Abstract
Aims
To identify distinct temporal likelihoods of age-related comorbidity (ARC) diagnoses: cardiovascular diseases (CVD), cancer, depression, dementia, and frailty-related diseases (FRD) in older men with type 2 diabetes (T2D) but ARC naïve initially, and assess the heterogeneous effects of metformin on ARCs and mortality.
Methods
We identified a clinical cohort of male veterans in the United States who were ≥ 65 years old with T2D and free from ARCs during 2002–2003. ARC diagnoses during 2004–2012 were analyzed using latent class modeling adjusted for confounders.
Results
The cohort consisted of 41,204 T2D men with age 74.6 ± 5.8 years, HbA1c 6.5 ± 0.97%, and 8393 (20.4%) metformin users. Four ARC classes were identified. ‘Healthy Class’ (53.6%): metformin reduced likelihoods of all ARCs (from 0.14% in dementia to 6.1% in CVD). ‘High Cancer Risk Class’ (11.6%): metformin reduced likelihoods of CVD (13.3%), cancer (45.5%), depression (5.0%), and FRD (13.7%). ‘High CVD Risk Class’ (17.4%): metformin reduced likelihoods of CVD (48.6%), cancer (3.2%), depression (2.8%), and FRD (6.3%). ‘High Frailty Risk Class’ (17.2%): metformin reduced likelihoods of CVD (18.8%), cancer (3.9%), dementia (3.8%), depression (15.6%), and FRD (23.8%).
Conclusions
Metformin slowed ARC development in old men with T2D, and these effects varied by ARC phenotype.
Keywords: Metformin, Type 2 diabetes, Comorbidity, Mortality, Frailty
1. Introduction
Currently, one in nine U.S. adults has type 2 diabetes (T2D); and, one in three is projected to have the disease by 2050 (National Center for Health Statistics). Among individuals with T2D, nearly 50% are over the age of 65 years, and these older adults are intrinsically vulnerable for age-related comorbidities such as cardiovascular diseases (CVD) (Bonora et al., 2007; Hanley, Williams, Stern, & Haffner, 2002; Isomaa et al., 2001), cancer (Djiogue et al., 2013; Novosyadlyy & LeRoith, 2010), depression (Haroon, Raison, & Miller, 2012; Stuart & Baune, 2012), dementia (De Felice & Ferreira, 2014), and frailty (Espinoza, Jung, & Hazuda, 2012; Wilson et al., 1998). These five age related comorbidities (ARCs) are also significant contributors to poor quality of life, psychological distress, disability, longer hospital stays, and mortality (Fortin et al., 2006; Gijsen et al., 2001; Wolff, Starfield, & Anderson, 2002). While no data are yet available in the literature, understanding the co-development of ARCs among older adults with T2D could enhance the identification of patients at higher risk for these conditions to permit early intervention.
Metformin is the most recommended first-line glucose-lowering medication (GLM) for treating T2D. Investigations of the pleiotropic effects of metformin have independently suggested its potential usefulness to prevent the development of ARC (Wnsperger, 2000). Based on previous studies (Barzilay et al., 2007; Currie, Poole, & Gale, 2009; Gupta, Bisht, & Dey, 2011; Holden, Jenkins-Jones, & Currie, 2016; Hsu, Wahlqvist, Lee, & Tsai, 2011; Imfeld, Bodmer, Jick, & Meier, 2012; Jacob, Kostev, Rathmann, & Kalder, 2016; Kickstein et al., 2010; Kowall, Rathmann, & Kostev, 2015; Lamanna, Monami, Marchionni, & Mannucci, 2011; Lehman, Lorenzo, Hernandez, & Wang, 2012; Liccini, Malmstrom, & Morley, 2016; Moore et al., 2013; Rao, Kuhadiya, Reynolds, & Fonseca, 2008; Sahra et al., 2010; Thakkar, Aronis, Vamvini, Shields, & Mantzoros, 2013; UK Prospective Diabetes Study (UKPDS) Group, 1998; Wang, Lorenzo, & Espinoza, 2014; Wang et al., 2012; Wright & Stanford, 2009; Zakikhani, Dowling, Fantus, Sonenberg, & Pollak, 2006) as we summarized below, these beneficial effects however are quite variable. It is not clear which target population is most likely to benefit from metformin’s ARC prevention effects. Further, whether metformin might be used as primary prevention for ARCs, which would subsequently lead to a life-extension benefit, remains to be seen.
The United Kingdom Prospective Diabetes Study (UKPDS) showed that long-term treatment with metformin substantially reduced cardiovascular morbidity and mortality compared to other glucose-lowering medications (GLMs) with similar glucose-lowering effect (UK Prospective Diabetes Study (UKPDS) Group, 1998). In a recent meta-analysis of clinical trials among individuals with T2D, Lamanna et al. (2011) observed that metformin significantly reduced CVD risk by 21% when compared to placebo, but not when compared to non-metformin GLMs. Lamanna et al. also indicated that the CVD prevention effect of metformin was more likely to be observed in longer trials (5% reduction per year longer of follow-up) with younger patients (2% reduction per year younger in age). However, the observational studies from the meta-analysis by Rao et al. (2008) suggested that metformin in combination with sulfonylureas (compared to no metformin use) could be associated with increased risk of CVD. Also, it is not clear about the conflicting findings seen in a large retrospective cohort study from the UK Clinical Practice Research Datalink, where metformin added to insulin was associated with 38% reduction in all-cause mortality, but 87% increase in major adverse cardiac events after 3.5 years of follow-up (Holden et al., 2016).
Cumulative evidence from clinical and preclinical studies has demonstrated metformin’s anti-cancer effects (Currie et al., 2009; Jacob et al., 2016; Kowall et al., 2015; Lehman et al., 2012; Sahra et al., 2010; Thakkar et al., 2013; Wright & Stanford, 2009; Zakikhani et al., 2006). A meta-analysis by Thakkar et al. (2013) showed that metformin use was associated with 30% reduced cancer risk in cohort studies and 10% reduced risk in case–control studies. However, this anti-cancer effect does not appear to be supported by randomized control trials (RCTs) that were not originally designed to examine cancer outcomes among more selected patient populations (Thakkar et al., 2013).
So far, whether metformin has an impact on the development of dementia is not clear. In vitro or animal studies have shown that metformin reduced phosphorylation of tau protein in cortical neurons of mice (Kickstein et al., 2010), and improved impaired neuronal insulin signaling and Alzheimer’s disease related neuropathological changes (Gupta et al., 2011). In clinical studies, however, the effect of metformin on cognitive impairment could vary by race/ethnicity or geographic origin. For example, the large cohort study from the Taiwan National Health Insurance Database showed that, compared with no medication, metformin use alone was associated with 24% reduction in dementia incidence, while the risk reduction was 35% when metformin was used in combination with other GLMs (Hsu et al., 2011). A small clinical study among patients with diabetes but without cognitive dysfunction initially also found that metformin use was marginally associated with 48% reduction in cognitive dysfunction (p = 0.05) (Liccini et al., 2016). In contrast, a case–control study derived from the United Kingdom General Practice Research Database among older adults showed that long-term use of metformin (compared with no use) was associated with 71% increased risk of developing Alzheimer’s disease (Imfeld et al., 2012). Similarly, in the Australia Primary Research in Memory study (~50% being cognitively impaired), patients with diabetes who were metformin users had 2.23 times worse cognitive performance compared to non-users (Moore et al., 2013).
Metformin’s anti-depression and anti-frailty effects are encouraging. However, the data are rather limited. With regard to depression, data suggest that metformin facilitates adult (hippocampal dentate gyrus) neurogenesis and enhanced spatial learning in mice (Wang et al., 2012). Regarding frailty, metformin’s insulin-sensitizing and anti-inflammation properties could in principle delay the onset of frailty (Barzilay et al., 2007). So far, only one existing clinical study has demonstrated that metformin use alone (compared to sulfonylureas alone) was associated with reduced likelihood of frailty-related diseases (FRD) in older adults with T2D (Wang, Lorenzo, et al., 2014).
The goal of the current study is to assess the heterogeneity of metformin’s effect on the co-development ARCs among healthy older adults with T2D by (i) identifying distinct strata of longitudinal (co)development of ARCs (including CVD, cancer, depression, dementia, and FRD) in older men with T2D; and (ii) estimating the differential effects of metformin on the developmental trajectory of each ARC and 9-year mortality rate by ARC class.
2. Subjects, material and methods
2.1. Study cohorts
The study cohort was derived from the Veterans Administration (VA) Electronic Medical Records (EMR) during 2002–2012 (Reiber & Boyko, 2004). The following inclusion and exclusion criteria were used to identify the study cohort: men who were ≥65 years old in 2003 and had (i) ≥1 diagnosis for T2D (International Classification of Diseases, Ninth Revision (National Center for Health Statistics & CDC) or ICD9 = 250.xx besides 250.01) in 2002 and 2003, and (ii) ≥1 outpatient visit per year during the follow-up period; but did not have any of the following: (i) any diagnoses for ARCs during 2002–2003, (ii) liver or renal diseases during the study period, (iii) any prescription of GLM prior to 2003 (i.e., patients were GLM naïve prior to 2003 to be sure of GLM initiation dates), and (iv) any prescription of thiazolidinedione or insulin during the study period (GLM classes used in the cohort included sulfonylureas, biguanides, meglitinides, and alpha-glucosidase inhibitors). That is, patients in this study were GLM naïve prior to 2003 and GLM classes used in the cohort included sulfonylureas, biguanides (metformin), meglitinides, and alpha-glucosidase inhibitors. This study was approved by the Institutional Review Board at the University of Texas Health Science Center at San Antonio and South Texas Veterans Health Care System.
2.2. Measurements and variables
Dependent variables are indicators of ARC diagnoses each year during 2004–2012:
Cancer: any diagnosis for ICD9:140–208 except for 173.
Dementia: any diagnosis for ICD9: 290.xx.
CVD: any diagnosis for ICD9: 414.x, 410, 411–414.9, 428–428.1, 430–434.9, 436, 443.8–443.9.
Depression: any diagnosis for ICD9 296.2, 296.3, 296.82, 298.0, 300.4, 301.12, 307.44, 309.0, 309.1, 309.28, 311.
FRD include: anemia (ICD9: 280.0, 280.8–280.9, 281, 285.9), fall (ICD9: E800–E804, E850–E854), head injury (ICD9: 800–804, 850–854), other injury (ICD9: 805.6, 805.7, 806.6, 806.7, 807.0, 807.1, 808, 810, 811–814, 818–819, 821–825, 827–829), coagulopathy (ICD9: 286, 287.1, 287.3, 287.4, 287.5), weight loss (ICD9: 260–263), gait disorder (ICD9: 781.3, 781.2, 719.7). These diagnoses are either primary frailty characteristics, or have demonstrated association with the frailty phenotype (Barzilay et al., 2007; Gremese & Ferraccioli, 2011), mortality (Wang, Lorenzo, et al., 2014) and healthcare utilization (Pugh et al., 2014).
Metformin exposure was a dichotomized variable indicating ≥180 days of prescription for metformin versus no prescription, where the metformin exposure cutpoint was determined based on prior studies (Currie et al., 2009; Lehman et al., 2012). Thus in this study cohort, the metformin users were those who had ≥180 days of prescriptions for metformin, and the non-metformin users were those who had no prescription for metformin during the study period, where each group could be exposed to any other GLMs besides thiazolidinedione or insulin. Covariates adjusted for in the analyses included age, race/ethnicity, Charlson comorbidity score (a validated scale for quantifying comorbidity) (Charlson, Pompei, Ales, & MacKenzie, 1987), means of body mass index (BMI), hemoglobin A1c (HbA1c), and low-density lipoprotein (LDL) during the follow-up period, and statin use.
2.3. Analysis
Following a two-step causal modeling technique (Wang, Jo, & Brown, 2014), we first identified distinct ARC trajectory strata using latent class analysis (LCA), and then assessed the effects of metformin within each ARC trajectory class. LCA is a statistical clustering technique to identify distinct unobserved subgroups within a population based on multivariate binary outcomes (such as multiple repeated measures of binary outcomes) such that individuals within each subgroup (or so called latent class) have similar probabilities of attaining the same value of those binary outcomes. In Step 1, we conducted initial LCA of repeated measures of yearly ARC diagnoses (binary outcomes) separately for metformin users and non-users, where each class was characterized by a distinct pattern of ARC diagnosis probabilities during 2004–2012. The LCA consisted of (i) pre-specifying the number of ARC trajectory classes; (ii) conditioned on each ARC class, modeling the log-odds of each ARC diagnosis as a linear function of a suitable time scale with trajectory parameters (such as intercept and slope) varying between classes and random effects associated with trajectory parameters to account for within class correlations; and (iii) multinomial logistic regression modeling of ARC class membership to identify predictors associated with the ARC classes. More specifically, in our LCA, conditioned on the ARC class membership, the log-odds of an ARC diagnosis each year during 2004–2012 was modeled as a linear function of logarithm of time (due to curvilinearity trend of the ARC trajectories), and predictors of ARC trajectory class membership included age, race/ethnicity, Charlson comorbidity score, statin use, and change in HbA1c, LDL, and BMI. Each ARC trajectory class identified by LCA represents a subgroup who shared similar probabilities of developing ARCs over time and similar correlations among ARCs conditioned on metformin use and covariates. Each LCA was run using Mplus 6.11 allowing 20 different start values to ensure global maximization of the model estimates. The best fitting LCA model was sought using Bayesian information criterion (BIC): models with smaller BIC values indicating better fit. We conducted LCA of ARCs assuming 2–5 classes since the model fit did not improve when assuming 5 or more classes.
Once the best-fit LCA models were obtained under metformin use and no use, we conducted subsequent LCA to estimate ARC classes under both metformin use and no use jointly using data from the entire cohort. To ensure unique solution of model estimates and comparable baseline ARC risk between metformin users and non-users within each ARC class, we constrained the model such that the intercept of each ARC trajectory (or the baseline prevalence of the ARC) for metformin users was similar to that of the non-users.
In Step 2 analysis, we first applied the pseudoclass technique to estimate each individual’s ARC class membership by drawing a random sample from the multinomial distribution based on posterior probabilities of ARC class membership conditioned on patients’ ARC diagnoses, metformin use, and covariates observed. To enhance the causal interpretation of the effects associated with metformin use in each ARC trajectory, we incorporated the inverse propensity score weighting technique to balance baseline covariates (including age, race/ethnicity, HbA1c, LDL, BMI, and Charlson comorbidity score at baseline) between metformin users and non-users within each ARC class. More specifically, in Step 2 analysis, conditioned on each ARC class (estimated from the pseudoclass technique) for both metformin users and non-users, we modeled the log-odds of each ARC as a linear function of log(time) and the interaction between metformin use and log(time) with the inverse propensity scores of metformin use being incorporated as weights to balance baseline covariates between metformin users and non-users and random effects to account for within class correlation.
Furthermore, we assessed the effect of metformin on mortality by ARC class using logistic regression analyses to compute the log-odds of mortality associated with metformin use adjusting for covariates (age, race/ethnicity, Charlson comorbidity score, BMI, HbA1c, statin use) and propensity scores of metformin use.
3. Results
Our analyses identified four distinct ARC trajectory classes over a 9-year period for both metformin users and non-users. Each ARC class was labeled to capitalize the most prevalent ARC diagnosis observed in that class. Table 1 contrasts the demographic and baseline clinical characteristics between metformin users and non-users. Fig. 1 shows the mean ARC trajectories (probabilities of ARC diagnoses over time) under metformin use versus no use for each ARC class. Table 2 summarizes the demographic and baseline and clinical characteristics of each ARC trajectory class. The log-odds of ARCs over time for each trajectory class and the associated metformin effects are shown in Table 3. Detailed results are described below.
Table 1.
Baseline characteristics by metformin use status.
| Metformin | Non-usersa
|
Usersa
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| N | 32,811 | 8393 | ||
| Age | 75.15 | 5.78 | 72.49 | 5.15 |
| Non-Hispanic White | 80% | 80% | ||
| Hispanics | 15% | 12% | ||
| African American | 5% | 8% | ||
| Charlson Scores | 3.02 | 2.45 | 2.93 | 2.11 |
| Statin use | 0.52 | 0.50 | 0.79 | 0.41 |
| Baseline HbA1c (%) | 6.41 | 0.96 | 6.96 | 0.86 |
| Baseline LDL(mg/dL) | 109.31 | 26.76 | 104.82 | 23.45 |
| Baseline BMI (kg/m2) | 30.38 | 5.58 | 30.05 | 4.73 |
| Baseline DBP (mg/L) | 72.92 | 9.12 | 72.30 | 8.24 |
| Baseline SBP (mg/L) | 140.44 | 16.08 | 136.74 | 14.06 |
Abbreviations: HbA1c: Hemoglobin A1c. BMI: body mass index. LDL: Low-density lipoprotein. DBP: diastolic blood pressure. SBP: systolic blood pressure.
The differences between metformin users and non-users are all statistically significant with p < 0.01.
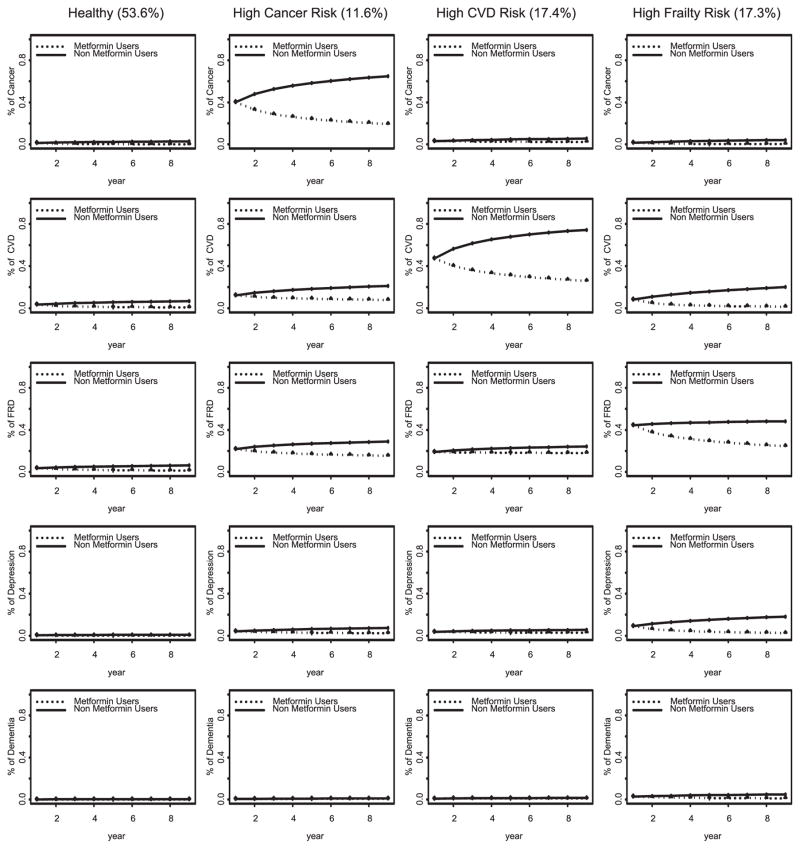
Fig. 1.
Effects of metformin on age-related comorbidity trajectory classes.
Table 2.
Cohort characteristics by age-related comorbidity class during study period.
| ARC Stratum | Healthy | High Cancer Risk | High CVD Risk | High Frailty Risk |
|---|---|---|---|---|
| N | 22,841 | 4669 | 6997 | 6697 |
| Agea | 74.3 (5.8) | 74.3 (5.6) | 75.2 (5.7) | 75.3 (5.8) |
| Non-Hispanic White | 82.2% | 75.9% | 82.6% | 74.5% |
| Hispanic | 5.3% | 6.1% | 5.0% | 7.5% |
| African American | 12.5% | 18% | 12.4% | 18% |
| Charlson Score | 2.1 (1.7) | 5.0 (3.2) | 4.1 (2.4) | 3.4 (2.3) |
| Metformin use | 20.1% | 19.7% | 19.2% | 22.9% |
| Statin use | 53.4% | 52.3% | 73.6% | 60.2% |
| HbA1c during study (%) | 6.6 (.87) | 6.5 (.86) | 6.5 (.80) | 6.4 (.84) |
| LDL during study (mg/dL)a | 92.0 (24.7) | 91.7 (26.3) | 85.2 (23.3) | 88.5 (23.5) |
| DBP during study | 70.5 (9.0) | 69.4 (8.3) | 68.5 (8.9) | 69.5 (8.7) |
| SBP during study | 133.7 (15.0) | 132.7 (14.2) | 132.4 (15.5) | 133.3 (14.6) |
| BMI during study (kg/m2)a,b | 29.9 (5.3) | 28.0 (4.8) | 29.3 (5.8) | 27.4 (5.1) |
| Death | 43.5% | 42.1% | 50.5% | 50.1% |
All other unmarked variables were significantly different between classes based on the χ2 test adjusting for multiple comparison (i.e., p-value < 0.0125).
Abbreviations: HbA1c: Hemoglobin A1c. BMI: body mass index. LDL: Low-density lipoprotein. DBP: diastolic blood pressure. SBP: systolic blood pressure.
No significant difference between the High Cancer Risk and Healthy classes.
No significant difference between the High CVD Risk and Healthy classes.
Table 3.
Trend of log-odds of age-related comorbidity (ARC) and metformin effect by ARC class.
| ARC Classa,b | Intercept | Slope (p-value) | Metformin effect | (p-valued) | |
|---|---|---|---|---|---|
| Healthy | Cancer | −4.31 | 0.35 (<0.01) | −1.66 | (0.02) |
| CVD | −3.36 | 0.33 (<0.01) | −1.12 | (<0.01) | |
| FRD | −3.29 | 0.26 (<0.01) | −0.77 | (<0.01) | |
| Depression | −5.28 | 0.30 (<0.01) | −1.46 | (<0.01) | |
| Dementia | −6.58 | 0.17c (0.12) | −0.54 | (<0.01) | |
| High Cancer Risk | Cancer | −0.40 | 0.46 (<0.01) | −0.93 | (<0.01) |
| CVD | −1.97 | 0.30 (<0.01) | −0.53 | (<0.01) | |
| FRD | −1.28 | 0.17 (<0.01) | −0.37 | (<0.01) | |
| Depression | −3.17 | 0.29 (<0.01) | −0.55 | (<0.01) | |
| Dementia | −5.41 | 0.42 (<0.01) | −0.19c | (0.50) | |
| High CVD Risk | Cancer | −3.49 | 0.30 (<0.01) | −0.42 | (<0.01) |
| CVD | −0.11 | 0.54 (<0.01) | −0.97 | (<0.01) | |
| FRD | −1.46 | 0.14 (<0.01) | −0.17 | (<0.01) | |
| Depression | −3.27 | 0.21 (<0.01) | −0.33 | (<0.01) | |
| Dementia | −4.74 | 0.25 (<0.01) | −0.15c | (0.45) | |
| High Frailty Risk | Cancer | −4.13 | 0.45 (<0.01) | −1.46 | (<0.01) |
| CVD | −2.43 | 0.48 (<0.01) | −1.37 | (<0.01) | |
| FRD | −0.22 | 0.07 (<0.01) | −0.48 | (<0.01) | |
| Depression | −2.31 | 0.36 (<0.01) | −0.98 | (<0.01) | |
| Dementia | −3.60 | 0.29 (<0.01) | −0.72 | (<0.01) |
Abbreviations: ARC: age-related comorbidities. CVD: cardiovascular diseases. FRD: frailty-related diseases.
The likelihood of an ARC diagnosis in year t of the study for non-users of metformin was estimated by: exp (intercept + slope*log(t))/{1+ exp (intercept + slope*log(t))}.
The likelihood of an ARC diagnosis in year t of the study for metformin users was estimated by: exp (intercept + [slope + Metformin effect]*log(t))/{1+ exp (intercept + [slope + Metformin effect]*log(t))}.
Not statistically significantly different from 0.
Statistical significance of the difference in the rate of change in the odds of ARC per time unit (log(year)) between metformin users and non-users.
3.1. Age-related comorbidity trajectory classes and effects of metformin
The Healthy Class consisted of 53.6% of the cohort who had relatively lower likelihood of all ARCs (see column 1 of Fig. 1). The log-odds of cancer, CVD, depression, and FRD diagnoses increased significantly over time, while the odds of dementia stayed stable over time. Among non-users of metformin, the likelihoods for a cancer diagnosis increased from 1.3% in year 1 to 2.8% in year 9, from 3.6% to 6.7% for CVD, from 3.6% to 6.2% for FRD, and from 0.5% to 1.0% for depression. Metformin use was associated with a significant reduction in the temporal increase of each ARC (on the log-odds scale, p ≤ 0.01). By year 9, metformin was associated with an absolute reduction of 2.77% in the diagnosis of cancer, 6.1% reduction in CVD, 5.0% reduction in FRD, 0.9% reduction in depression, and 0.14% reduction in dementia.
The High Cancer Risk Class consisted of 11.6% of the cohort who had a relatively higher likelihood of having been given a cancer diagnosis over time (see column 2 of Fig. 1). There was a significant temporal increase in each ARC diagnosis, and the magnitude of the increase was the greatest in cancer. Among non-users of metformin, the likelihood of a cancer diagnosis increased from 40.1% in year 1 to 64.7% in year 9, from 12.2% in year 1 to 21.0% in year 9 for CVD, from 21.7% to 28.9% for FRD, from 4.0% to 7.4% for depression, and from 0.4% to 1.1% for dementia. Metformin was associated with a significant reduction in the temporal increase in all ARCs except for dementia, and the magnitude of the metformin impact was greatest in cancer. By year 9, metformin was associated with a 45.5% reduction in cancer, 13.3% reduction in CVD, 13.7% reduction in FRD, and 5.0% reduction in depression diagnoses.
The High CVD Risk Class consisted of 17.6% of the cohort who had the highest likelihood of CVD diagnosis over time (see column 3 of Fig. 1). The likelihood of a diagnosis for FRD, depression, or dementia over time in this class was similar to that in the High Cancer Risk class. The temporal increase associated with ARC diagnoses in this class was the greatest for CVD. Among non-users of metformin, the proportion of cancer diagnosis increased from 2.9% in year 1 to 5.5% in year 9, CVD diagnosis increased from 47.1% in year 1 to 74.5% in year 9, 18.9% to 24.2% in FRD, 3.6% to 5.6% in depression, and 0.9% to 1.5% in dementia. Metformin was associated with a significant reduction in the rate of change in the diagnosis of all ARC (p < 0.01) except for dementia. By year 9, metformin was associated with 3.2% decrease in the cancer diagnosis, 48.6% decrease in CVD, 6.3% decrease in FRD, and 2.8% decrease in depression.
The High Frailty Risk Class consisted of 17.2% of the cohort who had the highest likelihood of FRD diagnoses over time, and a modest likelihood of CVD or cancer diagnosis (see column 4 of Fig. 1). There was a significant increase in the occurrence of all ARCs. Among non-users of metformin, the likelihood of acancer diagnosis increased from 1.6% in year 1 to 4.1% in year 9, 8.1% to 20.0 in CVD, 44.4% to 48.2% in FRD, 9.0% to 18.1% in depression, and 2.7% to 4.9% in dementia. Metformin was associated with a significant reduction in the diagnosis of all ARCs over time, and the magnitude of this impact was the greatest on FRD. By year 9, metformin was associated with a 3.9% decrease in cancer, 18.8% decrease in CVD, 23.8% decrease in FRD, 15.6% decrease in depression, and 3.8% decrease in dementia diagnoses.
3.2. Patient characteristics of ARC classes (see Table 2)
As shown in Table 2, compared to non-Hispanic white (NHW), both Hispanics and African Americans (AA) were more likely to be in the High Frailty Risk class but less likely to be in the High CVD Risk class. AA had a greater likelihood of being in the High Cancer Risk class compared to NHW or Hispanics. The derivation for the posterior probability of belonging to an ARC class conditioned on covariates is shown in the footnote of Table 2. The Charlson comorbidity score was significantly higher in both High Cancer Risk class and High CVD Risk class compared to the other ARC classes.
Both the High Frailty Risk class and High CVD Risk class were about 1 year older (yet statistically significant) than the Healthy or the High Cancer Risk class.
The mean HbA1c for the Healthy class was maintained at 6.6% throughout the study period. Similar HbA1c levels were observed in other ARC classes.
The baseline LDL was also similar across ARC classes: the mean LDL ranged between 106.7 mg/dL and 108.9 mg/dL (see Supplement Table 1). All classes had a significant LDL decline during the study period. The amount of decline in LDL over time was consistent with the between-class difference in statin use: the proportion of statin use was 53.4%, 52.3%, 60.2% and 73.6% for the Healthy, High Cancer Risk, High Frailty Risk, and High CVD Risk classes, respectively.
Mean BMI during the study period in the High Frailty Risk class was 2.2–2.6 kg/m2 (7%) lower compared to the mean BMI in the other ARC classes (with mean BMI falling between 28.0–29.9 kg/m2).
Although the differences in blood pressures between ARC classes were statistically significant, these differences were not clinically significant.
3.3. Effect of metformin on mortality by ARC class
As shown in Table 2, the unadjusted all-cause mortality rate was higher in both the High Frailty Risk class (50.1%) and the High CVD Risk class (50.5%) compared to the High Cancer Risk class (42.1%) and the Healthy class (43.5%). Metformin use was associated with a reduced mortality rate in all ARC classes: adjusted ORs of mortality associated with metformin use was 0.53 (95% CI = (0.50, 0.57)), 0.72 (95% CI = (0.62, 0.83)), 0.58 (95% CI = (0.52, 0.64)), 0.39 (95% CI = (0.35, 0.43)) for the Healthy, High Cancer Risk, High CVD Risk, and High Frailty Risk classes, respectively (see Table 4).
Table 4.
Effect of metformin on mortality (odds ratio of mortality associated with metformin use) by age-related comorbidity class.
| ARC Class | OR | 95% CI |
|---|---|---|
| Healthy | 0.53 | (0.50, 0.57) |
| High Cancer Risk | 0.72 | (0.62, 0.83) |
| High CVD Risk | 0.58 | (0.52, 0.64) |
| High Frailty Risk | 0.39 | (0.35, 0.43) |
Abbreviations: ARC: age-related comorbidities. CVD: cardiovascular diseases. FRD: frailty-related diseases.
4. Discussion
This study identified four distinct ARC trajectory phenotypes in healthy older male veterans with T2D over a 9-year period: the Healthy class (53.6%), the High Cancer Risk class (11.6%), the High CVD Risk class (17.6%), and the High Frailty Risk class (17.2%). We showed that metformin had differential effects on ARCs across ARC classes. In particular, the effect of metformin on reducing a specific ARC occurrence was the greatest in the class with the highest risk for that condition (e.g., the greatest reduction in CVD was found in the High CVD Risk class), and these benefits appeared to further reduce mortality (reduction in mortality ranged 28%–61% across ARC classes). Thus, these results suggest that metformin could be promising for ARC prevention and life extension in older adults with T2D. However, the timing of ARC intervention seems critical in these older patients among whom 47% become high risk for ARC (i.e., 47% were in the non Healthy classes) within a one-year period after two years of being free from any ARC diagnoses. The moderate to large effects associated with metformin use on ARCs observed in this study appear to be comparable with some existing studies (which did not require patients to be ARC free at baseline). For example, the meta-analysis by Gandini, Puntoni, Heckman-Stoddard, et al. (2014) found that metformin use was associated with 31% reduction cancer (RR = 0.69, 95% CI: 0.52–0.90), and 34% reduction in cancer mortality (RR = 0.66, 95% CI: 0.54–0.81; I2 = 21%); and the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction 2 Study (Mellbin, Malmberg, Norhammar, Wedel, & Ryden, 2011) showed that in patients with T2D and suspected acute myocardial infarction, metformin was associated with a 35% decrease in mortality rate (HR = 0.65, 95% CI 0.47–0.90; p = 0.01) and 75% reduction in death from malignancies (HR = 0.25, 95% CI 0.08–0.83; p = 0.02). Since statin use (50%–73%) was common in this cohort, it is likely that the relatively large metformin effect was partly due to the synergistic effect between metformin and statins (Lehman et al., 2012).
The variation of the effect of metformin on CVD between ARC classes observed in this study added further insight to the observation of Lamanna et al. regarding the heterogeneity of metformin’s CVD prevention effect. The meta-analysis by Lamanna et al. (2011) suggested that metformin may be more effective in studies with a longer follow-up period. Note that in the analysis by Lamanna et al., the UKPDS was the only long-term study that had a comparable follow-up period and comparison group to our study group. However, the UKPDS cohort was much younger than our study cohort (mean age of 58 vs. 74 years). Lamanna et al. (2011) also noticed that metformin was more effective for CVD outcomes in studies with participants of age ≤ 30 years (Fig. 4 in Smith et al.). However, these data were from studies of women with polycystic ovary syndrome as opposed to our cohort of older male veterans with T2D. Furthermore, in the analysis by Lamanna et al., only two studies had similar glycemic control as ours (mean HbA1c below 6.6%). However, these studies involved patients with impaired glucose tolerance and mean age of 45–50 years versus ours involving patients with T2D and mean age of 74–75 years old. These data collectively suggest that the heterogeneous effect of metformin on CVD could involve a more complex combination effect associated with age, age-related diseases, or other medication use.
Our study found the anti-cancer impact of metformin to be the greatest in the High Cancer Risk class (18% reduction over a 9-year period), fairly limited reduction (1.2%–1.8%) in the High Frailty Risk class and Healthy class, and non-significant reduction in the High CVD Risk class. The variation of the metformin’s anti-cancer effect between ARC classes shed new light on those previously reported in the literature which suggested that metformin’s anti-cancer effect could vary by (i) the concomitant medication use (attenuation by use of sulfonylureas (Thakkar et al., 2013) and enhancement by statins use (Lehman et al., 2012)), (ii) the comparison group (Thakkar et al., 2013), or (iii) the race/ethnicity composition of the study population (Chen et al., 2009; Jablonski, McAteer, de Bakker, et al., 2010). Note that in Table 2, statin use was less common in the High Cancer Risk class compared to other ARC classes. Thus the superior anti-cancer effect of metformin seen in the High Cancer Risk class compared to other ARC classes was likely due to other factors (e.g., clinical) beyond the enhancement of the statin’s anti-cancer effect that was reported previously (Lehman et al., 2012). Also, although both the High Cancer Risk class and the High Frailty Risk class had a higher proportion of race/ethnic groups (Hispanics and AA) who tended to respond better to metformin (Chen et al., 2009; Jablonski et al., 2010), the superior anti-cancer effect of metformin was observed only in the High Cancer Risk class but not the High Frailty Risk class. These data suggest that the cancer prevention effect of metformin could be modulated by biological and clinical (e.g., ARC phenotype) factors jointly.
The effect of metformin on reducing FRD was found to be the greatest in the High Frailty Risk class (20.1% reduction over a 9-year period). This finding was consistent with our previous study of veterans (97% men) with T2D who were not completely free from ARCs at baseline (Wang, Lorenzo, et al., 2014). The discrepancy of the metformin effect (magnitude-wise) between our previous study (Wang, Lorenzo, et al., 2014) and the present study could be due to their differences in the baseline characteristics and/or the composition of the metformin and non-metformin groups. In the previous study, metformin use alone was compared to sulfonylurea use alone (Wang, Lorenzo, et al., 2014). In the present study, the metformin group consisted of those who used metformin as the sole GLM (28.2%), those who used both metformin and sulfonylurea (71.5%), and those who used metformin and other non-sulfonylurea GLM (0.3%); while in the non-metformin group, 51.6% used sulfonylurea as the sole GLM, 48.1% did not use any oral GLM, and 0.3% used non-sulfonylurea GLM with or without sulfonylurea. In this High Frailty Risk class, metformin was also associated with a significant reduction in dementia and depression diagnoses. This result appeared to support the hypothesis of Guo et al. based on an analysis of a mid-life cohort with T2D (Guo et al., 2014) — the amelioration of depression by metformin treatment could be attributable to enhanced cognitive function.
It is worth noting the difference in mortality rates across ARC classes. Both the High CVD Risk and the High Frailty Risk classes had the highest mortality rates compared to the Healthy class and the High Cancer Risk class. On the other hand, the Charlson comorbidity score, a commonly used index for predicting mortality rate, was found to be higher in the High Cancer Risk and the High CVD Risk classes, but lower in the High Frailty Risk class. These data suggest that the combination use of the Charlson score with ARC phenotype could enhance the prediction of mortality rate in older men with T2D.
Regarding metformin’s life-extension effect, our previous study suggested that the reduced all-cause mortality by metformin use in men with T2D might be mediated by its frailty prevention effect (Wang, Lorenzo, et al., 2014). Our current data further suggested that metformin’s ARC prevention effect might be extended to increase lifespan: the life-extension impact of metformin was the greatest in the High Frailty Risk stratum (OR = 0.39, 95% CI = 0.35–0.43), similar between the Healthy Stratum (OR = 0.53, 95% CI = 0.50–0.57) and the High CVD Risk Stratum (OR = 0.58, 95% CI = 0.52–0.64), and the least in the High Cancer Risk Stratum (OR = 0.72, 95% CI = 0.62–0.73). These data could offer an explanation for the finding of Boussageon et al. (2012) of a significant heterogeneity of metformin’s life-extension effects in a meta-analysis of 13 RCTs with N = 13,110.
This study has limitations. First, recognizing the non-experimental nature of the study design, we have conducted state-of-art causal modeling to overcome the shortfall. This method, like any causal modeling, relies on the assumption of no unmeasured confounding (Wang, Jo, et al., 2014). Thus the validity of our results could depend on whether any unobserved predictors associated with ARCs or mortality (such as blood pressure lowering medication that was not available in our dataset) were balanced between metformin users and non-users. Second, the study cohort is exclusively men. Based on the data from the Diabetes Prevention Program (West, Elaine Prewitt, Bursac, & Felix, 2008), we expected that the beneficial effects of metformin on ARCs in this male cohort could be non-inferior to those in the female counterpart. Nevertheless, our results require verification by randomized trials with both men and women. Third, while it would be useful to assess the dose–response effects of metformin on ARCs, these data however were not available in this study.
In conclusion, this study identified distinct developmental trajectories of ARCs in older men with T2D who maintained good glycemic control over a 9-year period. We also found differential impacts of metformin on ARC prevention and life extension across these ARC classes. While the positioning of metformin among other GLMs in terms of comorbidities and mortality prevention requires further investigation (for example, empagliflozin has shown potential CVD prevention benefit in adults with T2D (Zinman et al., 2015)), this study provides important data for designing prospective studies aimed at examining the pleiotropic effects of metformin against other GLMs on ARCs.
Supplementary Material
Acknowledgments
This work is partly supported by the National Cancer Institute [grant number R21CA161180], National Institute on Aging [grant number P30AG04427], National Institute on Drug Abuse [grant number R01DA31698], and resources at the South Texas Veterans Health Care System, San Antonio, Texas.
Footnotes
The authors declare that there is no conflict of interest.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jdiacomp.2017.01.013.
References
- Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Archives of Internal Medicine. 2007;167(7):635–641. doi: 10.1001/archinte.167.7.635. http://dx.doi.org/10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, … Muggeo M. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: The Bruneck study. Diabetes Care. 2007;30:318–324. doi: 10.2337/dc06-0919. http://dx.doi.org/10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- Boussageon R, Supper I, Bejan-Angoulvant T, Kellou N, Cucherat M, Boissel JP, … Cornu C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: A meta-analysis of randomised controlled trials. PLoS Medicine. 2012;9(4):e1001204. doi: 10.1371/journal.pmed.1001204. http://dx.doi.org/10.1371/journal.pmed.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Teranishi K, Li S, Yee SW, Hesselson S, Stryke D, … Giacomini KM. Genetic variants in multidrug and toxic compound extrusion 1, hMATE1, Alter transport function. The Pharmacogenomics Journal. 2009;9:127–136. doi: 10.1038/tpj.2008.19. http://dx.doi.org/10.1038/tpj.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–1777. doi: 10.1007/s00125-009-1440-6. http://dx.doi.org/10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262–2272. doi: 10.2337/db13-1954. http://dx.doi.org/10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- Djiogue S, Nwabo Kamdje AH, Vecchio L, Kipanyula MJ, Farahna M, Aldebasi Y, Seke Etet PF. Insulin resistance and cancer: The role of insulin and IGFs. Endocrine-Related Cancer. 2013;20:R1–R17. doi: 10.1530/ERC-12-0324. http://dx.doi.org/10.1530/ERC-12-0324. [DOI] [PubMed] [Google Scholar]
- Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio longitudinal study of aging. Journal of the American Geriatrics Society. 2012;60(4):652–660. doi: 10.1111/j.1532-5415.2011.03882.x. http://dx.doi.org/10.1111/j.1532-5415.2011.03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Bravo G, Hudon C, Lapointe L, Almirall J, Dubois MF, Vanasse A. Relationship between multimorbidity and health-related quality of life of patients in primary care. Quality of Life Research. 2006;15:83–91. doi: 10.1007/s11136-005-8661-z. http://dx.doi.org/10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer prevention research (Philadelphia, Pa) 2014;7(9):867–885. doi: 10.1158/1940-6207.CAPR-13-0424. http://dx.doi.org/10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, van den Bos GA. Causes and consequences of comorbidity: A review. Journal of Clinical Epidemiology. 2001;54:661–674. doi: 10.1016/s0895-4356(00)00363-2. http://dx.doi.org/10.1016/S0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- Gremese E, Ferraccioli G. The metabolic syndrome: The crossroads between rheumatoid arthritis and cardiovascular risk. Autoimmunity Reviews. 2011;10(10):582–589. doi: 10.1016/j.autrev.2011.04.018. http://dx.doi.org/10.1016/j.autrev.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Guo M, Mi J, Jiang QM, Xu JM, Tang YY, Tian G, Wang B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clinical and Experimental Pharmacology & Physiology. 2014;41(9):650–656. doi: 10.1111/1440-1681.12265. [DOI] [PubMed] [Google Scholar]
- Gupta A, Bisht B, Dey CS. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology. 2011;60:910–920. doi: 10.1016/j.neuropharm.2011.01.033. http://dx.doi.org/10.1016/j.neuropharm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The San Antonio heart study. Diabetes Care. 2002;25(7):1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology Reviews. 2012;37:137–162. doi: 10.1038/npp.2011.205. http://dx.doi.org/10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden SE, Jenkins-Jones S, Currie CJ. Association between insulin monotherapy versus insulin plus metformin and the risk of all-cause mortality and other serious outcomes: A retrospective cohort study. PloS One. 2016;11(5):e0153594. doi: 10.1371/journal.pone.0153594. http://dx.doi.org/10.1371/journal.pone.0153594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Wahlqvist ML, Lee MS, Tsai HN. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. Journal of Alzheimer’s Disease. 2011;24:485–493. doi: 10.3233/JAD-2011-101524. http://dx.doi.org/10.3233/JAD-2011-101524. [DOI] [PubMed] [Google Scholar]
- Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: A population-based case–control study. Journal of the American Geriatrics Society. 2012;60(5):916–921. doi: 10.1111/j.1532-5415.2012.03916.x. http://dx.doi.org/10.1111/j.1532-5415.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, … Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. http://dx.doi.org/10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Jablonski KA, McAteer JB, de Bakker PIW, Franks PW, Pollin TI, Hanson RL … for the Diabetes Prevention Program Research Group. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes. 2010;59(10):2672–2681. doi: 10.2337/db10-0543. http://dx.doi.org/10.2337/db10-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob L, Kostev K, Rathmann W, Kalder M. Impact of metforminon metastases in patients with breast cancer and type 2 diabetes. Journal of Diabetes and its Complications. 2016;30(6):1056–1059. doi: 10.1016/j.jdiacomp.2016.04.003. http://dx.doi.org/10.1016/j.jdiacomp.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, … Schweiger S. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proceedings of the National Academy of Sciences of the United States of America (PNAS) 2010;107:21830–21835. doi: 10.1073/pnas.0912793107. http://dx.doi.org/10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall B, Rathmann W, Kostev K. Are sulfonylurea and insulin therapies associated with a larger risk of cancer than metformin therapy? A retrospective database analysis. Diabetes Care. 2015;38(1):59–65. doi: 10.2337/dc14-0977. [DOI] [PubMed] [Google Scholar]
- Lamanna C, Monami M, Marchionni N, Mannucci E. Effect of metformin on cardiovascular events and mortality: A meta-analysis of randomized clinical trials. Diabetes, Obesity and Metabolism. 2011;13(3):221–228. doi: 10.1111/j.1463-1326.2010.01349.x. http://dx.doi.org/10.1111/j.1463-1326.2010.01349.x. [DOI] [PubMed] [Google Scholar]
- Lehman DM, Lorenzo C, Hernandez J, Wang CP. Statin use as a moderator of metformin effect on risk for prostate cancer among type 2 diabetic patients. Diabetes Care. 2012;35:1002–1007. doi: 10.2337/dc11-1829. http://dx.doi.org/10.2337/dc11-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liccini A, Malmstrom TK, Morley JE. Metformin use and cognitive dysfunction among patients with diabetes mellitus. Journal of the American Medical Directors Association. 2016;17(11):1063–1065. doi: 10.1016/j.jamda.2016.08.026. http://dx.doi.org/10.1016/j.jamda.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Ryden L. Prognostic implications of glucose-lowering treatment in patients with acute myocardial infarction and diabetes: Experiences from an extended follow-up of the diabetes mellitus insulin-glucose infusion in acute myocardial infarction (DIGAMI) 2 study. Diabetologia. 2011;54:1308–1317. doi: 10.1007/s00125-011-2084-x. http://dx.doi.org/10.1007/s00125-011-2084-x. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty HWoodward M … Investigators, A. I. B. L. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36:2981–2987. doi: 10.2337/dc13-0229. http://dx.doi.org/10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics (d) Available at: http://www.cdc.gov/nchs.
- National Center for Health Statistics, CDC. ICD-9-CM guidelines, conversion table, and addenda. Classification of diseases, functioning, and disability. (Retrieved 2017–02-04) [Google Scholar]
- Novosyadlyy R, LeRoith D. Hyperinsulinemia and type 2 diabetes: Impact on cancer. Cell Cycle. 2010;9:1449–1450. doi: 10.4161/cc.9.8.11512. http://dx.doi.org/10.4161/cc.9.8.11512. [DOI] [PubMed] [Google Scholar]
- Pugh JA, Wang CP, Espinoza SE, Noel PH, Bollinger M, Amuan M, … Pugh MJ. Influence of frailty-related diagnoses, high-risk prescribing in elderly adults, and primary care use on readmissions in fewer than 30 days for veterans aged 65 and older. Journal of the American Geriatrics Society. 2014;62(2):291–298. doi: 10.1111/jgs.12656. [DOI] [PubMed] [Google Scholar]
- Rao AD, Kuhadiya N, Reynolds K, Fonseca VA. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality?: A meta-analysis of observational studies. Diabetes Care. 2008;31(8):1672–1678. doi: 10.2337/dc08-0167. http://dx.doi.org/10.2337/dc08-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber GE, Boyko EJ. Diabetes research in the Department of Veterans Affairs. Diabetes Care. 2004;27(Suppl 2):b95–b98. doi: 10.2337/diacare.27.suppl_2.b95. http://dx.doi.org/10.2337/diacare.27.suppl_2.B95. [DOI] [PubMed] [Google Scholar]
- Sahra IB, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, … Bost F. Targeting cancer cell metabolism: The combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Research. 2010;70:2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. http://dx.doi.org/10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, Baune BT. Depression and type 2 diabetes: Inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neuroscience & Biobehavioral Reviews. 2012;36:658–676. doi: 10.1016/j.neubiorev.2011.10.001. http://dx.doi.org/10.1016/j.neubiorev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Thakkar B, Aronis KN, Vamvini MT, Shields K, Mantzoros CS. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: A meta-analysis using primary data of published studies. Metabolism: Clinical and Experimental. 2013;62(7):922–934. doi: 10.1016/j.metabol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) group. Lancet. 1998;352(9131):854–865. http://dx.doi.org/10.1016/S0140-6736(98)07037-8. [PubMed] [Google Scholar]
- Wang CP, Jo B, Brown CH. Causal inference in longitudinal comparative effectiveness studies with repeated measures of a continuous intermediate variable. Statistics in Medicine. 2014;33(20):3509–3527. doi: 10.1002/sim.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CP, Lorenzo C, Espinoza SE. Frailty attenuates the impact of metformin on reducing mortality in older adults with type 2 diabetes. Journal of Endocrinology, Diabetes & Obesity. 2014;2(2):1031. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gallagher D, Devito LM, Cancino GI, Tsui D, He L, … Miller FD. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. http://dx.doi.org/10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the diabetes prevention program. Obesity. 2008;16(6):1413–1420. doi: 10.1038/oby.2008.224. http://dx.doi.org/10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factors categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. http://dx.doi.org/10.1161/01.CIR.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wnsperger NF. Metformin: Intrinsic vasculoprotective properties. Diabetes Technology & Therapeutics. 2000;2:259–272. doi: 10.1089/15209150050025230. [DOI] [PubMed] [Google Scholar]
- Wolff J, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. JAMA Internal Medicine. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. http://dx.doi.org/10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: Results from a population-based case–control study. Cancer Causes & Control. 2009;20(9):1617–1622. doi: 10.1007/s10552-009-9407-y. http://dx.doi.org/10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Research. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. The New England Journal of Medicine. 2015;373:2117–2128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.