Abstract
Linker histones (H1s) are a primary component of metazoan chromatin, fulfilling numerous functions, both in vitro and in vivo, including stabilizing wrapping of DNA around the nucleosome, promoting folding and assembly of higher order chromatin structures influencing nucleosome spacing on DNA and regulating specific gene expression. However, many molecular details of how H1 binds to nucleosomes and recognizes unique structural features on the nucleosome surface remain undefined. Numerous, confounding studies are complicated not only by experimental limitations, but the use of different linker histone isoforms and nucleosome constructions. This review summarizes the decades of research that has resulted in several models of H1 association with nucleosomes, and focuses on recent advances that suggest modes of binding may influence chromatin organization while also highlighting remaining questions.
Keywords: nucleosome, linker histone, DNA
Introduction
Linker histone family
Linker histones (H1s) are present with an abundance of about 1 per nucleosome in vivo (van Holde, 1989) and exhibit stoichiometric and preferential binding to nucleosomes in vitro (Hayes and Wolffe, 1993). [For a comprehensive review of nucleosome structure see (Cutter and Hayes, 2015)] The H1 family of proteins is less conserved between species than that of the core histones, varying in both sequence homology and number of non-allelic variants across eukaryotes (Happel and Doenecke, 2009; Talbert et al., 2012). Mammals for example have eleven subtypes, with seven that are found in somatic cell types (H1.0, H1.1 (H1a), H1.2 (H1.b), H1.3 (H1c), H1.4 (H1d), H1.5 (H1e), and H1X), and four that are found in embryonic or germ cells (H1t, H1T2, H1oo, and H1LS1), [for nomenclature ((Parseghian and Hamkalo, 2001; Talbert et al., 2012)], expressed uniquely during development and with tissue specificity (Millan-Arino et al., 2016; Pan and Fan, 2016; Parseghian and Hamkalo, 2001). Interestingly, individual H1 isotypes are more conserved across species than to other isotypes within a species, indicating selective pressure to maintain the diversity in H1s (Figure 1a).
Figure 1. Linker histone family and structure.
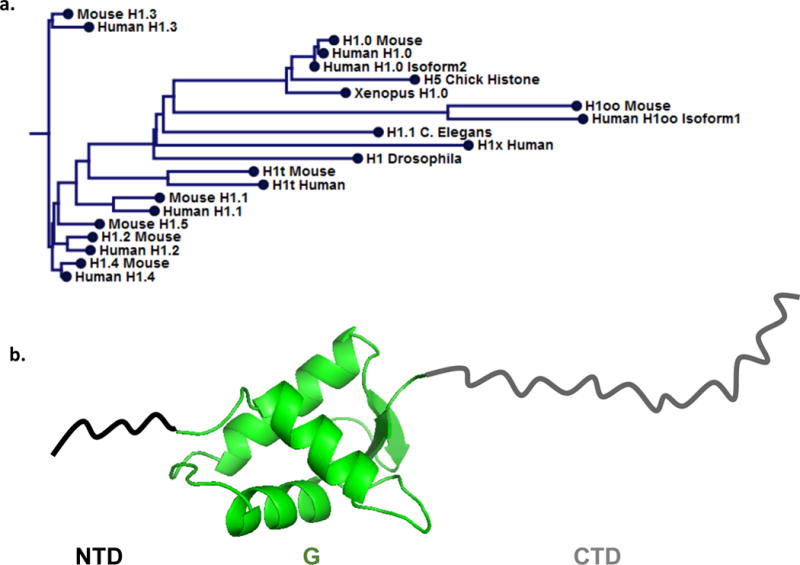
a. Phlyogram generated from multiple sequence alignment of indicated H1 sequences, both generated in CLC Sequence Viewer 7.0.2 from sequences obtained with the following Uniprot accession numbers: P02259, 78707158, P22844, P07305, P07305-2, 3878755, 4885373, 1426823, 4885375, 9845257, 4885377, 254588110, 4885379, 13430890, 4885381, 21426893, 5174449, 24475863, 19923865, 112807207, 20544168. The inferred evolutionary relationships show closer relationship of subtype across species than to other subtypes in the same species. b. Linker histone tripartite structure: structured (PDB ID: 1HST) globular domain (G-green), is flanked by a short N-terminal domain (NTD-black), and long C-terminal domain (CTD-grey), both unstructured.
Metazoan H1s have a tripartite structure, with a short, protease sensitive N-terminal tail domain (NTD), a central stably folded and protease resistant globular domain (G) and, a protease sensitive, highly basic, intrinsically disordered C-terminal domain (CTD), as illustrated in Figure 1b (Allan et al., 1980; Bohm and Mitchell, 1985). However, variations on this theme can be found, with some H1s exhibiting alternative domain structures, typically in lower eukaryotes (van Holde, 1989). For example, S. cerevisiae has a single H1-like protein, Hho1p, with a unique structure containing two globular domains connected by a short C-terminal tail-like domain (Landsman, 1996), whereas the somatic H1 of the ciliated protozoa T. thermophila resembles the C-terminal domain of metazoans and lacks a globular region (Hayashi et al., 1987; Wu et al., 1986). Though in higher eukaryotes the number of H1 variants within an organism is typically much larger, the tripartite domain structure is generally maintained, with differences in sequences primarily found in the C-terminal regions.
Although similar in overall characteristics, important differences distinguish H1 variants in metazoans. For example embryonic specific variants (i.e. H1oo, B4) feature both acidic and basic residues (mostly lysines) within their highly charged C-terminal regions, suggesting an attenuated ability to neutralize charge resulting in a less compacted chromatin structure. Somatic variants tend to be devoid or nearly devoid of acidic residues within the CTD, increasing the overall positive charge of this domain compared to embryonic variants, while variants associated with quiescent cell types (H1.0, H5) tend to have more arginines as well as the complement of lysine residues. This is consistent with biochemical evidence that the arginine binds more tightly to DNA than lysine, consistent with a more highly compacted chromatin in quiescent cells (Leng and Felsenfeld, 1966). Moreover, different H1 isotypes exhibit different motilities within live nuclei, which may be related to differential functions (Flanagan et al., 2016; Hendzel et al., 2004; Misteli et al., 2000; Stasevich et al., 2010).
Linker histone substructure
Early studies revealed that the globular domain is sufficient for structure-specific recognition of the nucleosome (Allan et al., 1980), though full length H1 was required for full compaction of chromatin. Though less conserved than core histones, the linker histone globular domain is more conserved and more hydrophobic than either N- or C-terminal regions. Comparison of the X-ray crystal structure of the H5 globular domain (GH5) (Ramakrishnan et al., 1993) to the tertiary structure of GH1 derived from NMR (Cerf et al., 1993; Cerf et al., 1994) demonstrates a remarkably similar 3D structure of the linker histone globular domains, albeit with slight differences in electrostatic potentials, which may relate to differences in function. Both globular domain structures contain a characteristic DNA-binding, winged-helix fold, similar to that found in several families of sequence-specific DNA binding transcription factors (Ramakrishnan et al., 1993), as well as two additional surfaces accessible for DNA binding,
The H1 NTD contains two unique regions, a proline and alanine rich subdomain and a shorter highly basic region near the globular domain (Bohm and Mitchell, 1985). Peptides derived from the NTD are unstructured in aqueous solutions but have the propensity to form α-helical structure in trifluoroethanol or in the presence of short DNA fragments (Vila et al., 2001). H1 peptides lacking the NTD are as effective as full-length H1 in inducing higher-order chromatin structure in vitro, but are less effective at conferring protection of 168 bp DNA from MNase digestion (Allan et al., 1986). It is also worth noting that H1 NTD alterations and deletions result in changes in binding affinities both in vitro and in vivo (Hendzel et al., 2004; Vyas and Brown, 2012). These observations suggest that although H1 NTD is not necessary for chromatin condensation, it may serve as an anchor for proper H1 positioning in such a way that seals off DNA entering and exiting the nucleosome.
The CTD of linker histones is sensitive to proteases and known to be disordered when the protein is free in solution (Bradbury et al., 1975). Moreover, the amino acid content of the CTD is characteristic of an intrinsically disordered protein domain (Hansen et al., 2006) and evidence suggests that the amino acid residue composition, rather than the specific sequence, is important for the chromatin condensing function of the CTD (Lu et al., 2009). Early studies indicated that CTD peptides exhibit secondary protein structural elements in the presence of DNA or structure- stabilizing solvents (Clark et al., 1988; Roque et al., 2005; Vila et al., 2001). Recent work employing FRET showed the H1 CTD undergoes a transition from a random coil conformation to a much more condensed structure upon H1 binding to nucleosomes, consistent with adoption of a defined fold or ensemble of folds (Caterino et al., 2011; Fang et al., 2012). Moreover, the CTD is required for high-affinity binding of H1 to nucleosomes in vitro and in vivo (Caterino et al., 2011; Hendzel et al., 2004) and for full compaction of chromatin (Allan et al., 1986; Lu et al., 2009; Lu and Hansen, 2004) and discontinuous regions of this domain have been identified as important for H1s chromatin condensation function (Lu et al., 2009; Lu and Hansen, 2004). The CTD also interacts with numerous nuclear factors, both in chromatin and in the nucleolus (Kalashnikova et al., 2016; Kalashnikova et al., 2013; Szerlong et al., 2015) and is subject to numerous post-translational modifications, reviewed elsewhere (Izzo and Schneider, 2016). Thus, one function of this intrinsically disordered region may be to accommodate disparate interactions with diverse macromolecular partners in the nucleus.
Linker histone interactions in chromatin
H1 binding to DNA
H1 binds to linear double stranded DNA in a salt-dependent, cooperative manner, to produce long filaments with multiple parallel strands of DNA, termed “tramtrack structures”(Clark and Thomas, 1986). This work indicated that H1s have multiple DNA-interacting surfaces and a propensity to organize nucleic acid into ordered structures. Trypsin digestion of H5 and subsequent analysis of DNA-GH5 complexes by electron microscopy reveal differences in DNA filaments bound by GH5, however centrifugation sedimentation assays of these complexes indicated cooperative modes of GH5 binding to DNA (Draves et al., 1992). The globular domain contains a characteristic DNA-binding, winged-helix domain with additional surfaces accessible for DNA interaction (Ramakrishnan et al., 1993). Mutation of residues in putative DNA binding domains reduced the ability of GH5 to form tram-track filaments characteristic of cooperative binding(Goytisolo et al., 1996). These GH5 DNA binding surfaces are important features for nucleosome structure-specific recognition by the globular domain, and will be discussed in further detail.
Linker histone-nucleosome interactions
Exploiting sensitivity of chromatin to nucleases, H1 was originally implicated as important for protecting linker DNA(Whitlock and Simpson, 1976). Subsequent analysis of micrococcal nuclease (MNase) digested chicken erythrocyte chromatin showed a defined digestion product larger than a nucleosome core particle, termed the chromatosome, which contains DNA fragments of ~168 bp, the four core histones and linker histone(Simpson, 1978). This early work indicated that H1 protects an additional 20 bp of DNA from nuclease digestion compared to nucleosome core particles, that is, on average, symmetrically disposed to either side of the 147 bp core DNA. While full length H1 may be required for full compaction of chromatin, the globular domain alone is sufficient to protect ~20 bp of DNA in the MNase digestion assay (Allan et al., 1980) and in the earliest model, GH1 was envisaged to bind at the nucleosome dyad, contacting the central wrap of nucleosome core DNA and the two 10 bp extensions of the linker DNA entering/exiting the structure. This 3-contact model was supported by DNase I footprinting studies of H1 and H5 within dinucleosomes, suggesting the globular domain contacts the DNA near nucleosomal DNA entry/exit points and the dyad axis, further proposing the NTD and CTD regions may account for partial protections of adjacent regions of linker DNA (Staynov and Crane-Robinson, 1988). However, a complicating factor in these studies was the use of dinucleosomes, so as to better preserve stoichiometric H1 binding, but it was unclear whether juxtaposed nucleosomes might contribute to the observed pattern of protection. Additional evidence for H1 protection at the dyad was provided by studies employing UV-dependent thymidine dimer production, which showed a clear effect of H1 occupancy on DNA at the nucleosome dyad in native chromatin (Pehrson, 1989).
In the following decades, many attempts to better characterize H1-nucleosome interactions resulted in additional models for how the globular domain interacts with the nucleosome surface (Crane-Robinson, 1997), summarized in Table 1. Nuclease protection, directed DNA cleavage, and chemical crosslinking studies of H1 and H5 bound nucleosomes, reconstituted on 5S RNA gene templates, indicated linker histones do not change the organization of DNA in the core, but did lead to protection of an additional 20 bp of DNA, asymmetrically distributed relative to the core particle, possibly contacting the inner surface of the DNA superhelix, near one end of the nucleosome core (Hayes et al., 1996; Hayes and Wolffe, 1993; Pruss et al., 1996). This data supported a model in which the globular domain inserts inside the superhelical DNA wrap, extending the ramp of charge provided by the core histones at one end of the nucleosome (Hayes et al., 1996; Pruss et al., 1996). An alternative bridging, 2-contact model was proposed based on site-directed protein-DNA photo-crosslinking to map GH5 on native chicken chromatosomes, wherein GH5 is positioned between the central turn of DNA near nucleosome dyad and one of the linker arms (Zhou et al., 1998).
Table 1. Linker histone-nucleosome interactions.
Table summarizes experimental system and resulting model of H1 globular domain location on nucleosome relative to dyad axis. Note the H1 subtype, nucleosome substrates and experimental techniques differ and information regarding H1 orientation and distinct interactions is unclear in many cases.
| Reference | Model | Experiment | H1 | Nucleosome |
|---|---|---|---|---|
| Allan et al., 1980 | On-axis, 3-contact | Mnase mapping | Calf thymus H1, GH1 Chicken H5, GH5 | chicken erythrocyte chromatin |
| Staynov and Crane-Robinson, 1988 | On-axis, 3-contact | Dnase I footprinting | H1, H5 | Dinucleosomes from chicken erythrocyte chromatin |
| Pruss et al., 1996 | Off-axis, inside DNA gyre | Site-directed crosslinking | Chicken GH5, Recombinant GH1°, Recombinant H1° ΔCTD | reconstituted with Xenopus borealis 5S RNA gene |
| (Hayes, 1996) | Off-axis, inside DNA gyre | Fe(II) EDTA conjugated linker histone directed cleavage | Recombinant H1° | reconstituted with Xenopus borealis 5S RNA gene |
| Zhou et al., 1998 | Bridging, 2-contact | Site-directed crosslinking | Recombinant GH5, | H1/H5 depleted chromatosomes from chicken erythrocytes |
| Brown et al., 2006 | major groove near the dyad and the minor groove of linker DNA | FRAP- intact cells, molecular modeling | Recombinant Mouse H1 GFP (CTD fusion)° | native chromatin |
| George et al., 2010M | inconsistent with H1° (Brown et al., 2006) model | FRAP- intact cells | Recombinant Mouse H1c GFP (CTD fusion) | native chromatin |
| Fan and Roberts, 2006 | On-axis, 3-contact | Computational docking | GH5 (PDB 1HST) | Nucleosome core particle (PDB 1AOI) with 16bp DNA extensions, one bent linker arm |
| Syed et al., 2010 | On-axis, 3-contact | ECM, hydroxyl radical footprinting, molecular modeling | Recombinant H1.5, Recombinant H1.5 ΔCTD | Mono-, di-, tri-, reconstituted with 200bp 601 DNA sequence |
| Zhou et al., 2013 | Off-axis, 2-contact | Solution, methyl-based NMR | Recombinant Drosophila Melanogaster H1 (H137–211) V53I, S56A, C81V, A97L | reconstituted with 167 bp 601 DNA sequence |
| Song et al., 2014 | Off-axis, 2-contact | Cryo-EM | Recombinant human H1.4 | reconstituted 12x 177-bp and 187 bp 601 DNA Sequence with recombinant Xenopus laevis histones |
| Zhou et al., 2015 | On axis, 3-contact | X-ray crystallography | Recombinant GH5 (H522–98) | reconstituted 167 bp 601 DNA sequence with recombinant Xenopus laevis histones |
Fluorescence recovery after photo-bleaching (FRAP) experiments to probe effects of mutations within the H1 globular domain on interaction with nucleosomes in living cells highlighted two important DNA binding regions in the globular domain (Misteli et al., 2000).These regions were previously identified as important for binding of the globular domain to nucleosomes in vitro (Goytisolo et al., 1996). In conjunction with molecular modeling, the results from these studies envision GH1° residues interacting with the major groove near the dyad and the minor groove of linker DNA, with NTD and CTD regions accessible for association with linker DNA (Brown et al., 2006; Misteli et al., 2000). Later work by the same group also indicates that different linker histone subtypes may exhibit different modes of associations with nucleosomes, as the interaction of H1c was observed to be distinct from that of the apparent 2-contact mode of interaction previously found for H1° (George et al., 2010). Moreover, such distinct modes of globular domain interaction may directly impact the ability of the CTD influences stability of higher order chromatin structure (see below).
Computational analysis of GH5 binding to DNA and nucleosomes by Fan and Roberts correlate well with earlier studies, though three distinct DNA binding sites were identified in GH5. Whereas the previous studies of GH5 and GH1 suggested two DNA-interacting sites (Goytisolo et al., 1996; Misteli et al., 2000) the modeling study suggested a primary DNA binding site is divided into two separate DNA interaction surfaces (Fan and Roberts, 2006). Thus the solutions resulting from these computational docking experiments suggested 3 clusters of GH5-nucleosome interactions with similar energies, with a single top-ranked solution consistent with previously proposed models of binding over the dyad axis (Allan et al., 1980) and Table 1.
A comprehensive study combining electron cryomicroscopy (ECM), and hydroxyl radical footprinting with mono-, di- and tri-, nucleosomes has provided further evidence for the globular domain located at the nucleosome dyad, contacting 3 DNA surfaces. ECM images provide evidence of reorganization of linker DNA upon H1 binding. Hydroxyl radical footprinting shows, with single base resolution, that GH1.5 protects minor groove DNA at the dyad and 10 bp of DNA beyond edge of the nucleosomal core (Syed et al., 2010). In addition, an alteration in the hydroxyl radical cleavage pattern of the linker DNA is observed when the footprinting is carried out with full length H1.5 or with an H1.5 truncation mutant containing a highly basic 7 amino acid residue region within the CTD, adjacent to the globular domain. Combining these results with molecular modeling provided insights into the H1-dependent organization of the linker DNA into a stem structure (Hamiche et al., 1996) in which the linker DNAs partially writhe around one another, with amino acid residues of CTD adjacent to the globular domain important for stem structure formation (Meyer et al., 2011; Syed et al., 2010). Interestingly, observations of CTD condensation and H1 interaction in mesoscale molecular modeling indicate that in low salt concentrations CTD condenses and H1 interacts with the nucleosome and one linker DNA. The modeling also predicts that in higher salt H1 interacts with the nucleosome body and both linker DNAs, promoting chromatin compaction through linker DNA stem formation (Luque et al., 2014).
Further attempts to clarify how the H1 globular domain binds and is oriented on the nucleosome surface include a solution NMR study of D. melanogaster H1-nucleosome complex using H1 and CTD truncation mutants. Paramagnetic relaxation enhancement (PRE) experiments suggest GH1 bridges the central superhelical turn of DNA in the nucleosome core and one 10bp segment of linker DNA asymmetrically, with the α3 helix facing DNA near the dyad (Zhou et al., 2013). This work also implicates both the H1 CTD and the H2A CTD in formation of H1-nucleosome complex. Additional work by Zhou and colleagues has led to a 3.5 Å crystal structure of the H5 globular domain in complex with a nucleosome containing 168 bp of DNA (Zhou et al., 2015). The reported structure shows on-dyad axis, 3-contact interactions between GH5 and the nucleosome, with additional support for the observed on-axis mode of binding for GH5 provided by PRE NMR data, in contrast to that obtained for Drosophila GH1. Consistent with differences in GH5 and Drosophila GH1 binding to nucleosomes, analyses of reconstituted nucleosome arrays containing 12 177 bp repeats and bound by either GH5 or GH1 showed differences in sedimentation, with GH5 having a higher sedimentation coefficient than GH1, suggesting differences in array compaction (Zhou et al., 2015). The authors propose that the off-axis contacts exhibited by Drosophila GH1 mediate a less condensed nucleosomal array, while H5 which binds on-axis, promotes additional contacts and a more condensed chromatin structure. These complementary studies extend the notion that multiple binding modes exist, and further suggest on and off-axis binding of different H1 globular domain subtypes contribute to unique H1-mediated condensation states of chromatin. However, the mechanism of oligonucleosome array condensation and how different proposed binding modes of the globular domain potentially alter the function of the H1 CTD, which is primarily responsible for stabilizing chromatin folding and higher order structure, remain unclear.
Linker histone interactions in the chromatin fiber
Beyond understanding how H1 interacts with individual nucleosomes, the location and function of H1 domains within the chromatin fiber remains a major question. Analysis of H1-depleted and native (H1-containing) chromatin indicate H1 is located near the entry/exit points of DNA and stabilizes the wrapping of nucleosome DNA near the edge of the nucleosome core region (Thoma et al., 1979). Additionally, H1 containing nucleosome arrays condense to more uniform folded states than arrays lacking H1 at various ionic conditions (Thoma and Koller, 1977; Thoma et al., 1983). The globular domain alone, though important for nucleosome binding, cannot stabilize folding of chromatin, (Allan et al., 1986; Losa et al., 1984) suggesting an importance of the CTD for chromatin compaction. Studies of mononucleosome binding suggest the globular domain of H1 can interact with several sites on the nucleosome (Table 1) but whether of these interactions are simultaneously prevalent in chromatin fibers, including native and reconstituted, remains unclear.
Though a thorough knowledge of higher-order chromatin structure remains elusive, the folding and condensation of nucleosomal arrays in the presence of H1 and physiological salts in vitro has been extensively studied. Nucleosomal arrays reconstituted with H1 behave similarly to isolated native chromatin, folding in the presence of monovalent or multivalent ions (Carruthers et al., 1998). H1-containing oligonucleosomes in vitro are decompacted in low salt buffers (≤10 mM Mg+) to form a ‘loose zig-zag’ structure where the nucleosomes do not contact one another at high frequency (Grigoryev et al., 2016; Thoma et al., 1979; Woodcock and Dimitrov, 2001). Elevation of the ionic strength induces folding and compaction, first to a ‘contacting zig-zag’ structure then to a more compact fiber. Several models of the chromatin fiber exist, falling mainly into two categories, a one-start helix in which the linker DNA is curved or bent in some fashion to permit juxtaposition of neighboring nucleosomes, or two-start helix models, where the linker DNA is more or less straight, with neighboring nucleosomes each initiating and adjoining stacks of nucleosomes. Arrays reconstituted on 167bp nucleosome repeat length (NRL) templates were shown via internucleosome crosslinking to condense in salt to fibers with near 30nm diameter by way of a two-start helix (Dorigo et al., 2004), though these assays were carried out in the absence of H1. It is interesting to note, however, that experiments with variations of linker DNA lengths show that nucleosome arrays with 167 bp NRL exhibit complete salt-dependent folding that is only marginally enhanced upon binding of linker histone while arrays with 172 bp NRL exhibit complete folding only in the presence of linker histone (Correll et al., 2012). Further support of the two-start helix model arises from the X-ray crystal structure of a 167-bp NRL tetranucleosome, also in the absence of linker histone (Schalch et al., 2005).
An interdigitated one-start helix model was proposed based on studies of long arrays reconstituted on tandemly repeated 601 nucleosome positioning sequences with NRLs ranging from 177 to 207 bp with stoichiometric quantities of H5 with folding achieved in 1.6 mM Mg2+ (Robinson et al., 2006). Other work later suggested that both NRL and H1 contribute significantly to the structure of chromatin fibers (Robinson and Rhodes, 2006). Arrays with 167-bp NRL, lacking H1 displayed an ordered structure consisting of stacked nucleosomes in two-start helix arrangement (Routh et al., 2008), reminiscent of previous observations (Dorigo et al., 2004), leading to the proposal that arrays with different linker lengths may fold in different arrangements.
Most recently, an 11 Å cryo-EM structure of 30 nm fibers in low salt provided a detailed model of reconstituted fibers containing H1 (H1.4) (Song et al., 2014). The structure utilized 177 and 187 bp NRLs, and the final calculated model has a structural repeat of tetra-nucleosomal subunits in the fiber, with each unit similar to the previously reported 167bp NRL X-ray crystal structure in the absence of H1 (Schalch et al., 2005). The difference in structure, however, occurs between stacks of repeated tetranucleosome units, presumably influenced by H1 interactions with linker DNA. Interestingly, in the cryo-EM structure H1 interacts in a three-contact mode, though asymmetrically as it is located off the dyad axis. The structure also reveals the NTD and CTD are positioned to interact with each linker DNA. Taken together, these results suggest the asymmetric, off-axis interaction with all three DNA surfaces (near the dyad and either linker) alters the entry-exit DNA angle, constraining the linker length and ultimately influencing the rotation between stacks of tetranucleosomes (Song et al., 2014). The observation of differences in GH5 and Drosophila GH1 binding to nucleosomes and arrays (see above), and H1.4 interactions in fibers further suggest that different subtypes could interact in distinct ways that affect linker DNA trajectories and influence overall chromatin architecture, though additional work to validate these structural studies is necessary. These studies also do not provide information on the location of the CTD, its interaction, or insights into how it facilitates higher order chromatin organization.
While H1 clearly plays an important role in the formation and stabilization of the 30-nm chromatin fiber, the exact position of the H1 on nucleosome within 30-nm fiber and its precise function remains to be determined. Antibody accessibility, protease probing and neutron diffraction have all been used to fix the location of H1 within the chromatin fiber, with the latter technique providing evidence that the largest fraction of H1’s mass is located toward the center of the chromatin fiber (Graziano et al., 1994). This would put the majority of the linker histone in contact with linker DNA traversing the center of the fiber in the crossed-linker model (Woodcock and Dimitrov, 2001). The contribution of H1 subtype heterogeneity and potential differences in modes of globular binding suggest that binding in alternative contact modes is one way H1 alters the path of DNA entering and exiting the nucleosome to subsequently effect compaction. However, the necessity of the H1 CTD for compact higher-order structure formation highlights the need to more precisely understand the structure and interactions of this domain in condensed chromatin.
Summary and Conclusion
In summary, although much data has been accumulated, resulting in significant refinement in our understanding of the structure specific recognition and nucleosome binding of H1, there is still much to learn regarding the structure and nucleosome interactions of this class of proteins. Moreover, the literature is complicated by differences in both in the DNA sequence and overall linker DNA length in nucleosome substrates, and the potential difference in binding characteristics of different H1 isoforms. In addition, it is still not established whether individual globular domains exhibit binding characteristics representative of those of the full-length proteins. Many of these studies are highlighted in Table 1. Some consistencies among available data suggest however, that the globular domain binds on the outside of the central superhelical DNA gyre in the nucleosome and interacts with both linkers. However whether both linker DNA interactions occur simultaneously may depend on linker histone subtype. In addition, the data indicate that the H1 tail domains, and especially the CTD strongly influence linker DNA geometry, both in nucleosomes and oligonucleosomal chromatin. Differences in globular domain binding modes of H1 subtypes however could very well be a biologically significant observation and subsequently affect chromatin fiber formation by altering orientation or position of the CTD, as shown in Figure 2. Additionally, domain swapping experiments in which N and C terminal regions were interchanged around the central globular domain showed that although the protein could still bind to nucleosomes, its ability to condense chromatin in vitro was compromised Moreover, the domain-swapped H1 exhibited abnormally increased mobility in nuclei of live cells as determined by fluorescence recovery after photobleaching (FRAP) (Hutchinson et al., 2015). It is interesting to note, however, little is known regarding interactions of the N- and C-terminal domains of H1 within the nucleosome or how posttranslational modifications may affect the structure and/or interactions of these domains. The crystal structure provides a detailed snapshot, but given the dynamic nature of H1 binding, it will be increasingly necessary for methods to fully interrogate H1 interactions in solution, including that of tail domains, which also contribute to subtype heterogeneity, to understand the functional implications in chromatin condensation. Moreover, the continual identification of post-translational modifications also adds to the dilemma, but raises important questions regarding contributions of H1 to a histone code. Linker histones, and relatedly, NRL and linker DNA trajectory likely impact subsequent nucleosome packing in higher order structures. The diverse modes of H1 binding likely contribute to dynamic and heterogeneous attributes of higher order chromatin structures in vivo but further work is needed to address these areas.
Figure 2. Potential mechanism for H1 binding mode influence on chromatin compaction.
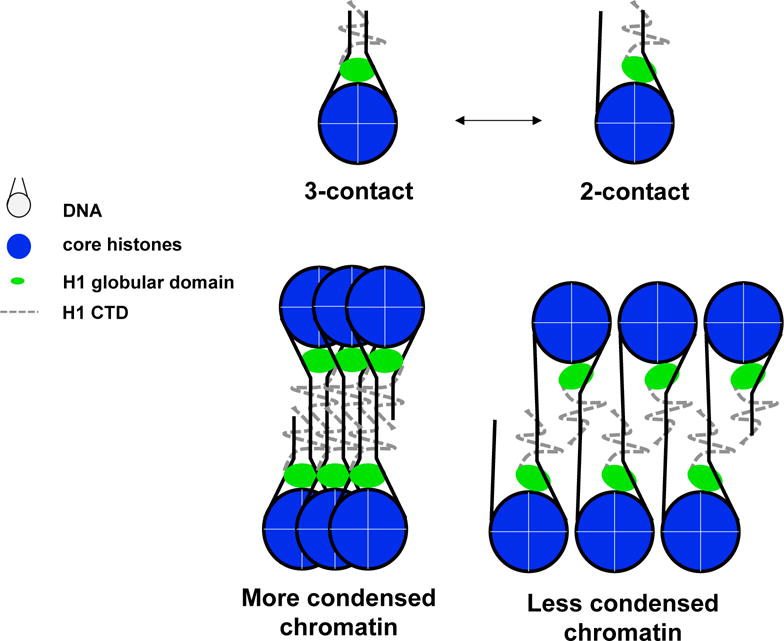
Model shows the 3-contact and 2-contact binding interactions might lead to more or less condensed chromatin.
References
- Allan J, Hartman PG, Crane-Robinson C, Aviles FX. The structure of histone H1 and its location in chromatin. Nature. 1980;288:675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Allan J, Mitchell T, Harborne N, Bohm L, Crane-Robinson C. Roles of H1 domains in determining higher order chromatin structure and H1 location. Journal of molecular biology. 1986;187:591–601. doi: 10.1016/0022-2836(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Bohm L, Mitchell TC. Sequence conservation in the N-terminal domain of histone H1. FEBS letters. 1985;193:1–4. doi: 10.1016/0014-5793(85)80067-3. [DOI] [PubMed] [Google Scholar]
- Bradbury EM, Chapman GE, Danby SE, Hartman PG, Riches PL. Studies on the role and mode of operation of the very-lysine-rich histone H1 (F1) in eukaryote chromatin. The properties of the N-terminal and C-terminal halves of histone H1. European journal of biochemistry / FEBS. 1975;57:521–528. doi: 10.1111/j.1432-1033.1975.tb02327.x. [DOI] [PubMed] [Google Scholar]
- Brown DT, Izard T, Misteli T. Mapping the interaction surface of linker histone H1(0) with the nucleosome of native chromatin in vivo. Nat Struct Mol Biol. 2006;13:250–255. doi: 10.1038/nsmb1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterino TL, Fang H, Hayes JJ. Nucleosome linker DNA contacts and induces specific folding of the intrinsically disordered H1 carboxyl-terminal domain. Mol Cell Biol. 2011;31:2341–2348. doi: 10.1128/MCB.05145-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf C, Lippens G, Muyldermans S, Segers A, Ramakrishnan V, Wodak SJ, Hallenga K, Wyns L. Homo- and heteronuclear two-dimensional NMR studies of the globular domain of histone H1: sequential assignment and secondary structure. Biochemistry. 1993;32:11345–11351. doi: 10.1021/bi00093a011. [DOI] [PubMed] [Google Scholar]
- Cerf C, Lippens G, Ramakrishnan V, Muyldermans S, Segers A, Wyns L, Wodak SJ, Hallenga K. Homo- and heteronuclear two-dimensional NMR studies of the globular domain of histone H1: full assignment, tertiary structure, and comparison with the globular domain of histone H5. Biochemistry. 1994;33:11079–11086. doi: 10.1021/bi00203a004. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Hill CS, Martin SR, Thomas JO. Alpha-helix in the carboxy-terminal domains of histones H1 and H5. The EMBO journal. 1988;7:69–75. doi: 10.1002/j.1460-2075.1988.tb02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Thomas JO. Salt-dependent co-operative interaction of histone H1 with linear DNA. Journal of molecular biology. 1986;187:569–580. doi: 10.1016/0022-2836(86)90335-9. [DOI] [PubMed] [Google Scholar]
- Correll SJ, Schubert MH, Grigoryev SA. Short nucleosome repeats impose rotational modulations on chromatin fibre folding. The EMBO journal. 2012;31:2416–2426. doi: 10.1038/emboj.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane-Robinson C. Where is the globular domain of linker histone located on the nucleosome? Trends in biochemical sciences. 1997;22:75–77. doi: 10.1016/s0968-0004(97)01013-x. [DOI] [PubMed] [Google Scholar]
- Cutter A, Hayes JJ. A Brief Review of Nucleosome Structure. FEBS letters. 2015 doi: 10.1016/j.febslet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigo B, Schalch T, Kulangara A, Duda S, Schroeder RR, Richmond TJ. Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science (New York, NY) 2004;306:1571–1573. doi: 10.1126/science.1103124. [DOI] [PubMed] [Google Scholar]
- Draves PH, Lowary PT, Widom J. Co-operative binding of the globular domain of histone H5 to DNA. Journal of molecular biology. 1992;225:1105–1121. doi: 10.1016/0022-2836(92)90108-v. [DOI] [PubMed] [Google Scholar]
- Fan L, Roberts VA. Complex of linker histone H5 with the nucleosome and its implications for chromatin packing. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8384–8389. doi: 10.1073/pnas.0508951103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Clark DJ, Hayes JJ. DNA and nucleosomes direct distinct folding of a linker histone H1 C-terminal domain. Nucleic acids research. 2012;40:1475–1484. doi: 10.1093/nar/gkr866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan TW, Files JK, Casano KR, George EM, Brown DT. Photobleaching studies reveal that a single amino acid polymorphism is responsible for the differential binding affinities of linker histone subtypes H1.1 and H1.5. Biology open. 2016 doi: 10.1242/bio.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EM, Izard T, Anderson SD, Brown DT. Nucleosome interaction surface of linker histone H1c is distinct from that of H1(0) The Journal of biological chemistry. 2010;285:20891–20896. doi: 10.1074/jbc.M110.108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goytisolo FA, Gerchman SE, Yu X, Rees C, Graziano V, Ramakrishnan V, Thomas JO. Identification of two DNA-binding sites on the globular domain of histone H5. The EMBO journal. 1996;15:3421–3429. [PMC free article] [PubMed] [Google Scholar]
- Graziano V, Gerchman SE, Schneider DK, Ramakrishnan V. Histone H1 is located in the interior of the chromatin 30-nm filament. Nature. 1994;368:351–354. doi: 10.1038/368351a0. [DOI] [PubMed] [Google Scholar]
- Grigoryev SA, Bascom G, Buckwalter JM, Schubert MB, Woodcock CL, Schlick T. Hierarchical looping of zigzag nucleosome chains in metaphase chromosomes. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:1238–1243. doi: 10.1073/pnas.1518280113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamiche A, Schultz P, Ramakrishnan V, Oudet P, Prunell A. Linker histone-dependent DNA structure in linear mononucleosomes. Journal of molecular biology. 1996;257:30–42. doi: 10.1006/jmbi.1996.0144. [DOI] [PubMed] [Google Scholar]
- Hansen JC, Lu X, Ross ED, Woody RW. Intrinsic protein disorder, amino acid composition, and histone terminal domains. The Journal of biological chemistry. 2006;281:1853–1856. doi: 10.1074/jbc.R500022200. [DOI] [PubMed] [Google Scholar]
- Happel N, Doenecke D. Histone H1 and its isoforms: contribution to chromatin structure and function. Gene. 2009;431:1–12. doi: 10.1016/j.gene.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hayashi H, Iwai K. Tetrahymena histone H1. Isolation and amino acid sequence lacking the central hydrophobic domain conserved in other H1 histones. J Biochem. 1987;102:369–376. doi: 10.1093/oxfordjournals.jbchem.a122063. [DOI] [PubMed] [Google Scholar]
- Hayes JJ. Site-directed cleavage of DNA by a linker histone–Fe(II) EDTA conjugate: localization of a globular domain binding site within a nucleosome. Biochemistry. 1996;35:11931–11937. doi: 10.1021/bi961590+. [DOI] [PubMed] [Google Scholar]
- Hayes JJ, Kaplan R, Ura K, Pruss D, Wolffe A. A putative DNA binding surface in the globular domain of a linker histone is not essential for specific binding to the nucleosome. The Journal of biological chemistry. 1996;271:25817–25822. doi: 10.1074/jbc.271.42.25817. [DOI] [PubMed] [Google Scholar]
- Hayes JJ, Wolffe AP. Preferential and asymmetric interaction of linker histones with 5S DNA in the nucleosome. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6415–6419. doi: 10.1073/pnas.90.14.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel MJ, Lever MA, Crawford E, Th’ng JP. The C-terminal domain is the primary determinant of histone H1 binding to chromatin in vivo. The Journal of biological chemistry. 2004;279:20028–20034. doi: 10.1074/jbc.M400070200. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Cheema MS, Wang J, Missiaen K, Finn R, Gonzalez Romero R, Th’ng JP, Hendzel M, Ausio J. Interaction of chromatin with a histone H1 containing swapped N- and C-terminal domains. Bioscience reports. 2015;35 doi: 10.1042/BSR20150087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A, Schneider R. The role of linker histone H1 modifications in the regulation of gene expression and chromatin dynamics. Biochimica et biophysica acta. 2016;1859:486–495. doi: 10.1016/j.bbagrm.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Kalashnikova AA, Rogge RA, Hansen JC. Linker histone H1 and protein-protein interactions. Biochimica et biophysica acta. 2016;1859:455–461. doi: 10.1016/j.bbagrm.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalashnikova AA, Winkler DD, McBryant SJ, Henderson RK, Herman JA, DeLuca JG, Luger K, Prenni JE, Hansen JC. Linker histone H1.0 interacts with an extensive network of proteins found in the nucleolus. Nucleic acids research. 2013;41:4026–4035. doi: 10.1093/nar/gkt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman D. Histone H1 in Saccharomyces cerevisiae: a double mystery solved? Trends in biochemical sciences. 1996;21:287–288. [PubMed] [Google Scholar]
- Leng M, Felsenfeld G. The preferential interactions of polylysine and polyarginine with specific base sequences in DNA. Proceedings of the National Academy of Sciences of the United States of America. 1966;56:1325–1332. doi: 10.1073/pnas.56.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa R, Thoma F, Koller T. Involvement of the globular domain of histone H1 in the higher order structures of chromatin. Journal of molecular biology. 1984;175:529–551. doi: 10.1016/0022-2836(84)90183-9. [DOI] [PubMed] [Google Scholar]
- Lu X, Hamkalo B, Parseghian MH, Hansen JC. Chromatin condensing functions of the linker histone C-terminal domain are mediated by specific amino acid composition and intrinsic protein disorder. Biochemistry. 2009;48:164–172. doi: 10.1021/bi801636y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Hansen JC. Identification of specific functional subdomains within the linker histone H10 C-terminal domain. The Journal of biological chemistry. 2004;279:8701–8707. doi: 10.1074/jbc.M311348200. [DOI] [PubMed] [Google Scholar]
- Luque A, Collepardo-Guevara R, Grigoryev S, Schlick T. Dynamic condensation of linker histone C-terminal domain regulates chromatin structure. Nucleic acids research. 2014;42:7553–7560. doi: 10.1093/nar/gku491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Becker NB, Syed SH, Goutte-Gattat D, Shukla MS, Hayes JJ, Angelov D, Bednar J, Dimitrov S, Everaers R. From crystal and NMR structures, footprints and cryo-electron-micrographs to large and soft structures: nanoscale modeling of the nucleosomal stem. Nucleic acids research. 2011;39:9139–9154. doi: 10.1093/nar/gkr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan-Arino L, Izquierdo-Bouldstridge A, Jordan A. Specificities and genomic distribution of somatic mammalian histone H1 subtypes. Biochimica et biophysica acta. 2016;1859:510–519. doi: 10.1016/j.bbagrm.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Misteli T, Gunjan A, Hock R, Bustin M, Brown DT. Dynamic binding of histone H1 to chromatin in living cells. Nature. 2000;408:877–881. doi: 10.1038/35048610. [DOI] [PubMed] [Google Scholar]
- Pan C, Fan Y. Role of H1 linker histones in mammalian development and stem cell differentiation. Biochimica et biophysica acta. 2016;1859:496–509. doi: 10.1016/j.bbagrm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parseghian MH, Hamkalo BA. A compendium of the histone H1 family of somatic subtypes: an elusive cast of characters and their characteristics. Biochem Cell Biol. 2001;79:289–304. [PubMed] [Google Scholar]
- Pehrson JR. Thymine dimer formation as a probe of the path of DNA in and between nucleosomes in intact chromatin. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9149–9153. doi: 10.1073/pnas.86.23.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss D, Bartholomew B, Persinger J, Hayes J, Arents G, Moudrianakis EN, Wolffe AP. An asymmetric model for the nucleosome: a binding site for linker histones inside the DNA gyres. Science (New York, NY) 1996;274:614–617. doi: 10.1126/science.274.5287.614. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V, Finch JT, Graziano V, Lee PL, Sweet RM. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature. 1993;362:219–223. doi: 10.1038/362219a0. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, Fairall L, Huynh VA, Rhodes D. EM measurements define the dimensions of the “30-nm” chromatin fiber: evidence for a compact, interdigitated structure. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6506–6511. doi: 10.1073/pnas.0601212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, Rhodes D. Structure of the ‘30 nm’ chromatin fibre: a key role for the linker histone. Current opinion in structural biology. 2006;16:336–343. doi: 10.1016/j.sbi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Roque A, Iloro I, Ponte I, Arrondo JL, Suau P. DNA-induced secondary structure of the carboxyl-terminal domain of histone H1. The Journal of biological chemistry. 2005;280:32141–32147. doi: 10.1074/jbc.M505636200. [DOI] [PubMed] [Google Scholar]
- Routh A, Sandin S, Rhodes D. Nucleosome repeat length and linker histone stoichiometry determine chromatin fiber structure. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8872–8877. doi: 10.1073/pnas.0802336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalch T, Duda S, Sargent DF, Richmond TJ. X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature. 2005;436:138–141. doi: 10.1038/nature03686. [DOI] [PubMed] [Google Scholar]
- Simpson RT. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978;17:5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Song F, Chen P, Sun D, Wang M, Dong L, Liang D, Xu RM, Zhu P, Li G. Cryo-EM study of the chromatin fiber reveals a double helix twisted by tetranucleosomal units. Science (New York, NY) 2014;344:376–380. doi: 10.1126/science.1251413. [DOI] [PubMed] [Google Scholar]
- Stasevich TJ, Mueller F, Brown DT, McNally JG. Dissecting the binding mechanism of the linker histone in live cells: an integrated FRAP analysis. The EMBO journal. 2010;29:1225–1234. doi: 10.1038/emboj.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staynov DZ, Crane-Robinson C. Footprinting of linker histones H5 and H1 on the nucleosome. The EMBO journal. 1988;7:3685–3691. doi: 10.1002/j.1460-2075.1988.tb03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SH, Goutte-Gattat D, Becker N, Meyer S, Shukla MS, Hayes JJ, Everaers R, Angelov D, Bednar J, Dimitrov S. Single-base resolution mapping of H1-nucleosome interactions and 3D organization of the nucleosome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9620–9625. doi: 10.1073/pnas.1000309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szerlong HJ, Herman JA, Krause CM, DeLuca JG, Skoultchi A, Winger QA, Prenni JE, Hansen JC. Proteomic characterization of the nucleolar linker histone H1 interaction network. Journal of molecular biology. 2015;427:2056–2071. doi: 10.1016/j.jmb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Ahmad K, Almouzni G, Ausio J, Berger F, Bhalla PL, Bonner WM, Cande WZ, Chadwick BP, Chan SW, et al. A unified phylogeny-based nomenclature for histone variants. Epigenetics & chromatin. 2012;5:7. doi: 10.1186/1756-8935-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma F, Koller T. Influence of histone H1 on chromatin structure. Cell. 1977;12:101–107. doi: 10.1016/0092-8674(77)90188-x. [DOI] [PubMed] [Google Scholar]
- Thoma F, Koller T, Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979;83:403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma F, Losa R, Koller T. Involvement of the domains of histones H1 and H5 in the structural organization of soluble chromatin. Journal of molecular biology. 1983;167:619–640. doi: 10.1016/s0022-2836(83)80102-8. [DOI] [PubMed] [Google Scholar]
- van Holde KE. Chromatin. New York: Springer Verlag; 1989. [Google Scholar]
- Vila R, Ponte I, Collado M, Arrondo JL, Jimenez MA, Rico M, Suau P. DNA-induced alpha-helical structure in the NH2-terminal domain of histone H1. The Journal of biological chemistry. 2001;276:46429–46435. doi: 10.1074/jbc.M106952200. [DOI] [PubMed] [Google Scholar]
- Vyas P, Brown DT. N- and C-terminal domains determine differential nucleosomal binding geometry and affinity of linker histone isotypes H1(0) and H1c. The Journal of biological chemistry. 2012;287:11778–11787. doi: 10.1074/jbc.M111.312819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JP, Jr, Simpson RT. Removal of histone H1 exposes a fifty base pair DNA segment between nucleosomes. Biochemistry. 1976;15:3307–3314. doi: 10.1021/bi00660a022. [DOI] [PubMed] [Google Scholar]
- Woodcock CL, Dimitrov S. Higher-order structure of chromatin and chromosomes. Current opinion in genetics & development. 2001;11:130–135. doi: 10.1016/s0959-437x(00)00169-6. [DOI] [PubMed] [Google Scholar]
- Wu M, Allis CD, Richman R, Cook RG, Gorovsky MA. An intervening sequence in an unusual histone H1 gene of Tetrahymena thermophila. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:8674–8678. doi: 10.1073/pnas.83.22.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BR, Feng H, Kato H, Dai L, Yang Y, Zhou Y, Bai Y. Structural insights into the histone H1-nucleosome complex. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19390–19395. doi: 10.1073/pnas.1314905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BR, Jiang J, Feng H, Ghirlando R, Xiao TS, Bai Y. Structural Mechanisms of Nucleosome Recognition by Linker Histones. Molecular cell. 2015;59:628–638. doi: 10.1016/j.molcel.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YB, Gerchman SE, Ramakrishnan V, Travers A, Muyldermans S. Position and orientation of the globular domain of linker histone H5 on the nucleosome. Nature. 1998;395:402–405. doi: 10.1038/26521. [DOI] [PubMed] [Google Scholar]


