Abstract
Varicella zoster virus (VZV) becomes latent in ganglionic neurons derived from neural crest cells. Because the adrenal gland also contains medullary chromaffin cells of neural crest origin, we examined human adrenal glands and medullary chromaffin cell tumors (pheochromocytomas) for VZV and herpes simplex virus type 1 (HSV-1). We found VZV, but not HSV-1, DNA in 4/63 (6 %) normal adrenal glands. No VZV transcripts or antigens were detected in the 4 VZV DNA-positive samples. No VZV or HSV-1 DNA was found in 21 pheochromocytomas.
Keywords: VZV, Latency, Adrenal gland, Pheochromocytoma
Introduction
Varicella zoster virus (VZV) is an exclusively human, neurotropic alphaherpesvirus that causes varicella (chickenpox), after which virus becomes latent in ganglionic neurons along the entire neuraxis. As cell-mediated immunity to VZV declines with age or in immunocompromised individuals, VZV reactivates to produce herpes zoster (shingles), frequently followed by chronic pain (postherpetic neuralgia). VZV reactivation also causes cranial nerve palsies, meningoencephalitis, vasculopathy, and multiple ocular disorders. Importantly, all these conditions may develop without zoster rash (Gilden et al. 2010). Herpes simplex virus type-1 and type-2 (HSV-1, HSV-2) are latent in the cranial nerve ganglia and sacral ganglia, respectively. HSV-1 reactivation produces recurrent mouth sores (herpes labialis) and rarely encephalitis, while HSV-2 reactivation produces genital herpes and less often meningitis, both of which may be recurrent. Furthermore, most thoracic sympathetic ganglia contain VZV DNA while only a small fraction contains HSV-1 (Nagel et al. 2014). Because adrenal glands contain medullary chromaffin cells which, like ganglionic neurons, are of neural crest origin, we examined normal adrenal glands and adrenal pheochromocytomas (medullary chromaffin cell tumors) for the presence of VZV and HSV-1.
Materials and methods
Human adrenal glands
Specimens (n = 63) were acquired as follows: (1) when pheochromocytoma was resected, normal adjacent adrenal gland tissue was removed; (2) during kidney transplantation, a small piece of adrenal gland on top of the donor kidney was removed; (3) 2 adrenal glands were removed during routine autopsy from 2 men, age 81 and 51 years; and (4) 21 adrenal pheochromocytoma tumors were obtained at surgical removal. The adrenal gland tissue was flash-frozen in liquid nitrogen and stored at −80 °C until analysis.
PCR amplification of VZV DNA in human adrenal glands
DNAwas extracted from the adrenal glands using the DNeasy blood and tissue kit (Qiagen, Valencia, CA) and quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). DNA was then analyzed by qPCR using primers corresponding to sequences in VZV ORF 68, in the non-coding region of HSV-1 genome between UL42 and UL43, and in cellular GAPdH (Table 1) as described (Gilden et al. 2015). The adrenal glands were considered positive for viral DNA if (1) no virus amplification was detected in wells without any target DNA; (2) GAPdH was detected in wells with adrenal DNA; and (3) both of 2 PCR replicates amplified viral target DNA. To determine the viral copy number from the adrenal glands, each PCR reaction contained DNA standards with known concentrations of viral DNA.
Table 1.
Primer and probe sequences
| Gene name | Forward sequence (5′-3′) | Reverse sequence (5′-3′) | Probe sequence (5′-3′) |
|---|---|---|---|
| Smart GAPdH | CACATGGCCTCCAAGGAGTAA | TGAGGGTCTCTCTCTTCCTCTTGT | VIC/CTGGACCACCAGCCCCAGCAAG |
| VZV ORF 68 | GTACATTTGGAACATGCGCG | TCCACATATGAAACTCAGCCC | FAM/AAAACAAGAAACCCTACGCCCGC |
| Unique HSV-1a | TGGTATTGCCCAACACTTTCC | GCGCCAGGCACACACAT | FAM/CGTGTCGCGTGTGGT/BHQ |
Amplifies the non-coding region of the HSV-1 genome between UL42 and UL43
RNA extraction and cDNA synthesis from human adrenal glands
Total RNA from the adrenal glands was extracted using the TriPure isolation agent (Sigma-Aldrich, St. Louis, MO) and the Direct-zol RNA mini prep kit (Zymo Research, Irvine, CA). Before cDNA synthesis, the RNA was treated with RNAse-free DNAse (Thermo Fischer Scientific) to eliminate any residual genomic DNA, according to the manufacturer’s instructions. cDNAwas synthesized from the RNA by reverse transcription using the Transcriptor First-Strand cDNA Synthesis kit (Roche Diagnostics, Indianapolis, IN) and analyzed by qPCR for the presence of VZV immediate-early, early, and late genes (ORF 63, 29, and 68, respectively) using TaqMan primers as described (Gilden et al. 2015).
Immunohistochemical analysis of human adrenal glands
Of the 4 normal adrenal glands that contained VZV DNA, 3 had corresponding optimal cutting temperature (OCT) tissue blocks. For each block, ten 5-μm sections were cut on a cryostat, fixed in acetone for 5 min, and rehydrated in 1× sterile PBS for 15 min. Six alternating sections from each block were analyzed by immunohistochemistry using a 1:10,000 dilution of rabbit monospecific polyclonal anti-VZV IE63 and a 1:500 dilution of mouse monoclonal anti-VZV gE IgG1 (Santa Cruz Biotechnology, Dallas, TX) antibodies; positive controls were provided by VZV-infected cadaveric cerebral arteries maintained for 14 days in vitro followed by immunostaining with the above antibodies. Substitution of mouse IgG1 (Dako, Carpenteria, CA) and rabbit anti-HSV-1 (Dako) for primary anti-VZV antibodies served as negative controls as described (Gilden et al. 2015). Two readers (D.G. and M.A.N.) examined each section for the presence of VZV antigen by light microscopy.
Results
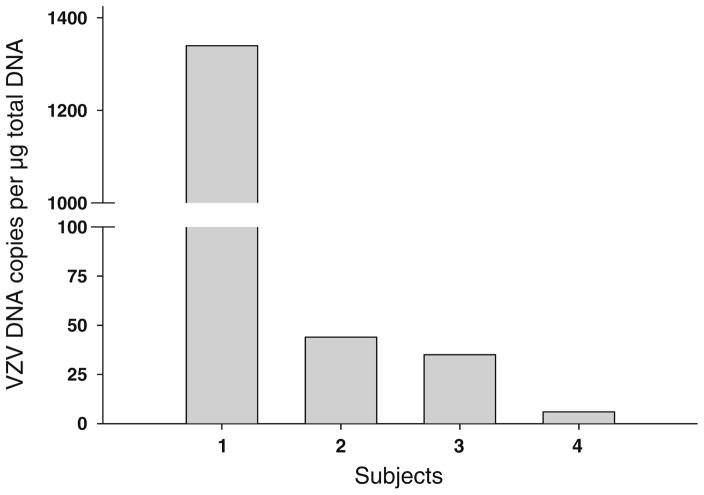
PCR amplified cellular GAPdH DNA in 63 normal adrenal glands and in 21 pheochromocytomas. In the 63 normal adrenal glands, VZV DNA was found in 4 (6 %) which contained 1339, 44, 35 and 6 VZV DNA copies per microgram total DNA (Fig. 1). No VZV DNA was detected in the 21 pheochromocytomas. HSV-1 DNA was not detected in any normal adrenal gland or pheochromocytomas. Of the 4 normal adrenal glands that contained VZV DNA, all had tissue sufficient for RNA extraction; analysis by RT-qPCR revealed transcripts for GAPdH, but none corresponding to VZV ORFs 63, 29, or 68. Immunohistochemical analysis of the 3 OCT tissue blocks of normal adrenal glands revealed no VZV IE63 or VZV glycoprotein E antigen.
Fig. 1.
Viral DNA load in normal human adrenal glands. Of 63 normal human adrenal glands, 4 contained VZV DNA. The VZV DNA copy number was 1339, 44, 35, and 6 per microgram total DNA, respectively
Discussion
In the only other analysis of human adrenal glands for alphaherpesviruses, VZV DNA, but not HSV-1 or HSV-2 DNA, was found in one of 8 adrenal glands (Chen and Hudnall, 2006). Our current study of 63 normal adrenal glands and 21 pheochromocytomas revealed the presence of VZV DNA, but not HSV-1, in 4 (6 %) normal adrenal glands. The presence of VZV DNA combined with the absence of VZV transcripts and antigen in the same adrenal gland indicates VZV latency. The absence of VZV DNA in any of 21 pheochromocytomas is not surprising. Since VZV is latent in only 2–5 % of neurons of latently infected ganglia (Kennedy et al. 1998), and all tumors reflect proliferation of a single cell, the likelihood that a pheochromocytoma would develop from a latently infected neuron is small.
Since VZV is latent in neural crest-derived ganglionic neurons, the presumed site of latency is neural crest-derived chromaffin cells of the adrenal medulla, although definitive assignment awaits the search for VZV in chromaffin cells of freshly dissected adrenal glands instead of flash-frozen specimens. The finding of VZV DNA in the adrenal glands further expands sites of VZV latency from the cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia to the adrenal gland.
Importantly, the presence of latent VZV in the adrenal glands raises the possibility that virus reactivation may disrupt production of catecholamines (e.g., epinephrine, norepinephrine), glucocorticoids, and mineralocorticoids which might play a role in the development of dysautonomia and fatigue syndromes. Future studies will involve analysis of chromaffin cells in dissected adrenal glands, as well as co-localization of VZV in chromaffin cells, and effects of VZV infection in adrenal function.
Acknowledgments
This work was supported by Public Health Service grants AG032958 (D.G., M.A.N.), NS093716 (D.G.), and NS094758 (M.A.N.) from the National Institutes of Health. Hussain Badani was supported by training grant NS007321 to D.G. from the National Institutes of Health. We thank Marina Hoffman for editorial review and Cathy Allen for manuscript preparation.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no competing interests.
References
- Chen T, Hudnall SD. Anatomical mapping of human herpesvirus reservoirs of infection. Mod Pathol. 2006;19:726–737. doi: 10.1038/modpathol.3800584. [DOI] [PubMed] [Google Scholar]
- Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by varicella zoster virus reactivation without rash. Curr Top Microbiol Immunol. 2010;342:243–253. doi: 10.1007/82_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D, White T, Khmeleva N, Heintzman A, Choe A, Boyer PJ, Grose C, Carpenter JE, Rempel A, Box N, Kandasamy B, Lear-Kaul K, Holmes DB, Bennett JL, Cohrs RJ, Mahalingam R, Mandava N, Eberhart CG, Bockelman B, Poppiti RJ, Tamhankar MA, Fogt F, Amato M, Wood E, Durairaj V, Rasmussen S, Petursdottir V, Pollak L, Mendlovic S, Chatelain D, Keyvani K, Brueck W, Nagel MA. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015;84:1948–1955. doi: 10.1212/WNL.0000000000001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PGE, Grinfeld E, Gow JW. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci. 1998;95:4658–4662. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel MA, Rempel A, Huntington J, Kim F, Choe A, Gilden D. Frequency and abundance of alphaherpesvirus DNA in human thoracic sympathetic ganglia. J Virol. 2014;88:8189–8192. doi: 10.1128/JVI.01070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]