Abstract
Quantification of cell-associated replication-competent HIV, in blood samples from patients with undetectable plasma viremia, requires specialized culture conditions that include exogenous pan T cell stimulation. Different research groups have used several stimuli for this purpose; however, the relative efficacies of these T cell stimuli to induce productive HIV replication from latently infected cells ex vivo have not been systematically evaluated. To this end, we compared four commonly used T cell stimuli: 1) irradiated allogeneic cells plus phytohaemagglutinin (PHA); 2) PHA alone; 3) phorbol myristate acetate plus Ionomycin; and 4) immobilized αCD3 plus αCD28 antibodies. End-point dilutions of patient CD4 T cells were performed, using virion RNA production to quantify HIV induction. Our results demonstrated that these activation approaches were not equivalent and that antibody cross-linking of CD3 and CD28 membrane receptors was the most effective means to activate HIV replication from a resting cell state, closely followed by stimulation with irradiated allogeneic cells plus PHA.
Keywords: HIV latency, cell-associated infectious units, T cell stimuli, assay optimization
Introduction
The latent cellular reservoir of human immunodeficiency virus (HIV) is recognized as the major barrier to cure (Richman et al., 2009). This reservoir is highly stable (Siliciano et al., 2003; Strain et al., 2003), necessitating life-long adherence to combination antiretroviral therapy (cART) to prevent HIV rebound. The “shock and kill” strategy has been envisioned as a controlled induction of virus reactivation to reveal latently infected cells for immune system recognition and destruction (Demonté et al., 2004). Small molecule compounds that activate HIV expression, but do not cause cellular activation, have been tested, including histone deacetylase inhibitors (HDACi) (Archin et al., 2009a; Archin et al., 2009b; Archin et al., 2012; Contreras et al., 2009; Rasmussen et al., 2014; Wei et al., 2014), protein kinase C (PKC) pathway agonists (Abreu et al., 2014; Beans et al., 2013; Mehla et al., 2010; Williams et al., 2004), histone methyltransferase inhibitors (Bouchat et al., 2012; Friedman et al., 2011), DNA methylase inhibitors (Kauder et al., 2009) and bromodomain inhibitors (Banerjee et al., 2012; Boehm et al., 2013; Li et al., 2013). HDACi, valproic acid and vorinostat, were tested in clinical trials (Archin et al., 2014; Archin et al., 2012; Elliott et al., 2014; Lehrman et al., 2005) with limited success, while a recently completed clinical trial of a more potent HDACi Romidepsin (Rasmussen et al., 2014; Søgaard et al., 2015), in combination with therapeutic HIV immunization provided the first evidence of feasibility of combination “shock and kill” strategy (Leth et al., 2016). A substantial challenge to the development and evaluation of such treatment strategies is the limited ability of current assays to accurately quantify the in vivo latent cell reservoir in peripheral blood samples from HIV-infected individuals on suppressive cART.
None of the existing assays measures the true size of the latent reservoir (reviewed in (Bruner et al., 2015; Massanella and Richman, 2016)). The present standard is the quantitative viral outgrowth assay (qVOA) (Chun et al., 1997a; Finzi et al., 1997; Laird et al., 2013; Siliciano and Siliciano, 2005; Wong et al., 1997), which measures replication-competent provirus, induced in a single round of T cell activation. Because not all non-inducible proviruses are defective, qVOA tends to underestimate the size of the reservoir approximately 60-fold (Ho et al., 2013). An approach to improve the accuracy of qVOA involves sequential rounds of T cell activation (Hosmane et al., 2017). While this may result in a more accurate measurement, multiple activation rounds make this approach very time consuming. HIV DNA assays that measure integrated or total DNA (O’Doherty et al., 2002; Rouzioux et al., 2014; Strain et al., 2013) are relatively quick to perform. However, they tend to overestimate the true reservoir size by detecting mutated proviruses that can never be expressed, even upon cessation of cART. Available RNA assays using unstimulated cells (Bullen et al., 2014; Pasternak et al., 2008) tend to produce intermediate results. A comparative study evaluating performance of these various assays (Eriksson et al., 2013) has demonstrated poor correlation between most of the measurements obtained for the same set of samples from HIV-infected patients, and a 300-fold discrepancy between qVOA and DNA-based assays. The only significant correlation observed was between the measurement of integrated HIV DNA by Alu PCR and qVOA, which was consistent with one of the earlier reports (Mendoza et al., 2012). However, this correlation may not be preserved when following reservoir size after HIV reactivation therapy (e.g. HDACi), as cells bearing replication-competent provirus are expected to be cleared and not show up in a qVOA, while cells bearing mutated provirus will remain and be measurable in DNA-based PCR assay (Eriksson et al., 2013).
Most recently, culture-based assays were developed to measure inducible RNA from stimulated cells (Cillo et al., 2014; Procopio et al., 2015; Richman, 2015). While these assays are faster and easier than the standard qVOA, the inability to induce all intact proviruses in a single round of T cell activation still remains a limitation to this new generation of assays. It is unknown whether reactivation of all intact proviruses ex vivo is possible, and if not, what stimulus would maximally reactivate the latent reservoir (Massanella and Richman, 2016). Several in vitro methods for T cell activation to induce HIV from latently infected CD4 lymphocytes have been employed by independent research groups (Chun et al., 1997b; Dornadula et al., 2001; Finzi et al., 1997; Procopio et al., 2015; Wong et al., 1997); however, such methods have not been systematically compared. In this present study, we compared the efficacy of 4 different T cell stimulation protocols to induce productive HIV replication ex vivo in blood samples taken from 5 patients, successfully treated and maintained with suppressive cART.
Results and Discussion
CD8 lymphocyte depletion creates optimal conditions for viral outgrowth during long-term culture
Because viral replication can be inhibited by soluble factors produced by CD8 T cells (Chang et al., 2002; Walker et al., 1989), we sought initially in our experiments to test several culture conditions, in the presence or absence of CD8 T cells, for viral outgrowth. Inhibition of HIV replication by CD8 T cells occurs primarily at the level of transcription (Mackewicz et al., 2000); therefore, the presence of these cells in culture may interfere with both qVOA and RNA-based methods of reservoir quantification. In addition, use of CD8 T cell depleted peripheral blood lymphocytes (PBL) leads to normalization of the absolute input of patient CD4 cells.
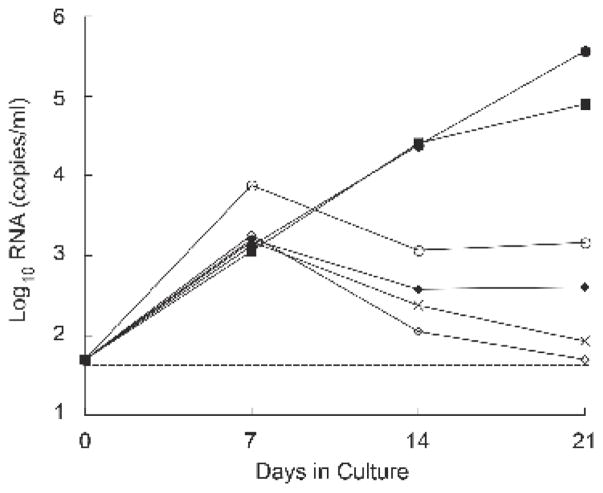
A variety of culture conditions were compared for their influence on viral outgrowth, using cells from cART-suppressed patients who began treatment during chronic infection. Figure 1 shows the dynamics of HIV RNA production for one patient (F); the results are representative of 6 experiments, performed with different donor cells. Levels of HIV RNA in culture supernatants increased between day 0 and day 7 with all co-culture cell combinations (Figure 1). However, continued expansion of HIV replication beyond day 7 was seen only in those conditions where the CD8 cells were depleted from both the patient and healthy donor PBL preparations. In this particular experiment, soluble p24 antigen became detectable between 11 and 21 days of culture (range 36–10,630 pg/ml). Thus, the inhibitory role of CD8 T cells seen in our experiments was consistent with previous observations (Chang et al., 2002; Mackewicz et al., 2000; Walker et al., 1989), and depletion of CD8 T cells appeared to be a requirement for recovery of replication-competent HIV from the small numbers of latently infected CD4 lymphocytes, typically present in patients on cART (Chun et al., 1997a; Finzi et al., 1997). Therefore, in the subsequent experiments of this study, CD4 T cells were enriched (CD8 and monocyte-depleted) in both patient and healthy donor control (HC) cell preparations for the propagation of virus (Figure 2A).
Figure 1. Quantification of HIV RNA levels in co-culture supernatants during the first 21 days of culture for patient F.
(○) PHA-stimulated HC total PBL plus αCD3 + αCD28-stimulated patient PBL. (X) PHA-stimulated HC total PBL plus αCD3 + αCD28-stimulated patient CD8-depleted PBL. (◇) PHA-stimulated HC total PBL plus αCD3 + αCD28-stimulated patient CD8-depleted PBL plus monocytes. (◆) PHA-stimulated HC CD8-depleted PBL plus αCD3 + αCD28-stimulated patient total PBL. (■) PHA-stimulated HC CD8-depleted PBL plus αCD3 + αCD28-stimulated patient CD8-depleted PBL. (●) PHA-stimulated HC CD8-depleted PBL plus αCD3 + αCD28-stimulated patient CD8-depleted PBL plus monocytes. (-----) Threshold of detection, 50 copies of HIV RNA/ml. Results from patient F are representative of experiments done with cultured cells from 6 different cART-suppressed patients.
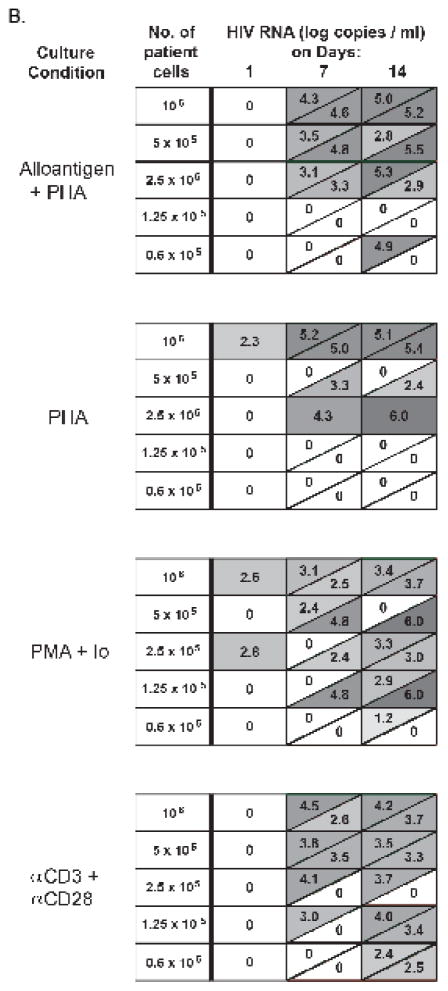
Figure 2. Diagram of co-culture method and detailed results of HIV RNA quantification for patient B.
A) CD4-enriched cells from patients were exposed to four different stimulation conditions. Alloantigen consisted of irradiated allogeneic PBMC added to the patient CD4 cells at a ratio of 10:1. Healthy control (HC) donor cells, stimulated with PHA for 48 hours prior, were added to patient cells on days 0 and 7 of culture. Io, Ionomycin. B) HIV RNA concentrations (log10 copies/ml) were assayed in supernate taken from duplicate wells for each co-culture condition for patient B, on days 1, 7 and 14. Each row depicts a cell concentration, with decreasing cell numbers from top to bottom. Each box represents duplicate cultures; a diagonal within a box indicates a result for 1 of 2 replicates. Zero indicates undetectable viral RNA.
Interestingly, even though HIV induction was the highest on day 21 when cultures were depleted of CD8 T cells, RNA levels were similar on day 7, with or without CD8 depletion (Figure 1). These results raise a possibility that in resource limited settings, where patients are more likely to have larger HIV reservoirs due to delayed initiation of cART during chronic infection, performing such assays for a shorter time without CD8 T cell depletion may be feasible; albeit, the latent HIV reservoir will be underestimated.
Choice of T cell activation stimuli for the recovery of replication-competent HIV from latently infected cells and the analysis approach
To determine the most effective method to activate HIV replication from latently infected cell reservoirs, CD4 lymphocyte preparations from 5 different patients with viral suppression were activated in vitro using 4 different stimuli (Figure 2A), which were selected based on the published methods from several different laboratories: (1) irradiated allogeneic peripheral blood mononuclear cells (PBMC) plus phytohaemagglutinin (PHA) (“alloantigen + PHA”) (Siliciano and Siliciano, 2005); (2) PHA alone, in the absence of allogeneic PBMC (“PHA”) (Dornadula et al., 2001); (3) phorbol myristate acetate (PMA) plus Ionomycin (“PMA + Io”) (Procopio et al., 2015); and (4) immobilized αCD3 plus αCD28 antibodies (“αCD3 + αCD28”) (Wong et al., 1997). The efficiency of viral induction was examined by performing co-cultures (in duplicate wells), using serial limiting dilution of the patients’ cells. Conditions for virus propagation throughout the experiment were kept constant (CD4 T-enriched HC) (Figure 2A). Due to the complexity of the experimental design that involved multiple stimuli performed in parallel and prolonged culture time, the experiment was performed using cells from a limited number of patients (N=5). To ensure the validity of the statistical analysis with the small sample size, estimates of frequencies of infectious units per million cells in limiting dilution assays were obtained using a χ2 statistic, assuming single-hit Poisson kinetics, which was shown to be the most accurate and precise frequency estimator (Taswell, 1981). The stimuli were then compared within each donor and assessed for consistency of observation across donors.
Patient characteristics and positive culture definition
The general clinical characteristics of the participating patients are summarized in Table 1. In all patients, the plasma HIV RNA level was undetectable (<50 copies/ml) at the time of blood collection for this study. CD4 T cells isolated from patients were stimulated for 24 hours prior to the addition of HC CD4 cells to the co-culture. A positive culture was defined as having a rising RNA level between days 1 and 14, with a concurrent positive p24 result (>10 pg/ml). HIV RNA levels at day 21 were used to confirm the persistence of a positive culture or a spreading infection (data not shown). An example of detailed results for the quantification of HIV RNA, produced from duplicate culture wells in the limiting dilution protocol, is shown for patient B in Figure 2B. In this particular patient, virus replication could be detected even in the culture wells originally seeded with as few as 60,000 CD8-depleted PBL.
Table 1. Clinical characteristics of the participating patients.
HIV RNA levels in plasma were first assayed using the Amplicore assay (Roche). When values were less than 400 copies/ml, the Ultrasensitive assay was used, with a limit of detection of 50 copies/ml. All patients, except for Patient B (D4T/3TC/IDV), were treated with the triple drug regimen of AZT/3TC/IDV. All patients, except for Patient E, began treatment during chronic infection. Patient E had acute HIV infection and began treatment prior to seroconversion, at approximately 4 months after his estimated date of infection. Prior, time point immediately prior to initiation of therapy. Current, at the time of blood sample draw for the study.
| Patients | Months on therapy | CD4 cells/cm3 | RNA copies/ml | ||
|---|---|---|---|---|---|
|
| |||||
| Prior | Current | Prior | Current | ||
| A | 19 | 185 | 265 | 16,487 | <50 |
| B | 10 | 243 | 509 | 21,633 | <50 |
| C | 17 | 178 | 448 | 13,127 | <50 |
| D | 34 | 11 | 538 | 10,242 | <50 |
| E | 19 | 840 | 974 | 1,506,600 | <50 |
| F | 27 | 274 | 579 | 19,200 | <50 |
T cell signaling through the CD3/T Cell Receptor (TCR) complex and alloantigen + PHA stimulus are the most effective means to activate HIV replication from latently infected CD4 lymphocytes
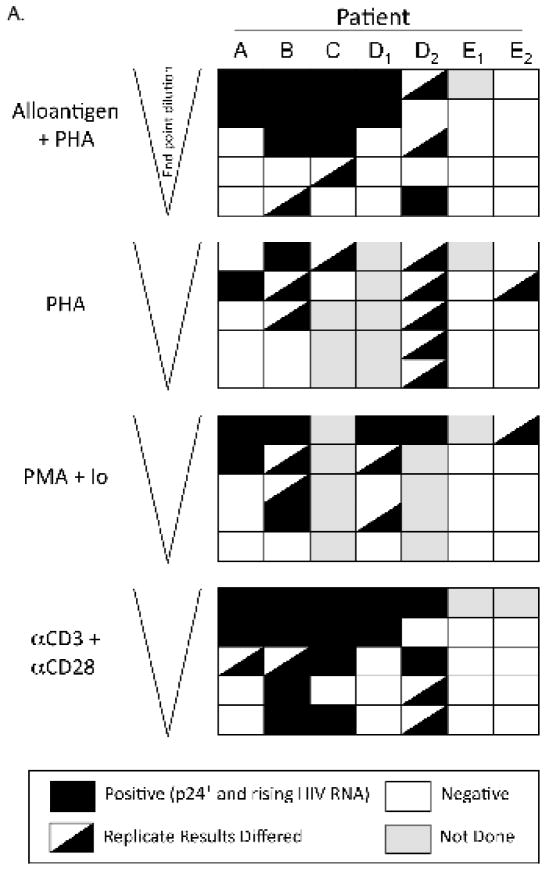
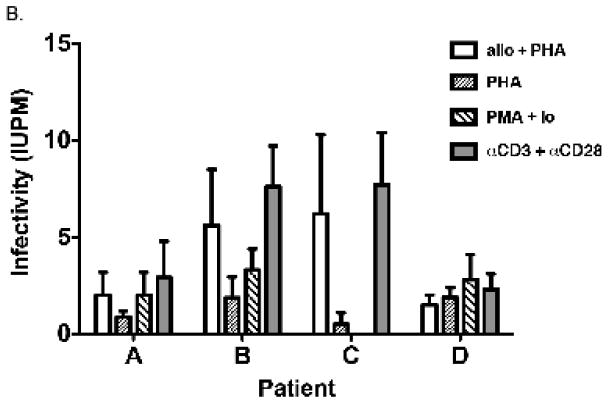
The results obtained from all 5 patients (including patient B, Figure 2B), are displayed in Figure 3(A, B) to compare different pan T cell stimulation conditions. In culture wells with the greatest number of patient cells (1×106 per well), virus was recovered from all patients tested; and in one case (patient D), on two separate occasions. In 4 of the 5 patients, the different stimuli appeared equivalent in producing detectable HIV replication when the co-cultures contained the maximum number (1×106) of CD4-enriched patient cells. However, as the number of patient CD4 cells decreased with dilution, stimulation with αCD3 + αCD28 or with alloantigen + PHA proved to be the most efficient means to induce viral replication (Figure 3A). For patient A, stimulation with αCD3 + αCD28 was significantly more effective at inducing viral replication than PHA, which was the least effective culture condition (P = 0.034, Figure 3B). No other comparisons between treatments achieved significance for this patient. For patient B, αCD3 + αCD28 stimulation was significantly better than both PHA and PMA + Io (P = 0.009 and 0.020, respectively). Both alloantigen + PHA (P = 0.008) and αCD3 + αCD28 (P = 0.002) stimulation were significantly more effective than PHA at inducing HIV expression from CD4 T cells of patient C. No treatment comparisons achieved significance for patients D and E with the number of replicates used. Overall, αCD3 + αCD28 stimulation appeared to induce HIV more effectively than all the other stimuli compared (highest frequency of infectious units per million cells for patients A, B and C, Figure 3B); however, there was no significant statistical difference between αCD3 + αCD28 and alloantigen + PHA stimuli for either of the patients (P=0.312 for patient A, P=0.283 for patient B, P=0.370 for patient C and P=0.184 for patient D).
Figure 3. Summary of results obtained from HIV RNA quantification of culture supernatants taken at day 14 (patients A–E).
Positive cultures were defined as those with: 1) a positive p24 result (>10 pg/ml) within 21 days of co-culture, and 2) rising RNA concentrations between days 1 and 14. HIV RNA at day 21 was used to confirm a spreading infection. D1, D2 and E1, E2 are the first and second cultures from patient D and E, respectively, which were performed using fresh blood samples taken approximately 2 months apart. A) Each row depicts a cell concentration, with decreasing cell numbers from top to bottom. Each box represents duplicate cultures; a diagonal within a box indicates a result for 1 of 2 replicates. B) Infectivity was estimated from the data shown in (A). Second culture of patient D was used for these analyses, for which data for all the treatments were available. Error bars indicate the estimated infectivity +1 SEM.
Activation of HIV replication from latently infected CD4 lymphocytes in one patient who initiated cART during acute infection
Patient E was remarkable for having a minimal, if not undetectable, response to all induction methods tested. The lower frequency of inducible cells did not appear to be explained by variation in the duration of suppressive therapy; patient E had 19 months of therapy at the time of testing compared with a mean of 20 months for the other 4 patients. Because this patient initiated cART prior to seroconversion, these results are consistent with the likelihood of a smaller cell reservoir size, such as that demonstrated for similar patients in previous studies (Schmid et al., 2010; Strain et al., 2005). Alternatively, it is possible that the reservoir of this patient contained a majority of HIV integrated into nonresponsive gene sites, or mutated virus, and only rare inducible provirus. Curiously, for PHA and PMA + Io, the replicate results differed, even in the co-cultures with high cell number (0.5 – 1×106 per well) (Figure 3A). It is possible that in this individual, the number of CD4 cells infected with replication-competent HIV was close to the limit of detection and results were affected randomly by sampling error variations. Such stochastic events, as those seen with the induction strategies used in the present study, may suggest that alternative methods, which do not require virus reactivation, need to be developed in order to monitor latent reservoir size in patients who initiate cART during the acute phase of infection.
Practical and theoretical implications of the findings
The results of our study indicate that commonly employed major T cell stimuli are not equally effective in reactivating latent HIV replication from patient CD4 T lymphocytes in vitro. Induction of detectable p24 antigen or viral RNA appears to vary, depending on the stimulation procedure used. We found that cross-linking of CD3 and CD28 cell surface receptors was the most efficient method for induction of detectable productive HIV replication from latently infected T cells, closely followed by stimulation with γ-irradiated allogeneic PBMCs and PHA. These results are in agreement with an earlier study of in vitro HIV infection of primary T cells from our group (Spina et al., 1997), which demonstrated that induced virus production from central memory cells was greater than that from naïve cells with αCD3 + αCD28 stimulation, but was equal in these two cell subsets with PHA induction. Since the latent viral reservoir is comprised predominantly of infected central memory T cells (Chomont et al., 2009), it is not surprising that αCD3 + αCD28 stimulation performed better for virus activation. Alloantigen + PHA stimulus would also be suitable for conducting HIV reservoir measurement assays, since its efficacy of inducing HIV provirus was close to that of αCD3 + αCD28 stimulation. However, αCD3 + αCD28 stimulation is by far simpler to administer, as compared to preservation and irradiation of allogeneic PBMC; thus using αCD3 + αCD28 stimulus will reduce the complexity of the assay (Kuzmichev et al., 2017). Prior studies that have relied upon stimulation conditions other than CD3 and CD28 cross-linking or alloantigen + PHA stimulation may have underestimated the quantity of replication competent latent HIV present.
Because T cell activation and division alone are not sufficient to support HIV replication (Hosmane et al., 2017; Moran et al., 1993; Spina et al., 1997), it is possible that virus reactivation may be induced more potently by simultaneous engagement of alternate cell signaling mechanisms. Treatment strategies using HDACi, combined with small molecule compounds of other classes, have been shown to be more potent than HDACi alone for HIV reactivation, but less potent than methods that induce robust T cell activation (Bartholomeeusen et al., 2013; Burnett et al., 2010). Unfortunately, the effects of αCD3 + αCD28 treatment were shown to be antagonized by the HDACi vorinostat at the level of gene expression; and translation of viral transcripts was not improved by vorinostat, beyond the effects of T cell activation alone (Mohammadi et al., 2014). Further exploration of such combinatorial strategies with host cell factors that contribute to HIV induction may lead to discovery of more potent HIV reactivators. This will be important for treatment protocol development, and will get us closer to measuring the true size of the replication-competent proviral reservoir.
In conclusion, we demonstrate that cross-linking of the CD3 and CD28 cell membrane receptors and treatment with allogeneic PBMC plus PHA are currently the most effective means to induce HIV replication ex vivo from patient CD4 T lymphocytes that harbor latent provirus, the former stimulus being easier and faster to administer. These results have the practical implication to improve the reliability and reduce the time required to conduct qVOA and culture-based assays to measure the true latent reservoir size. In addition, our findings are consistent with the notion that T cells containing latent HIV infection are composed of heterogeneous subpopulations with varying thresholds for reactivation of productive viral replication, and with the idea that a combination of therapeutic agents will be required to activate HIV to a level sufficient to eradicate the latent reservoir.
Materials and Methods
Patients
The patients selected for study had received a standard triple-drug regimen for >10 months and had sustained reductions in HIV plasma RNA to <50 copies/ml for at least 6 months prior to further testing (Table 1). All patients were treated with the triple drug regimen of AZT/3TC/IDV, except for Patient B who received D4T/3TC/IDV. Patient E was diagnosed during acute infection, as defined previously (Gulick et al., 1997), and began treatment prior to seroconversion. All other patients were in the chronic infection phase when treatment was initiated. Written informed consent was obtained from all participants in accordance with local investigational review board guidelines. To determine the relative efficiency of the different stimulation conditions, enriched CD4 cell preparations from these patients were tested using end-point cell dilution into a constant co-culture cell background. Cells from patient F were used for testing CD8 T cell depletion culture conditions; while, cells from patients A–E were used in the HIV reactivation studies.
Cell preparation, CD8 T cell depletion and co-culture
PBMC were prepared by Ficoll-hypaque density gradient centrifugation, and plasma was separated and stored at −80° C. Monocytes were removed and isolated by fibronectin adhesion (Freundlich and Avdalovic, 1983). CD8 cells were depleted by treatment with αCD8 antibody (OKT-8; Ortho Diagnostics, Raritan, NJ) and panning on tissue culture plates coated with goat anti-mouse antibody (Spina et al., 1994). Patient cells were stimulated with immobilized αCD3 + αCD28 (Leu-4; Becton Dickinson ImmunoDiagnostics, San Jose, CA and CD28.2; PharMingen, San Diego, CA, respectively) for 24 hours prior to the addition to co-culture (Spina et al., 1997). Either 5×106 total PBL or CD8-depleted PBL from a healthy donor, following 24–48 hours of stimulation with PHA (3 μg/ml; Sigma chemical Co., St. Louis, MO), were added to each well of a 6-well plate that contained 2×106 stimulated patient cells (day 1 of culture). Co-cultures were initiated using the following cell combinations: 1) patient PBL + donor PBL; 2) patient CD8-depleted PBL + donor PBL; 3) patient CD8-depleted PBL + patient monocytes + donor PBL; 4) patient PBL + donor CD8-depleted PBL; 5) patient CD8-depleted PBL + donor CD8-depleted PBL; 6) patient CD8-depleted PBL + patient monocytes + donor CD8-depleted PBL (Figure 1). Cultures were maintained in the presence of rIL-2, (10 U/ml, Dupont, New England Nuclear Research Products, Boston, MA). At days 1, 7, 14, and 21 of culture, 1 ml of medium was collected from each well without disturbing the cell layer and was frozen for subsequent HIV quantification by ELISA for p24 antigen (Coulter, Hialeah, FL) and by real time quantitative polymerase chain reaction (RT-qPCR) for viral RNA (Roche Molecular Systems, Branchberg, NJ).
T cell activation stimuli and terminal-dilution co-culture assay
The conditions used to initially stimulate the cell preparations from each patient included: 1) irradiated allogeneic PBMC (10:1, HC:Pt) + PHA (3 μg/ml); 2) PHA (3 μg/ml) in the absence of allogeneic PBMC; 3) PMA (1 nM) + Ionomycin (0.25 μM); and 4) immobilized αCD3 (Leu-4, 31 ng/ml, Becton-Dickinson Immunocytometry Systems, San Jose, CA) + αCD28 (CD28.2, 0.2 μg/ml, BD-Pharmingen, San Diego, CA). Healthy donor PBMC were inactivated by γ-irradiation (5,000 rads) to serve as a source of alloantigen in condition 1 (Chun et al., 1997b; Finzi et al., 1997). For virus propagation in culture, CD4-enriched HC donor cells were stimulated with PHA (3 μg/ml) for 48 hours prior to addition to co-culture. End-point dilution of patient cells was performed in duplicate series of 2-fold dilutions in a 6-well flat-bottom plate, and a constant number of CD4-enriched HC cells (2×106) was added to each well at days 0 and 7 (Figure 2A). Cultures were maintained in RPMI 1640 medium supplemented with L-glutamine (1 mM), penicillin (50 U/ml), streptomycin (50 μg/ml), 10% fetal bovine serum and rIL-2 (10 U/ml). On days 1, 7, 14 and 21, half of the culture medium was removed from each well and stored at −80° C for later batch testing of HIV RNA and p24 antigen, and an equal volume of fresh medium was added. For the purpose of this study, all cultures were terminated after the day 21 harvest.
Statistics
Terminal dilution assay data were converted to estimated infectious units per million cells using a χ2 statistic, assuming single-hit Poisson kinetics (Taswell, 1981). Assay results for different treatment conditions were compared by integrating the Gaussian confidence intervals computed in the χ2 analysis.
Highlights.
Four in vitro methods for T cell activation to induce latent HIV were compared.
CD3 and CD28 receptor cross-linking was the most effective and simplest approach.
Its use may improve the reliability of assays to measure the true latent reservoir.
Acknowledgments
We wish to thank the patients who contributed blood for this study, and L. Terry and C. Ignacio for excellent technical support. The work was supported by grants from the NIH (AI 43752 to JKW; AI 27670 and AI 43638 to DDR; U19 AI 096113 to CAS and DDR), the Swiss National Science Foundation (823A-50434 to MH), and through the research infrastructure provided by the UCSD Center for AIDS Research (AI 36214). This material is based upon work supported in part by the Department of Veterans Affairs (VA Merit Review, I01 BX001160 to CAS, and VA Career Developmental Award-2, I01 BX007080 to NBB), Veterans Health Administration, Office of Research and Development. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. The sponsors of this research were not involved in the study design, collection or interpretation of the data, manuscript preparation, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu CM, Price SL, Shirk EN, Cunha RD, Pianowski LF, Clements JE, Tanuri A, Gama L. Dual role of novel ingenol derivatives from euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR. PLoS ONE. 2014;9:e97257. doi: 10.1371/journal.pone.0097257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang K-H, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, Richman DD, Robertson KR, Kashuba AD, Bosch RJ, Hazuda DJ, Kuruc JD, Eron JJ, Margolis DM. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis. 2014;210:728–735. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009a;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Keedy KS, Espeseth A, Dang H, Hazuda DJ, Margolis DM. Expression of latent human immunodeficiency type 1 is induced by novel and selective histone deacetylase inhibitors. AIDS. 2009b;23:1799–1806. doi: 10.1097/QAD.0b013e32832ec1dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee C, Archin N, Michaels D, Belkina AC, Denis GV, Bradner J, Sebastiani P, Margolis DM, Montano M. BET bromodomain inhibition as a novel strategy for reactivation of HIV-1. J Leukoc Biol. 2012;92:1147–1154. doi: 10.1189/jlb.0312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeeusen K, Fujinaga K, Xiang Y, Peterlin BM. Histone deacetylase inhibitors (HDACis) that release the positive transcription elongation factor b (P-TEFb) from its inhibitory complex also activate HIV transcription. J Biol Chem. 2013;288:14400–14407. doi: 10.1074/jbc.M113.464834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beans EJ, Fournogerakis D, Gauntlett C, Heumann LV, Kramer R, Marsden MD, Murray D, Chun TW, Zack JA, Wender PA. Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci U S A. 2013;110:11698–11703. doi: 10.1073/pnas.1302634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D, Calvanese V, Dar RD, Xing S, Schroeder S, Martins L, Aull K, Li PC, Planelles V, Bradner JE, Zhou MM, Siliciano RF, Weinberger L, Verdin E, Ott M. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell Cycle. 2013;12:452–462. doi: 10.4161/cc.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchat S, Gatot JS, Kabeya K, Cardona C, Colin L, Herbein G, De Wit S, Clumeck N, Lambotte O, Rouzioux C, Rohr O, Van Lint C. Histone methyltransferase inhibitors induce HIV-1 recovery in resting CD4+ T cells from HIV-1-infected HAART-treated patients. AIDS. 2012;26:1473–1482. doi: 10.1097/QAD.0b013e32835535f5. [DOI] [PubMed] [Google Scholar]
- Bruner KM, Hosmane NN, Siliciano RF. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol. 2015;23:192–203. doi: 10.1016/j.tim.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014;20:425–429. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett JC, Lim Kl, Calafi A, Rossi JJ, Schaffer DV, Arkin AP. Combinatorial latency reactivation for HIV-1 subtypes and variants. J Virol. 2010;84:5958–5974. doi: 10.1128/JVI.00161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TL, Mosoian A, Pine R, Klotman ME, Moore JP. A soluble factor(s) secreted from CD8(+) T lymphocytes inhibits human immunodeficiency virus type 1 replication through STAT1 activation. J Virol. 2002;76:569–581. doi: 10.1128/JVI.76.2.569-581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel M-R, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy J-P, Haddad EK, Sékaly R-P. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997a;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997b;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo AR, Sobolewski MD, Bosch RJ, Fyne E, Piatak M, Coffin JM, Mellors JW. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2014;111:7078–7083. doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras X, Schweneker M, Chen C-S, McCune JM, Deeks SG, Martin J, Peterlin BM. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonté D, Quivy V, Colette Y, Van Lint C. Administration of HDAC inhibitors to reactivate HIV-1 expression in latent cellular reservoirs: implications for the development of therapeutic strategies. Biochem Pharmacol. 2004;68:1231–1238. doi: 10.1016/j.bcp.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Dornadula G, Nunnari G, Vanella M, Roman J, Babinchak T, DeSimone J, Stern J, Braffman M, Zhang H, Pomerantz RJ. Human immunodeficiency virus type 1–infected persons with residual disease and virus reservoirs on suppressive highly active antiretroviral therapy can be stratified into relevant virologic and immunologic subgroups. J Infect Dis. 2001;183:1682–1687. doi: 10.1086/320715. [DOI] [PubMed] [Google Scholar]
- Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G, Roney J, Watson J, Prince MH, Hoy JF, Chomont N, Fromentin R, Procopio FA, Zeidan J, Palmer S, Odevall L, Johnstone RW, Martin BP, Sinclair E, Deeks SG, Hazuda DJ, Cameron PU, Sékaly R-P, Lewin SR. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, Abdel-Mohsen M, Hoh R, Hecht F, Hunt P, Somsouk M, Wong J, Johnston R, Siliciano RF, Richman DD, O’Doherty U, Palmer S, Deeks SG, Siliciano JD. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Freundlich B, Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983;62:31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Friedman J, Cho WK, Chu CK, Keedy KS, Archin NM, Margolis DM, Karn J. Epigenetic silencing of HIV-1 by the histone H3 lysine 27 methyltransferase enhancer of zeste 2. J Virol. 2011;85:9078–9089. doi: 10.1128/JVI.00836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, Richman DD, Valentine FT, Jonas L, Meibohm A, Emini EA, Chodakewitz JA, Deutsch P, Holder D, Schleif WA, Condra JH. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- Ho YC, Shan L, Hosmane Nina N, Wang J, Laskey Sarah B, Rosenbloom Daniel IS, Lai J, Blankson Joel N, Siliciano Janet D, Siliciano Robert F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DIS, Keele BF, Ho Y-C, Siliciano JD, Siliciano RF. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J Exp Med. 2017;214:959–972. doi: 10.1084/jem.20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5:e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmichev YV, Veenhuis RT, Pohlmeyer CW, Garliss CC, Walker-Sperling VEK, Blankson JN. A CD3/CD28 microbead-based HIV-1 viral outgrowth assay. J Virus Erad. 2017;3:85–89. doi: 10.1016/S2055-6640(20)30292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird GM, Eisele EE, Rabi SA, Lai J, Chioma S, Blankson JN, Siliciano JD, Siliciano RF. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog. 2013;9:e1003398. doi: 10.1371/journal.ppat.1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, Coffin JM, Bosch RJ, Margolis DM. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth S, Schleimann MH, Nissen SK, Højen JF, Olesen R, Graversen ME, Jørgensen S, Kjær AS, Denton PW, Mørk A, Sommerfelt MA, Krogsgaard K, Østergaard L, Rasmussen TA, Tolstrup M, Søgaard OS. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. The Lancet HIV. 2016;3:e463–e472. doi: 10.1016/S2352-3018(16)30055-8. [DOI] [PubMed] [Google Scholar]
- Li Z, Guo J, Wu Y, Zhou Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 2013;41:277–287. doi: 10.1093/nar/gks976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackewicz CE, Patterson BK, Lee SA, Levy JA. CD8+ cell noncytotoxic anti-human immunodeficiency virus response inhibits expression of viral RNA but not reverse transcription or provirus integration. J Gen Virol. 2000;81:1261–1264. doi: 10.1099/0022-1317-81-5-1261. [DOI] [PubMed] [Google Scholar]
- Massanella M, Richman DD. Measuring the latent reservoir in vivo. J Clin Invest. 2016;126:464–472. doi: 10.1172/JCI80567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla R, Bivalkar-Mehla S, Zhang R, Handy I, Albrecht H, Giri S, Nagarkatti P, Nagarkatti M, Chauhan A. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS ONE. 2010;5:e11160. doi: 10.1371/journal.pone.0011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza D, Johnson SA, Peterson BA, Natarajan V, Salgado M, Dewar RL, Burbelo PD, Doria-Rose NA, Graf EH, Greenwald JH, Hodge JN, Thompson WL, Cogliano NA, Chairez CL, Rehm CA, Jones S, Hallahan CW, Kovacs JA, Sereti I, Sued O, Peel SA, O’Connell RJ, O’Doherty U, Chun TW, Connors M, Migueles SA. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood. 2012;119:4645–4655. doi: 10.1182/blood-2011-10-381996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi P, Di Iulio J, Muñoz M, Martinez R, Beerenwinkel N, Telenti A, Ciuffi A. SAHA dampens TCR-mediated reactivation of latently infected cells. Program and abstracts of the 21st Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. [Google Scholar]
- Moran PA, Diegel ML, Sias JC, Ledbetter JA, Zarling JM. Regulation of HIV production by blood mononucear cells from HIV-infected donors: lack of correlation between HIV-1 production and T cell activation. AIDS Res Hum Retroviruses. 1993;9:455–464. doi: 10.1089/aid.1993.9.455. [DOI] [PubMed] [Google Scholar]
- O’Doherty U, Swiggard WJ, Jeyakumar D, McGain D, Malim MH. A sensitive, quantitative assay for human immunodeficiency virus type 1 integration. J Virol. 2002;76:10942–10950. doi: 10.1128/JVI.76.21.10942-10950.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak AO, Adema KW, Bakker M, Jurriaans S, Berkhout B, Cornelissen M, Lukashov VV. Highly sensitive methods based on seminested real-time reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 unspliced and multiply spliced RNA and proviral DNA. J Clin Microbiol. 2008;46:2206–2211. doi: 10.1128/JCM.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procopio FA, Fromentin R, Kulpa DA, Brehm JH, Bebin AG, Strain MC, Richman DD, O’Doherty U, Palmer S, Hecht FM, Hoh R, Barnard RJO, Miller MD, Hazuda DJ, Deeks SG, Sékaly RP, Chomont N. A novel assay to measure the magnitude of the inducible viral reservoir in HIV-infected individuals. EBioMedicine. 2015;2:874–883. doi: 10.1016/j.ebiom.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. The Lancet HIV. 2014;1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- Richman D. Detection and quantification of HIV reservoirs: Measuring the latent reservoir. Keystone Symposia - Mechanisms of HIV persistence: implication for a cure; Boston, MA. 2015. [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda DJ, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Rouzioux C, Mélard A, Avéttand-Fénoël V. Quantification of total HIV1-DNA in peripheral blood mononuclear cells. In: Vicenzi E, Poli G, editors. Human Retroviruses. Humana Press; 2014. pp. 261–270. [DOI] [PubMed] [Google Scholar]
- Schmid A, Gianella S, von Wyl V, Metzner KJ, Scherrer AU, Niederöst B, Althaus CF, Rieder P, Grube C, Joos B, Weber R, Fischer M, Günthard HF. Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection. PLoS ONE. 2010;5:e13310. doi: 10.1371/journal.pone.0013310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano J, Siliciano R. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. In: Zhu T, editor. Human Retrovirus Protocols. Humana Press; 2005. pp. 3–15. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Østergaard L, Tolstrup M. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 2015;11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina C, Kwoh T, Chowers M, Guatelli J, Richman D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina CA, Prince HE, Richman DD. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain MC, Günthard HF, Havlir DV, Ignacio CC, Smith DM, Leigh-Brown AJ, Macaranas TR, Lam RY, Daly OA, Fischer M, Opravil M, Levine H, Bacheler L, Spina CA, Richman DD, Wong JK. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: Intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci U S A. 2003;100:4819–4824. doi: 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, Spina CA, Woelk CH, Richman DD. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS ONE. 2013;8:e55943. doi: 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain MC, Little SJ, Daar ES, Havlir DV, Günthard HF, Lam RY, Daly OA, Nguyen J, Ignacio CC, Spina CA, Richman DD, Wong JK. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- Walker CM, PJM, Stites DP, Levy JA. CD8+ T lymphocyte control of HIV replication in cultured CD4+ T cells varies among infected individuals. Cell Immunol. 1989;119:470. doi: 10.1016/0008-8749(89)90259-1. [DOI] [PubMed] [Google Scholar]
- Wei DG, Chiang V, Fyne E, Balakrishnan M, Barnes T, Graupe M, Hesselgesser J, Irrinki A, Murry JP, Stepan G, Stray KM, Tsai A, Yu H, Spindler J, Kearney M, Spina CA, McMahon D, Lalezari J, Sloan D, Mellors J, Geleziunas R, Cihlar T. Histone deacetylase inhibitor Romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog. 2014;10:e1004071. doi: 10.1371/journal.ppat.1004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Samuel A, Chen L-F, Kwon H, Fenard D, Bisgrove D, Verdin E, Greene WC. Prostratin antagonizes HIV latency by activating NF-κB. J Biol Chem. 2004;279:42008–42017. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]