Abstract
Osteoporosis and periodontitis are both diseases characterized by bone resorption. Osteoporosis features systemic degenerative bone loss that leads to loss of skeletal cancellous microstructure and subsequent fracture, whereas periodontitis involves local inflammatory bone loss, following an infectious breach of the alveolar cortical bone, and it may result in tooth loss. Most cross-sectional studies have confirmed the association of osteoporosis and periodontitis primarily on radiographic measurements and to a lesser degree on clinical parameters. Multiple shared risk factors include age, genetics, hormonal change, smoking, as well as calcium and vitamin D deficiency. Both diseases could also be risk factors for each other and have a mutual impact that requires concomitant management. Suggested mechanisms underlying the linkage are disruption of the homeostasis concerning bone remodeling, hormonal balance, and inflammation resolution. A mutual interventional approach is emerging with complex treatment interactions. Prevention and management of both diseases require interdisciplinary approaches and warrants future well-controlled longitudinal and interventional studies for evidence-based clinical guidelines.
Keywords: Periodontitis, Osteoporosis, Risk factor, Mechanism, Bone, Inflammation, Estrogen
Introduction
Osteoporosis and periodontitis are prevalent conditions. Osteoporosis impacts half of the elderly over 65 [1], and periodontal disease affects half of the adult population [2]. The number of patients who suffer from these diseases is expected to increase as the population advances in age. Both health problems require expensive, long-term medical and dental care. Osteoporosis is a systemic skeletal disorder with compromised bone density and strength that leads to increased risk of bone fracture [3]; whereas periodontitis is considered a local infection with a host inflammatory response within the supporting tissues of the teeth that results in alveolar bone loss [4]. Both diseases are defined by a preponderance of bone resorption, and their progression or severity is assessed systemically and/or locally. It is reasonable to argue that systematic skeletal change inevitably impacts the jaws and alveolar bone. As early as 1960, there were discussions about periodontal disease also being a degenerative disease termed “presenile osteoporosis” [5], but the primary etiology of periodontitis was later demonstrated as bacterial plaque [6, 7]. The multifactorial nature of the host response to the periodontal disease, as well as its association and mutual skeletal impact are still an interesting debate in the field [8–10]. This report updates the current understanding and scientific advancement that is pertinent to these two bone-resorbing diseases. Their updated association, shared risk factors, potential mechanisms, mutual impact, and interactive treatment will be discussed.
Association Between Osteoporosis and Periodontitis
The association of osteoporosis and periodontitis in clinical studies is defined by the subjects and measuring methods. In general, osteoporosis affects more women [1] and periodontitis affects more men [2]. Most of the cross-sectional association studies were conducted within elderly post-menopausal women, where a general positive correlation was found [8, 10], indicating such association may exist in this subset of the population. In addition, depending on the clinical and radiographic parameters used for both diseases, the strength of the association may vary significantly.
The techniques currently used to assess osteoporosis include dual-photon absorptiometry (DPA), dual-energy absorptiometry (DXA), and quantitative computerized tomography (QCT). The definition of osteoporosis proposed by the World Health Organization (WHO) [11] is a bone mineral density (BMD) score of 2.5 standard deviations or more below (T-score) of the average peak in young adults. Preferred locations for diagnosing osteoporosis with BMD are the spine and hip and femur. Some studies use a dichotomous diagnosis system while others use continuous BMD for statistical assessment, which can also result in variability. On the other hand, methods for assessing periodontal conditions and oral bone loss include clinical probing depth (PD), attachment levels (CAL), tooth loss, radiographic measures of alveolar crest height (ACH), absolute bone density (DXA, DPA, QCT), and computer-assisted densitometry image analysis (CADIA). In summary, the parameters used for periodontal disease can be divided into clinical periodontal examinations (PD, CAL, tooth loss) and radiographic examinations (ACH, DXA, DPA, QCT). A recent systematic review has shown that the association between osteoporosis and the clinical parameters of periodontitis are still inconclusive (six positive vs. five negative studies) but there is a significant association with tooth loss [10]. However, if we measure radiographic findings (osteoporotic change of the jaw or alveolar bone), there is a very strong correlation (18 positive vs. 3 negative studies) [10]. These results suggest a specific mechanistic relationship between the two bone-resorbing disorders.
A hypothesis that relates systemic osteoporosis to local osteoporotic changes in the jaws and loss of tooth-supporting alveolar bone is supported by the observations from various studies. Cross-sectional plus a longitudinal study demonstrate that total bone mass or skeletal density are closely correlated with the resorption of the alveolar crest [12–15]. However, there is a larger study that did not show a correlation between the BMD and alveolar bone height [16]. The latter method involved calibration with a standardized mineral solution equivalent to hydroxyl-apatite (HA) to conventional film. The sensitivity of the technique may have contributed to the result [16]. These results suggest that systemic osteoporosis also has a local impact via osteoporotic changes in the jaws.
Resorption of the alveolar bone may influence clinical periodontal parameters, such as tooth loss, probing depth (PD), or clinical attachment loss (CAL) [17]. For instance, Elders et al. [16] showed a significant inverse correlation of mean alveolar bone density to tooth loss and mean probing depth in dentate subjects. However, the association of systemic osteoporosis with tooth loss, supported by positive correlations from two longitudinal studies [18, 19], may be a more sensitive indicator compared to other clinical measures, such as PD and CAL [8, 10]. The value of PD and CAL appears to depend significantly on the number of teeth retained. Since osteoporotic subjects may present with premature tooth loss, the clinical parameters are limited in sensitivity for typical cross-sectional studies. On the other hand, tooth loss may be a cumulative result of progressing periodontal pockets and attachment loss. For example, Klementti et al. [20] reported that among 227 post-menopausal women, nearly half only had teeth in the mandibular arch, a jaw with a higher BMD. This may be one of the reasons why they failed to find a positive association between osteoporosis and the Community Periodontal Index of Treatment Needs (CPITN) [21], a method that at least partially reflects PD. This also raises a suggestion for future research to clarify the number of the retained teeth, and whether clinical attachment loss is associated with gingival recession or deep periodontal pockets. In addition, bleeding upon probing (BOP), as a predictor for periodontal disease progression [22], should also be noted. Because inflammation may be a mechanism that links the two disorders, it is important to investigate that association in the future. Deep periodontal pockets with signs of inflammation that require further treatment may have an impact on the outcome and complications of the patients who share both conditions.
Although there is some inconsistency among studies, most data suggests there is an association, especially related to bone densitometric measurements on radiographs. Whether the relationship is gender, race, or age, the association of shared risk factors warrants well-controlled longitudinal study. Defining the relationship between osteoporosis and periodontitis will help identify patients with one disorder who are at a higher risk for developing the other, and thereby could benefit from coordinated interdisciplinary care.
Shared Risk Factors
Although a causal relationship is yet to be established, there are numerous shared risk factors between osteoporosis and periodontitis. These established or potential risk factors may also provide important information regarding etiologies and contributing factors that will assist clinicians in preventing or managing these two diseases simultaneously.
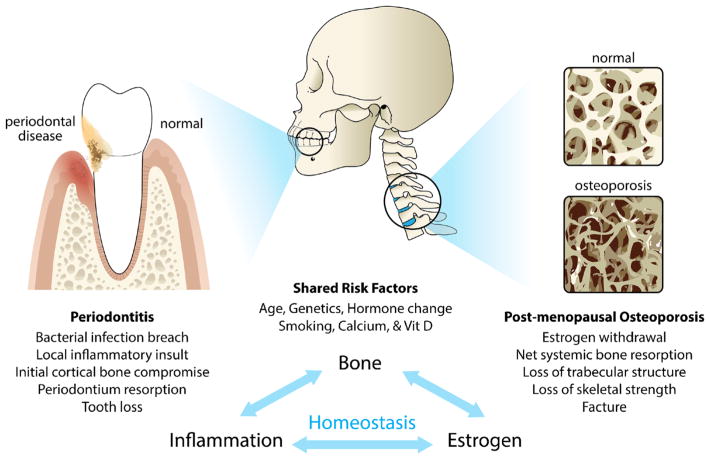
There are several shared risk factors for both diseases as listed in Fig. 1. Osteoporosis has been proposed as a risk factor for periodontal disease [8, 10, 23•], but prospective longitudinal studies are needed to establish a solid cause and effect relationship. Based on the data from the third National Health and Nutrition Examination Survey (NHANES III) regarding adults above the age of 50, it is estimated that 13–18 % of women (3–6 % of men) have osteoporosis in addition to 37–50 % of women and 28–47 % of men who have osteopenia [24]. An established casual relationship would pose these subjects at a higher risk for periodontal bone loss. Although both diseases are more prevalent among elderly population [1, 25], the association is more significant in women [26], and thus, most studies focused on elderly menopausal women. The main risk factor for osteoporosis in women is menopause, which is associated with a reduced production of estrogen [27]. The latter is associated with decreased protection from bone resorption [27] as well as a suppression in calcium absorption [28]. Studies of estrogen deficiency and periodontitis are limited in humans, but animal experiments have shown that ovariectomy-induced estrogen deficiency enhances alveolar bone loss caused by experimental periodontitis [29]. Furthermore, a gonadal hormone deficiency in men can also have an impact on maintaining bone mineral density [30, 31].
Fig. 1.
Schematic illustration of the association between periodontitis and osteoporosis. The diagram highlights the osseous target for periodontal disease (alveolar cortical bone) and osteoporosis (trabecular bone). Shared risk factors and potential mechanisms underlying both diseases are also listed
Calcium and vitamin D deficiency are major risk factors for osteoporosis [32], and more recent evidence suggests a similar role for periodontitis [33–35]. Data from NHANES demonstrates that women with a low intake of dietary calcium have more severe periodontal disease [33], and a more modest relationship is suggested for men [34, 35]. The genetic component for both diseases was widely investigated and supports a future personalized medicine approach for prevention and management [36, 37, 38•, 39, 40]. Although genetic studies specifically targeting both diseases are limited, some evidence suggests the polymorphisms of the vitamin D receptor [41, 42].
Smoking is a dose-dependent, major risk factor for periodontal disease [25], and has also been implicated in osteoporosis, especially as a predictor for facture risk [43]. Since osteoporosis may also involve inflammatory bone loss [44] (See Section “Potential Mechanisms Between Osteoporosis and Periodontitis”), it is of interest that a focal infection such as periodontitis could be a risk factor for osteoporosis. Could “presenile osteoporosis of the jaw” be an early sign of osteoporosis? Currently, it is still unclear whether there is an inverse interaction of periodontal disease to systemic bone remodeling [9]. A large prospective cohort study by Persson et al. [45] reported that osteoporotic patients with periodontitis have a higher risk for hip/hand fracture. More recently, experimental periodontitis aggravated systemic ovariectomy -induced bone loss [46••]. Although indirect evidence continues to accumulate, prospective and interventional clinical studies are needed to clearly establish the relationship to serve as an evidence base for future clinical management.
Potential Mechanisms Between Osteoporosis and Periodontitis
Systemic to Local Bone Resorptive Disease
Osteoporosis is a systemic bone-resorbing disease affecting mostly cancellous bone, whereas periodontal disease involves a local infection of the periodontium that first attacks the cortical bone and results in a dimensional change of the alveolar ridge. Since there is a strong association between systematic and local osteoporotic changes in the jaw [12], osteoporosis of the alveolar bone may constitute a “weakened resistance” of the periodontium to infectious challenge. This mechanism is well described in the literature and is consistent with the earlier proposal that osteopenia in the jaw (a non-inflammatory form of dental disease), is a sign of presenile osteoporosis [5]. However, given that the link between alveolar bone resorption and tooth loss is stronger than other clinical measurements of periodontal disease [8], it is possible that the osteoporotic change of the alveolar (tooth-supporting) bone directly contributes to premature loss of teeth through non-infectious mechanisms. It has been proposed that the mechanical challenge from occlusal forces causes an increased prevalence of micro-fractures in the osteoporotic alveolar bone that may lead to fatigue failure [47, 48]. The concept of noninflammatory degenerative disease of the periodontium was once considered obsolete; however, the latter potential mechanism encourages further investigation of this potential systemic factor from the host perspective.
Hormonal Impact on Bone Homeostasis and Inflammation
Several hormones may play important roles in regulating bone homeostasis, including estrogen, testosterone, cortisol, as well as parathyroid and thyroid hormones. The imbalance of these hormones may impact the metabolism of calcium/phosphate and bone homeostasis. Recently studies have also demonstrated that some of these hormones can also regulate inflammation [49–51].
Estrogen deficiency following menopause in women is a major risk factor for osteoporosis [27]. Hormonal changes impact systemic bone homeostasis and inflammatory responses. Estrogen deficiency also compromises calcium absorption and increases calcium excretion that leads to additional calcium requirements [28]. Reduced levels of estrogen also induce osteocyte apoptosis, which disrupts the homeostasis of bone [52]. The role of estrogen in inflammation is gaining attention, and the action of estrogen may be both pro-inflammatory and anti-inflammatory depending on the physiologic context [49]. In lipopolysaccharide (LPS)-stimulated human monocytes, lower levels of estrogen stimulate IL-1 mRNA expression [53], yet higher levels of estrogen attenuate IL-6 secretion and oxidative stress [54]. Therefore, estrogen may have an impact on both bone homeostasis and inflammation.
In animal models, estrogen deficiency aggravates the severity of experimental periodontitis [29]. Ovariectomized rats have higher expression of IL-6, RANKL, osteoprotegerin (OPG), to a lesser extent, and downregulation of IL-10 in the periodontal tissue, suggesting the impact of estrogen hormone on inflammatory bone resorption [55•]. Hormone replacement therapy (HRT) in humans improves mandibular bone density and reduces gingival bleeding and the number of teeth lost due to periodontitis [56, 57]. These results suggest a potential role of estrogen deficiency in periodontal disease.
Another essential hormone for bone homeostasis is parathyroid hormone (PTH), which increases bone resorption to ensure sufficient calcium in the blood. Intermittent PTH application improves periodontal healing and promotes bone regeneration in extraction sites [58, 59]. Most recently, PTH was found to regulate pro-resolving lipid mediators, which promoted macrophage efferocytosis for bone homeostasis [51]. These findings suggest that PTH can regulate bone homeostasis through inflammation-related pathways and highlights an important role of PTH in promoting bone healing and regeneration.
Together, these results suggest that the interaction of hormones related to bone remodeling and inflammation may be a mechanism that links osteoporosis and periodontitis.
Inflammation and Bone Homeostasis
Although osteoporosis was not considered an immunological disorder in the past, recent studies are indicative of the overlapping relationship between osteoporosis and inflammation [44, 60–62]. Patients with osteoporosis have elevated systemic levels of pro-inflammatory cytokines: IL-1, IL-6, and TNF-α [63, 64], which are all considered osteoclastogenic bone resorption-inducing cytokines [65]. Among these cytokines, the levels of IL-6 predict bone mineral density change [66] as well as fracture rate [67].
These inflammatory cytokines and other factors in the circulation not only impact systemic bone remodeling but also act locally to compromise the tissue response to periodontal disease (e.g., TNF-α also induces collagenase activity) [68]. Focal infection of the periodontium in turn may also release these inflammatory cytokines into the system and broadly impact inflammatory diseases [69]. It is very likely that one of the mechanisms underlying both diseases is through inflammatory pathways.
With the advances in understanding inflammation, it is now known that the resolution phase of inflammation is an active process orchestrated by pro-resolving lipid mediators (SPMs) [70]. Homeostatic bone remodeling involves “physiologic inflammation” to recruit non-phlogistic macrophages for clearance of apoptotic bone cells [51, 71]. Understanding the role of SPMs in bone homeostasis is a new field in osteoimmunology and can lead to further elucidation of the links between osteoporosis, inflammation, and periodontitis.
It is possible that the potential mechanisms are all linked to each other in various pathways. Patients with both osteoporosis and periodontitis may serve as a disease model in the future to study the etiologies and the mechanisms between bone disorders and inflammatory diseases.
Interdisciplinary Management of Osteoporosis and Periodontitis
Given the shared risks and mutual impact of both diseases, it is of interest to know whether the strategies for prevention and treatment of osteoporosis and periodontitis are shared. Osteoporosis is considered a “modifiable risk factor” for periodontitis with regard to host modulation therapy [23•, 72••, 73]. Osteoporotic elderly women who were not treated for the condition have a higher risk for severe periodontal disease [74•].
Early diagnosis of osteoporosis is also essential for managing the disease and for suppressing facture incidence. In elderly women, a significant association was found between mandibular basal bone and hip bone density [73]. In a study of 525 women, assessing the trabecular pattern features from dental panoramic radiographs can predict the measurement of DXA bone mineral density with high internal consistency [75]. It is possible that regular dental images could serve as a low-cost screening tool for osteoporosis.
Regarding the shared risk factor from calcium and vitamin D deficiency, oral supplementation of these two elements shows a positive impact on both diseases. Clinical studies, including RCT, have reported oral supplementation of calcium (1000 mg or more daily) and vitamin D (400 IU) improves periodontal conditions and helps to retain the teeth [18, 76–78]. However, a recent longitudinal study indicated that patients with lower (<30 nmol/L) 25-hydroxyvitamin D [25 (OH) D] levels could still be maintained periodontally stable for 5 years [79, 80•]. Therefore, future studies may need to include both the blood test readings, and all the sources for vitamin D for better elucidation of the metabolic dynamics. On the other hand, vitamin D deficiency (<20 nmol/L) at the time of periodontal surgery negatively affected treatment outcomes for up to 1 year [59]. In terms of managing osteoporosis, a meta-analysis concluded that oral supplementation of 700–800 instead of 400 IU vitamin D per day can reduce the risk of fracture [32]. In general, oral supplementation with calcium and vitamin D appear to have a positive impact on both osteoporosis and periodontal disease.
Smoking has been established as a major risk factor for periodontitis and osteoporotic fractures [25, 43]. Reduced smoking is associated with a suppressed incidence of fractures [43], especially in the vertebral column [43, 81] and reduced prevalence of periodontal disease [82]. Smoking is the most detrimental modifiable risk factor that should be pro-actively managed [83]. Although hormone replacement therapy (HRT) is not frequently recommended for the management of osteoporosis, it has demonstrated its beneficial effect on tooth retention [84]. PTH, teriparatide, and administration may also improve the treatment outcome for osteoporosis [85] and promote periodontal regeneration [86].
The benefits of oral bisphosphonates on periodontal alveolar bone loss was demonstrated in randomized controlled, double-blinded clinical trials for type II diabetic patients as an adjunctive measure to conventional anti-infective therapy through debridement [87]. In addition in this study, a decrease in hemoglobin A1c levels was noted for both control and experimental groups after the combination treatment. Although anti-resorptive medications such as bisphosphonates and RANK ligand inhibitor have shown beneficial impact on both diseases [73], the emerging risk for medication-related osteonecrosis of the jaw (MRONJ) is a concern. Patients receiving these antiresorptive medications, especially when taken for more than 4 years orally or through intravenous injection, have a higher risk of developing exposed bony lesions in the oral cavity [88]. Because the benefit of anti-resorptive medication for preventing osteoporotic fractures far outweighs the risk of MRONJ, taking these medications is still recommended. Since the population of patients with a history of bisphosphonate therapy continues to increase, dentists should be familiar with the most recent clinical guidelines for managing these patients [88, 89].
Osteoporotic patients with periodontitis require treatment for both conditions. Current trends in the treatment of osteoporosis are focused more on fracture prevention than on BMD levels. Recent association studies have chosen to investigate pathological fractures relative to the status of periodontal disease, and the presence of periodontitis is associated with an increased risk of fractures [90, 91]. Another more convincing prospective study showed that elderly patients with generalized severe periodontitis have an odds ratio (OR) of hip/hand fracture of 1.8:1 and after adjustment for age, the OR increased to 12 [45]. Experimental periodontitis was found to aggravate systemic ovariectomy-induced bone loss [46••]. Moreover, data from a large national longitudinal health insurance database showed that the incidence positively correlated with the severity of periodontitis. In addition, the risk of osteoporosis in patients with periodontitis increased dramatically (OR = 6.37) when these patients do not have professional periodontal maintenance [92••]. Therefore, it is important that patients with osteoporosis and periodontitis be treated concomitantly as well as have regular recall visits for monitoring and maintenance care.
Osteoporosis patients may also present with premature loss of teeth and a partially edentulous dentition that requires dental implant restoration. Osteoporosis was once considered a relative contraindication for dental implant therapy. However, recent data indicate that patients with osteoporosis have similar high success rates [93]. In addition, studies have shown that dental implants placed in fully edentulous patients may stimulate bone growth [94]. Therefore, osteoporotic patients may still be good candidates for dental implant therapy.
There are several considerations in managing patients with both osteoporosis and periodontitis. Treating both diseases simultaneously may have a positive synergistic effect for improved outcomes for interdisciplinary care. More longitudinal and interventional clinical studies are needed to recommend clinical guidelines for co-managing both diseases. Clinicians should be aware and knowledgeable of both conditions to make the proper referrals for co-management of the diseases.
Conclusion
Osteoporosis and periodontitis are both diseases with excessive bone resorption. There may be a disruption of the homeostasis involving bone remodeling, hormonal balance, as well as inflammation progression and resolution. Several shared risk factors exist, and their interactive impact is emerging. Well-controlled clinical studies are needed to establish an evidence base for efficient interdisciplinary management of both diseases.
Acknowledgments
Partial support for this work was provided by the NIH: DK053904. The authors thank Victoria Zakrzewski for drawing Figure 1.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Chin-Wei Wang and Laurie McCauley declare no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res Off J Am Soc Bone Miner Res. 2014;29(11):2520–6. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–20. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 3.Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement. 2000;17(1):1–45. [PubMed] [Google Scholar]
- 4.Periodontology AAP. Glossary of periodontal terms. 4. Chicago: American Academy of Periodontology; 2001. pp. 1–45. [Google Scholar]
- 5.Groen JJ, Duyvensz F, Halsted JA. Diffuse alveolar atrophy of the jaw (non-inflammatory form of paradental disease) and pre-senile osteoporosis. Gerontol Clin (Basel) 1960;2:68–86. doi: 10.1159/000244610. [DOI] [PubMed] [Google Scholar]
- 6.Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–87. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 7.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34(3):235–49. [PubMed] [Google Scholar]
- 8.Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann Periodontol. 2001;6(1):197–208. doi: 10.1902/annals.2001.6.1.197. [DOI] [PubMed] [Google Scholar]
- 9.Guiglia R, Di Fede O, Lo Russo L, Sprini D, Rini GB, Campisi G. Osteoporosis, jawbones and periodontal disease. Med Oral Patol Oral Cir Bucal. 2013;18(1):e93–9. doi: 10.4317/medoral.18298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Maestre MA, Gonzalez-Cejudo C, Machuca G, Torrejon R, Castelo-Branco C. Periodontitis and osteoporosis: a systematic review. Climacteric. 2010;13(6):523–9. doi: 10.3109/13697137.2010.500749. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–81. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 12.Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent. 1990;63(2):218–22. doi: 10.1016/0022-3913(90)90108-o. [DOI] [PubMed] [Google Scholar]
- 13.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10(1):34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 14.Wactawski-Wende J, Grossi SG, Trevisan M, Genco RJ, Tezal M, Dunford RG, et al. The role of osteopenia in oral bone loss and periodontal disease. J Periodontol. 1996;67(10 Suppl):1076–84. doi: 10.1902/jop.1996.67.10s.1076. [DOI] [PubMed] [Google Scholar]
- 15.Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71(9):1492–8. doi: 10.1902/jop.2000.71.9.1492. [DOI] [PubMed] [Google Scholar]
- 16.Elders PJ, Habets LL, Netelenbos JC, van der Linden LW, van der Stelt PF. The relation between periodontitis and systemic bone mass in women between 46 and 55 years of age. J Clin Periodontol. 1992;19(7):492–6. doi: 10.1111/j.1600-051x.1992.tb01162.x. [DOI] [PubMed] [Google Scholar]
- 17.Weyant RJ, Pearlstein ME, Churak AP, Forrest K, Famili P, Cauley JA. The association between osteopenia and periodontal attachment loss in older women. J Periodontol. 1999;70(9):982–91. doi: 10.1902/jop.1999.70.9.982. [DOI] [PubMed] [Google Scholar]
- 18.Krall EA, Garcia RI, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip, and spine. Calcif Tissue Int. 1996;59(6):433–7. doi: 10.1007/BF00369206. [DOI] [PubMed] [Google Scholar]
- 19.Astrom J, Backstrom C, Thidevall G. Tooth loss and hip fractures in the elderly. J Bone Joint Surg (Br) 1990;72(2):324–5. doi: 10.1302/0301-620X.72B2.2312583. [DOI] [PubMed] [Google Scholar]
- 20.Klemetti E, Collin HL, Forss H, Markkanen H, Lassila V. Mineral status of skeleton and advanced periodontal disease. J Clin Periodontol. 1994;21(3):184–8. doi: 10.1111/j.1600-051x.1994.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 21.Ainamo J, Barmes D, Beagrie G, Cutress T, Martin J, Sardo-Infirri J. Development of the World Health Organization (WHO) community periodontal index of treatment needs (CPITN) Int Dent J. 1982;32(3):281–91. [PubMed] [Google Scholar]
- 22.Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. Bleeding on probing. A predictor for the progression of periodontal disease? J Clin Periodontol. 1986;13(6):590–6. doi: 10.1111/j.1600-051x.1986.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 23•.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62(1):59–94. doi: 10.1111/j.1600-0757.2012.00457.x. This is a recent comprehensive review of the risk factors associated with periodontal disease. Not only osteoporosis was discussed; other shared risk factors, including dietary calcium and vitamin D were also addressed. [DOI] [PubMed] [Google Scholar]
- 24.Looker AC, Orwoll ES, Johnston CC, Jr, Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res Off J Am Soc Bone Miner Res. 1997;12(11):1761–8. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 25.Grossi SG, Genco RJ, Machtei EE, Ho AW, Koch G, Dunford R, et al. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J Periodontol. 1995;66(1):23–9. doi: 10.1902/jop.1995.66.1.23. [DOI] [PubMed] [Google Scholar]
- 26.Lin TH, Lung CC, Su HP, Huang JY, Ko PC, Jan SR, et al. Association between periodontal disease and osteoporosis by gender: a nationwide population-based cohort study. Medicine (Baltimore) 2015;94(7):e553. doi: 10.1097/MD.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Recker R, Lappe J, Davies KM, Heaney R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res Off J Am Soc Bone Miner Res. 2004;19(10):1628–33. doi: 10.1359/JBMR.040710. [DOI] [PubMed] [Google Scholar]
- 28.Nordin BE, Wishart JM, Clifton PM, McArthur R, Scopacasa F, Need AG, et al. A longitudinal study of bone-related biochemical changes at the menopause. Clin Endocrinol (Oxf) 2004;61(1):123–30. doi: 10.1111/j.1365-2265.2004.02066.x. [DOI] [PubMed] [Google Scholar]
- 29.Xu XC, Chen H, Zhang X, Zhai ZJ, Liu XQ, Zheng XY, et al. Effects of oestrogen deficiency on the alveolar bone of rats with experimental periodontitis. Mol Med Rep. 2015;12(3):3494–502. doi: 10.3892/mmr.2015.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khosla S, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86(8): 3555–61. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 31.Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston CC. Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. J Clin Invest. 1997;100(7):1755–9. doi: 10.1172/JCI119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–64. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 33.Nishida M, Grossi SG, Dunford RG, Ho AW, Trevisan M, Genco RJ. Dietary vitamin C and the risk for periodontal disease. J Periodontol. 2000;71(8):1215–23. doi: 10.1902/jop.2000.71.8.1215. [DOI] [PubMed] [Google Scholar]
- 34.Al-Zahrani MS. Increased intake of dairy products is related to lower periodontitis prevalence. J Periodontol. 2006;77(2):289–94. doi: 10.1902/jop.2006.050082. [DOI] [PubMed] [Google Scholar]
- 35.Shimazaki Y, Shirota T, Uchida K, Yonemoto K, Kiyohara Y, Iida M, et al. Intake of dairy products and periodontal disease: the Hisayama Study. J Periodontol. 2008;79(1):131–7. doi: 10.1902/jop.2008.070202. [DOI] [PubMed] [Google Scholar]
- 36.Seeman E, Hopper JL, Bach LA, Cooper ME, Parkinson E, McKay J, et al. Reduced bone mass in daughters of women with osteoporosis. N Engl J Med. 1989;320(9):554–8. doi: 10.1056/NEJM198903023200903. [DOI] [PubMed] [Google Scholar]
- 37.Soroko SB, Barrett-Connor E, Edelstein SL, Kritz-Silverstein D. Family history of osteoporosis and bonemineral density at the axial skeleton: the Rancho Bernardo Study. J Bone Miner Res Off J Am Soc Bone Miner Res. 1994;9(6):761–9. doi: 10.1002/jbmr.5650090602. [DOI] [PubMed] [Google Scholar]
- 38•.Karasik D, Rivadeneira F, Johnson ML. The genetics of bone mass and susceptibility to bone diseases. Nat Rev Rheumatol. 2016;12(6):323–34. doi: 10.1038/nrrheum.2016.48. This is a review paper summarizes key advances in genetics, including GWAS and functional studies that contributes to bone phenotype. Future target for drug development and personalized medicine was also discussed. [DOI] [PubMed] [Google Scholar]
- 39.Michalowicz BS, Diehl SR, Gunsolley JC, Sparks BS, Brooks CN, Koertge TE, et al. Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol. 2000;71(11):1699–707. doi: 10.1902/jop.2000.71.11.1699. [DOI] [PubMed] [Google Scholar]
- 40.Kornman KS, di Giovine FS. Genetic variations in cytokine expression: a risk factor for severity of adult periodontitis. Ann Periodontol. 1998;3(1):327–38. doi: 10.1902/annals.1998.3.1.327. [DOI] [PubMed] [Google Scholar]
- 41.Chen LL, Li H, Zhang PP, Wang SM. Association between vitamin D receptor polymorphisms and periodontitis: a meta-analysis. J Periodontol. 2012;83(9):1095–103. doi: 10.1902/jop.2011.110518. [DOI] [PubMed] [Google Scholar]
- 42.Kiel DP, Demissie S, Dupuis J, Lunetta KL, Murabito JM, Karasik D. Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S14. doi: 10.1186/1471-2350-8-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155–62. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 44.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65(12 Pt 2):S147–51. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 45.Persson GR, Berglund J, Persson RE, Renvert S. Prediction of hip and hand fractures in older persons with or without a diagnosis of periodontitis. Bone. 2011;48(3):552–6. doi: 10.1016/j.bone.2010.09.237. [DOI] [PubMed] [Google Scholar]
- 46••.Anbinder AL, Moraes RM, Lima GM, Oliveira FE, Campos DR, Rossoni RD, et al. Periodontal disease exacerbates systemic ovariectomy-induced bone loss in mice. Bone. 2016;83:241–7. doi: 10.1016/j.bone.2015.11.014. This is a recent animal study that demonstrates experimental periodontitis can aggrevate systemic ovareictomy-induced bone loss. [DOI] [PubMed] [Google Scholar]
- 47.Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech. 1985;18(3): 189–200. doi: 10.1016/0021-9290(85)90204-0. [DOI] [PubMed] [Google Scholar]
- 48.Mori S, Burr DB. Increased intracortical remodeling following fatigue damage. Bone. 1993;14(2):103–9. doi: 10.1016/8756-3282(93)90235-3. [DOI] [PubMed] [Google Scholar]
- 49.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 50.Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, et al. Thyroid hormones, oxidative stress, and inflammation. Mediat Inflamm. 2016;2016:6757154. doi: 10.1155/2016/6757154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCauley LK, Dalli J, Koh AJ, Chiang N, Serhan CN. Cutting edge: parathyroid hormone facilitates macrophage efferocytosis in bone marrow via proresolving mediators resolvin D1 and resolvin D2. J Immunol. 2014;193(1):26–9. doi: 10.4049/jimmunol.1301945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res Off J Am Soc Bone Miner Res. 1998;13(8):1243–50. doi: 10.1359/jbmr.1998.13.8.1243. [DOI] [PubMed] [Google Scholar]
- 53.Shanker G, Sorci-Thomas M, Register TC, Adams MR. The inducible expression of THP-1 cell interleukin-1 mRNA: effects of estrogen on differential response to phorbol ester and lipopolysaccharide. Lymphokine Cytokine Res. 1994;13(1):1–7. [PubMed] [Google Scholar]
- 54.Jain SK, Rogier K, Prouty L, Jain SK. Protective effects of 17beta-estradiol and trivalent chromium on interleukin-6 secretion, oxidative stress, and adhesion of monocytes: relevance to heart disease in postmenopausal women. Free Radic Biol Med. 2004;37(11):1730–5. doi: 10.1016/j.freeradbiomed.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 55•.Luo K, Ma S, Guo J, Huang Y, Yan F, Xiao Y. Association between postmenopausal osteoporosis and experimental periodontitis. Biomed Res Int. 2014;2014:316134. doi: 10.1155/2014/316134. This recent animal study demonstrates the impact of ovariectomy-induced postmenopausal osteoporosis on the pro-inflammatory and bone resorbing mediators in periodontal tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paganini-Hill A. The benefits of estrogen replacement therapy on oral health. The Leisure World cohort. Arch Intern Med. 1995;155(21):2325–9. [PubMed] [Google Scholar]
- 57.Grodstein F, Colditz GA, Stampfer MJ. Post-menopausal hormone use and tooth loss: a prospective study. J Am Dent Assoc. 1996;127(3):370–7. doi: 10.14219/jada.archive.1996.0208. quiz 92. [DOI] [PubMed] [Google Scholar]
- 58.Kuroshima S, Kovacic BL, Kozloff KM, McCauley LK, Yamashita J. Intra-oral PTH administration promotes tooth extraction socket healing. J Dent Res. 2013;92(6):553–9. doi: 10.1177/0022034513487558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. The impact of vitamin D status on periodontal surgery outcomes. J Dent Res. 2011;90(8):1007–12. doi: 10.1177/0022034511407771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ginaldi L, Di Benedetto MC, DeMartinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2:14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfeilschifter J. Role of cytokines in postmenopausal bone loss. Curr Osteoporos Rep. 2003;1(2):53–8. doi: 10.1007/s11914-003-0009-4. [DOI] [PubMed] [Google Scholar]
- 62.McLean RR. Proinflammatory cytokines and osteoporosis. Curr Osteoporos Rep. 2009;7(4):134–9. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 63.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res. 1996;11(8):1043–51. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 64.Brincat SD, Borg M, Camilleri G, Calleja-Agius J. The role of cytokines in postmenopausal osteoporosis. Minerva Ginecol. 2014;66(4):391–407. [PubMed] [Google Scholar]
- 65.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115(2):282–90. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R, et al. Serum interleukin 6 is amajor predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86(5):2032–42. doi: 10.1210/jcem.86.5.7445. [DOI] [PubMed] [Google Scholar]
- 67.Barbour KE, Lui LY, Ensrud KE, Hillier TA, LeBlanc ES, Ing SW, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res Off J Am Soc Bone Miner Res. 2014;29(9):2057–64. doi: 10.1002/jbmr.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golub LM, Payne JB, Reinhardt RA, Nieman G. Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. J Dent Res. 2006;85(2):102–5. doi: 10.1177/154405910608500201. [DOI] [PubMed] [Google Scholar]
- 69.Pizzo G, Guiglia R, Lo Russo L, Campisi G. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med. 2010;21(6):496–502. doi: 10.1016/j.ejim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinder BP, Pettit AR, McCauley LK. Macrophages: their emerging roles in bone. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015;30(12):2140–9. doi: 10.1002/jbmr.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72••.Reddy MS, Morgan SL. Decreased bone mineral density and periodontal management. Periodontol 2000. 2013;61(1):195–218. doi: 10.1111/j.1600-0757.2011.00400.x. This article specifically reviews the imaging test and therapies for osteoporosis and periodontitis. Relationship and risk factors of both diseases are also addressed with proposed management for shared patients. [DOI] [PubMed] [Google Scholar]
- 73.Jeffcoat MK. Osteoporosis: a possible modifying factor in oral bone loss. Ann Periodontol. 1998;3(1):312–21. doi: 10.1902/annals.1998.3.1.312. [DOI] [PubMed] [Google Scholar]
- 74•.Penoni DC, Torres SR, Farias ML, Fernandes TM, Luiz RR, Leao AT. Association of osteoporosis and bone medication with the periodontal condition in elderly women. Osteoporos Int. 2016;27(5): 1887–96. doi: 10.1007/s00198-015-3437-y. This paper shows a larger cross-sectional study regarding the association of BMD with clinical attachment level (CAL) and demonstrates the higher risk of severe periodontitis in subjects who do not control the osteoporotic condition. [DOI] [PubMed] [Google Scholar]
- 75.Geraets WG, Verheij JG, van der Stelt PF, Horner K, Lindh C, Nicopoulou-Karayianni K, et al. Osteoporosis and the general dental practitioner: reliability of some digital dental radiological measures. Community Dent Oral Epidemiol. 2007;35(6):465–71. doi: 10.1111/j.1600-0528.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- 76.Miley DD, Garcia MN, Hildebolt CF, Shannon WD, Couture RA, Anderson Spearie CL, et al. Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol. 2009;80(9):1433–9. doi: 10.1902/jop.2009.090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dixon D, Hildebolt CF, Miley DD, Garcia MN, Pilgram TK, Couture R, et al. Calcium and vitamin D use among adults in periodontal disease maintenance programmes. Br Dent J. 2009;206(12):627–31. doi: 10.1038/sj.bdj.2009.519. discussion 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia MN, Hildebolt CF, Miley DD, Dixon DA, Couture RA, Spearie CL, et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol. 2011;82(1):25–32. doi: 10.1902/jop.2010.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Millen AE, Andrews CA, LaMonte MJ, Hovey KM, Swanson M, Genco RJ, et al. Vitamin D status and 5-year changes in periodontal disease measures among postmenopausal women: the Buffalo OsteoPerio Study. J Periodontol. 2014;85(10):1321–32. doi: 10.1902/jop.2014.130686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80•.Pavlesen S, Mai X, Wactawski-Wende J, LaMonte MJ, Hovey KM, Genco RJ, et al. Vitamin D status and prevalent and incident tooth loss in postmenopausal women: the buffalo osteoporosis and periodontal disease (OsteoPerio) study. J Periodontol. 2016;87(8):1–17. doi: 10.1902/jop.2016.150733. A 5 years longitudinal clinical study shows that patients with lower levels of 25(OH) D at baseline may still be periodontally stable with appropriate maintenance. Some limitations in addressing the progression of periodontal disease in longitudinal studies were discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thorin MH, Wihlborg A, Akesson K, Gerdhem P. Smoking, smoking cessation, and fracture risk in elderly women followed for 10 years. Osteoporos Int. 2016;27(1):249–55. doi: 10.1007/s00198-015-3290-z. [DOI] [PubMed] [Google Scholar]
- 82.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71(5):743–51. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 83.Reynolds MA. Modifiable risk factors in periodontitis: at the intersection of aging and disease. Periodontol 2000. 2014;64(1):7–19. doi: 10.1111/prd.12047. [DOI] [PubMed] [Google Scholar]
- 84.Krall EA, Dawson-Hughes B, Hannan MT, Kiel DP. Postmenopausal estrogen replacement and tooth retention. Compend Contin Educ Dent Suppl. 1998;22:S17–22. [PubMed] [Google Scholar]
- 85.Fahrleitner-Pammer A, Burr D, Dobnig H, Stepan JJ, Petto H, Li J, et al. Improvement of cancellous bone microstructure in patients on teriparatide following alendronate pretreatment. Bone. 2016;89:16–24. doi: 10.1016/j.bone.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010;363(25):2396–405. doi: 10.1056/NEJMoa1005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rocha M, Nava LE, Vazquez de la Torre C, Sanchez-Marin F, Garay-Sevilla ME, Malacara JM. Clinical and radiological improvement of periodontal disease in patients with type 2 diabetes mellitus treated with alendronate: a randomized, placebo-controlled trial. J Periodontol. 2001;72(2):204–9. doi: 10.1902/jop.2001.72.2.204. [DOI] [PubMed] [Google Scholar]
- 88.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 89.Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015;30(1):3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 90.Moedano DE, Irigoyen ME, Borges-Yanez A, Flores-Sanchez I, Rotter RC. Osteoporosis, the risk of vertebral fracture, and periodontal disease in an elderly group in Mexico City. Gerodontology. 2011;28(1):19–27. doi: 10.1111/j.1741-2358.2009.00342.x. [DOI] [PubMed] [Google Scholar]
- 91.Clark P, Cons-Molina F, Deleze M, Ragi S, Haddock L, Zanchetta JR, et al. The prevalence of radiographic vertebral fractures in Latin American countries: the Latin American Vertebral Osteoporosis Study (LAVOS) Osteoporos Int. 2009;20(2):275–82. doi: 10.1007/s00198-008-0657-4. [DOI] [PubMed] [Google Scholar]
- 92••.Huang YF, Chang CT, Liu SP, Muo CH, Tsai CH, Hong HH, et al. The impact of oral hygiene maintenance on the association between periodontitis and osteoporosis: a nationwide population-based cross sectional study. Medicine (Baltimore) 2016;95(6):e2348. doi: 10.1097/MD.0000000000002348. This paper from a large national insurance database concludes that the risk for osteoporosis positively correlates with the severity of periodontal disease; and the risk was further increased in patients without regular periodontal maintenance treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeffcoat MK. Safety of oral bisphosphonates: controlled studies on alveolar bone. Int J Oral Maxillofac Implants. 2006;21(3):349–53. [PubMed] [Google Scholar]
- 94.Reddy MS, Geurs NC, Wang IC, Liu PR, Hsu YT, Jeffcoat RL, et al. Mandibular growth following implant restoration: does Wolff’s law apply to residual ridge resorption? Int J Periodontics Restorative Dent. 2002;22(4):315–21. [PubMed] [Google Scholar]