Abstract
The discovery of a third type of photoreceptors in the mammalian retina, intrinsically photosensitive retinal ganglion cells (ipRGCs), has had a revolutionary impact on chronobiology. We can now properly account for numerous non-vision-related functions of light, including its effect on the circadian system. Here, we give an overview of ipRGCs and their function as it relates specifically to mood and biological rhythms. Although circadian disruptions have been traditionally hypothesized to be the mediators of light’s effects on mood, here we present an alternative model that dispenses with assumptions of causality between the two phenomena and explains mood regulation by light via another ipRGC-dependent mechanism.
Keywords: mood, light, circadian, rhythms, ipRGC, photoperiod
One of the most constant features of the biosphere during the evolution of life has been the daily solar cycle. Because there is a clear selective advantage in anticipating changes in the environment, it is not surprising that all life forms exposed to the Sun exhibit circadian rhythms in their physiology and behavior. Locomotor activity and sleep are segregated to different phases of the light/dark cycle. Endocrine processes have strong rhythms: Cortisol secretion peaks in the morning, and many hormones involved in metabolism, such as leptin and ghrelin, peak at night (Huang et al. 2011). Researchers have also observed daily rhythms of urine excretion (Noh et al. 2011), perception [nociception (Pickard 1987) and temperature sensation (Refinetti 1995)], alertness (Lockley et al. 2006), and even cognitive abilities (memory and mathematical performance) (Johnson et al. 1992, Foster & Kreitzman 2014). Even mood appears to cycle in a circadian manner (Boivin et al. 1997, Birchler-Pedross et al. 2009), with stronger evidence for a role in so-called positive (versus negative) affect (Murray et al. 2002).
Some of these rhythms are endogenous (circadian) and persist even in constant environmental conditions. However, time-cues called zeitgebers can reset the internal circadian clock and synchronize it to external time. Light is one such cue. Indeed, the eye, far from being purely an organ involved in the formation and tracking of images, provides sensory information for a variety of other purposes: the resetting of the circadian clock (circadian photoentrainment), the constriction of pupils [pupillary light reflex (PLR)], the suppression of activity in nocturnal animals (masking), and the direct modulation of physiological functions. This direct role can interact in more or less complex ways with circadian rhythmicity. Melatonin secretion exemplifies this concept: Although it is regulated in a circadian manner, it is also directly suppressed by light exposure (Czeisler et al. 1995). These unconventional (non-image-related) functions of light have their own unconventional source of sensory information in a photoreceptor that long eluded scientists.
DISCOVERY OF ipRGCs
For more than a century, rods and cones were thought to be the retina’s only light detectors, their properties characterized, their functions familiar. Rods are the exquisitely sensitive cells primarily responsible for nighttime black-and-white vision. Cones are less sensitive to light and come in varieties with different light absorption spectra, making them, in combination, responsible for color perception. Rods and cones are found in the outer nuclear layer of the retina, farthest from the pupil, and connect to bipolar cells, whose soma is located in the inner nuclear layer, halfway through the thickness of the retina; bipolar cells in turn synapse with retinal ganglion cells (RGCs), arrayed in their eponymous layer closest to the incoming light. RGCs send their axons, bundled into the optic nerve, to the brain. This circuitry, with rods and cones as sole light detectors, was held to account for all of the eyes’ biological functions.
The first cracks in this harmonious theory began to appear as early as 1927 (Keeler 1927) but multiplied to the point they could no longer be ignored in the 1990s, when several groups observed a phenomenon as remarkable as it was puzzling: Image-blind humans could still align their sleep/wake cycle to the solar day and, when moving to different time zones, could adapt their circadian rhythms (e.g., melatonin secretion) to the new setting; some, despite their utter inability to form images, could also have a tenuous conscious awareness of the presence of light, particularly blue-shifted light (Zaidi et al. 2007, Van Gelder & Buhr 2016). In addition, mice genetically engineered to have complete degeneration of rods and cones were found to be still capable of circadian photoentrainment (Foster et al. 1991, Vandewalle et al. 2013). The mystery was solved approximately a decade later with the discovery that a small subset of RGCs [now known as intrinsically photosensitive RGCs (ipRGCs)] can be excited by light directly, even in the absence of functional rods and cones (Berson et al. 2002). How is this possible? The answer, it turned out, lay in a new blue light–sensitive photopigment, melanopsin (Provencio et al. 2000, Fu et al. 2005). Melanopsin is expressed in ipRGCs but not in nonphotosensitive RGCs. Introducing it into heterologous systems (e.g., kidney cells) makes them photosensitive (Qiu et al. 2005). And, most notably, mice lacking rods, cones, and melanopsin are blind and do not photoentrain, mask, constrict their pupils or otherwise respond functionally to light (Hattar et al. 2003).
Researchers now know that ipRGCs constitute 4–5% of total RGCs in mice and that this diminutive population is the source of light signaling to the brain for a variety of both image- and non-image-forming purposes.
Melanopsin and rod/cone phototransduction pathways are integrated in ipRGCs, as they participate in the same retinal circuitry as ordinary RGCs. Their different biophysical responses dovetail with each other, and this is reflected in behavior. For instance, melanopsin knockout mice (Opn4−/−) have a normal PLR at low light intensities, with reduced responses only in high brightness. The low sensitivity of melanopsin to light makes rod input necessary for circadian photoentrainment and the PLR in dim light conditions (Altimus et al. 2010). Cones contribute with their faster kinetics, and color is also a signal for fine-tuning photoentrainment and the PLR in mammals (Walmsley et al. 2015). Intrinsic melanopsin phototransduction predominates at high light intensities and for long time spans (Butler & Silver 2011, Jain et al. 2016).
Notably, rods and cones contribute to the regulation of the circadian system and the other non-image-forming functions of the eye exclusively via ipRGCs, as selective ablation of ipRGCs results in the loss of circadian photoentrainment, masking, phase-shifting, and endocrine responses to light pulses, whereas ordinary vision is almost completely spared (Güler et al. 2007, Göz et al. 2008).
MORPHOLOGICAL, PHYSIOLOGICAL, AND TARGET DIVERSITY OF ipRGCs
Researchers now know that ipRGCs, initially thought to be homogeneous, are a highly diverse population. The variety of biological functions they are known to modulate has expanded steadily since their discovery in 2002, and their axons keep being spotted in the most unsuspected nooks of the brain beyond the classic visual areas (Hattar et al. 2006).
At the time of writing, there are five recognized classes of ipRGCs, named M1–5. ipRGC subtypes differ in their size, dendritic stratification, and electrophysiological characteristics, as well as the brain regions they project to (Schmidt et al. 2011a,b). M1 were the first ipRGCs to be characterized. They are the smallest, and their dendrites stratify in the outer inner plexiform layer (IPL) (also known as the OFF sublayer of the IPL). They have the highest level of melanopsin expression and consequently the highest sensitivity to light. M2, M4, and M5 are larger (with M4 being the largest subtype); they stratify their dendrites in the inner (or ON) sublayer of the IPL and have lower light sensitivity. M3 are distinguished by their sprawling dendritic tree, which stratifies in both sublayers of the IPL (Schmidt & Kofuji 2009; Schmidt et al. 2011a,b).
ipRGCs differ substantially in their connectivity and function. M1 ipRGCs project to the suprachiasmatic nucleus (SCN), the shell of the olivary pretectal nucleus (OPN), the intergeniculate leaflet (IGL), and the ventral part of the lateral geniculate nucleus (LGN), as well as to limbic, mood-regulating regions, including the medial amygdala and the boundary region between the lateral habenula (LHb) and the adjacent dorsal thalamus. Non-M1 ipRGCs project to the SCN but also to the core of the OPN, to mood- and pain-modulating regions such as the periaqueductal gray and amygdala, and to more traditionally visual areas such as the dorsal LGN and the superior colliculus (Hattar et al. 2002, 2006; Schmidt et al. 2011a,b).
Perhaps the most general concept to emerge from the study of the central projections of ipRGCs is an endlessly fascinating, protean corrective to a historical prejudice: We have long been used to considering the eye purely as a visual organ. Yet spooring ipRGC axons to the posterior thalamus, we came upon a neural network mediating light’s effect of exacerbating migraines (Noseda et al. 2010) (a phenomenon experienced by some blind people, too, with blue light being the worst offender); projections to the ventrolateral preoptic area have been implicated in sleep induction (Altimus et al. 2008, Tsai et al. 2009), whereas inputs to the subparaventricular zone and lateral hypothalamus may affect these regions’ modulatory role in general activity levels, feeding, and hormone secretion.
It would be a mistake, however, to interpret the distinction between ipRGCs and RGCs as the physical embodiment of the functional dichotomy between image and nonimage vision. Indeed, a traditional RGC subtype, ON alpha, was recently found to be intrinsically photosensitive, and melanopsin signaling in these cells contributes to contrast detection (Schmidt 2014). The real distinction is between those functions of light that require sustained signaling of environmental luminance over long time spans versus those predicated on abrupt, short-term changes.
THE EFFECT OF LIGHT ON MOOD
One of the most remarkable roles of light is mood regulation. Shift work, jet lag, and seasonal affective disorder (SAD) are common human experiences (Foster & Kreitzman 2014). Traveling across time zones causes our (unchanged) internal clock to become desynchronized relative to the (changed) light/dark cycle, so that there is light exposure during the subjective night (scotophase) and darkness during the subjective day (photophase). This is accompanied by symptoms of malaise and irritability, as well as cognitive impairment and constipation or diarrhea. Jet lag persists until the internal circadian rhythm entrains to the new light cycle, with glucocorticoids likely involved in the resynchronization process (Mohawk et al. 2005, Kiessling et al. 2010).
Workers on night shifts (or frequently rotating shifts) suffer from changed internal activity rhythms in a constant light/dark cycle. The symptoms are similar to jet lag, but the impact on health is more severe because entrainment to the new schedule is more difficult or downright unattainable. Aged mice subjected to repeated phase shifts simulating chronic jet lag or shift work suffer an increase in mortality from all causes (Davidson et al. 2006).
SAD is a form of depression recurring in late autumn or winter, when the photoperiod (daylength) is short and rapidly getting shorter. Initially, the obvious therapy seemed to be an artificial extension of the photoperiod, achieved by flooding the sufferer with bright light in the morning and evening. This phototherapy is effective (Wehr et al. 1986). However, so is a single noonday session, which leaves the photoperiod quite unextended (Jacobsen et al. 1987). Researchers then proposed that SAD patients are phase shifted, specifically that their sleep/wake cycle is delayed or advanced relative to their circadian cycle (as revealed by the time course of melatonin secretion) (Lewy et al. 1987). Unfortunately, there is no agreement in the literature as to the actual existence of phase differences in SAD patients, let alone the direction (delay or advance). A typical study reported that the only difference in circadian parameters between SAD patients and controls was the amplitude of body temperature rhythms (Koorengevel et al. 2002).
The mystery as to how light exposure affects mood in SAD endures, but animal studies have begun to provide some clues. Researchers have deployed a plethora of inventive lighting regimes to try to isolate the contributions of sleep and circadian changes to light’s mood-altering effects. Such effects have been confirmed to be marked and pervasive. Both constant light and constant darkness lead to higher anxiety and depression-like behavior, with concomitant effects on sleep or circadian activity. Keeping adult diurnal rodents in a very short (5 h light, 19 h darkness) photoperiod for 6 weeks is similarly depressive (Ashkenazy-Frolinger et al. 2010). Recent results suggest that signaling encoding photoperiod information can coax interneurons in the hypothalamus that regulate the release of corticotropin-releasing hormone to switch between dopamine and somatostatin expression, with an accompanying change in the postsynaptic dopamine receptors. Intriguingly, blockade or ablation of these neurons adversely affects mood (Dulcis et al. 2013). Whether the observed phenomena are determined by the absolute duration of daylength or the total irradiance level over the light/dark cycle (a question that bears on SAD etiology) remains unclear. Experiments with an equinoctial photoperiod using dim light in the photophase have revealed depressive effects analogous to those seen with short photoperiods (Deats et al. 2015). The converse experiment, dim light at night, impairs cognition and causes depressive behavior in diurnal rodents without circadian alterations (Fonken et al. 2012), whereas the same intervention in mice leads to mood deficits with attenuation of SCN rhythms (Fonken et al. 2013). An aberrant light cycle consisting of 3.5 h of light followed by 3.5 h of darkness produced one of the clearest dissociations between circadian rhythmicity (with only a minor lengthening of SCN period) and sleep (unchanged) on one hand and mood (marked depressive effects) on the other (LeGates et al. 2012).
The traditional interpretation in the field holds that disruptions in circadian rhythms are causally linked to mood disorders (and vice versa). However, many of the recent findings we have just reviewed show that light can affect mood even without gross circadian alterations. This aporia prompted researchers to devise a new model, rather in the way of Ptolemy’s epicycles, whereby ipRGCs affect mood via two distinct pathways, an indirect one impinging on the circadian clock as well as a direct one (LeGates et al. 2012, 2014) that has not been characterized.
We submit here a provocative alternative interpretation: Circadian disruptions may not be the cause of mood alterations but rather may be an epiphenomenon, with light affecting mood by a noncircadian mechanism. To evaluate this possibility, we must preliminarily plunge ourselves into the inner workings of the circadian system, and address the evidence linking it with affective states.
CIRCADIAN OSCILLATORS OF THE BRAIN
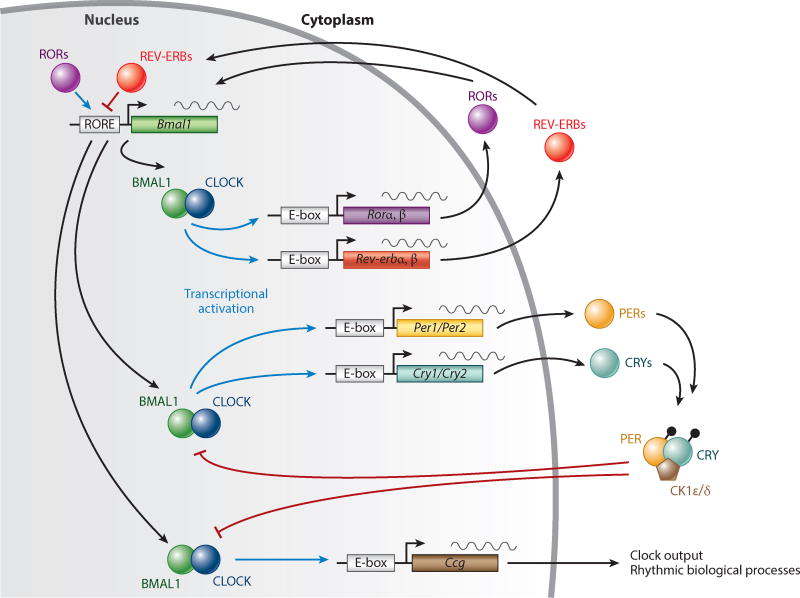
How does the body generate its circadian rhythms? Every cell has a molecular clock based on delayed reciprocal inhibitory loops (Figure 1).
Figure 1.
The molecular clock in every cell consists of reciprocal positive and negative feedback loops. CLOCK and BMAL1 form heterodimers that activate the transcription of Cry and Per genes. PER dimerizes with CRY to inhibit CLOCK-BMAL1–mediated transcription. The timing of dimerization, complex formation, and translocation into the nucleus determines the cellular rhythms. Abbreviations: Ccg, clock controlled gene; CK1ε/δ, casein kinase 1ε/δ; CRY, cryptochrome; E-box, enhancer-box; PER, period; ROR, retinoic acid–related orphan receptor; RORE, RevDR2 and ROR-binding element. Figure adapted from Takahashi (2017).
The SCN is so internally organized that its neurons synchronize their electrical activity spontaneously in a nucleus-wide rhythm extraordinarily resistant to external perturbations. This spontaneous organization is exceedingly rare: The olfactory bulbs are the only other region that maintains coherent, tissue-wide rhythms ex vivo. By both secreted signals (such as glucocorticoids, AVP, and PK2) (Cheng et al. 2002, Hastings et al. 2007) and its own rhythmic electrical output, the SCN is thought to synchronize all the other cellular oscillators, which in turn drive the circadian rhythm of local transcriptomes. ipRGCs are connected to the SCN by two major pathways: directly, via the retinohypothalamic tract (RHT), which packs glutamate and PACAP as neurotransmitters (Hannibal et al. 2004), and indirectly, via the IGL (geniculohypothalamic tract), which results in neuropeptide Y release (Smale & Boverhof 1999). Innumerable potential multirelay circuits exist; we limit ourselves to mentioning that the raphe nuclei, whose activity is modulated by direct ipRGC targets such as the lateral habenula, send one of their densest serotoninergic efferents to the SCN (Hay-Schmidt et al. 2003).
The SCN is made up of two distinct domains: a ventrolateral region (core) that is the recipient of most of the direct photic input and a dorsomedial (shell) region that is the major intrinsic rhythm generator and also receives light input. The RHT is necessary and sufficient for photoentrainment, a phenomenon thought to be based on a dazzling choreography of gene expression involving Period (Per) paralogs. ipRGC signaling causes increased Per1 expression in the SCN core irrespective of circadian phase; stimulation during the early subjective night (which causes circadian phase delays) causes Per2 overexpression in both the shell and core, whereas stimulation during the late subjective night (which induces phase advances) leads to Per1 overexpression in the shell. These transient per overexpressions alter the duration of the cellular clock loop and result in phase-shifting and resynchronization of the internal clock with external time.
Circadian rhythms could, in principle, affect mood by way of the SCN’s connections to mood regulatory centers. Bilateral SCN lesions result in loss of circadian rhythms (arrhythmicity), but the consequences on mood are underwhelming (a minor decrease in immobility time in the forced swim test) (Tataroğlu et al. 2004) and could be accounted for by the extra-SCN effects of inadvertent damage to the optic chiasm. Incidentally, we would like to highlight that immobility tests in rodents are not a model for human depression, but they are valuable at delineating circuits for motivational states or hedonic tone. The SCN also regulates the hypothalamo-pituitary-adrenal (HPA) axis via the periventricular nucleus. Circadian disruptions are often accompanied by HPA axis upregulation, and glucocorticoids have complex effects both directly on the brain and on the immune system. Their role in depression is controversial (Wolkowitz et al. 2009). Circadian genes also have immunoregulatory roles of their own (Edgar et al. 2016). Circadian disruptions are associated with immune dysregulation, including increased inflammatory cytokines (Narasimamurthy et al. 2012), and these in turn affect circadian gene expression, via nuclear factor κB (Gibbs et al. 2012). Inflammation is known to be associated with depression (Miller & Raison 2015), particularly the atypical subtype (Lamers et al. 2013), although there are confounding factors such as body mass index and smoking (Chocano-Bedoya et al. 2014), and even the direction of the presumed cause-effect relationship is far from established [depression itself could ramp up inflammation (Glaus et al. 2014)].
Neurogenesis in the hippocampus appears to have a circadian rhythm as well, with a mitotic peak at night (Tamai et al. 2008). Impaired neurogenesis may play a role in depression (Miller & Hen 2015), an association underscored by the recent development of what is purported to be a purely neurogenic antidepressant (Fava et al. 2015).
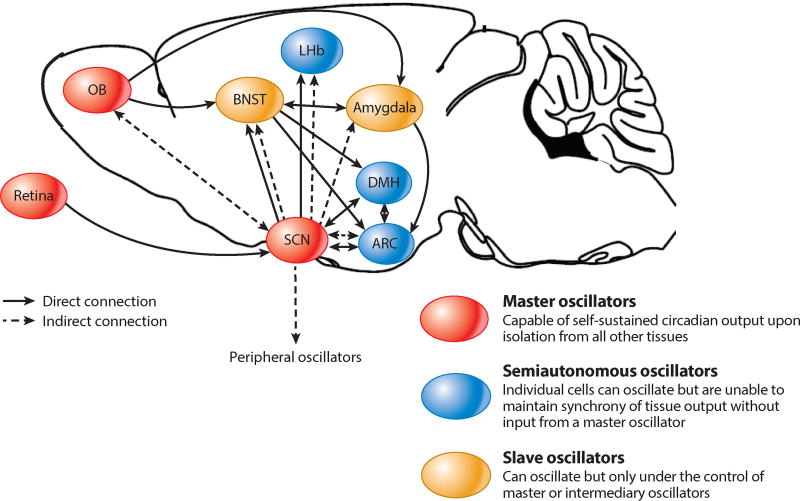
Other brain nuclei more obviously connected than the SCN with affective states display a circadian output in vivo (e.g., the nucleus accumbens, habenula, locus coeruleus, and medial and dorsal raphe) (Figure 2). The LHb, which has daily firing rhythms with higher baseline during the subjective day, receives input from both the SCN (de Vries et al. 1981) and ipRGCs (and harbors cells excited or inhibited by light) (Zhao & Rusak 2005), a convergence that, as we see below, may be significant for detecting specific changes in environmental illumination for mood-setting purposes. These ipRGC-innervated secondary brain oscillators (rather than, or in addition to, the SCN) could be the link between mood, circadian rhythms, and light exposure, given both their well-established functions in reward processing and mood regulation and the fact that they are less impervious to perturbations (Welsh et al. 2010) than the master pacemaker.
Figure 2.
The mammalian brain teems with circadian oscillators. The SCN is the master pacemaker. Semiautonomous oscillators, such as the LHb, contain cells individually characterized by rhythmic activity, but only the input from a master oscillator can synchronize them into a coherent, nucleus-wide rhythm. Finally, slave oscillators, such as the BNST, display rhythms only in response to input from other oscillators. Abbreviations: ARC, arcuate nucleus; BNST, bed nucleus of the stria terminalis; DMH, dorsomedial hypothalamus; LHb, lateral habenula; OB, olfactory bulb; SCN, suprachiasmatic nucleus. Figure adapted from Guilding & Piggins (2007).
A profusion of other potential mechanisms are conceivable to link mood and the circadian system (McClung 2013), but what is the evidence for causality?
CIRCADIAN COMPONENT OF MOOD DISORDERS
Many circadian alterations have been reported in humans with major depressive disorder (MDD). The typical finding is a small reduction in the amplitude of the daily oscillation of certain hormones, notably cortisol (Yehuda et al. 1996, Souêtre et al. 1989), whose trough level at night is slightly elevated. Others have reported phase delays or phase advances relative to healthy controls. For instance, a study of serum melatonin concentration in depressed versus nondepressed humans concluded that depressed patients were phase delayed (i.e., the peak levels of melatonin occur later) (Crasson et al. 2004). In a separate study, researchers tested the concentration in urine of a metabolite of norepinephrine with circadian variation, and the depressed subjects were found to be phase advanced (Wehr & Wirz-Justice 1982). However, a subsequent reanalysis of the data from both of these studies showed that there was in fact no statistically significant difference between groups (Refinetti 2016).
Sleep abnormalities are also commonly associated with MDD (Benca et al. 1992, Peterson & Benca 2006), although there is no established causal relationship, and indeed, the association between sleep and mood is complex and not always dependent on circadian rhythmicity. For instance, acute sleep deprivation alters circadian activity rhythms and the expression of circadian genes (Edwards & Waterhouse 2009) and is well known to have a rapid antidepressant effect. It can in fact induce hypomania even in healthy humans. However, a short 1-h nap the day after the onset of sleep deprivation is enough to cause a relapse of depression without affecting circadian rhythms (Riemann et al. 1993, Monk et al. 2001). Noncircadian mechanisms may be at work, such as changes in cytokines, cortisol, or brain-derived neurotrophic factor (Gorgulu & Caliyurt 2009, Voderholzer et al. 2012).
Some patients suffering from bipolar disorder (BD) have a shortened circadian period (and only this subset seems to respond to lithium therapy) (Kripke et al. 1978). Lengthening the photoperiod, after the winter solstice, can elicit a switch to mania, the origin of the expression March madness, but photoperiod changes are a potential signal for infradian/circannual as well as circadian processes.
Another line of evidence comes from the circadian effects of drugs used to treat mood disorders. Agomelatine is a weak antidepressant acting as a melatonin receptor (MT) 1 and 2 agonist; it increases the amplitude of the sleep/wake cycle (Gorwood 2010) and, in animal models, restores sleep and circadian rhythms induced by certain types of stress (Mairesse et al. 2013); however, agomelatine is also a 5-hydroxytryptamine 2C (5-HT2C) receptor antagonist, and melatonin is not exclusively involved in the synchronization of circadian rhythms (besides their role in sleep and encoding photoperiod information, MTs are also present on immune cells). More troubling still, melatonin displays no antidepressant properties on its own, and agomelatine itself failed to meet the standards of efficacy required by US regulators (although it is approved and marketed in Europe).
Given the massive serotoninergic input to the SCN, it is not surprising to find that selective serotonin reuptake inhibitors alter (shorten) the circadian period (Nomura et al. 2008), and fluoxetine can also cause phase advances (Sprouse et al. 2006). Some nonserotoninergic antidepressants, such as imipramine, do not affect circadian rhythms at all (Refinetti & Menaker 1993). Furthermore, the very existence of the raphe-hypothalamic tract invites the question of whether alterations in the serotoninergic system might not be actually the cause (rather than the consequence) of the circadian disruptions (such as they are) in MDD.
Drugs used to treat bipolar disorder, such as lithium or valproate, cause phase delays, increase the amplitude of daily oscillations of per2 expression (Johansson et al. 2011), and lengthen the activity period (Li et al. 2012). These effects are thought to be mediated by lithium’s activity as an inhibitor of GSK3β, an enzyme with multifarious cellular roles, including the phosphorylation of clock proteins Bmal1, Cry2, Per2, and Rev-Erbα. This is controversial, however, as specific GSK3β inhibitors actually shorten the circadian period. Lithium also reduces the suppressive effect of light on melatonin secretion (Hallam et al. 2005), inviting the suspicion of potential interference with other noncircadian functions of light.
Another interesting and seemingly dispositive finding was the correlation between polymorphisms of many clock genes (such as cry2, clock, npas2, and bmal1) with bipolar disorder and depression (Mansour et al. 2006, Lavebratt et al. 2010, McCarthy et al. 2012). The enthusiasm, however, proved short-lived, as the source studies were based on discredited candidate gene approaches and were severely underpowered; subsequent iterations with better methodology have failed to yield any association between human mood disorders and clock gene loci (CONVERGE Consortium 2015, Hyde et al. 2016).
MOOD AND THE CIRCADIAN SYSTEM IN ANIMAL MODELS
Transgenic animals with mutations in certain clock genes display remarkable mood phenotypes. We focus on the ClockΔ19 mouse because its saga is exemplary. ClockΔ19 is a dominant negative mutation of clock. In homozygosity, mice have a lengthened circadian period of 27 h that is lost to arrhythmicity under prolonged constant dark conditions. During the day, the ClockΔ19 mouse exhibits marked hyperactivity, increased reward-seeking behavior, reduced immobility in the forced swim test, and decreased anxiety. It recapitulates the most salient features of mania in humans (Roybal et al. 2007). At night, it is euthymic. This mouse has an increased expression of tyrosine hydroxylase (TH) and increased ventral tegmental area (VTA) dopaminergic tone. Normally, the VTA harbors a subset of dopaminergic neurons that display, in vivo, circadian activity (Baltazar et al. 2013). This rhythm ceases ex vivo, suggesting a dependence on the SCN, which is connected to the VTA indirectly by way of the medial preoptic nucleus. Therefore, since the creation of the ClockΔ19 mouse, researchers have speculated as to the causal relationship between its mood and circadian phenotypes. The denouement is instructive in that it epitomizes how clock genes have pleiotropic effects: clock also acts as a negative regulator of TH expression, and pharmacological inhibition of TH during the day reverses the manic phenotype, whereas optogenetic chronic stimulation that increases tonic VTA activity triggers manic behavior (Sidor et al. 2015). Furthermore, Clock/Bmal1 also activate transcription of cholecystokinin (CCK), which is a digestive hormone but also a neuropeptide that negatively regulates dopaminergic signaling, and the ClockΔ19 phenotype shows reduced CCK levels (Arey et al. 2014). This pleiotropy provides a model to explain epiphenomenal circadian alterations. In other words, the same factor can produce a circadian phenotype and an affective phenotype without there being any causal link between them. Sometimes pleiotropic noncircadian effects can be unmasked. To reprise our example, the manic phenotype of ClockΔ19 is ameliorated by CK1δ/ε inhibitors (Arey & McClung 2012) (CK1δ/ε participate in the phosphorylation-mediated degradation of Per1, 2, and 3). However, CK1δ/ε inhibition causes period lengthening (Loudon et al. 2007), therefore aggravating ClockΔ19’s circadian phenotype.
Pleiotropic effects are by no means exclusive to clock. Monoaminoxidase-A, an enzyme that metabolizes 5-HT, norepinephrine, and dopamine, is under direct transcriptional (circadian) regulation by Npas2, Bmal1, and Per2 in the striatum, and these proteins are regulated not only by circadian signals from the SCN (with no evidence that their rhythmic expression is important for mood regulation) but also by extrinsic factors, such as chronic stress (Spencer et al. 2013).
The fact that the cellular circadian machinery is so evolutionarily ancient and so intertwined with cell metabolism, as well as the fact that so much of the genome is under circadian regulation (up to 10% in mice) (Doherty & Kay 2014), suggests that pleiotropy could be pervasive.
In mutants and genetic models, circadian and mood alterations are generally incongruent: For instance, Per1 mutants have no gross mood disruption (exhibiting only slight differences in reward seeking after stress) but do show altered circadian rhythms (smaller amplitude, shorter period, and a tendency to become arrhythmic in constant darkness). Per2 mutants have inconsistent mood phenotypes depending on the specific mutation but have similar circadian alterations (shorter period, arrhythmicity) (Landgraf et al. 2014).
These discrepancies, taken together with the results of experimental light exposure regimes, could indicate that the mood and circadian systems are indeed not causally linked (Bechtel 2015).
AN ALTERNATIVE: PHOTOPERIOD HISTORY MODELS
If circadian alterations in mood disorders are indeed epiphenomena, is it possible to account for light’s effects on mood without introducing distinct ad hoc direct and indirect pathways?
Whereas daily mood oscillations in humans are fairly minor, there is a powerful seasonal rhythm for depressive mood (even in the non-SAD population) and feelings of hostility, anger, anxiety, and irritability (Harmatz et al. 2000). Most finally, suicides also display a clear circannual cycle, which peaks in late spring and early summer (Coimbra et al. 2016)—a rhythm that flattened over the twentieth century as artificial light spread (Woo et al. 2012). Recurrent depression is seasonal in 10–20% of cases, with autumn and winter onset most common (Roecklein & Rohan 2005). From an evolutionary standpoint, it is easy to imagine the adaptive advantage of modulating mood to engender preparatory behaviors for the ecological changes (e.g., weather) that accompany quite reliably the passage of seasons.
Endogenous circannual clocks exist in humans and other primates, although they are less well understood than circadian clocks (Wehr 2001). Many animals display circannual rhythms of body mass and reproductive competence, which free run in the absence of environmental cues with endogenous periods of 10–12 months. These rhythms can be synchronized to sidereal time by zeitgebers, photoperiod being the preeminent one. Other seasonal rhythms can be evoked by environmental cues without the existence of an endogenous rhythm (Peacock et al. 2004).
A plausible scenario is one in which absolute daylength is used as a cue to determine season for mood-setting purposes (critical daylength model). All days shorter than the critical threshold are interpreted as winter, all days longer as summer (Paul et al. 2007). The SCN does encode the photoperiod in its output and undergoes characteristic seasonal changes (Coomans et al. 2015).
We saw above the effect of photoperiod in adult animals; the perinatal photoperiod also has long-lasting effects on mood, with short (versus long) photoperiods producing more depressive, anxious behavior in adulthood. This is accompanied by an imprinting effect on the SCN (with persisting differences in the properties of the circadian oscillator) (Ciarleglio et al. 2011) and dorsal raphe: The serotoninergic system undergoes abiding changes in firing rate and excitability via an MT1-dependent mechanism (Green et al. 2015).
Although the brain presumably requires in this case information about the absolute duration of daytime, this is not necessary in principle for detecting the passage of seasons. Indeed, an equinoctial photoperiod may be foretokening either summer or winter, and distinguishing between the two contingencies has adaptive value. What is required is a way to determine photoperiodic history.
A possibility (drawn from studies of insect photoperiodism) is an internal coincidence model in which two brain regions with circadian output exist: a morning oscillator with a longer period entrained to dawn and an evening oscillator with a shorter period synchronized to dusk (Paul et al. 2007). When the photoperiod changes, a phase change arises between these two regions’ circadian output, decoded by a comparison area as photoperiod change information.
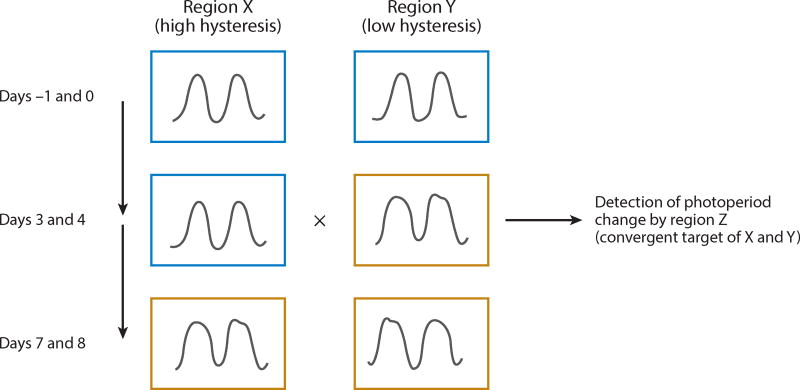
Along these lines, we advance an alternative hypothesis: The circadian output of different brain regions characterized by different speed of entrainment to ipRGC signaling (i.e., different hysteresis) could be compared and integrated to detect waveform (e.g., period or phase) discrepancies that arise dynamically as daylength expands or contracts (Figure 3). The reductio of this concept would be an area that compares the circadian output of a different region with direct ipRGC signaling. Subdomains within the SCN itself could play this role; The fabled robustness of the circadian output of the SCN shell (Welsh et al. 2010) relative to the core immediately suggests a source of the differential hysteresis posited by this model: Ventral oscillators adjust within a day to an abrupt light shift, whereas dorsal ones take several days (Nagano et al. 2003). However, the mechanism mediating this adaptive response to light shifts is likely not the same as that underlying detection of gradual changes in the photoperiod, the two phenomena being fundamentally different. For instance, acute desynchrony of the SCN’s core and shell produces a bimodal output that is speculated to be involved in the onset of two distinct circadianmotor activity rhythms (so-called splitting) (de la Iglesia et al. 2004). No such behavior is observed following abrupt changes of the photoperiod, suggesting that at least one non-SCN region might be implicated.
Figure 3.
Photoperiod history calculation based on differential speed of entrainment to an increase in daylength. The plots represent the output of two different circadian oscillators. The first row shows the state of the system in a constant short [e.g., light:dark (LD) 8:16 h] photoperiod (framed in blue); on day 0, the animal is switched from a short to a long (e.g., LD 16:8) photoperiod. The bottom row shows the state of the system after both oscillators have entrained to the new photoperiod (framed in yellow). The middle row shows the transient state during which a discordance arises between the two.
We point out that this model provides a way for the brain to compute photoperiod history based on transients elicited by photoperiod changes, independent of the final steady states of circadian oscillators (as they would be detected in constant light regimens). Our hypothesis is testable by investigating the hysteresis to photoperiod change of the circadian output of various brain regions and the activation of nuclei receiving afferents from regions of different hysteresis.
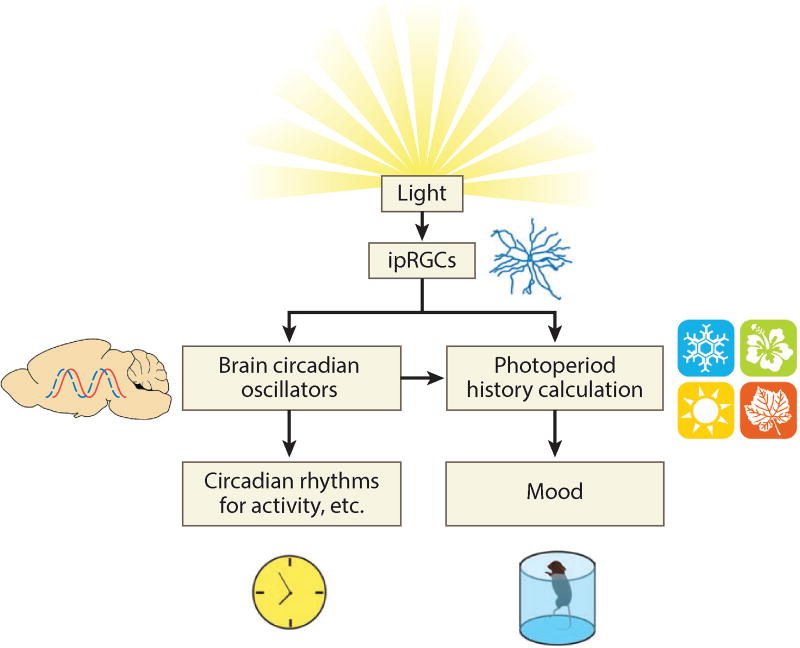
This model provides a mechanism to link circadian rhythms with circannual or seasonal rhythms (Figure 4). Furthermore, it could account for light’s modulation of mood and explain the epiphenomenal alterations in circadian rhythms without invoking the existence of multiple pathways for light to act on.
Figure 4.
ipRGC signaling affects both circadian rhythms and mood. This diagram illustrates how the two phenomena can appear correlated while not being causally linked. Circadian oscillators of the brain orchestrate the circadian rhythms of physiology and behavior directly, but mood is affected by a comparison of incidental properties of their output. Abbreviation: ipRGC, intrinsically photosensitive retinal ganglion cell.
More conservatively, if one does not wish to disavow entirely the traditional notion of a causal nexus between mood and circadian rhythmicity, photoperiodism may be one mechanism whereby ipRGC signaling affects mood alongside true circadian effects, thereby supplying the identity of the direct pathway.
The identification of a brain circuit extracting photoperiod history information for the setting of mood would be a theoretical breakthrough of considerable interest in its own right. It would also have tantalizing medical implications, given the prevalence of mood disorders in humans and artificial light’s continued relentless erosion of the night.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Altimus CM, Güler AD, Alam NM, Arman AC, Prusky GT, et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat. Neurosci. 2010;13(9):1107–12. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus CM, Güler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. PNAS. 2008;105(50):19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arey RN, Enwright JF, III, Spencer SM, Falcon E, Ozburn AR, et al. An important role for cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors. Mol. Psychiatry. 2014;19(3):342–50. doi: 10.1038/mp.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arey RN, McClung CA. An inhibitor of casein kinase 1 ε/δ partially normalizes the manic-like behaviors of the ClockΔ19 mouse. Behav. Pharmacol. 2012;23(4):392–96. doi: 10.1097/FBP.0b013e32835651fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazy-Frolinger T, Kronfeld-Schor N, Juetten J, Einat H. It is darkness and not light: depression-like behaviors of diurnal unstriped Nile grass rats maintained under a short photoperiod schedule. J. Neurosci. Methods. 2010;186(2):165–70. doi: 10.1016/j.jneumeth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Baltazar RM, Coolen LM, Webb IC. Diurnal rhythms in neural activation in the mesolimbic reward system: critical role of the medial prefrontal cortex. Eur. J. Neurosci. 2013;38(2):2319–27. doi: 10.1111/ejn.12224. [DOI] [PubMed] [Google Scholar]
- Bechtel W. Circadian rhythms and mood disorders: Are the phenomena and mechanisms causally related? Front. Psychiatry. 2015;6:118. doi: 10.3389/fpsyt.2015.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch. Gen. Psychiatry. 1992;49(8):651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–73. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Birchler-Pedross A, Schröder CM, Münch M, Knoblauch V, Blatter K, et al. Subjective well-being is modulated by circadian phase, sleep pressure, age, and gender. J. Biol. Rhythm. 2009;24(3):232–42. doi: 10.1177/0748730409335546. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch. Gen. Psychiatry. 1997;54(2):145–52. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Butler MP, Silver R. Divergent photic thresholds in the non-image-forming visual system: entrainment, masking and pupillary light reflex. Proc. R. Soc. B. 2011;278(1706):745–50. doi: 10.1098/rspb.2010.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417(6887):405–10. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Chocano-Bedoya PO, Mirzaei F, O’Reilly EJ, Lucas M, Okereke OI, et al. C-reactive protein, interleukin-6, soluble tumor necrosis factor α receptor 2 and incident clinical depression. J. Affect. Disord. 2014;163:25–32. doi: 10.1016/j.jad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio CM, Axley JC, Strauss BR, Gamble KL, McMahon DG. Perinatal photoperiod imprints the circadian clock. Nat. Neurosci. 2011;14(1):25–27. doi: 10.1038/nn.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra DG, Pereira E Silva AC, de Sousa-Rodrigues CF, Barbosa FT, de Siqueira Figueredo D, et al. Do suicide attempts occur more frequently in the spring too? A systematic review and rhythmic analysis. J. Affect. Disord. 2016;196:125–37. doi: 10.1016/j.jad.2016.02.036. [DOI] [PubMed] [Google Scholar]
- CONVERGE Consort. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523(7562):588–91. doi: 10.1038/nature14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomans CP, Ramkisoensing A, Meijer JH. The suprachiasmatic nuclei as a seasonal clock. Front. Neuroendocrinol. 2015;37:29–42. doi: 10.1016/j.yfrne.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Crasson M, Kjiri S, Colin A, Kjiri K, L’Hermite-Baleriaux M, et al. Serum melatonin and urinary 6-sulfatoxymelatonin in major depression. Psychoneuroendocrinology. 2004;29(1):1–12. doi: 10.1016/s0306-4530(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. J. Med. 1995;332(1):6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr. Biol. 2006;16(21):R914–16. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Cambras T, Schwartz WJ, Díez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr. Biol. 2004;14(9):796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- de Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain—presence of a sex difference in the lateral septum. Brain Res. 1981;218(1–2):67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- Deats SP, Adidharma W, Yan L. Hypothalamic dopaminergic neurons in an animal model of seasonal affective disorder. Neurosci. Lett. 2015;602:17–21. doi: 10.1016/j.neulet.2015.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty CJ, Kay SA. Circadian control of global gene expression patterns. Annu. Rev. Genet. 2014;44:419–44. doi: 10.1146/annurev-genet-102209-163432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340(6131):449–53. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, et al. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. PNAS. 2016;113(36):10085–90. doi: 10.1073/pnas.1601895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BJ, Waterhouse J. Effects of one night of partial sleep deprivation upon diurnal rhythms of accuracy and consistency in throwing darts. Chronobiol. Int. 2009;26(4):756–68. doi: 10.1080/07420520902929037. [DOI] [PubMed] [Google Scholar]
- Fava M, Johe K, Ereshefsky L, Gertsik LG, English BA, et al. A Phase 1B, randomized, double blind, placebo controlled, multiple-dose escalation study of NSI-189 phosphate, a neurogenic compound, in depressed patients. Mol. Psychiatry. 2015;21(10):1372–80. doi: 10.1038/mp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and affects metabolism. J. Biol. Rhythm. 2013;28(4):262–71. doi: 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythm. 2012;27(4):319–27. doi: 10.1177/0748730412448324. [DOI] [PubMed] [Google Scholar]
- Foster RG, Kreitzman L. The rhythms of life: What your body clock means to you! Exp. Physiol. 2014;99(4):599–606. doi: 10.1113/expphysiol.2012.071118. [DOI] [PubMed] [Google Scholar]
- Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd) J. Comp. Physiol. A. 1991;169(1):39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, et al. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. PNAS. 2005;102(29):10339–44. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. PNAS. 2012;109(2):582–87. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaus J, Vandeleur CL, von Känel R, Lasserre AM, Strippoli MP, et al. Associations between mood, anxiety or substance use disorders and inflammatory markers after adjustment for multiple covariates in a population-based study. J. Psychiatr. Res. 2014;58:36–45. doi: 10.1016/j.jpsychires.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Gorgulu Y, Caliyurt O. Rapid antidepressant effects of sleep deprivation therapy correlates with serum BDNF changes in major depression. Brain Res. Bull. 2009;80(3):158–62. doi: 10.1016/j.brainresbull.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Gorwood P. Restoring circadian rhythms: a new way to successfully manage depression. J. Psychopharmacol. 2010;24(2 Suppl):15–19. doi: 10.1177/1359786810372981. [DOI] [PubMed] [Google Scholar]
- Göz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLOS ONE. 2008;3(9):e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green NH, Jackson CR, Iwamoto H, Tackenberg MC, McMahon DG. Photoperiod programs dorsal raphe serotonergic neurons and affective behaviors. Curr. Biol. 2015;25(10):1389–94. doi: 10.1016/j.cub.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: Are circadian oscillators present throughout the mammalian brain? Eur. J. Neurosci. 2007;25(11):3195–216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- Güler AD, Altimus CM, Ecker JL, Hattar S. Multiple photoreceptors contribute to nonimage-forming visual functions predominantly through melanopsin-containing retinal ganglion cells. Cold Spring Harb. Symp. Quant. Biol. 2007;72:509–15. doi: 10.1101/sqb.2007.72.074. [DOI] [PubMed] [Google Scholar]
- Hallam KT, Olver JS, Horgan JE, McGrath C, Norman TR. Low doses of lithium carbonate reduce melatonin light sensitivity in healthy volunteers. Int. J. Neuropsychopharmacol. 2005;8(2):255–59. doi: 10.1017/S1461145704004894. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Østergaard J, Georg B, Heegaard S, et al. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the retinohypothalamic tract. Investig. Ophthalmol. Vis. Sci. 2004;45(11):4202–9. doi: 10.1167/iovs.04-0313. [DOI] [PubMed] [Google Scholar]
- Harmatz MG, Well AD, Overtree CE, Kawamura KY, Rosal M, Ockene IS. Seasonal variation of depression and other moods: a longitudinal approach. J. Biol. Rhythm. 2000;15(4):344–50. doi: 10.1177/074873000129001350. [DOI] [PubMed] [Google Scholar]
- Hastings M, O’Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J. Endocrinol. 2007;195(2):187–98. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006;497(3):326–49. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay-Schmidt A, Vrang N, Larsen PJ, Mikkelsen JD. Projections from the raphe nuclei to the suprachiasmatic nucleus of the rat. J. Chem. Neuroanat. 2003;25(4):293–310. doi: 10.1016/s0891-0618(03)00042-5. [DOI] [PubMed] [Google Scholar]
- Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet. 2016;48(9):1031–36. doi: 10.1038/ng.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011;121(6):2133–41. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen FM, Wehr TA, Skwerer RA, Sack DA, Rosenthal NE. Morning versus midday phototherapy of seasonal affective disorder. Am. J. Psychiatry. 1987;144(10):1301–5. doi: 10.1176/ajp.144.10.1301. [DOI] [PubMed] [Google Scholar]
- Jain V, Srivastava I, Palchaudhuri S, Goel M, Sinha-Mahapatra SK, Dhingra NK. Classical photoreceptors are primarily responsible for the pupillary light reflex in mouse. PLOS ONE. 2016;11(6):e0157226. doi: 10.1371/journal.pone.0157226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson AS, Brask J, Owe-Larsson B, Hetta J, Lundkvist GBS. Valproic acid phase shifts the rhythmic expression of PERIOD2::LUCIFERASE. J. Biol. Rhythm. 2011;26(6):541–51. doi: 10.1177/0748730411419775. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J. Sleep Res. 1992;1(1):24–29. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Keeler CE. Iris movements in blind mice. Am. J. Physiol. 1927;81(1):107–12. [Google Scholar]
- Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J. Clin. Investig. 2010;120(7):2600–9. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koorengevel KM, Beersma DG, den Boer JA, van den Hoofdakker RH. A forced desynchrony study of circadian pacemaker characteristics in seasonal affective disorder. J. Biol. Rhythm. 2002;17(5):463–75. doi: 10.1177/074873002237140. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Mullaney DJ, Atkinson M, Wolf S. Circadian rhythm disorders in manic-depressives. Biol. Psychiatry. 1978;13(3):335–51. [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry. 2013;18(6):692–99. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- Landgraf D, McCarthy MJ, Welsh DK. The role of the circadian clock in animal models of mood disorders. Behav. Neurosci. 2014;128(3):344–59. doi: 10.1037/a0036029. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Sjöholm LK, Soronen P, Paunio T, Vawter MP, et al. CRY2 is associated with depression. PLOS ONE. 2010;5(2):e9407. doi: 10.1371/journal.pone.0009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–98. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014;15(7):443–54. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235(4786):352–54. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- Li J, Lu WQ, Beesley S, Loudon ASI, Meng QJ. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLOS ONE. 2012;7(3):e33292. doi: 10.1371/journal.pone.0033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29(2):161–68. [PubMed] [Google Scholar]
- Loudon ASI, Meng QJ, Maywood ES, Bechtold DA, Boot-Handford RP, Hastings MH. The biology of the circadian Ck1ε tau mutation in mice and Syrian hamsters: a tale of two species. Cold Spring Harb. Symp. Quant. Biol. 2007;72:261–71. doi: 10.1101/sqb.2007.72.073. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Silletti V, Laloux C, Zuena AR, Giovine A, et al. Chronic agomelatine treatment corrects the abnormalities in the circadian rhythm of motor activity and sleep/wake cycle induced by prenatal restraint stress in adult rats. Int. J. Neuropsychopharmacol. 2013;16(2):323–38. doi: 10.1017/S1461145711001970. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, et al. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5(2):150–57. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Nievergelt CM, Kelsoe JR, Welsh DK. A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PLOS ONE. 2012;7(2):e32091. doi: 10.1371/journal.pone.0032091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. How might circadian rhythms control mood? Let me count the ways. Biol. Psychiatry. 2013;74(4):242–49. doi: 10.1016/j.biopsych.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2015;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 2015;30:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Cashen K, Lee TM. Inhibiting cortisol response accelerates recovery from a photic phase shift. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288(1):R221–28. doi: 10.1152/ajpregu.00272.2004. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Carrier J, Billy BD, Rose LR. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24(6):680–87. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- Murray G, Allen NB, Trinder J. Mood and the circadian system: investigation of a circadian component in positive affect. Chronobiol. Int. 2002;19(6):1151–69. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- Nagano M, Adachi A, Nakahama K, Nakamura T, Tamada M, et al. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J. Neurosci. 2003;23(14):6141–51. doi: 10.1523/JNEUROSCI.23-14-06141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. PNAS. 2012;109(31):12662–67. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh JY, Han DH, Yoon JA, Kim MH, Kim SE, et al. Circadian rhythms in urinary functions: possible roles of circadian clocks? Int. Neurourol. J. 2011;15(2):64–73. doi: 10.5213/inj.2011.15.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Castanon-Cervantes O, Davidson A, Fukuhara C. Selective serotonin reuptake inhibitors and raft inhibitors shorten the period of Period1-driven circadian bioluminescence rhythms in rat-1 fibroblasts. Life Sci. 2008;82(23–24):1169–74. doi: 10.1016/j.lfs.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, et al. A neural mechanism for exacerbation of headache by light. Nat. Neurosci. 2010;13(2):239–45. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: the internal calendars of vertebrates. Philos. Trans. R. Soc. B. 2007;363(1490):341–61. doi: 10.1098/rstb.2007.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock WL, Król E, Moar KM, McLaren JS, Mercer JG, Speakman JR. Photoperiodic effects on body mass, energy balance and hypothalamic gene expression in the bank vole. J. Exp. Biol. 2004;207(Pt. 1):165–77. doi: 10.1242/jeb.00719. [DOI] [PubMed] [Google Scholar]
- Peterson MJ, Benca RM. Sleep in mood disorders. Psychiatr. Clin. N. Am. 2006;29(4):1009–32. doi: 10.1016/j.psc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Pickard GE. Circadian rhythm of nociception in the golden hamster. Brain Res. 1987;425(2):395–400. doi: 10.1016/0006-8993(87)90529-4. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J. Neurosci. 2000;20(2):600–5. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433(7027):745–49. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Rhythms of temperature selection and body temperature are out of phase in the golden hamster. Behav. Neurosci. 1995;109(3):523–27. doi: 10.1037//0735-7044.109.3.523. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Circadian Physiology. 3 Boca Raton, FL: CRC Press; 2016. [Google Scholar]
- Refinetti R, Menaker M. Effects of imipramine on circadian rhythms in the golden hamster. Pharmacol. Biochem. Behav. 1993;45(1):27–33. doi: 10.1016/0091-3057(93)90081-4. [DOI] [PubMed] [Google Scholar]
- Riemann D, Wiegand M, Lauer CJ, Berger M. Naps after total sleep deprivation in depressed patients: Are they depressiogenic? Psychiatry Res. 1993;49(2):109–20. doi: 10.1016/0165-1781(93)90099-3. [DOI] [PubMed] [Google Scholar]
- Roecklein KA, Rohan KJ. Seasonal affective disorder: an overview and update. Psychiatry. 2005;2(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, et al. Mania-like behavior induced by disruption of CLOCK. PNAS. 2007;104(15):6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Alam NM, Chen S, Kofuji P, Li W, et al. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron. 2014;82(4):781–88. doi: 10.1016/j.neuron.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011a;34(11):572–80. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J. Neurosci. 2011b;31(45):16094–101. doi: 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J. Neurosci. 2009;29(2):476–82. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, et al. Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol. Psychiatry. 2015;20(11):1406–19. doi: 10.1038/mp.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale L, Boverhof J. The suprachiasmatic nucleus and intergeniculate leaflet of Arvicanthis niloticus, a diurnal murid rodent from East Africa. J. Comp. Neurol. 1999;403(2):190–208. doi: 10.1002/(sici)1096-9861(19990111)403:2<190::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Souêtre E, Salvati E, Belugou JL, Pringuey D, Candito M, et al. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28(3):263–78. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, et al. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. Eur. J. Neurosci. 2013;37(2):242–50. doi: 10.1111/ejn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse J, Braselton J, Reynolds L. Fluoxetine modulates the circadian biological clock via phase advances of suprachiasmatic nucleus neuronal firing. Biol. Psychiatry. 2006;60(8):896–99. doi: 10.1016/j.biopsych.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Tamai S, Sanada K, Fukada Y. Time-of-day-dependent enhancement of adult neurogenesis in the hippocampus. PLOS ONE. 2008;3(12):e3835. doi: 10.1371/journal.pone.0003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18:164–79. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataroğlu O, Aksoy A, Yilmaz A, Canbeyli R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Res. 2004;1001(1–2):118–24. doi: 10.1016/j.brainres.2003.11.063. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, et al. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4−/− mice. PLOS Biol. 2009;7(6):e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelder RN, Buhr ED. Ocular photoreception for circadian rhythm entrainment in mammals. Annu. Rev. Vis. Sci. 2016;2:153–69. doi: 10.1146/annurev-vision-111815-114558. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Collignon O, Hull JT, Daneault V, Albouy G, et al. Blue light stimulates cognitive brain activity in visually blind individuals. J. Cogn. Neurosci. 2013;25(12):2072–85. doi: 10.1162/jocn_a_00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voderholzer U, Fiebich BL, Dersch R, Feige B, Piosczyk H, et al. Effects of sleep deprivation on nocturnal cytokine concentrations in depressed patients and healthy control subjects. J. Neuropsychiatry Clin. Neurosci. 2012;24(3):354–66. doi: 10.1176/appi.neuropsych.11060142. [DOI] [PubMed] [Google Scholar]
- Walmsley L, Hanna L, Mouland J, Martial F, West A, et al. Colour as a signal for entraining the mammalian circadian clock. PLOS Biol. 2015;13(4):e1002127. doi: 10.1371/journal.pbio.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA. Photoperiodism in humans and other primates: evidence and implications. J. Biol. Rhythm. 2001;16(4):348–64. doi: 10.1177/074873001129002060. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Jacobsen FM, Sack DA, Arendt J, Tamarkin L, Rosenthal NE. Phototherapy of seasonal affective disorder: Time of day and suppression of melatonin are not critical for antidepressant effects. Arch. Gen. Psychiatry. 1986;43(9):870–75. doi: 10.1001/archpsyc.1986.01800090060008. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A. Circadian rhythm mechanisms in affective illness and in antidepressant drug action. Pharmacopsychiatria. 1982;15(1):31–39. doi: 10.1055/s-2007-1019506. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 2010;72:551–77. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann. N. Y. Acad. Sci. 2009;1179:19–40. doi: 10.1111/j.1749-6632.2009.04980.x. [DOI] [PubMed] [Google Scholar]
- Woo JM, Okusaga O, Postolache TT. Seasonality of suicidal behavior. Int. J. Environ. Res. Public Health. 2012;9(2):531–47. doi: 10.3390/ijerph9020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol. Psychiatry. 1996;40(2):79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr. Biol. 2007;17(24):2122–28. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132(2):519–28. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]