Abstract
Importance
Trastuzumab is life-saving but is associated with symptomatic and asymptomatic left ventricular ejection fraction (LVEF) decline. Here we report the low cardiac toxicity of a non-anthracycline and trastuzumab based treatment for patients with early stage HER2-positive breast cancer (BCA).
Objective
To determine the cardiac safety of paclitaxel and trastuzumab in patients with node-negative, HER2-positive BCA.
Design
Enrollment occurred from October 2007 to September 2010, and patients received adjuvant paclitaxel for 12 weeks with trastuzumab, and trastuzumab was continued for one year. The median follow-up was 4.0 years.
Setting
Institutional practice
Participants
406 patients were enrolled. Eligibility included patients with ≤3 cm and node-negative (N1mi allowed), HER2-positive BCA with baseline LVEF of ≥ 50%. Median age was 55 years, 118 (29%) had hypertension, and 30 (7%) had diabetes.
Intervention
Treatment consisted of weekly paclitaxel (80 mg/m2) with trastuzumab (4mg/kg IV bolus → 2 mg/kg weekly) × 12 → 39 weeks of trastuzumab. LVEF was assessed at baseline, 12 weeks, 6 months, and 1 year
Main Outcome Measures
Cardiac safety data, including grade 3-4 left ventricular systolic dysfunction (LVSD) and significant asymptomatic LVEF decline, as defined by study, were reported.
Results
Overall, 2 (0.5%) (95% CI: 0.1-1.8%) developed grade 3 LVSD and came off study. Thirteen (3.2%) (95% CI: 1.9-5.4%) had significant asymptomatic LVEF decline, 11 of whom completed study treatment. Median LVEF was 65%, 64%, 64%, and 64% at baseline 12 weeks, 6 months, and 1 year, respectively.
Conclusion
Cardiac toxicity, manifested by symptomatic or asymptomatic LV dysfunction, from paclitaxel with trastuzumab, was low. Our findings suggest that LVEF monitoring during trastuzumab therapy without anthracyclines could be simplified for many individuals.
Trial Registration
Clinicaltrials.gov, NCT00542451
Keywords: paclitaxel, trastuzumab, paclitaxel, breast cancer
Introduction
In the United States, breast cancer is the most common female cancer and the second most common cause of cancer death in women (1). Amplification or overexpression of the human epidermal growth factor receptor 2 (HER2/neu) oncogene is present in approximately 20-25% of primary invasive breast cancers (2). Trastuzumab has demonstrated a significant improvement in outcomes of women with early stage breast cancer in key adjuvant trials, but most of these trials contained an anthracycline-based therapy followed by trastuzumab (with or without a taxane) in women with either node-positive or node-negative high-risk breast cancer (usually defined as tumor size >1 cm or >2 cm) (3-8). However, several studies from the pre-trastuzumab era suggest a higher risk of recurrence for patients with HER2-positive, node-negative tumors compared to those with HER2-negative tumors of the same size (9-11). Recent retrospective studies have demonstrated a benefit from the combination of chemotherapy and trastuzumab in those with node-negative disease and a time trend increase in the use of these agents (12-14).
The most significant toxicity of trastuzumab, especially following an anthracycline-based therapy, is symptomatic congestive heart failure (CHF) that has been reported on the order of 0.9%-4% and significant asymptomatic decline ranging from 4%-19% in clinical trials (3,5-8). After the anthracycline phase, most of the events [symptomatic CHF and asymptomatic left ventricular ejection fraction decline (LVEF) decline] occurred during the period of trastuzumab administration (3-6, 15-18). Unlike cardiotoxicity associated with anthracyclines, cardiac dysfunction (LVEF decline) following trastuzumab therapy is not dose-related and is considered to be mostly reversible (19). Most previous clinical trials involving trastuzumab included patients who were exposed to anthracyclines where it is not clear how much the anthracyclines contributed to trastuzumab-mediated cardiac dysfunction. In order to reduce cardiac and non-cardiac toxicity with the hope of maintaining a high degree of efficacy, we conducted a trial of weekly paclitaxel with trastuzumab in patients with early stage HER2+ breast cancer. The overall study results have been previously reported and this manuscript highlights the detailed cardiac data that have been collected (20).
Methods
This was a phase 2 study across 14 centers of weekly paclitaxel with trastuzumab in patients with node-negative breast cancer (Figure 1). The study was approved by the institutional review board at each site. Written informed consent was obtained from each patient. Race, ethnicity, and sex were defined by each patient, to demonstrate the enrolled patient population (Table 1). The primary endpoint was disease-free survival (DFS). Grade 3 and 4 left ventricular systolic dysfunction (LVSD), as defined by the National Cancer Institute Common Toxicity Criteria Adverse Events (NCI CTCAE) version 3.0, was a secondary endpoint. Grade 3 LVSD is symptomatic CHF responsive to intervention and grade 4 LVSD is refractory CHF. Other adverse cardiac events were also classified per NCI CTCAE grading system version 3.0. This study protocol required longitudinal assessment of left ventricular systolic function using LVEF quantified by transthoracic echocardiogram (ECHO) or radionuclide multi-gated acquisition scan (MUGA) at baseline, 12 weeks, 6 months, and 1 year after starting protocol specified therapy. Patients who went off treatment early due to CHF were also required to have follow-up LVEF assessments 3, 6, and 12 months after the CHF event.
Figure. 1. Schema Phase II Study of Paclitaxel + Trastuzumab N =406.
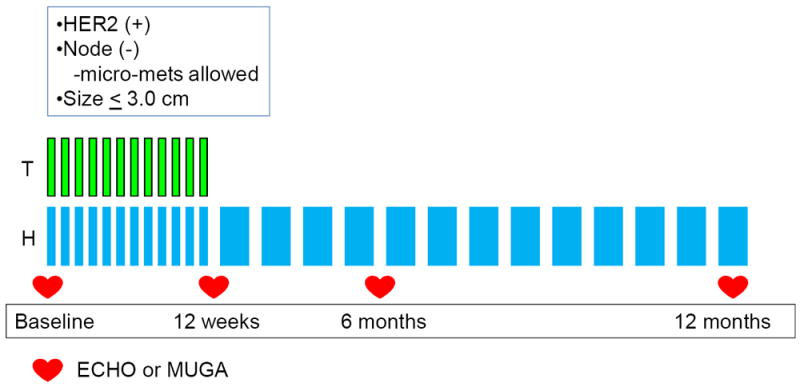
T=paclitaxel weekly at 80 mg/m2
H=trastuzumab 4 mg/kg load → 2 mg/kg weekly during TH phase → 6 mg/kg
q 3 weeks (or 2 mg/kg weekly) during H monotherapy
Table 1.
Patient Characteristics
| Characteristic | N=406 | % |
|---|---|---|
| . | ||
| Median Age (Range) | 55 (24-85) | . |
| Sex | ||
| Male | 1 | <1 |
| Female | 405 | 100 |
| Race | ||
| White | 351 | 86 |
| African American | 28 | 7 |
| Asian | 11 | 3 |
| Other | 16 | 4 |
| Ethnicity | ||
| Hispanic or latino | 9 | 2 |
| Non-Hispanic | 374 | 92 |
| Ethnicity Not Known | 23 | 6 |
| History of Hypertension | ||
| Yes | 118 | 29 |
| No | 288 | 71 |
| History of Diabetes | ||
| Yes | 30 | 7 |
| No | 376 | 93 |
The analysis population was defined as all patients who received any amount of protocol therapy. Diagnosis of grade 3-4 LVSD (symptomatic CHF) during protocol therapy required cessation of trastuzumab therapy. For these patients, we reported the registration date, protocol therapy starting date, off-treatment date, number of cycles administered, and LVEF percentages. Trastuzumab was interrupted for asymptomatic declines in LVEF within the following categories: 1) a decrease of 10-15 percentage points from a baseline LVEF that was less than the local lower limit of normal (LLN) for LVEF, or 2) a decrease ≥16 percentage points from a baseline LVEF that was greater than or equal to local LLN for LVEF (eTable 1). All patients with interval development of either symptomatic or asymptomatic LVSD described above requiring interruption of trastuzumab underwent repeat LVEF assessment using the same modality after an interval of 4 weeks. If the LVEF did not recover to a “continue” category as defined by study guidelines (eTable 1) and if two consecutive “holds” were required, then the patient would be withdrawn from study treatment. Investigators were strongly urged to schedule MUGA scans or ECHOs at the same radiology facility where the patient’s baseline scan was done, and we used the baseline LLN to compare to LVEF assessed at 12 weeks, 6 months, and 1 year. The incidence of grade 3-4 LVSD was determined with the 95% confidence interval (CI). The median LVEF for each time point and proportion of patients within each category of LVSD are presented.
Statistical Analysis
The planned sample size of 400 was based on the primary endpoint of disease-free survival. The statistical design and sample size considerations have been described elsewhere (20). The incidence of LVSD and asymptomatic LVEF decline were secondary endpoints. For this analysis, the incidence of LVSD and asymptomatic LVEF decline were analyzed as binary outcomes. Rates of LVSD and asymptomatic LVEF decline and 95% confidence intervals (CI) were calculated using Wilson’s method. With a planned sample size of 400 patients, the estimated half-width of the 95% Wilson confidence interval was 0.5%, 1.1%, 14%, 1.7%, 2.0% and 2.2% with 0, 4, 8, 12, 16, and 20 cases of LVSD observed.
To explore the association between baseline characteristics and occurrence of cardiac toxicity, relative risks (RR) and 95% confidence intervals (CI) were calculated using robust variance estimates (21-22). Statistical analyses were performed using SAS® 9.3 and R version 2.6.1.
Patients
Eligible patients had HER2 IHC 3+ or FISH-amplified (ratio ≥ 2.0) breast cancer, with node-negative breast cancer and a tumor size of ≤ 3 cm, were enrolled. Patients with a micrometastasis in a lymph node were later allowed with study amendment on May 19, 2009. Patients with a baseline LVEF < 50%, history of myocardial infarction (MI), CHF, uncontrolled hypertension (systolic blood pressure of > 200 mmHg or diastolic blood pressure of > 100 mmHg), or hemodynamically significant pericardial effusion were excluded from this study.
Treatment
Treatment consisted of weekly paclitaxel (80 mg/m2) administered intravenously (IV) concurrently with trastuzumab (4mg/kg IV bolus followed by 2 mg/kg weekly) × 12 followed by trastuzumab monotherapy × 39 weeks. During the monotherapy phase, trastuzumab could be given weekly or every 3 weeks (6 mg/kg) (Figure 1). Radiation and hormonal therapy were administered per standard guidelines after completion of the 12 weeks of chemotherapy.
Results
From October 9, 2007 to September 3, 2010, 410 patients enrolled and 406 started protocol therapy. All 406 patients completed treatment as of September 8, 2011. The median follow-up was 4.0 years. The median age was 55 years of age, and 118 (29%) and 30 (7%) patients had a history of hypertension and diabetes, respectively (Table 1). The majority of patients with hypertension (84%) and diabetes (83%) were at least 50 years of age (Table 2). There were 356 (88%) patients who completed about a year of protocol therapy and 50 (12%) who did not. The 50 patients were taken off study for the following reasons: 2 for grade 3 LVSD, 2 for persistent grade 2 LVSD, 1 for grade 3 arrhythmia, 1 for grade 3 sinus tachycardia, 1 for grade 2 palpitations, 42 for non-cardiac reasons, and 1 for an unknown reason. Overall, 7 (1.7%) patients discontinued protocol treatment for all cardiovascular reasons but only 4 (1.0%) patients discontinued due to LVSD (95% CI 0.3%-2.5%).
Table 2.
Distribution of Age in Patients with HTN and DM
| Age (years) | All patients | Patients with HTN | Patients with DM |
|---|---|---|---|
| N | 406 | 118 | 30 |
| Median (Range) | 55 (24-85) | 61 (40-85) | 59 (40-76) |
| Age Group (years) | N (%) | N (%) | N (%) |
| <50 | 132 (33) | 19 (16) | 5 (17) |
| 50-59 | 137 (34) | 32 (27) | 10 (33) |
| 60-69 | 96 (24) | 45 (38) | 11 (37) |
| 70+ | 41 (10) | 22 (19) | 4 (13) |
HTN=hypertension; DM=diabetes
Changes in Left Ventricular Ejection Fraction
Baseline LVEF values were between 50%-55% in 40 (10%), and > 55% in 366 (90%) patients (eTable 2). Of the 40 patients with baseline LVEF of ≤ 55%, 26 (65%) were at least 50 years of age. Overall, the majority of patients had a minimal decline in LVEF of < 10% (84%, 80% and 74%), and only a minority of patients had a decline in LVEF to 10-15% (7%, 9%, and 9%) and ≥ 16% (< 1%, 1%, and 2%) from baseline at 12 weeks, 6 months, and 1 year, respectively. The median LVEF values were well preserved throughout treatment as 65% (50%-81%), 64% (45%-81%), 64% (45%-83%), and 64% (41%-90%) at baseline, 12 weeks, 6 months, and 1 year, respectively (Table 3).
Table 3.
Summary of LVEF at protocol specified time points and changes from baseline values
| Baseline | 12 weeks | 6 months | 1 year | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N=406 | % | N=406 | % | N=406 | % | N=406 | % | |
| LVEF reduction from baseline | ||||||||
| <10% | . | 343 | 84 | 325 | 80 | 302 | 74 | |
| 10-15% | . | 29 | 7 | 36 | 9 | 35 | 9 | |
| ≥16% | . | 2 | < 1 | 5 | 1 | 7 | 2 | |
| Required, not evaluated 1 | . | 7 | 1 | 9 | 2 | 22 | 5 | |
| Not required2 | . | 25 | 6 | 31 | 8 | 40 | 10 | |
| LVEF level (%) | ||||||||
| Median(Range) | 65 (50-81) | . | 64 (45-81) | . | 64 (45-83) | . | 64 (41-90) | |
For each time point, patients for whom cardiac evaluation was required but reported as not done were counted as “required but not evaluated” at that time point
Cardiac evaluations were not required for patients who went off protocol therapy due to non-cardiac toxicity. For patients who went off treatment before completing 1-year protocol specified therapy due to non-cardiac-toxicity reasons, assessments after the off-treatment visit were counted as “not required”.
LVEF=left ventricular ejection fraction
Symptomatic Left Ventricular Systolic Dysfunction
Out of 406 patients who started protocol therapy, 2 patients (#257 and #309) developed grade 3 LVSD (0.5%, 95% CI: 0.1%-1.8%). Patient #257 was a 67 year-old female on medications for hyperlipidemia upon study entry who developed symptomatic CHF at 11 months after starting treatment. Her LVEF assessments were as follows: 55% at baseline, 54% at 12 weeks, 60% at 6 months, and 37% at 11 months. She did not resume trastuzumab per study stipulation. Subsequent treatment with lisinopril achieved full resolution of CHF symptoms and a restoration of LVEF to normal: 60% at 16 months and 66% at 27 months (eTable 3eFigure). Patient #309 was a 56 year-old female on a beta-blocker for a diagnosis of arrhythmogenic right ventricular dysplasia. Her LVEF was 66% at baseline and 61% at 12 weeks, and it declined to 50% at 6 months, which coincided with the onset of symptoms of CHF. Trastuzumab was terminated. Medical management with a regimen of furosemide, ramipril, and carvedilol was initiated, and she became asymptomatic with an improvement in subsequent LVEF assessments: 51% at 7 months, 54% at 8 months, and 56% at 18 months (eTable 3, eFigure). Both patients were medically managed by their cardiologists for CHF. Overall, these 2 patients had at least 2 potential risk factors for cardiac dysfunction. Both patients were older than 50 years of age. Patient #257 also had a baseline LVEF of only 55%, and patient #309 had arrhythmogenic right ventricular dysplasia. This is due to fatty infiltration of the RV, and most of these patients will have LV involvement over time resulting in bi-ventricular failure.
Incidence of Asymptomatic Decrease in Left Ventricular Ejection Fraction
Overall, 13 (3.2%) (95% CI: 1.9-5.4%) of patients experienced an asymptomatic LVEF decline significant enough to require trastuzumab interruption as per study criteria (eTable 3). Two patients (# 168 and # 325) discontinued protocol therapy. Patient # 254 had significant asymptomatic LVEF decline that did not recover during the study timeframe (within 4 weeks). However, she was not taken off study and trastuzumab was continued (study violation). Of the 3 patients with “persistent” (lasting for at least 4 weeks) asymptomatic LVEF decline (grade 2 LVSD), two (# 168 and # 325) had at least 2 potential risk factors (at least 50 years of age and baseline LVEF of only 55%); patient # 168 also had hypertension and diabetes. Patient # 168 was on cardiac medications (amlodipine, atenolol) at baseline for hypertension and did not have additional medications at time of trastuzumab cessation; patient # 325 had cardiac medications (lisinopril, losartan, carvedilol) added (eTable 3).
In the remaining 10 patients with significant asymptomatic LVEF decline during treatment, 6 had already completed one year of therapy when a significant asymptomatic LVEF decline occurred at the end of treatment (# 27, # 50, # 227, # 279, # 338, and # 360), and 2 patients had cardiac medications added (# 279 and # 360). As these patients completed therapy already, any follow-up LVEF monitoring was at the physician’s discretion. The other 4 patients (# 71, # 97, # 147, and # 173) experienced significant asymptomatic LVEF decrement and recovered appropriately before completing one year of therapy; only 1 patient (# 173) had cardiac medication added but other 3 did not. In these 10 patients only 3 had 2 cardiovascular risk factors (at least 50 years of age and hypertension) (# 50, # 147, and # 173), and 2 patients were on anti-hypertensive medication at baseline and 1 was not. Of note in the 10 patients, all but 1 patient had LVEF > 55%. Overall, in these 13 (3.2%) patients with significant asymptomatic LVEF decline, only 5 had at least 2 cardiovascular risk factors and notably only 3 had LVEF of 55%. We performed an exploratory analysis to assess the relationship between baseline characteristics and cardiac outcomes and there appeared to be a correlation between a low baseline LVEF of ≤ 55% and higher incidence of significant asymptomatic LVEF decline or symptomatic LVSD (RR 0.30, 95% CI: 0.10-0.90)(p = 0.05) (Table 4).
Table 4.
Cross-tabulation of baseline characteristics and LVSD/asymptomatic LVEF decline
| Total number of patients | No cardiac toxicity | LVSD or Asymptomatic LVEF Decline | RR (95% CI) | p | |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Age at study entry (yrs) | |||||
| <50 | 132 | 126 (95) | 6 (5) | - | 0.58 |
| ≥50 | 274 | 265 (97) | 9 (3) | 0.72 (0.26-1.99) | |
| Baseline LVEF (%) | |||||
| ≤55 | 40 | 36 (90) | 4 (10) | - | 0.05 |
| >55 | 366 | 355 (97) | 11 (3) | 0.30 (0.10-0.90) | |
| History of Hypertension | |||||
| Yes | 118 | 113 (96) | 5 (4) | - | 0.77 |
| No | 288 | 278 (97) | 10 (3) | 0.82 (0.29-2.35) | |
| History of Diabetes | |||||
| Yes | 30 | 27 (90) | 3 (10) | - | 0.09 |
| No | 376 | 364 (97) | 12 (3) | 0.32 (0.10-1.07) |
LVSD: Left ventricular systolic dysfunction
LVEF: Left ventricular ejection fraction
Discussion
This regimen of paclitaxel and trastuzumab (without an anthracycline) has already demonstrated exceptional outcomes with 3-year DFS exceeding 98.7% (20). In this analysis we also demonstrate that it is well-tolerated, with incidence of symptomatic CHF of only 0.5% (95% CI: 0.1-1.8%). This is consistent with the data from BCIRG 006 and the phase II trial by Jones et al on docetaxel and cyclophosphamide with trastuzumab (7, 23). The incidence of grade 3-4 LVSD for both studies was 0.4% with a non-anthracycline taxane/trastuzumab combination (5, 23). In this study, the two patients who developed grade 3 LVSD had at least 2 cardiovascular risk factors and experienced CHF during active therapy at 6 and 11 months, respectively, with subsequent recovery. Only 13 (3.2%) (95% CI: 1.9-5.4%) of 406 patients demonstrated a significant asymptomatic decline in LVEF requiring trastuzumab interruption per protocol. Of these 13 patients, 3 had “persistent” LVEF decline; 2 patients were removed from study and 1 continued therapy (study violation). The remaining 10 patients completed trastuzumab as planned. In these 13 patients, 5 had at least 2 cardiovascular risk factors.
These results compared favorably to the combined analysis of NSABP B-31/N9831, in which 14.2% of patients did not complete one year of trastuzumab due to significant asymptomatic LVEF deterioration (3). The low incidence of LVEF decline in our study was most likely attributable to the absence of the anthracycline. In addition, over the last decade, there has been a higher threshold to stop trastuzumab as a result to asymptomatic LV dysfunction given the growing appreciation of the benefits of trastuzumab therapy and collaboration between cardiologists and oncologists (24). In our study, 88% of patients completed one year of therapy, which included the patients who resumed therapy after a temporary interruption for asymptomatic LVEF decline. This was favorable when compared to the phase II study by Jones et al in which only 82% of patient completed the full year of therapy (23).
Previous data suggest that late cardiac toxicity from trastuzumab therapy is rare in oncology clinical trials. Seven and eight year follow-up data from the NSABP B-31 and HERA trials, respectively, described no increase in the cardiac event (NYHA Class III-IV heart failure/cardiac death) rate, with events occurring mainly during active therapy (6, 25). In addition, cardiac events reported for two trials of dose-dense chemotherapy with anti-HER2 agents occurred mainly during active therapy, with 5-year and 7-year follow-up data, respectively (18). When extrapolating these data to the current study with a median follow-up of 4.0 years, it is likely that the low incidence of grade 3 LVSD of 0.5% with paclitaxel and trastuzumab will not change over time with a longer follow-up.
In contrast, recent claims-based reports have shown heart failure/cardiomyopathy rates exceeded those reported by other prospective studies with long-term follow-up durations (6, 18, 25). For example, Bowles et al conducted a population-based retrospective cohort study of 12,500 women with invasive breast cancer who were treated with no chemotherapy, anthracycline, trastuzumab, anthracycline + trastuzumab, or other chemotherapies. In this study, only 0.9% of patients received trastuzumab (without an anthracycline). At 1, 3, and 5 years, the respective rates of heart failure/cardiomyopathy were 3.6%, 7.8%, and 12.1% with trastuzumab (without an anthracycline) (26). These findings were similar to those reported in papers by Chen et al, Chavez-MacGregor and colleagues, and Ezaz et al (27-29). The patient populations in these retrospective studies were much older with the mean ages ranging from 60-76 years old (26-29). The higher rates in these retrospective studies, when compared to well-designed prospective trials, could be due to an overestimation of the true risk of cardiac toxicity in claims-based studies and/or that the patients in prospective clinical trials were healthier and younger by selection. Finally, retrospective claims-based data were also limited due to a lack of rigorous adjudication of events.
Risk factors associated with trastuzumab related cardiac toxicity include anthracycline exposure and age ≥ 50 years (25, 31-34). Our exploratory analysis suggested an association between low baseline LVEF of ≤ 55% and incidence of asymptomatic LVEF decline and symptomatic LVSD. In addition, multivariate analyses identified borderline low LVEF of < 55%, hypertension, and high body mass index as predisposing risk factors for trastuzumab-induced cardiotoxicity, while influences of diabetes, valvular heart disease, and coronary artery disease were not statistically significant (25, 32, 34-35). Concurrent trastuzumab with radiation does not increase cardiac toxicity (25, 34, 36). In our study of 406 patients, 40 (10%) had baseline LVEF of ≤ 55%; 26 (6%) had baseline LVEF of ≤ 55% and were ≥ 50 years of age. Additionally, 118 (29%) had a history of hypertension, 30 (7%) had diabetes, and the majority of patients with hypertension (84%) and diabetes (83%) were at least 50 years of age. Given the low incidence of cardiac dysfunction in our study, where patients did not receive an anthracycline, perhaps serial monitoring may be reserved for patients considered at a higher risk of developing cardiotoxicity and those with signs and symptoms of CHF or other cardiac symptoms. If this approach to screening was implemented in this study, many LVEF assessments would have been avoided for the majority of asymptomatic patients with baseline LVEF > 55% without coexisting cardiovascular risk factors. The extent to which such rationalized LVEF surveillance might reduce interruption of trastuzumab, reduce cost, and impact upon cardiovascular and cancer prognoses requires investigation. Moreover, we advocate for closer collaboration between cardiologists and oncologists in determining the best strategies in identifying patients who are at risk of developing heart failure such that the patient can complete the course of trastuzumab therapy without interruption.
This study had some limitations. First, LVEF quantifications by ECHO or MUGA in this study did not follow a standard protocol and a core lab was not used for analyses; thus, data on inter- and intra-observed variability and reproducibility of LVEF reports in this study were lacking. However, this limitation was common to many chemotherapy trials that included cardiovascular safety end-points. Second, certain patient groups that were considered at higher risk for trastuzumab-associated cardiotoxicity, such as those with a history of MI or CHF, were excluded from this study. As such, study findings and recommendations for a reduced number of LVEF assessments cannot be generalized to such patients. Third, it is increasingly clear from emerging cardiac literature that LVEF may not be the best marker of cardiac contractility (37). Fourth, data for certain cardiovascular risk factors such as hyperlipidemia, cerebrovascular disease, and prior coronary revascularizations were not consistently collected. Fifth, management of deteriorations in LVEF during treatment was directed at the physician discretion; variation in management likely influenced likelihood of recovery in LVEF.
In conclusion, the incidence of symptomatic and asymptomatic deteriorations in LVEF during treatment with paclitaxel and trastuzumab was low, 0.5% and 3.2%, respectively. The favorable cardiovascular safety profile of trastuzumab in this non-anthracycline setting suggests that baseline LVEF assessment may be sufficient for the majority of patients, with serial LVEF assessments reserved for patients considered at a higher risk for cardiotoxicity. A prospective trial to include a uniform assessment and management of cardiovascular risk factors and a central review of LVEF data in patients receiving a non-anthracycline regimen with anti-HER2 therapy will be needed to help further define which patients may have less intensive LVEF monitoring.
Supplementary Material
Acknowledgments
We would like to specially acknowledge Drs. Linda Vahdat, Susan Burdette-Radoux, and Thomas Budd for their contribution to this study and manuscript.
1) Linda Vahdat, MD
New York Presbyterian Hospital
Weill Cornell Medical College, Cornell University, New York, NY
Contribution: Patient enrollment and editing manuscript
Compensation: None
2) Susan Burdette-Radoux, MD
Maimonides Medical Center, Brooklyn, NY
Contribution: Patient enrollment and editing manuscript
Compensation: None
3) Thomas Budd, MD
Cleveland Clinic, Cleveland, OH
Contribution: Patient enrollment and editing manuscript
Compensation: None
Dr. Chau Dang and Dr. Sara Tolaney had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Genentech provided funding but was not involved in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Research Support: Roche/Genentech
Footnotes
Presented: San Antonio Breast Cancer Symposium 2011
- Dr. Chau Dang reports research funding from Genentech and GlaxoSmithKline.
- D r. Kathy Miller reports research funding from Genentech/Roche, AVEO, Merrimack, ImClone Systems, EntreMed Taiho Pharmaceutical, Geron, Macrogenics, Medivation, Novartis, Seattle Genetics, Eli Lilly, BiPar Sciences and Pfizer.
- Dr. Javid Moslehi reports having served as a consultant to Novartis, Pfizer, Takeda, Ariad, Bristol Myers Squibb, Millenum, ARIAD and Acceleron.
- Dr. Sara Tolaney reports research funding from Genentech.
- Dr. Antonio Wolff reports John Hopkins has received funds from Genentech for a clinical trial where he serves as a site Primary Investigator and research funding from Myriad Genetics. Dr. Antonio Wolff reports consulting for Mersana.
- Dr. Denise Yardley reports consulting for Genentech.
- Dr. Kelly Marcom reports research funding from Abbvie, Novartis, Genentech/Roche and Veridex.
- Dr. Kathy Albain reports honoraria from Genomic Health and consulting for bioTheranostics, Genentech and Genomic Health, NanoString Technologies, Novartis. Dr. Kathy Albain reports travel, accommodations and/or expenses from Genentech, Genomic Health and Pfizer.
- Dr. Hope Rugo reports research funding from Plexxikon, Macrogenics, OBI, Eisai, Pfizer, Novartis, Lilly, GlaxoSmithKline, Genentech, Celsion, Nektar, and Merck. Dr. Hope Rugo reports honoraria from Genomic Health and travel, accomedations and/or expenses from Novartis, Nektar, Roche/Genentech, OBI and Mylan.
- Dr. Lisa Carey reports research funding from Genentech and GlaxoSmithKline.
- Dr. Ian Krop reports research funding from Genentech and Stock or Other Ownership in Vertex Pharmaceuticals.
- Dr. Clifford Hudis reports relationship with Breast Cancer Research Foundation.
- Dr. Eric Winer reports Unrecognized Companies: Gerson Lehman Group; McKinsey. Dr. Eric Winer reports research funding and travel, accommodations and/or expenses from Genentech and Novartis.
- No other disclosures were reported.
References
- 1.http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013
- 2.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Romond E, Suman VJ, Jeong J-H, et al. Trastuzumab Plus Adjuvant Chemotherapy for HER2-Positive Breast Cancer: Final Planned Joint Analysis of Overall Survival (OS) from NSABP B-31 and NCCTG N9831. San Antonio Breast Cancer Symposium; 2012. Abstract S5-5 (oral presentation) [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in Herceptin adjuvant trial (BIG 1-01) J Clin Oncol. 2014;32(20):2159–2165. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27(34):5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 9.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27(34):5693–5699. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27(34):5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26(35):5697–5704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 12.McArthur HL, Mahoney KM, Morris PG, et al. Adjuvant trastuzumab with chemotherapy is effective in women with small, node-negative, HER2-positive breast cancer. Cancer. 2011;117:5461–5468. doi: 10.1002/cncr.26171. [DOI] [PubMed] [Google Scholar]
- 13.Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer. 2012;118:1982–1988. doi: 10.1002/cncr.26484. [DOI] [PubMed] [Google Scholar]
- 14.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol. 2014 Jul 10;32(20):2142–2150. doi: 10.1200/JCO.2013.53.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23(13):2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 16.Spielmann M, Roche H, Humblet Y, et al. 3 year follow-up of trastuzumab following adjuvant chemotherapy in node positive HER2-positive breast cancer patients: results of the PACS-04 trial. Breast Cancer Res Treat. 2007;106(suppl 1):S72. [Google Scholar]
- 17.Dang C, Fornier M, Sugarman S, et al. The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER-2/neu overexpressed/amplified breast cancer. J Clin Oncol. 2008;26(8):1216–1222. doi: 10.1200/JCO.2007.12.0733. [DOI] [PubMed] [Google Scholar]
- 18.Morris PG, Iyengar NM, Patil S, et al. Long-Term Cardiac Safety and Outcomes of Dose Dense Doxorubicin and Cyclophosphamide followed by Paclitaxel and Trastuzumab with and without Lapatinib in Patients with Early Breast Cancer. Cancer. 2013;119:3943–3951. doi: 10.1002/cncr.28284. [DOI] [PubMed] [Google Scholar]
- 19.Ky B, Vejpongsa P, Yeh ET, et al. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circulation Research. 2013;113(6):754–764. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative HER2+ breast cancer. N Engl J Med. 372(2):134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. American journal of epidemiology. 2004;160:301–305. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 22.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. American journal of epidemiology. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 23.Jones S, Collea R, Paul D, et al. Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: a single-group, open-label, phase 2 stduy. Lancet Oncol. 2013;14(11):1121–1128. doi: 10.1016/S1470-2045(13)70384-X. [DOI] [PubMed] [Google Scholar]
- 24.Francis SA, Cheng S, Arteaga CL, et al. Heart failure and breast cancer therapies: moving towards personalized risk assessment. J Am Heart Assoc. 2014 Feb 28;3(1):e000780. doi: 10.1161/JAHA.113.000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Long JB, Hurria A, et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–2512. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 28.Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–4228. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezaz G, Long JB, Gross CP, et al. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. 10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 31.Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–3421. doi: 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 32.Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf. 2008;31(6):459–67. doi: 10.2165/00002018-200831060-00002. [DOI] [PubMed] [Google Scholar]
- 33.Suter TM, Procter M, van Veldhusein DJ, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25(25):3859–3865. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 34.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-3. J Clin Oncol. 2005;23(31):7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 35.Perez EA, Suman VJ, Davidson NE, et al. Cardiac Safety Analysis of Doxorubicin and Cyclophosphamide Followed by Paclitaxel With or Without Trastuzumab in the North Central Cancer Treatment Group N9831 Adjuvant Breast Cancer Trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halyard MY, Pisansky TM, Dueck AC, et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol. 2009;27(16):2638–2644. doi: 10.1200/JCO.2008.17.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circulation Res. 2014;115(1):79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


