Synopsis
Asthma and chronic obstructive pulmonary disease are two common chronic respiratory disorders in primary care that cause considerable morbidity and mortality. This article reviews disease pathophysiology and outlines an integrative, multi-dimensional approach to evaluation and management of these conditions, including pharmacotreatment, nutrition, supplements, self-care strategies, mind-body therapies, and other integrative modalities.
Keywords: asthma, COPD, respiratory disease, integrative medicine
INTRODUCTON
ASTHMA AND CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Asthma and chronic obstructive pulmonary disease (COPD) are both common chronic respiratory disorders in primary care that cause considerable morbidity and mortality. Although typically classified as distinct entities, they both involve obstructive airflow limitation and inflammation, and some describe them as existing along the same spectrum. In the U.S., asthma affects 7.7% of the population (over 24 million people), a prevalence that has been rising for the past several decades.1 Although asthma can occur at any age, over a quarter of asthma sufferers in the U.S. are children. COPD tends to have an older demographic. It has an estimated prevalence of 6.5% (14 million people) in the U.S., but the condition is thought to be greatly underdiagnosed.2,3,4 COPD is the third most common cause of death in the U.S., behind heart disease and cancer.5
While evidence-based complementary approaches to asthma and COPD can be very helpful, they are but one component of a comprehensive care plan that should include conventional therapies and regular follow-up, and should never be used in place of appropriate medical care.
ASTHMA
PATHOPHYSIOLOGY
Early in the 1990’s, asthma was categorized as either “allergic” or “non-allergic.” Since then, advanced diagnostic techniques have allowed for a marked expansion of these categories, resulting in the identification of dozens of asthma phenotypes.6 Most of these have defined discrete triggers, rather than unique pathophysiologic mechanisms. At its root, in people with asthma, the responses of the asthmatic airway to provocative stimuli remain constant: bronchoconstriction, bronchial hyperresponsiveness, and inflammation. Of these, airway inflammation has proven to be the most amenable to Integrative modalities.
Allergic triggers are among the most predominant initiators of the asthma exacerbation, and much of the pathophysiology of asthma has been delineated within the allergic model. In an atopic individual, with characteristic airway hyperresponsiveness, the introduction of an allergen into the airway triggers a well-defined series of reactions, beginning with mast cell degranulation and the release of vasoactive amines, enzymes, and leukotrienes, that trigger smooth muscle bronchoconstriction. Allergic T helper cells, or Th2 cells, then secrete cytokines and chemokines that further recruit other cells to the airway. The cellular influx results in mucus production that further contributes to the inflammatory cascade. The recognition of an inflammatory component of asthma in the 1970’s allowed for successful therapies using broad spectrum anti-inflammatory drugs, such as oral corticosteroids, albeit with severe and long-term adverse effects. The recent discovery of the discrete constituents of the inflammatory pathway has allowed for targeted biological therapies using monoclonal antibodies that reduce adverse events, but may have long-term sequelae that have not been observed yet.
EVALUATION
The diagnosis and continuing care of the patient with asthma are standard elements of medical education and training, however, some aspects are often forgotten. Initial evaluation should characterize the frequency and severity of symptoms, the degree of airway compromise and reversibility (using spirometry with/without a bronchodilator), and any identifiable triggers. Asthma triggers are typically individualized, however it is worth exploring the more common triggers, which include: upper respiratory infections, exercise, allergen exposure, cold weather, laughing or crying, or other emotional stress. In addition, an environmental history and food diary may be helpful in discerning dietary triggers or location-specific allergen exposures (i.e., dust mites in the home or office). Allergy testing is also important, especially with seasonal symptoms, as the effectiveness of immunotherapy in atopic individuals has been demonstrated.7
Asthma diagnosis and classification relies upon the presence of a set of discrete symptoms, including recurrent and reversible cough, wheezing, dyspnea, or chest tightness. Objective evidence includes spirometry that demonstrates reversal of airway obstruction following administration of a bronchodilator medication (e.g., albuterol). Asthma classification is based upon frequency of symptoms (including nocturnal symptoms), need and weekly use of rescue medication, interference with daily activities, lung function, and frequency of severe exacerbations. Therapeutic decisions are based upon this classification. Details on assessment and classification can be found in the NHLBI Expert panel review.8
Follow-up care in asthma is critical, as it can uncover new triggers and identify the need for more or less intensive therapy. It is always prudent to use the lowest dose of drug needed to achieve and sustain control—especially when a patient is chronically using oral corticosteroids. Finally, the use of objective lung function data (spirometry) along with a standard, validated measure of asthma control is encouraged at each appointment. Two such accepted tools are the Asthma Control Test (ACT) and the Asthma Control Questionnaire (ACQ).9,10 These brief forms are filled out by the patient, and provide objective, quantifiable indicators of asthma symptoms, and take only minutes to administer and interpret.
PREVENTION
Prevention of the development of asthma is not yet possible. Studies examining genetic and epigenetic contributors, both pre-natal and post-natal, have proven difficult. Much evidence points to upper respiratory viral infections early in childhood as potent catalysts to the development of asthma.11 No dietary or environmental interventions have been shown to effectively eliminate the risk of developing asthma in susceptible or at-risk individuals. There are, however, interventions that may be useful in preventing/reducing asthma exacerbations. Avoidance of airborne triggers is difficult, given the ubiquitous nature of windblown pollens, but some commonsense recommendations may be of use, including avoidance of: raking leaves (alternaria exposure), vacuuming (dust mite exposure), pet ownership, or at least pet access to the bedroom (cat, dog allergens). High-efficiency particulate air (HEPA) filters can be useful for individuals with allergic rhinitis.
TREATMENT
Pharmaceuticals
The conventional management of asthma focuses on both immediate relief of symptoms and long-term control. Step therapy is based upon asthma disease classification, and degree of symptom control.8 The most common medications for immediate relief are the bronchodilators, mainly short-acting metered dose inhalers, including the short-acting beta agonists (SABA), and anti-cholinergics. The medications for long-term control include inhaled corticosteroid preparations, long-acting bronchodilators, leukotriene antagonists and modifiers, and newer biological modifiers (monoclonal antibodies given through I.V.).8 Inhaled corticosteroids have become a mainstay of conventional therapy, and newer formulations greatly reduce systemic corticosteroid absorption and adverse effects. Long-acting beta agonists (LABA) have a high beta-2 receptor affinity and bronchodilate for 12 hours or more with a single administration. However, multiple studies have raised concerns about using a LABA as monotherapy, with higher numbers of asthma related-deaths observed. Studies using concomitant LABA/inhaled corticosteroid did not result in increased asthma-related deaths. Leukotriene modifiers have demonstrated efficacy in the treatment of both asthma and allergic rhinitis, but only in those patients with high levels of leukotrienes.8 The newest medications are biologically engineered antibodies designed to interfere with discrete steps in the allergic inflammatory cascade.
Cromolyn is a prime example of a drug whose active ingredient was extracted from a botanical source with a historical record of effectiveness. Isolated from an extract of the khella plant (Ammi visnaga), cromolyn demonstrates potent mast cell–stabilizing activity in vitro. When used prophylactically, in advance of allergenic exposure, cromolyn can markedly reduce the rate and degree of mast cell degranulation, and thus allergic symptoms. Cromolyn is available by prescription in a nebulized form, as a liquid for oral use in gastrointestinal allergic conditions, and without a prescription as a nasal preparation for allergic rhinitis. This drug is regarded as a steroid-sparing anti-inflammatory, as opposed to a rescue inhaler.
Diet and Nutritional Supplements
The avoidance of specific foods or food additives for gastrointestinal or anaphylactic allergic reactions is an obvious and effective intervention. For asthma, however, the use of specific or elimination diets has been controversial. While elimination diets are specific to an individual, some common classes of foods including dairy products, wheat, and even certain animal proteins, have been popularly linked to allergic exacerbations, although published clinical data in this area are scant.
The association between milk intake and mucus production in people with allergies and asthma is a very popular belief among patients, but is refuted by a small number of studies.12 However, several biologically plausible hypotheses may support such an association. For example, patients with asthma have higher levels of a specific mucin in their airways (MUC5AC) relative to non-asthma patients.13 Certain types of milk (from specific breeds of cow) contain a protein called β-CM-7, which has been shown to stimulate MUC5AC production. It has been hypothesized that milk ingestion may lead to stimulation of respiratory mucin production in the airway, and thus increase phlegm production.14 A brief trial (4 to 6 weeks) of dairy avoidance may be helpful to discern such an association in selected individuals.
An important initial step in most inflammatory conditions, including asthma, is the catabolism of cell membrane–derived fats for entry into the arachidonic acid pathway. The cell utilizes these fatty acids to synthesize many significant inflammatory mediators in an asthma exacerbation, including the leukotrienes. Alterations in the dietary intake of fats are known to affect the fatty acid composition of cell membranes.15,16 Omega-3 supplementation decreases the ratio of omega-6 to omega-3 fatty acids in the inflammatory cell lipid membrane, thus creating less substrate for inflammatory mediator production (Table 1). This process, in turn, decreases the production of many potent bioactive compounds (e.g., leukotrienes) that are intimately involved in allergic inflammation.17 Clinical trials of omega-3 acid supplementation from fish and plant sources in the treatment of asthma and allergic diseases have been inconsistent.18 In one small clinical trial, patients with asthma who consumed a diet with an elevated omega-3 to omega-6 content showed marked improvement in airway hyperresponsiveness.19 These responders to dietary interventions could be readily identified through analysis of the leukotriene composition in the urine, a measure that predicted which patients were likely to improve with dietary intervention.19 Unfortunately, such testing is not currently feasible for routine practice. Thus increased dietary omega-3 intake (or a trial of omega-3 supplementation) in some patients may be a useful clinical intervention.
Table 1.
Selected Oral Supplements in Asthma and COPD
| Supplement | Asthma (A) COPD (C) |
Possible mechanism of action | Dosing* | Comments |
|---|---|---|---|---|
| Omega-3 fatty acids (fish or plant-derived) | A, C | Decreases inflammatory mediator production | 1500–2000 mg EPA daily in divided doses | Fishy breath and GI side effects most common. Lower doses being studied in COPD. |
| Quercetin | A | Mast cell stabilizer; anti-inflammatory | 400–600 mg up to TID | No human clinical study data available |
| Pycnogenol | A | Anti-inflammatory; decreased leukotriene production | 100–200 mg BID daily in divided doses | Proprietary antioxidant- bioflavonoid mixed extract |
| Fisetin | A | Anti-inflammatory; NF- kB inhibition | 100–200 mg daily | No human clinical study data available |
| Magnesium | A | Airway smooth muscle relaxation; potentiation of beta agonist activity | 300–500 mg daily | Use limited by diarrhea. Mg glycinate may have less laxative effect. Caution in kidney disease |
| Vitamin D-3 cholecalciferol | A, C | Immunomodulation; anti-inflammatory | 800–2000IU daily | May enhance effectiveness of corticosteroid treatment |
| N-Acetyl Cysteine | C | Mucoactive; anti- inflammatory; anti- oxidant | 600–1,200 mg daily in divided doses | Avoid nebulized form due to potential acute bronchospasm. Caution with anticoagulants, such as warfarin |
Vitamin D deficiency has been associated with increased symptoms, exacerbations, medication use, and reduced lung function in both adults and children. Although studies have been mixed regarding the value of supplementation, a recent Cochrane meta-analysis reported that Vitamin D is likely to reduce both risk of severe asthma exacerbation and healthcare use. It remains unclear whether this effect is limited to those with baseline vitamin deficiencies.20 The role of antioxidants and other vitamins such as Vitamin C has also received considerable attention, although the clinical value of supplementation is still largely unknown.
Magnesium is a standard of care in the emergency treatment of acute asthma exacerbations, and is administered intravenously, or in nebulized form.30 Magnesium has been shown to improve forced expiratory volume in 1 second (FEV1) and reduce ICU admissions in a hospital setting.31 In chronic asthma, inverse associations are also reported between intracellular (RBC) magnesium levels and asthma severity.32 Despite this, little convincing literature supports a role for long-term magnesium supplementation in mild to moderate asthma. Some reports note an improvement in asthma symptoms for those subjects with a higher magnesium intake,33 while others link dietary magnesium intake with an increased risk of asthma and wheezing in children.34 A 6-month study of magnesium supplementation (340 mg of magnesium citrate daily) in adults with mild-to-moderate asthma produced improvements in bronchial hyperreactivity, peak flow, and quality of life, without significant effects on other markers of asthma control nor inflammatory markers.35
Other Supplements
The most promising of the many other supplements purported for use in asthma are the bioflavonoids. Benefits of these compounds have been demonstrated in both in vitro and animal studies, however human studies are just beginning. It is very difficult to absorb orally administered flavonoids owing to their unique chemistry, so several compounds are routinely added to bioflavonoid formulations (e.g., bromelain, vitamin C) to increase systemic absorption. Some flavonoids, such as cromolyn, have already been successfully marketed by the pharmaceutical industry and are available as prescription medications. Others are available over-the-counter including Pycnogenol®, quercetin, and fisetin. As these compounds have shown remarkable in vitro activity against allergic inflammation, they are worth discussing in some detail.
Pycnogenol is a proprietary antioxidant-bioflavonoid mixed extract isolated from the bark of the maritime pine (Pinus pinaster). It has a variety of biological activities, ranging from blood pressure reduction to mitigation of elevated blood glucose. In terms of asthma, it has been shown to be efficacious in a murine model of airway inflammation,26 as well as in a clinical trial of children with asthma.27 In this study, Pycnogenol improved asthma symptoms and pulmonary function, and decreased rescue inhaler use. In addition, there was a significant reduction of urinary leukotriene production in the treated group.27 Pycnogenol can be considered for use in adolescents and adults with persistent, mild-moderate asthma.
Quercetin is a bioflavonoid found in diverse foods, including apples, buckwheat, onions, and citrus fruits. In vitro, quercetin stabilizes the membranes of mast cells and reduces the release of preformed histamine.21,22 In animal models, quercetin suppresses anaphylactic responses in sensitized rats,23 and it inhibits asthmatic inflammation in guinea pigs and rats.24 Quercetin also inhibits the production of enzymes responsible for synthesizing the leukotrienes.25 Due to its ubiquity in nature, and potential benefit, quercetin can be considered for patients with allergies or allergic asthma.
Fisetin is found primarily in strawberries, but also in apples, persimmons, and onions. Bioavailability is the biggest issue with fisetin, as with other flavonoids. Most studies of fisetin involving asthma animal models were done using IV or intraperitoneal formulations, although some used oral preparations.28 This compound potently inhibits the transcription factor, nuclear factor-kappaB (NF-κB), responsible for the expression of many inflammatory genes (e.g., IL-1, IL-2, IL-6, IL-8, tumor necrosis factor, adhesins)29 and may produce broad anti-inflammatory effects that transcend allergic disease. In animal models, the compound inhibited asthma flares with an anti-inflammatory effect that rivaled corticosteroids.29 Human studies of fisetin are to date lacking, thus no formal clinical recommendations can be made; however, it is commonly used for asthma patients by integrative medicine practitioners.
An exciting area of future innovation worth noting is the ongoing work in the domain of Chinese herbal formulations. One herbal preparation, tested in animal models of asthma, was shown to be as effective an anti-inflammatory agent as corticosteroids, but via a novel mechanism and with potentially fewer adverse effects.40 Using a series of carefully designed experiments, the group tested the compound ASHMI (Anti-Asthma Herbal Medicine Intervention) in adults with mild-moderate asthma, compared to daily prednisone over a 4-week period.. Both ASHMI and prednisone resulted in improved peak flow, FEV1, symptoms, and reduced need for rescue bronchodilators. However, those taking ASHMI did not experience prednisone-related weight gain and increased serum cortisol.41
Mind-Body Therapy
Numerous studies document the value of mind-body approaches in the treatment of asthma. This is not unexpected considering the association of anxiety and stress with asthma exacerbations. Recall of anxious and stressful experiences adversely affect expiratory flow rates in children with chronic asthma with no pre-existing psychopathology.42 These emotionally-induced decreases in expiratory flow rates were reversed with relaxation and self-hypnosis. In addition, poor asthma control is highly associated with greater symptoms of anxiety and depression.43 Individuals with high chronic stress exhibit a greater cortisol response to an acute stressor, as well as increases in blood and sputum eosinophils, whereas these relationships are opposite in those with low chronic stress.43 Thus, a true psychoneuroimmunological association has been established between asthma and stress/anxiety. While external stress can trigger or worsen chronic asthma, research has shown that intrinsic stress can have the same effect.
Interestingly, classic studies from the late 1960s demonstrated the power of the mind-body connection in asthma, Patients with moderate to severe asthma exhibited severe symptoms when they were exposed to saline mists that they believed were potent allergens. Even more remarkable was their prompt recovery with use of a saline inhaler that they believed to be a beta agonist.36,37 Even standard skin test reactions that produce classic wheal-and-flare reactions to subcutaneously introduced allergens can be modulated by mind-body techniques. In one study, patients with dust mite sensitivity who were skin tested after viewing a humorous video demonstrated lower wheal-and-flare reactivity to dust mite allergen than did patients viewing a control video (weather documentary).38
Some mind-body therapies that have been studied in both adults and children with asthma include breathing exercises, relaxation, guided imagery, biofeedback, journaling, tai chi/qigong and yoga.39,44 Clinical hypnosis also has a long history of successful use in asthma. Another popular mind-body therapy is mindfulness meditation. One randomized controlled trial of an 8-week MBSR program versus an educational program in adults with asthma showed clinically significant improvements in quality of life and perceived stress, however without demonstrable improvements in lung function.45
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
PATHOPHYSIOLOGY
Chronic obstructive pulmonary disease (COPD) is a syndrome of progressive airflow limitation which, in contrast to asthma, is not fully reversible. It is characterized by abnormal inflammatory responses of the lung to noxious particles or gases leading to structural changes and narrowing of the small airways. This pathophysiology results in hyperinflation of the lungs (i.e., an increase of end expiratory lung volume due to airflow limitation), weakened respiratory muscles, and inefficient gas exchange.46 Early definitions of COPD distinguished different types (e.g., chronic bronchitis, emphysema). This distinction is not in the current definition, but individual patients present along a spectrum. An asthma-COPD overlap syndrome is also recently recognized.47
Toxic environmental exposures are central to pathogenesis, with cigarette smoking being the single most important risk factor for developing COPD. Up to 90% of all deaths from COPD are attributable to smoking.48 Environmental exposures to occupational dusts, chemicals, and air pollution (particularly in developing worlds) also contribute to incidence. Other influences such as infections in early life, genetic predispositions (e.g., alpha-1 antitrypsin deficiency), and pre-existing asthma may also play a role. The mechanistic basis underlying COPD is complex and involves inflammation, oxidative stress, protease/antiprotease imbalance, environmental insult and host genetics.5
In general, COPD is slowly progressive with periods of exacerbations. The cardinal symptoms are dyspnea (first present with physical activity, but later also at rest) and exercise intolerance. Other major symptoms are chronic cough and increased sputum production. Exacerbations worsen these symptoms in addition to wheezing, chest tightness, tachycardia, and fevers. Skeletal muscle dysfunction, nutritional abnormalities, and systemic inflammation are important players. Fatigue, weight loss and anorexia are common in patients with severe disease. Associated comorbidities include cardiovascular disease, metabolic syndrome, osteoporosis, lung cancer, depression, anxiety, and sleep disorders.5
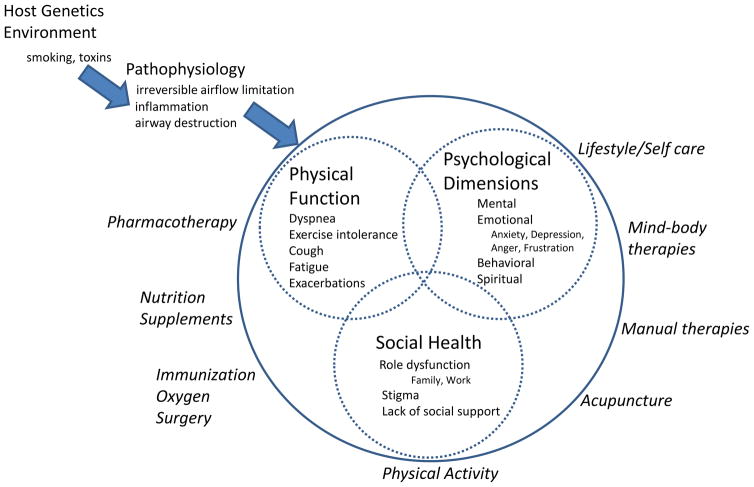
There is increasing appreciation that COPD is a complex, multidimensional chronic disease whereby the patient experience is a consequence of not only physical and functional problems, but overlaid with a myriad of emotional, behavioral, spiritual considerations and psychosocial stressors. This multidimensional framework (Figure 1) can be considered in all aspects of COPD management from pathophysiology to evaluation to treatment.
Figure 1.
Multidimensional Integrative Framework of COPD Pathophysiology, Evaluation and Management
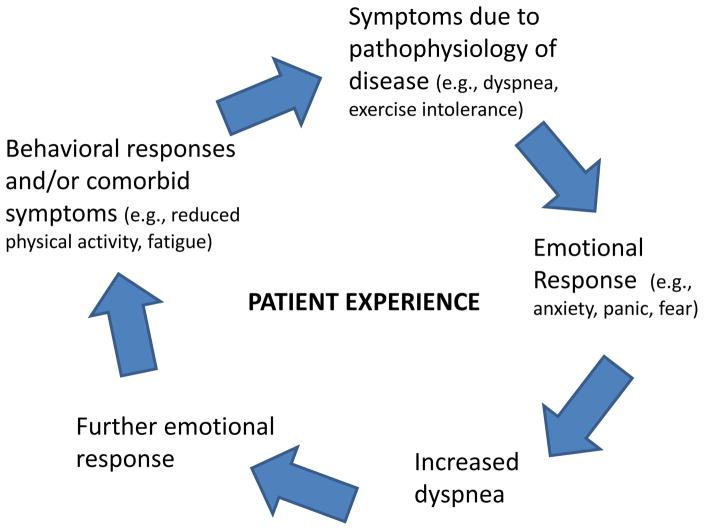
A conceptual model that considers one simple aspect of this framework is the anxiety/breathlessness cycle. This model (Figure 2) describes an interplay between emotions and the cardinal symptom of dyspnea which is inherently multifaceted, including physiological, sensory and affective dimensions.49,50 While this model was initially described in COPD, it is similarly relevant in asthma.
Figure 2. Anxiety-Breathlessness Cycle.
The cycle starts with disease pathophysiology leading to dyspnea, which then exacerbates anxiety and emotional responses, which then leads to more dyspnea, further exacerbating emotional dysfunction, comorbid symptoms or behavioral responses (e.g., fatigue, reduced activity). This leads to worsening disease state, which manifests as increased dyspnea, and the cycle continues.
EVALUATION
The 2016 updated Global Obstructive Lung Disease (GOLD) guidelines for the evaluation of COPD consider the following domains: severity of airflow limitation (pulmonary function testing, in particular spirometry), current level of patient’s symptoms, exacerbation risk, and presence of comorbidities.5 Degree of airflow limitation, symptoms and impairment of quality of life are not well correlated, so independent consideration of each is needed. With spirometry, the presence of a post-bronchodilator FEV1/FVC < 0.70 confirms the presence of persistent airflow limitation. GOLD severity staging is based on degree of airflow limitation ranging from Stage 1 (mild, FEV ≥80% predicted) to Stage 4 (very severe, FEV1 <30% predicted). Symptom assessment can include short questionnaires, such as the Comprehensive Assessment Test (CAT) or the COPD Control Questionnaire (CCQ).51,52 Comorbidities are common at all stages of COPD, and can influence mortality and hospitalizations independently. Assessment should include routine screening for comorbidities, in particular anxiety and depression.53 In addition to physiologic and functional evaluation, a detailed assessment should explore psychosocial and behavioral issues. Important questions may probe mental, emotional, and spiritual health, including the impact of disease on daily activities, missed work, financial burdens, family routines, and social role. Assessment of lifestyle factors, e.g., smoking status and motivation to quit, dietary patterns and physical activity, is also important.
TREATMENT
Pharmacotherapy
Pharmacological treatments aim to improve quality of life, control symptoms and reduce exacerbation risk. No medications for COPD have been conclusively shown to modify long-term decline in lung function although research is ongoing with long-acting bronchodilators and/or inhaled corticosteroids.54,55 COPD pharmacotreatment is guided by GOLD stage. As disease severity increases, treatment step-ups occur and therapies are added to provide additional symptom control and reduce the risk of exacerbations. Medications include bronchodilators (short acting and long acting beta2 agonists and anticholinergics; methylxanthines are less frequently used), anti-inflammatory agents (inhaled and systemic corticosteroids and phosphodiesterase-4 inhibitors (leukotriene modifiers), and antibiotics for respiratory infections. Some of these medications also have a mucoactive effect (facilitating clearance or decreasing hypersecretion of mucous).5
Diet and nutritional supplements
Vitamins and Antioxidants
As with any other chronic condition, proper nutrition and a healthy diet are extremely important. For COPD in particular, inflammation and oxidative stress and the potential role of antioxidants have received considerable attention.56 Most available data are observational and suggest there is a protective effect on COPD associated with antioxidant vitamins, particularly Vitamin C and to a lesser extent Vitamin E, beta carotenoids, and omega-3 FA.57 There may be an association of individual vitamins with decreased symptoms, infections, and exacerbation. Some have suggested possible benefits of omega-3 fatty acids for muscle oxidative metabolism as this relates to exercise performance.59,60 Low levels of certain minerals including potassium, magnesium, selenium, and zinc are also associated with risk of COPD and poor lung function. However, intervention studies for supplementation are lacking or inconclusive. Higher consumption of fruits and vegetables is associated with higher FEV and reduced rate of lung function decline, and diets rich in oily fish may protect against COPD.58,60 One recent RCT showed that a shift to a higher antioxidant diet improved lung function.61 Thus, it may be reasonable at this point to consider antioxidant supplementation only in those with extreme oxidative stress challenges, e.g., exposure to high levels of air pollution. Otherwise, for most patients with COPD it is best to recommend a prudent diet rich in antioxidants, magnesium, other minerals, and omega-3 fatty acids (including fruits, vegetables, and fish).
Also of note, Vitamin D deficiency is highly prevalent in COPD and associated with disease severity, decreased lung function, muscle dysfunction, osteoporosis and immune dysfunction. There are hypothesized genetic subgroups of COPD patients with different responses to supplementation and COPD exacerbations. Current studies are ongoing.62,63
It is also important to note that anorexia and protein-calorie malnutrition is present in more than 30% of patients with severe disease and this is associated with increased mortality, impaired respiratory muscle function, and immune dysfunction.60 Oral nutritional supplementation in malnourished patients with COPD improves nutritional status, respiratory muscle strength, and quality of life but the benefits are less clear in the average nourished patient with stable disease.64
Mucoactive agents
Mucoactive agents facilitate clearance or decrease hypersecretion of mucous. For patients with bothersome sputum production that is refractory to smoking cessation and routine therapies for COPD, N-acetyl cysteine (NAC), an oral thiol preparation, has had some reported benefits.65,66 NAC may also have anti-inflammatory and antioxidant properties. Although some practices use nebulized NAC, this is best avoided due to the potential for acute bronchospasm.
Hypertonic saline inhalation, oral expectorants (e.g, guaifenesin), iodide preparations (e.g., saturated solution of potassium iodide), inhaled cromoglycate and DNase have also been suggested as mucoactive agents, but currently lack evidence of clinical efficacy. Other agents that decrease sputum adhesiveness, viscosity or production are currently being studied, such as surfactants, actin-severing agents, and prostaglandin inhibitors.67
Other potential supplements
Several other supplements have purported benefits, although further clinical studies are needed before any recommendations in COPD can be made. Among these are: L-carnitine; echinacea-containing formulas; polyphenols such as curcumin and reservatrol; and Chinese herbs (e.g., Astragalus membranaceus, panax ginseng, cordyceps sinensis, and combination formulas made from isolated herbal compounds).56,64,68,69,70
Immunization
Yearly influenza vaccination is recommended and reduces COPD exacerbations. The Centers for Disease Control and Prevention also recommends pneumococcal vaccination for adults with chronic lung disease, although the benefit on pneumonia rates, hospital admissions and ER visits in COPD is unclear.5
Lifestyle/Self-Care
Environmental exposures/toxins
Smoking cessation should be targeted as one of the most important self-care interventions, with counseling, nicotine replacement, and other medications should be recommended as appropriate for individual patients.5 The American Academy of Family Physicians’ Ask and Act Tobacco Cessation Program provides excellent online resources for physicians and patients.71 Acupuncture, hypnosis, meditation and mind-body practices may be considered as helpful adjunctive therapies to aid in smoking cessation.72,73
Physical Activity
Physical inactivity has been demonstrated to be the strongest predictor of all-cause mortality in COPD.76 This finding underscores the importance of exercise in the management of COPD.5,74 Conventional exercise programs, both high and low-intensity, improve endurance, dyspnea, and quality of life, even in patients with severe disease and poor exercise tolerance. Optimal regimens are not known, however, a combination of endurance and strength training has been proposed to best treat peripheral muscle dysfunction. Current guidelines recommend formal pulmonary rehabilitation for all patients. These programs are heterogeneous, however, typically include both lower extremity (e.g., stationary cycle ergometer, treadmill, walking) as well as upper extremity exercise (e.g., arm cycle ergometer, free weights, elastic bands), breathing exercises (e.g., pursed lip technique, diaphragmatic breathing, spirometry), and education. Guidelines also recognize the importance of psychosocial stressors and include stress reduction as a recommended component of pulmonary rehabilitation. Symptoms of depression and anxiety can limit the benefits of exercise training due to poor motivation or fear of symptoms such as exertional dyspnea and should therefore be addressed concomitantly.75 Mind-body exercise, such as tai chi and yoga, may be helpful to engage deconditioned patients in physical activity either as a bridge, adjunct, and/or alternative to conventional choices.77,78
Mind-Body Therapy
As COPD is a chronic disease that affects not only physical and functional but behavioral, emotional, and spiritual domains in a patient’s life, addressing the whole person in the course and management of the disease is crucial. Mind-body therapies may play a role in multiple levels of impairment in COPD. Inherent in these therapies is often an attention to the breath which may be particularly salient to a population whose main symptom is breathlessness. There is some overlap between controlled breathing exercises and respiratory muscle training used conventionally with techniques of diaphragmatic breathing or yogic breathing. Mind-body approaches may also play a role in addressing a myriad of psychosocial aspects of living with the disease, including complex emotions surrounding loss of independence and self-efficacy, anger, depression, frustration, guilt, family and social role dysfunction.77
Mindfulness has been shown to be effective for anxiety in many populations through promoting self-awareness and body awareness. Interestingly, in COPD, some have suggested that mindfulness may trigger anxiety, and that perhaps other meditation styles may be better suited.78 In practice, however, elements of mindfulness are common to many styles, and it is unclear why patients with COPD would be different from other chronic disease populations, including asthma. In fact, studies have shown that mindfulness programs in other chronic disease populations can promote self-care, improve sleep and well-being, and increase adherence to medical recommendations. Mind-body exercise such as tai chi and yoga may theoretically provide both gentle exercise as well as the benefits of mindfulness, meditative breathing, and stress reduction. There are limited data to suggest benefit of tai chi for exercise capacity, quality of life, symptoms of dyspnea, and pulmonary function.79,80 Data in other chronic diseases suggest mind-body exercise may promote self-efficacy and empowerment.81
Cognitive behavioral therapy (CBT) includes cognitive restructuring, behavioral activation, competency enhancement through skill-building exercises, and psycho-education. Some studies suggest CBT may reduce symptoms of depression or anxiety, lower dyspnea, as well as improve walking distance, quality of life, and hospital admissions in patients with COPD.77
Other small studies in COPD have suggested benefits of progressive muscle relaxation (improved fatigue and sleep quality) and various physiologic biofeedback techniques.77
Other Integrative modalities
Acupuncture
Data on the use of acupuncture in COPD suggest benefit but is still limited, and thus recommendations for use are premature. Several RCTs have reported safety.82 One promising relatively small RCT investigated acupuncture vs. placebo needling in Stage 2–4 COPD and reported improved dyspnea, 6 minute walk distance, oxygenation, quality of life, nutritional status, spirometry and chest wall biomechanics.83
Manual Therapies
While there is a proposed theoretical rationale for manual therapies like spinal manipulation and soft tissue therapies to reduce chest wall rigidity and thereby improve breathing mechanics, the data on the use of manual therapies in COPD are sparse. One systematic review of spinal manipulation and osteopathic manipulation suggested improved symptoms, lung function and exercise.84
Other considerations
Oxygen
Supplemental oxygen improves endurance and exercise capacity in patients with moderate to severe COPD. Long-term therapy is recommended for patients with severe hypoxemia (oxygen saturation less than 88% or partial arterial oxygen pressure less than 55 mg Hg). Prescriptions are tailored based on need (e.g., differential flow delivery and timing of use [continuous, nocturnal, with exercise]).5
Surgery
Lung volume reduction surgery may be helpful in select patients with severe COPD and heterogeneous distribution of emphysema with upper lobe predominance. Criteria for referral include a score greater than 5 on the BODE Index (body mass index, obstruction, dyspnea, exercise).5
SUMMARY
For both asthma and COPD, an integrative approach may emphasize a more holistic strategy of patient management and recognizes the multidimensional aspects of disease. Undoubtedly, there are well-studied and effective conventional medications and treatments. However, integrative medicine may offer beneficial adjuncts or address areas that are more often overlooked. An integrative approach may help to bring commonsense self-care to the fore, including healthy lifestyle modifications, allergen avoidance, smoking cessation, physical activity, and a prudent diet. Mind-body therapies may help to address not only psychosocial aspects, but benefits in physical and functional dimensions are closely intertwined. Several supplements or herbal compounds may have benefit to consider further as research grows. Open communication between patients and providers is strongly emphasized. It is important to stress that the foundational tenet of integrative medicine is that it promotes a treatment plan-- together with, and not as replacement for-- appropriate conventional care.
Key Points.
An integrative approach for asthma and COPD considers a multi-dimensional, biopsychosocial model.
Integrative medicine in the management of asthma and COPD includes appropriate conventional care, medications, lifestyle and self-care strategies (smoking cessation, allergen avoidance, physical activity, nutrition), attention to mental and emotional health, and consideration of select evidence-based complementary modalities.
Mind-body therapies may help to address psychosocial aspects of chronic disease, improve quality of life and symptoms.
Select supplements or herbal compounds may be promising to improve symptoms and decrease exacerbations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gloria Y. Yeh, Associate Professor of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center, Division of General Medicine and Primary Care, 1309 Beacon Street, Brookline, MA 02446.
Randy Horwitz, Medical Director, Arizona Center for Integrative Medicine, Associate Professor of Medicine, University of Arizona College of Medicine, P.O. Box 245153, Tucson, AZ 85724-5153.
References
- 1.CDC. [Accessed October 29, 2016];Most Recent Asthma Data Website. http://www.cdc.gov/asthma/most_recent_data.htm. Updated: April 14, 2016.
- 2.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance—United States, 1999–2011. Chest. 2013;144(1):284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–32. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 4.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 5.From the Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016 doi: 10.3760/cma.j.issn.0376-2491.2016.34.001. Available from: http://goldcopd.org/ [DOI] [PubMed]
- 6.Hekking PW, Bel EH. Developing and Emerging Clinical Asthma Phenotypes. J Allergy Clin Immunol Pract. 2014;2:671–80. doi: 10.1016/j.jaip.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Ross RN, Nelson HS, Finegold I. Effectiveness of specific immunotherapy in the treatment of asthma: a meta-analysis of prospective, randomized, double-blind, placebo-controlled studies. Clin Ther. 2000;22:329–341. doi: 10.1016/S0149-2918(00)80037-5. [DOI] [PubMed] [Google Scholar]
- 8.National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. [Accessed October 29, 2016];Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007 Aug 28; http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.
- 9.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–6. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Medicine. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125(6):1202–1205. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Wüthrich B, Schmid A, Walther B, Sieber R. Milk consumption does not lead to mucus production or occurrence of asthma. J Am Coll Nutr. 2005;24(suppl):547S–555S. doi: 10.1080/07315724.2005.10719503. [DOI] [PubMed] [Google Scholar]
- 13.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002 Feb 1;361(Pt 3):537–46. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartley J, McGlashan SR. Does milk increase mucus production? Med Hypotheses. 2010;74:732–734. doi: 10.1016/j.mehy.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Lee TH, Hoover RL, Williams JD, et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985;312:1217–1223. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- 16.Arm JP, Horton CE, Mencia-Huerta JM, et al. Effect of dietary supplementation with fish oil lipids on mild asthma. Thorax. 1988;43:84–92. doi: 10.1136/thx.43.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz R. Controlling asthma: the role of nutrition. Explore (NY) 2005;1:393–395. doi: 10.1016/j.explore.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015 Jan;64(1):27–34. doi: 10.1016/j.alit.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Broughton KS, Johnson CS, Pace BK, et al. Reduced asthma symptoms with w-3 fatty acid ingestion are related to 5-series leukotriene production. Am J Clin Nutr. 1997;65:1011–1017. doi: 10.1093/ajcn/65.4.1011. [DOI] [PubMed] [Google Scholar]
- 20.Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, Sheikh A, Griffiths CJ. Vitamin D for the management of asthma. Cochrane Database of Systematic Reviews. 2016;9:CD011511. doi: 10.1002/14651858.CD011511.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otsuka H, Inaba M, Fujikura T, et al. Histochemical and functional characteristics of metachromic cells in the nasal epithelium in allergic rhinitis: studies of nasal scrapings and their dispersed cells. J Allergy Clin Immunol. 1995;96:528–536. doi: 10.1016/s0091-6749(95)70297-0. [DOI] [PubMed] [Google Scholar]
- 22.Haggag EG, Abou-Moustafa MA, Boucher W, Theoharides TC. The effect of a herbal water-extract on histamine release from mast cells and on allergic asthma. J Herb Pharmacother. 2003;3:41–54. [PubMed] [Google Scholar]
- 23.Shishehbor F, Behroo L, Broujerdnia G, et al. Quercetin effectively quells peanut-induced anaphylactic reactions in the peanut sensitized rats. Iran J Allergy Asthma Immunol. 2010;9:27–34. [PubMed] [Google Scholar]
- 24.Moon H, Choi HH, Lee JY, et al. Quercetin inhalation inhibits the asthmatic responses by exposure to aerosolized-ovalbumin in conscious guinea-pigs. Arch Pharm Res. 2008;31:771–778. doi: 10.1007/s12272-001-1225-2. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimoto T, Furukawa M, Yamamoto S, et al. Flavonoids: potent inhibitors of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun. 1983;116:612–618. doi: 10.1016/0006-291x(83)90568-5. [DOI] [PubMed] [Google Scholar]
- 26.Shin I, Shin N, Jeon C, et al. Inhibitory effects of Pycnogenol (French maritime pine bark extract) on airway inflammation in ovalbumin-induced allergic asthma. Food and Chemical Toxicology. 2013;62:681–686. doi: 10.1016/j.fct.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Lau B, Riesen SK, Truong KP, et al. Pycnogenol® as an Adjunct in the Management of Childhood Asthma. The Journal of Asthma: Official Journal of the Association for the Care of Asthma. 2004;41(8):825–832. doi: 10.1081/jas-200038433. [DOI] [PubMed] [Google Scholar]
- 28.Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci USA. 2006;103(44):16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goh FY, Upton N, Guan S, et al. Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-κB. Eur J Pharmacol. 2012;679:109–116. doi: 10.1016/j.ejphar.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Powell CV. The role of magnesium sulfate in acute asthma: does route of administration make a difference? Current Opinion in Pulmonary Medicine. 2014;20(1):103–8. doi: 10.1097/MCP.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 31.Powell CV, Kolamunnage-Dona R, Lowe J the MAGNETIC study group. MAGNEsium Trial In Children (MAGNETIC): a randomised, placebo-controlled trial and economic evaluation of nebulised magnesium sulphate in acute severe asthma in children. Health Technology Assessment (Winchester, England) 2013;17(45):v–vi. 1–216. doi: 10.3310/hta17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominguez LJ, Barbagallo M, Di Lorenzo M, et al. Bronchial reactivity and intracellular magnesium: a possible mechanism for the bronchodilating effects of magnesium in asthma. Clin Sci. 1998;95:137–142. [PubMed] [Google Scholar]
- 33.Britton J, Pavord I, Richards K, et al. Dietary magnesium, lung function, wheezing, and airway hyperreactivity in a random adult population sample. Lancet. 1994;344:357–362. doi: 10.1016/s0140-6736(94)91399-4. [DOI] [PubMed] [Google Scholar]
- 34.Emmanouil E, Manios Y, Grammatikaki E, et al. Association of nutrient intake and wheeze or asthma in a Greek pre-school population. Pediatr Allergy Immunol. 2010;21:90–95. doi: 10.1111/j.1399-3038.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 35.Kazaks AG, Uriu-Adams JY, Albertson TE, et al. Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma: a randomized placebo controlled trial. J Asthma. 2010 Feb;47(1):83–92. doi: 10.3109/02770900903331127. [DOI] [PubMed] [Google Scholar]
- 36.Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. [update of Cochrane Database Syst Rev. 2003;(2):CD002893] Cochrane Database Syst Rev. 2010;(12):CD002893. doi: 10.1002/14651858.CD002893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFadden ER, Luparello T, Lons HA, et al. The mechanism of suggestion in the induction of acute asthma attacks. Psychosom Med. 1969;31:134–143. doi: 10.1097/00006842-196903000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Luparello TJ, Lyons HA, Bleeker ER, et al. Influence of suggestion on airways reactivity in asthmatic subjects. Psychosom Med. 1968;30:819. doi: 10.1097/00006842-196811000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Smyth JM, Stone AA, Hurewitz A, Kaell A. Effects of writing about stressful experiences on stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: a randomized trial. JAMA. 1999;281(14):1304–9. doi: 10.1001/jama.281.14.1304. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava K, Teper AA, Zhang T, et al. Immunomodulatory effect of the antiasthma Chinese herbal formula MSSM-002 on TH2 cells. J Allergy Clin Immunol. 2004;113:268–276. doi: 10.1016/j.jaci.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 41.Kelly-Pieper K, Patil SP, Busse P, et al. Safety and Tolerability of an Antiasthma Herbal Formula (ASHMI™) in Adult Subjects with Asthma: A Randomized, Double-Blinded, Placebo-Controlled, Dose-Escalation Phase I Study. J Altern Complement Med. 2009 Jul;15(7):735–743. doi: 10.1089/acm.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tal A, Miklich DR. Emotionally induced decreases in pulmonary flow rates in asthmatic children. Psychosom Med. 1976;38:190–200. doi: 10.1097/00006842-197605000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Rosenkranz MA, Esnault S, Christian BT, Crisafi G, Gresham LK, Higgins AT, Moore MN, Moore SM, Weng HY, Salk RH, Busse WW, Davidson RJ. Mind-body interactions in the regulation of airway inflammation in asthma: A PET study of acute and chronic stress. Brain, Behavior, and Immunity. 2016;58:18–30. doi: 10.1016/j.bbi.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClafferty H. An overview of integrative therapies in asthma treatment. Curr Allergy Asthma Rep. 2014;14:464. doi: 10.1007/s11882-014-0464-2. [DOI] [PubMed] [Google Scholar]
- 45.Pbert L, Madison JM, Druker S, et al. Effect of mindfulness training on asthma quality of life and lung function: A randomised controlled trial. Thorax. 2012;67:769–76. doi: 10.1136/thoraxjnl-2011-200253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutherland ER1, Cherniack RM. Management of chronic obstructive pulmonary disease. N Engl J Med. 2004 Jun 24;350(26):2689–97. doi: 10.1056/NEJMra030415. [DOI] [PubMed] [Google Scholar]
- 47.Postma DS, Rabe KF. The Asthma-COPD Overlap Syndrome. N Engl J Med. 2015;373:1241. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 48.Chen JC, Mannino DM. Worldwide epidemiology of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 1999;5:93–9. doi: 10.1097/00063198-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Bailey PH. The Dyspnea-Anxiety-Dyspnea Cycle – COPD patients’ stories of breathlessness: “It’s scary/when you can’t breathe”. Qualitative Health Research. 2005;14(6):760–778. doi: 10.1177/1049732304265973. [DOI] [PubMed] [Google Scholar]
- 50.Smoller JW, et al. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. American Journal of Respiratory and Critical Care Medicine. 1996;154(1):6–17. doi: 10.1164/ajrccm.154.1.8680700. [DOI] [PubMed] [Google Scholar]
- 51.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 52.Reda AA, Kotz D, Kocks JW, Wesseling G, van Schayck CP. Reliability and validity of the clinical COPD questionniare and chronic respiratory questionnaire. Respir Med. 2010 Nov;104(11):1675–82. doi: 10.1016/j.rmed.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 53.Pumar MI1, Gray CR1, Walsh JR1, Yang IA1, Rolls TA1, Ward DL1. Anxiety and depression-Important psychological comorbidities of COPD. J Thorac Dis. 2014 Nov;6(11):1615–31. doi: 10.3978/j.issn.2072-1439.2014.09.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171–8. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 55.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–8. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 56.Fischer BM, Voynow JA, Ghio AJ. COPD: balancing oxidants and antioxidants. Int J COPD. 2015;10:261–276. doi: 10.2147/COPD.S42414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsigliani IG, van der Molen T. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Resp Res. 2010;11:171. doi: 10.1186/1465-9921-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romieu I, Trenga C. Diet and obstructive lung diseases. Epidemiol Rev. 2001;23(2):268–87. doi: 10.1093/oxfordjournals.epirev.a000806. [DOI] [PubMed] [Google Scholar]
- 59.Broekhuizen R1, Wouters EF, Creutzberg EC, Weling-Scheepers CA, Schols AM. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax. 2005;60:376–82. doi: 10.1136/thx.2004.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schols AM. Nutrition as a metabolic modulator in COPD. Chest. 2013;144:1340–1345. doi: 10.1378/chest.13-0326. [DOI] [PubMed] [Google Scholar]
- 61.Keranis E, Makris D, Rodopoulou P, Martinou H, Papamakarios G, Danill Z, Zintzaras E, Gourgoulianis KI. Impact of dietary shift to hgher antioxidant foods in COPD: a randomized trial. Eur Resp J. 2010;36:774–80. doi: 10.1183/09031936.00113809. [DOI] [PubMed] [Google Scholar]
- 62.de Tena JG, El Hachem Debek A, Hernandez Guttierez C, Izquierdo Alonso JL. The role of vitamin D in chronic obstructive pulmonary disease, asthma, and other respiratory diseases. Arch Bronconeumologia. 2014;50:179–184. doi: 10.1016/j.arbres.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 63.Janssens W1, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010 Mar;65(3):215–20. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira IM1, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012 Dec 12;12:CD000998. doi: 10.1002/14651858.CD000998.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cazzola M1, Calzetta L2, Page C3, Jardim J4, Chuchalin AG5, Rogliani P2, Matera MG6. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015 Sep;24(137):451–61. doi: 10.1183/16000617.00002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tse HN1, Tseng CZ1. Update on the pathological processes, molecular biology, and clinical utility of N-acetylcysteine in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:825–36. doi: 10.2147/COPD.S51057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rogers DF1. Mucoactive agents for airway mucus hypersecretory diseases. Chemotherapy. 2002 Dec;48:259–66. [PubMed] [Google Scholar]
- 68.Hauke W1, Köhler G, Henneicke-Von Zepelin HH, Freudenstein J. Esberitox N as supportive therapy when providing standard antibiotic treatment in subjects with a severe bacterial infection (acute exacerbation of chronic bronchitis). A multicentric, prospective, double-blind, placebo-controlled study. Respir Care. 2007;52:1176–93. doi: 10.1159/000066763. discussion 1193–7. [DOI] [PubMed] [Google Scholar]
- 69.Chen HY, Ma CH, Cao KJ, Chung-Man Ho J, Ziea E, Wong VT, Zhang ZJ. A systematic review and meta-analysis of herbal medicine on chronic obstructive pulmonary diseases. Evid Based Complement Alternat Med. 2014;2014:925069. doi: 10.1155/2014/925069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J, Zhao P, Li Y, Tian Y, Wang Y. Systems pharmacology-based dissection of mechanisms of Chinese medicinal formula Bufei Yishen as an effective treatment for chronic obstructive pulmonary disease. Sci Rep. 2015 Oct 15;5:15290. doi: 10.1038/srep15290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.The American Academy of Family Physicians’ Ask and Act Tobacco Cessation Program. [Accessed 10/31/2016]; http://www.aafp.org/patient-care/public-health/tobacco-nicotine/ask-act.html.
- 72.Tahiri M1, Mottillo S, Joseph L, Pilote L, Eisenberg MJ. Alternative smoking cessation aids: a meta-analysis of randomized controlled trials. Am J Med. 2012;125:576–84. doi: 10.1016/j.amjmed.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 73.Carim-Todd L1, Mitchell SH, Oken BS. Mind-body practices: an alternative, drug-free treatment for smoking cessation? A systematic review of the literature. Drug Alcohol Depend. 2013;132:399–410. doi: 10.1016/j.drugalcdep.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garvey C1, Bayles MP, Hamm LF, Hill K, Holland A, Limberg TM, Spruit MA. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines: an official statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2016;36:75–83. doi: 10.1097/HCR.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 75.von Leupoldt A, Taube K, Lehmann K, Fritzsche A, Magnussen H. The impact of anxiety and depression on outcomes of pulmonary rehabilitation in patients with COPD. Chest. 2011;140:730–6. doi: 10.1378/chest.10-2917. [DOI] [PubMed] [Google Scholar]
- 76.Waschki B, Kirsten A, Holz O, Muller K-C, Meyer T, Watz H, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest. 2011;140:331–42. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 77.von Leupoldt A1, Fritzsche A, Trueba AF, Meuret AE, Ritz T. Behavioral medicine approaches to chronic obstructive pulmonary disease. Ann Behav Med. 2012;44:52–65. doi: 10.1007/s12160-012-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan RR1, Giardino N2, Larson JL3. A pilot study: mindfulness meditation intervention in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:445–54. doi: 10.2147/COPD.S73864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ngai SP1, Jones AY, Tam WW. Tai Chi for chronic obstructive pulmonary disease (COPD) Cochrane Database Syst Rev. 2016;(6):CD009953. doi: 10.1002/14651858.CD009953.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu XC1, Pan L1, Hu Q1, Dong WP1, Yan JH1, Dong L1. Effects of yoga training in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Thorac Dis. 2014;6:795–802. doi: 10.3978/j.issn.2072-1439.2014.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farver-Vestergaard I1, Jacobsen D, Zachariae R. Efficacy of psychosocial interventions on psychological and physical health outcomes in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Psychother Psychosom. 2015;84:37–50. doi: 10.1159/000367635. [DOI] [PubMed] [Google Scholar]
- 82.Coyle ME, Shergis JL, Huang ET, Guo X, Di YM, Zhang A, Xue CC. Acupuncture therapies for chronic obstructive pulmonary disease: a systematic review of randomized, controlled trials. Altern Ther Health Med. 2014;20:10–23. [PubMed] [Google Scholar]
- 83.Suzuki M1, Muro S, Ando Y, Omori T, Shiota T, Endo K, Sato S, Aihara K, Matsumoto M, Suzuki S, Itotani R, Ishitoko M, Hara Y, Takemura M, Ueda T, Kagioka H, Hirabayashi M, Fukui M, Mishima M. A randomized, placebo-controlled trial of acupuncture in patients with chronic obstructive pulmonary disease (COPD): the COPD-acupuncture trial (CAT) Arch Intern Med. 2012;172:878–86. doi: 10.1001/archinternmed.2012.1233. [DOI] [PubMed] [Google Scholar]
- 84.Wearing J1, Beaumont S2, Forbes D1, Brown B1, Engel R1. The Use of Spinal Manipulative Therapy in the Management of Chronic Obstructive Pulmonary Disease: A Systematic Review. J Altern Complement Med. 2016;22:108–14. doi: 10.1089/acm.2015.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarah-Egert S, Wolffram S, Bosy-Westphal A, et al. Daily Quercetin Supplementation Dose-Dependently Increases Plasma Quercetin Concentrations in Healthy Humans. J Nutr. 2008;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 86.Hosseini S, Pishnamazi S, Sadrzadeh SM, Farid F, Farid R, Watson RR. Pycnogenol((R)) in the Management of Asthma. J Med Food. 2001;4:201–209. doi: 10.1089/10966200152744472. [DOI] [PubMed] [Google Scholar]
- 87.Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39–49. doi: 10.1378/chest.129.1.39. [DOI] [PubMed] [Google Scholar]