binding cellular activities augmenting fungal virulence are induced [25], namely enhanced adhesion to HIV-infected cells, a decreased phagocytosis of the yeast and an increased Sap release. The reduced binding of HIV-gp160 to the hgt1Δ/Δ mutants indicates that CaHgt1p may also function as an HIV receptor.
The ability to switch between different morphological forms is thought to be an important feature of C. albicans, relevant for its pathogenesis and virulence [26]. Because the hyphal form is considered to be the more virulent phenotype, the expression library was made from C. albicans growing in that form. Our results revealed a reduced ability of C. albicans hgt1Δ/Δ mutants to form hyphae, which suggests a role of the HGT1 gene for C. albicans in modulating hyphae formation. However, this reduced ability did not affect the testing: FH-binding was detected for both hyphal and yeast forms by immunofluorescence (only yeast forms depicted, Figure 2); for flow cytometry yeast cells must be taken to avoid a blockage of the nozzle, whereas rosette and HIV-binding studies were done with hyphae. In both latter experiments, the incubation period was long enough to allow hyphal formation, so the effects are likely not due to an inability of the mutant to generate hyphae, but we cannot fully exclude that some Hgt1 functions are displayed indirectly via an altered cellular morphology because a possibly inferior carbohydrate update may influence the cell wall structure.
Interestingly, the quality of Hgt1p matters more than the quantity, so if Hgt1p is present (as in wild-type or restored strains), the phenotype is always similar. The restored strain is not intermediate between normal and mutant.
In conclusion, CaHgt1p appears to be a multifunctional complement-evasion molecule that causes downregulation of complement activation by acquisition of FH and C4BP and thus functions as a complement inhibitor and that may also represent both a CR3 analogue and an HIV-binding molecule on C. albicans. CaHgt1p or derivates may thus be used as prototypes for the development of complement inhibitors.
Acknowledgments
The help from Denes Hnisz and Rolando Colonia-Silva is gratefully acknowledged.
Funding
This work was supported by Austrian Science Foundation (FWF-P17043) and EU-Projects QLG1–CT2001–01039 and LSHB-CT-2005-512061 (support to R. W.); Christian Doppler Research Society; and in part by ERA-Net Pathogenomics project FunPath through the Austrian Science Foundation (FWF-I125-B09, support to K. K.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Whiteway M, Bachewich C. Morphogenesis in Candida albicans. Ann Rev Microbiol. 2007;61:529–53. doi: 10.1146/annurev.micro.61.080706.093341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munro CA, Hube B. Anti-fungal therapy at the HAART of viral therapy. Trends Microbiol. 2002;10:173–7. doi: 10.1016/s0966-842x(02)02330-2. [DOI] [PubMed] [Google Scholar]
- 3.Richardson M, Lass-Flörl C. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect. 2008;14:5–24. doi: 10.1111/j.1469-0691.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 4.Speth C, Rambach G, Würzner R, Lass-Flörl C. Complement and fungal pathogens: An update. Mycoses. 2008;51:477–96. doi: 10.1111/j.1439-0507.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 5.Józsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29:380–7. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Kraiczy P, Würzner R. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol Immunol. 2006;43:31–44. doi: 10.1016/j.molimm.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Meri T, Hartmann A, Lenk D, et al. The yeast Candida albicans binds complement regulators factor H and FHL-1. Infect Immun. 2002;70:5185–92. doi: 10.1128/IAI.70.9.5185-5192.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meri T, Blom AM, Hartmann A, Lenk D, Meri S, Zipfel PF. The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein. Infect Immun. 2004;72:6633–41. doi: 10.1128/IAI.72.11.6633-6641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogl G, Lesiak I, Jensen DB, et al. Immune evasion by acquisition of complement inhibitors: The mould Aspergillus binds both factor H and C4b binding protein. Mol Immunol. 2008;45:1485–93. doi: 10.1016/j.molimm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathieson PW, Würzner R, Oliveria DB, Lachmann PJ, Peters DK. Complement-mediated adipocyte lysis by nephritic factor sera. J Exp Med. 1993;177:1827–31. doi: 10.1084/jem.177.6.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt SP, Livesey R, editors. Functional genomics. A practical approach. Oxford: Oxford University Press; 2000. [Google Scholar]
- 13.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Nat Acad Sci U S A. 1977;74:5463–7. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Würzner R, Langgartner M, Spötl L, et al. Temperature-dependent surface expression of the ß2-integrin analogue of Candida albicans and its role in adhesion to the human endothelium. Exp Clin Immunogenet. 1996;13:162–73. [PubMed] [Google Scholar]
- 15.Fan J, Chaturvedi V, Shen SH. Identification and phylogenetic analysis of glucose transporter gene family from the human pathogenic yeast Candida albicans. J Mol Evol. 2002;55:336–46. doi: 10.1007/s00239-002-2330-4. [DOI] [PubMed] [Google Scholar]
- 16.Poltermann S, Kunert A, von der Heide M, Eck R, Hartmann A, Zipfel PF. Gpm1p is a factor H-, FHL-1-, and plasminogen-binding surface protein of Candida albicans. J Biol Chem. 2007;282:37537–44. doi: 10.1074/jbc.M707280200. [DOI] [PubMed] [Google Scholar]
- 17.Luo S, Poltermann S, Kunert A, Rupp S, Zipfel PF. Immune evasion of the human pathogenic yeast Candida albicans: Pra1 isa Factor H, FHL-1 and plasminogen binding surface protein. Mol Immunol. 2009;47:541–50. doi: 10.1016/j.molimm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Lukasser-Vogl E, Gruber A, Lass-Flörl C, et al. Membrane attack complex formation on yeast as trigger of selective release of terminal complement proteins from human polymorphonuclear leukocytes. FEMS Immunol Med Microbiol. 2000;28:15–23. doi: 10.1111/j.1574-695X.2000.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 19.Losse J, Zipfel PF, Józsi M. Factor H and factor H-related protein 1 bind to human neutrophils via complement receptor 3, mediate attachment to Candida albicans, and enhance neutrophil antimicrobial activity. J Immunol. 2010;184:912–21. doi: 10.4049/jimmunol.0901702. [DOI] [PubMed] [Google Scholar]
- 20.DiScipio RG, Daffern PJ, Schraufstätter IU, Sriramarao P. Human polymorphonuclear leukocytes adhere to complement factor H through an interaction that involves alphaMbeta2 (CD11b/CD18) J Immunol. 1998;160:4057–66. [PubMed] [Google Scholar]
- 21.Heidenreich F, Dierich MP. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985;50:598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ollert MW, Wadsworth E, Calderone RA. Reduced expression of the functionally active complement receptor for iC3b but not for C3d on an avirulent mutant of Candida albicans. Infect Immun. 1990;59:909–13. doi: 10.1128/iai.58.4.909-913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale CA, Bendel CM, McClellan M, et al. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–8. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 24.Würzner R, Gruber A, Stoiber H, et al. Human immunodeficiency virus type 1 gp41 binds to Candida albicans via complement C3-like regions. J Infect Dis. 1997;176:492–8. doi: 10.1086/514069. [DOI] [PubMed] [Google Scholar]
- 25.Gruber A, Lukasser-Vogl E, Borg-von Zepelin M, Dierich MP, Würzner R. Human immunodeficiency virus type 1 gp160/gp41 binding to Candida albicans selectively enhances candidal virulence in vitro. J Infect Dis. 1998;177:1057–63. doi: 10.1086/515231. [DOI] [PubMed] [Google Scholar]
- 26.Brown AJ, Gow NA. Regulatory networks controlling morphogenesis. Trends Microbiol. 1999;7:333–8. doi: 10.1016/s0966-842x(99)01556-5. [DOI] [PubMed] [Google Scholar]