Abstract
State-Dependent Learning (SDL) is a phenomenon relating to information storage and retrieval restricted to discrete states. While extensively studied using psychopharmacological approaches, SDL has not been subjected to rigorous neuroscientific study. Here we present an overview of approaches historically used to induce SDL, and highlight some of the known neurobiological mechanisms, in particular those related to inhibitory neurotransmission and its regulation by microRNAs (miR). We also propose novel cellular and circuit mechanisms as contributing factors. Lastly, we discuss the implications of advancing our knowledge on SDL, both for most fundamental processes of learning and memory as well as for development and maintenance of psychopathology.
Introduction
SDL is a phenomenon related to information processing wherein information acquired in a certain state requires a similar state for best recall. Because such information cannot be reliably accessed under baseline conditions, SDL is manifested as a memory retrieval deficit, however this deficit can be reversed with techniques that reinstate the conditions that were present at encoding.
The phenomenon of SDL was first demonstrated by Girden and Culler [1**], who noticed that leg flexion conditioned in dogs under curare could only be elicited when the animals were drugged with curare again. However, when the reflex was conditioned in the non drugged state, it disappeared under curare, and reappeared under a non drugged state. They also referred to the phenomenon as “dissociation of learning” to indicate the separation of memory encoding and recall between the drugged and non-drugged state.
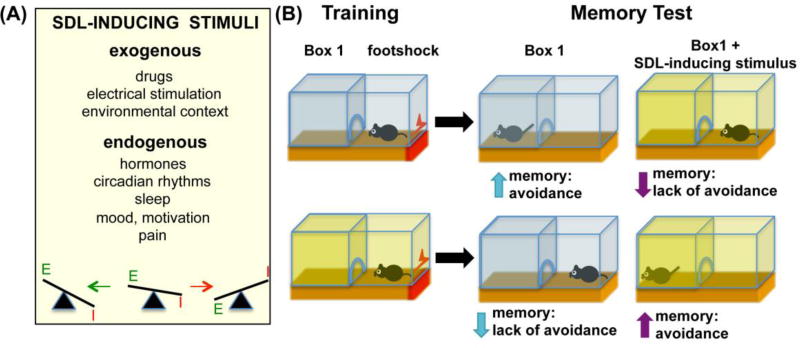
SDL has since been demonstrated in a wide variety of organisms, including invertebrates, goldfish, mice, rats, rabbits, cats, dogs, monkeys, and man [2*],[3],[4*],[5**]. Furthermore, in addition to drugs [6–8], a number of exogenous and endogenous stimuli have proved capable of supporting SDL [9]. These include electrical stimulation (e.g., electroconvulsive seizures, cortical spreading depression) [10, 11], hormones [12], mood and motivation [13, 14], circadian rhythms [15], sleep [16], pain [17], and environmental contexts [18]. With this in mind, it is reasonable to suppose that as a result of affective states, implicit and explicit motives, and interaction with the environment, all memories are to some degree state-dependent (Figure 1).
Figure 1.
Inducing SDL by stimuli that change the excitatory/inhibitory balance. (A) Exogenous and endogenous stimuli known to induce SDL. (B) SDL in an example of a passive avoidance paradigm, where the presence of memory is reflected by avoidance of the shock compartment at test. Top, memories learned under normal conditions are easily retrieved under similar conditions, but not if SDL-inducing stimuli are applied before the test. Bottom, memories learnt under SDL-inducing stimuli are not accessible for retrieval under normal conditions but can be retrieved if the same stimuli are reapplied. E, excitation; I, inhibition.
To date, SDL has most extensively been studied using drugs, which has led to the identification of many conditions that support SDL, as well as some constraints. Under some drugs such as phentobarbital, dissociation or state-dependency can be complete, meaning that there is no information transfer between the drug and non drug states, however, such transfer can occur among drug-induced states which share similarities [4*]. In animal experiments, recovery of memory has also been found during increased arousal [19], with the presentation of a salient reminder [20], or after overtraining [21]. Examples of recovery in humans can also arise as a result of experimental cueing or prompting [7, 8].
State-dependency of learning and memory under various psychoactive drugs has been extensively reported with rodent models of reinforcement learning and passive avoidance [22**] [23, 24]. However, many of these drugs, such as benzodiazepines, NMDAR antagonists, amphetamine, and scopolamine have, until recently [25**], proved ineffective in fear conditioning [26–29]. The reasons for these task-related differences are not known, but some possibilities will be discussed below.
Extensive research in the 1960s -1980s resulted in an impressive breath but limited depth of our knowledge of SDL both in respect to the definition of a state as well as to the underlying neurobiological mechanisms. The term “state” has been broadly used to describe a condition of the brain, the mind, or individual as a whole. Nevertheless, at the most fundamental level it refers to changes of timing and routing of neuronal firing within specific networks [4*]. These changes can alter the processing of distinct stimulus features at encoding [30, 31], and possibly the function of neuronal comparators (whose role is to match sensory inputs with encoded information) at retrieval [32]. When it comes to candidate mechanisms of SDL, there are all kinds of possibilities because state- dependency is inherent to every component of neuronal activity, from molecular, cellular, circuit, and global network activity, to consciousness itself [33]. Therefore, determinants of discrete neuronal states will likely be found at all of these levels. This may best be illustrated with the example of sleep, an altered state of information processing, which entails well-defined changes of the balance among key neurotransmitter systems, redistribution of activity within subcortical and cortical circuits, and generation of slow oscillatory rhythms [34*]. Similar levels of analyses applied to SDL are likely to identify the defining features of the various brain states that support the encoding and retrieval of long-term memories.
Molecular mechanisms of SDL
Under normal awake conditions, memory processes predominantly depend on excitatory transmission, in particular N-methyl-D-aspartate receptor (NMDAR) and α- amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), whose activity somewhat predominates in the overall excitatory/inhibitory balance. However, changes of this balance in either direction can support SDL. For example, cholinergic mechanisms of SDL involve both blocking cholinergic function with scopolamine and increasing cholinergic function with physostigmine [35]. In humans and rodents, SDL is frequently reported with psychostimulants, such as, amphetamine [6], meprobamate [36], cocaine [37], and caffeine [38]. Opiates also support SDL and of all classes of opioid receptors, morphine-activated µ receptors seem to be the most effective [39].
Notwithstanding the above, most of the evidence for SDL comes from activation of GABAergic transmission and shifting the excitatory/inhibitory balance towards inhibition. The ionotropic GABAAR is a pentamer composed of two α, two β, and one γ or δ subunit. Many drugs bind to GABAAR and alter its conductance for chloride ions, which regulates the degree of neuronal inhibition. However, drug effects are also unique because they bind to distinct sites of the receptor complex. In rodents, SDL has been found with a variety of GABAAR agonists and positive allosteric modulators, including barbiturates [9]. GABABR agonists, such as baclofen are ineffective [40*], supporting the view that SDL is primarily GABAAR-mediated phenomenon. Similar effects have been found in humans [2*], [6, 41] except that diazepam’s actions were less clear [42]. An important condition for the ability of GABAergic drugs to induce SDL is the applied dose. Contrary to the initial assumption that SDL requires high drug doses, Colpaert [43] demonstrated that relatively low, therapeutic doses of the benzodiazepine chlordiazepoxide also give rise to SDL, and that doses required for recall can be much lower than those applied at encoding. This differs from initial observations with pentobarbital, where the highest degree of recall was found with the same dose of drug whereas the amnestic barrier became stronger the more the dose at test deviated from the dose at training [4*]. This could explain some of the inconsistent findings in the field and suggests that research on SDL warrants careful consideration of dose responses for particular drugs and learning tasks.
Studies examining the ability of GABAAR agonists to substitute for one another in recovering state-dependent memories have revealed that substitution is asymmetrical, suggesting that discrete GABAAR mechanisms underlie SDL. In general, ethanol, the least specific GABAAR agonist, could recover memories encoded under the GABAAR agonists diazepam or muscimol, but neither diazepam nor muscimol were effective when SDL occurred under ethanol [44]. Similarly, amobarbital, which binds to all GABAAR, recovered the memory [45] whereas diazepam, which predominantly binds to synaptic GABAAR [46], did not yield consistent results [42]. This suggests that extrasynaptic, αβδ GABAAR, could be particularly important for SDL. Unlike most of γ subunit-containing GABA receptors, αβδ receptors have a very low sensitivity to benzodiazepines, but are highly sensitive to low concentrations of alcohol [47] and the drug gaboxadol [48]. These GABAAR are extrasynaptic, regulating tonic inhibition [49*], and they mediate the sensitivity of mice to the sedative, hypnotic, and anxiolytic effects of neuroactive steroids [50]. In our own work, gaboxadol strongly supported SDL [25**], and this was shown with the contextual fear conditioning paradigm, in which state-dependent effects are usually difficult to observe.
Bioinformatic analyses have recently revealed that in addition to their regulation by various endogenous and exogenous agents, GABAAR are also targeted by many microRNAs (miRNA). miRNAs regulate protein levels through the degradation or translation block of their target mRNA. Unlike transcriptional regulation, which causes substantial changes in the protein level, miRNAs cause subtle changes in protein levels and their role is seen more as fine-tuning the amounts of the target proteins [51, 52]. However, although their effect on individual targets is small, their overall physiological effect is strong due to the simultaneous regulation of many functionally related proteins. We have recently found that miR-33, which targets several GABA-related proteins, has a strong influence on the ability of gaboxadol to induce SDL. Unlike miRNAs that directly regulate learning and memory [53], miR-33 increased the threshold for gaboxadol’s actions, and shifted the dose-response curve to the right [25**]. Interestingly, the levels of several extrasynaptic GABAAR and GABAAR-targeting miRNAs, including miR-33, are consistently dysregulated in patients suffering from major psychiatric disorders [54, 55], such as major depression and schizophrenia. It remains to be determined whether the observed molecular abnormalities contribute to the generation and maintenance of state-dependent information processing characteristic of these disorders.
In summary, several neurotransmitter systems, most notably the GABAergic system, support SDL, allowing for the formation of memories that are not readily retrievable. Much work needs to be done to better understand the mechanisms and significance of memory formation under different states. One of the important remaining questions is whether GABAergic mechanisms mediate SDL or, alternatively, whether they induce states that allow for different interpretation of ongoing glutamatergic transmission.
Cellular and circuit mechanisms of SDL
In addition to GABAergic drugs, GABA receptors also mediate the SDL induced by other drugs such as morphine [24]. This is not surprising given that µ opioid receptors are primarily expressed on interneurons [56], and suggests that GABA receptors can be the downstream effectors of other receptor mechanisms involving interneurons. Innervation of pyramidal excitatory neurons by interneurons is domain-specific, allowing for the coordination of multiple glutamatergic inputs on different parts of pyramidal cells [57**]. This is achieved through temporally distinct activity of GABAergic interneurons, which change their firing during different network states [58]. Although interneuron-specific firing has so far been mainly implicated in segregating cell assemblies and establishing the temporal order of assemblies during behaviors, it is also likely that some of these mechanisms contribute to SDL (see below).
Already in some of the first SDL studies, it was shown that brain regions differently support SDL. For example, for the caudate nucleus and hippocampus, low intensity electrical stimulation is sufficient for SDL, whereas the amygdala produces SDL effects only after strong stimulation that induces overt seizures [59].
In their early work on SDL, Girden and Culler, [1] suggested that conditioning under curare is subcortical in nature and does not require, or is even suppressed by cortical activity. The first study to address this question was by Girden [60]. He found that bilateral ablation of the auditory cortex in dogs eliminated dissociation between a curare- induced drug state and the nondrug state. However, this was not replicated by Bliss, Sledjeski, and Leiman [61] who demonstrated intact SDL when monkeys with bilateral dorsolateral frontal ablation were on pentobarbital. Robust circuit effects were next shown with the finding of lateralization of state-dependency in split brain rats, but not in intact rats [62]. In our own lab, we have examined the role of the extended hippocampal circuit in gaboxadol-induced SDL. Normally, contextual fear conditioning depends on the hippocampus as well as the retrosplenial cortex [63], but as predicted by Girden and Culler, SDL under gaboxadol was independent of this cortical area, and even showed enhancement following retrosplenial cortical inactivation [25**]. Analyses of suppressed cortical and elevated subcortical activation of immediate early genes further support this view [25**] and suggest that changes of neuronal states also involve changes of the routing of neuronal signals within broader brain circuits.
Brain states supporting learning processes are often defined by the rhythmic neuronal activity of various frequencies [64**]. Many drugs that give rise to SDL also induce changes of the electroencephalogram (EEG), as first reported for phentobarbital [65]. Subsequently, Sadowski and Longo [66] found that the synchronization of the EEG after injection of scopolamine closely paralleled the disruption of a response learned under the nondrug state. Leiman, Bliss, Powers, and Rosenzweig [67] showed that when rats were injected with pentobarbital at a dosage capable of producing dissociation, the EEG activity changed from the normal arousal portrait of low-voltage desynchronized activity to high-amplitude synchronized waves. It is now well established that most drugs that support SDL, including gaboxadol, scopolamine, and opiates, induce changes of oscillatory neuronal activity, measured by EEG or local field potentials [68–70]. These large changes in electrical activity may well be correlated with the behavioral findings of drug dissociation. Thus, a process initiated at a molecular level, such as activation of extrasynaptic GABAAR, could change local and global network activity that enables state-dependent encoding and retrieval of memories (Figure 2).
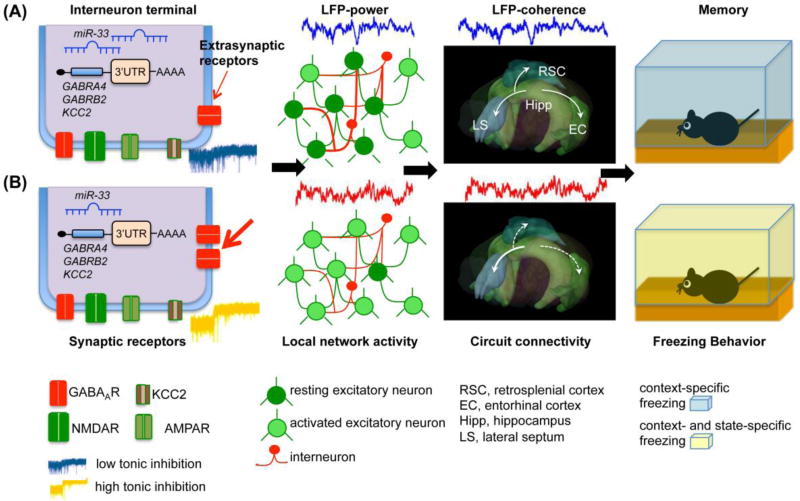
Figure 2.
Molecular, cellular, and circuit mechanisms of SDL. A model of SDL based on activation of extrasynaptic GABAAR on hippocampal dentate gyrus interneurons [28**]. (A) Conditions reflecting normal tonic inhibition (thin red arrow) allow for activation of some excitatory granule cells and induce changes of local network activity as well as coherent activity between the hippocampus and its cortical and subcortical targets. These changes are correlated with successful memory retrieval in a contextual fear conditioning paradigm, revealed as freezing behavior during a memory test. (B) Increasing tonic inhibition via extrasynaptic GABAAR (thick red arrow) on interneurons increases the number of active granule cells via disinhibition, and induces changes of local and global oscillatory activities. This results in disrupted hippocampal-cortical and enhanced hippocampal subcortical processing of context memories. Such memories are best retrieved when extrasynaptic GABAAR are reactivated, recreating the state at encoding. KCC2, chloride symporter.
Conclusion and implications of SDL
The SDL phenomenon has received little recent attention, which is somewhat surprising given that a number of advantages will accrue from a better understanding of the neurobiology of SDL. These relate to (i) the fundamental principles of information processing, (ii) the impact of inaccessible memories on behavior, (iii) the role of SDL in transition to and maintenance of psychopathology, and (iv) information processing under psychiatric conditions.
Although, in research settings, SDL is most frequently studied using drugs, it may well be a routine aspect of information processing. Recent work is exploring the idea that spatiotemporal patterns of synchronized spontaneous activity in neuronal networks serve as memories [71], and it has recently been suggested that state-dependency accounts for the variety of such patterns [72**]. From an evolutionary perspective, SDL has also been conceptualized as a way to organize memories so that they can influence decision-making only under constrained conditions when access to specific information is particularly advantageous [5]. Finally, SDL is regarded as a protective mechanism that helps to temporarily avoid negative affect triggered by distressing memories [73].
In contrast to these generally beneficial effects, relying on SDL as a predominant learning strategy has many adverse consequences partly because memories and their associated emotions are not properly integrated at encoding. This could place individuals at risk for a wide variety of psychiatric disorders, especially dissociative disorders and post-traumatic stress-disorder [74], because despite the fact that state-dependent (often traumatic) memories cannot be fully retrieved, they nevertheless strongly influence social and affective behavior [75, 76]. Accordingly, emotion processing, an important domain of social cognition, is state-dependent in patients with schizophrenia [77*]. SDL has been implicated in the persistence of drug addiction, because being on drugs can be a strategy for gaining better access to information learned while in a drug state [78]. This putative role of SDL may prove of particular relevance given the widespread and increasing use and abuse of recreational and prescription drugs. Taken together, understanding the mechanisms of SDL could help us to better understand both the phenomenology of psychiatric states and actions of psychotropic drugs. By facilitating transfer of information across different states, we might be able to generate more effective treatment approaches for various psychiatric and neurological disorders.
Highlights.
-
▪
SDL can be induced by a variety of endogenous and exogenous stimuli
-
▪
Many drugs supporting SDL converge on GABAergic transmission
-
▪
GABAergic induction of SDL is regulated by microRNAs
-
▪
SDL entails changes of circuit and global network activities
-
▪
SDL is a fundamental mechanism of learning and a gateway to psychopathology
Acknowledgments
Research in the authors’ laboratory was supported by grants MH078064 and MH108837 from the National Institutes of Mental Health to J.R and the Neurobiology of Information Storage Training Grant MH067564 to M.A.A.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
**of outstanding interest
- 1**.Girden E, Culler E. Conditioned responses in curarized striate muscle in dogs. Journal of Comparative Psychology. 1937;23:261–274. This is the first demonstration of SDL. [Google Scholar]
- 2*.Goodwin DW, Powell B, Bremer D, Hoine H, Stern J. Alcohol and recall: state-dependent effects in man. Science. 1969;163:1358–1360. doi: 10.1126/science.163.3873.1358. This study reports SDL under alcohol in humans. [DOI] [PubMed] [Google Scholar]
- 3.Bliss DK. Dissociated learning and state-dependent retention induced by pentobarbital in rhesus monkeys. J Comp Physiol Psychol. 1973;84:149–161. doi: 10.1037/h0035025. [DOI] [PubMed] [Google Scholar]
- 4*.Overton DA. State-Dependent or "Dissociated" Learning Produced with Pentobarbital. J Comp Physiol Psychol. 1964;57:3–12. doi: 10.1037/h0048023. The first implication of GABAAR in SDL comes from this seminl study with a barbiturate. [DOI] [PubMed] [Google Scholar]
- 5**.Pompilio L, Kacelnik A, Behmer ST. State-dependent learned valuation drives choice in an invertebrate. Science. 2006;311:1613–1615. doi: 10.1126/science.1123924. This is the first study implicating SDL in decision making. [DOI] [PubMed] [Google Scholar]
- 6.Bustamante JA, Jordan A, Vila M, Gonzalez A, Insua A. State dependent learning in humans. Physiol Behav. 1970;5:793–796. doi: 10.1016/0031-9384(70)90281-7. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC. Retrieval failures in alcohol state-dependent learning. Psychopharmacology (Berl) 1977;55:141–146. doi: 10.1007/BF01457849. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC. Scopolamine state-dependent memory processes in man. Psychopharmacology (Berl) 1979;64:309–314. doi: 10.1007/BF00427515. [DOI] [PubMed] [Google Scholar]
- 9.Overton DA. In: State dependent learning and drug discriminations. Iversen LL, Iverse SD, Snyder SH, editors. Plenum Press; 1984. [Google Scholar]
- 10.Greenwood PM, Singer JJ. Cortical spreading depression induced state dependency. Behav Biol. 1974;10:345–351. doi: 10.1016/s0091-6773(74)91934-8. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre DC, Gunter JL. State-dependent learning induced by low intensity electrical stimulation of the caudate or amygdala nuclei in rats. Physiol Behav. 1979;23:449–454. doi: 10.1016/0031-9384(79)90042-8. [DOI] [PubMed] [Google Scholar]
- 12.Gray P. Effect of adrenocorticotropic hormone on conditioned avoidance in rats interpreted as state-dependent learning. J Comp Physiol Psychol. 1975;88:281–284. doi: 10.1037/h0076206. [DOI] [PubMed] [Google Scholar]
- 13.Bower GH, Monteiro KP, Gilligan SG. Emotional Mood as a Context for Learning and Recall. Journal of Verbal Learning and Verbal Behavior. 1978;17:573–585. [Google Scholar]
- 14.Woike B, McLeod S, Goggin M. Implicit and explicit motives influence accessibility to different autobiographical knowledge. Pers Soc Psychol Bull. 2003;29:1046–1055. doi: 10.1177/0146167203254504. [DOI] [PubMed] [Google Scholar]
- 15.Holloway FA. State dependent retrieval based on time of day. In: Ho B, Richards D, Chute D, editors. Drug discriminations and state dependent learning. Academic Press; 1978. pp. 319–343. [Google Scholar]
- 16.Evans FJ. Hypnosis and sleep: Techniques for exploring cognitive activity during sleep. Chicago: Aldine; 1972. [Google Scholar]
- 17.Pearce SA, Isherwood S, Hrouda D, Richardson PH, Erskine A, Skinner J. Memory and pain: tests of mood congruity and state dependent learning in experimentally induced and clinical pain. Pain. 1990;43:187–193. doi: 10.1016/0304-3959(90)91072-Q. [DOI] [PubMed] [Google Scholar]
- 18.Godden DR, Baddeley AD. Context-Dependent Memory in 2 Natural Environments - Land and Underwater. British Journal of Psychology. 1975;66:325–331. [Google Scholar]
- 19.Connelly JF, Connelly JM, Phifer R. Disruption of state-dependent learning (memory retrieval) by emotionally-important stimuli. Psychopharmacologia. 1975;41:139–143. doi: 10.1007/BF00421071. [DOI] [PubMed] [Google Scholar]
- 20.Connelly JF, Connelly JM, Timmons JK. Disruption of drug-dependent learning (memory retrieval) using an ethanol drug state: a replication. Psychopharmacology (Berl) 1979;65:319–320. doi: 10.1007/BF00492222. [DOI] [PubMed] [Google Scholar]
- 21.Iwahara S, Noguchi S. Drug-State Dependency as a Function of Overtraining in Rats. Japanese Psychological Research. 1972;14:141–144. [Google Scholar]
- 22**.Overton DA. Historical context of state dependent learning and discriminative drug effects. Behav Pharmacol. 1991;2:253–264. This review article provides a summary of key animal and human studies demonstrating SDL. [PubMed] [Google Scholar]
- 23.Koek W. Drug-induced state-dependent learning: review of an operant procedure in rats. Behav Pharmacol. 2011;22:430–440. doi: 10.1097/FBP.0b013e328348ed3b. [DOI] [PubMed] [Google Scholar]
- 24.Zarrindast MR, Noorbakhshnia M, Motamedi F, Haeri-Rohani A, Rezayof A. Effect of the GABAergic system on memory formation and state-dependent learning induced by morphine in rats. Pharmacology. 2006;76:93–100. doi: 10.1159/000089934. [DOI] [PubMed] [Google Scholar]
- 25**.Jovasevic V, Corcoran KA, Leaderbrand K, Yamawaki N, Guedea AL, Chen HJ, Shepherd GM, Radulovic J. GABAergic mechanisms regulated by miR-33 encode state-dependent fear. Nat Neurosci. 2015;18:1265–1271. doi: 10.1038/nn.4084. This study demonstrates an important contribution of extrasynaptic GABAAR regulated by miR in SDL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anagnostaras SG, Maren S, Fanselow MS. Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem. 1995;64:191–194. doi: 10.1006/nlme.1995.0001. [DOI] [PubMed] [Google Scholar]
- 27.Davis M. Diazepam and flurazepam: effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology (Berl) 1979;62:1–7. doi: 10.1007/BF00426027. [DOI] [PubMed] [Google Scholar]
- 28.Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- 29.Wood SC, Anagnostaras SG. Memory and psychostimulants: modulation of Pavlovian fear conditioning by amphetamine in C57BL/6 mice. Psychopharmacology (Berl) 2009;202:197–206. doi: 10.1007/s00213-008-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weingartner H, Murphy D, Stillman RC. Drug and mood state-specific encoding and retrieval of experience. In: Peterson R, editor. The International Challenge of Drug Abuse, Volume 19 of NIDA Research Monograph. Department of Health, Education, and Welfare, Public Health Service, Alcohol Abuse and Mental Health Administration, National Institute on Drug Abuse, Division of Research; 1978. pp. 210–223. [PubMed] [Google Scholar]
- 31.Nolen TG, Hoy RR. Initiation of behavior by single neurons: the role of behavioral context. Science. 1984;226:992–994. doi: 10.1126/science.6505681. [DOI] [PubMed] [Google Scholar]
- 32.Reus VI. A neuroanatomic perspective on state-dependent learning: the role of the striatum. Acta Neurol Scand Suppl. 1986;109:31–36. doi: 10.1111/j.1600-0404.1986.tb04862.x. [DOI] [PubMed] [Google Scholar]
- 33.Kahn D, Pace-Schott EF, Hobson JA. Consciousness in waking and dreaming: the roles of neuronal oscillation and neuromodulation in determining similarities and differences. Neuroscience. 1997;78:13–38. doi: 10.1016/s0306-4522(96)00550-7. [DOI] [PubMed] [Google Scholar]
- 34*.Weber F, Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538:51–59. doi: 10.1038/nature19773. This article summarizes the defining neurobiological features of sleep states. [DOI] [PubMed] [Google Scholar]
- 35.Gardner EL, Glick SD, Jarvik ME. ECS dissociation of learning and one-way cross-dissociation with physostigmine and scopolamine. Physiol Behav. 1972;8:11–15. doi: 10.1016/0031-9384(72)90121-7. [DOI] [PubMed] [Google Scholar]
- 36.Barnhart SS, Abbott DW. Dissociation of learning and meprobamate. Psychol Rep. 1967;20:520–522. doi: 10.2466/pr0.1967.20.2.520. [DOI] [PubMed] [Google Scholar]
- 37.Romieu P, Lucas M, Maurice T. Sigma1 receptor ligands and related neuroactive steroids interfere with the cocaine-induced state of memory. Neuropsychopharmacology. 2006;31:1431–1443. doi: 10.1038/sj.npp.1300885. [DOI] [PubMed] [Google Scholar]
- 38.Sanday L, Zanin KA, Patti CL, Fernandes-Santos L, Oliveira LC, Longo BM, Andersen ML, Tufik S, Frussa-Filho R. Role of state-dependent learning in the cognitive effects of caffeine in mice. Int J Neuropsychopharmacol. 2013;16:1547–1557. doi: 10.1017/S1461145712001551. [DOI] [PubMed] [Google Scholar]
- 39.Bruins Slot LA, Colpaert FC. Opiate states of memory: receptor mechanisms. J Neurosci. 1999;19:10520–10529. doi: 10.1523/JNEUROSCI.19-23-10520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Nakagawa Y, Iwasaki T, Ishima T, Kimura K. Interaction between benzodiazepine and GABA-A receptors in state-dependent learning. Life Sci. 1993;52:1935–1945. doi: 10.1016/0024-3205(93)90634-f. This paper shows that GABAAR are the main receptor type mediating SDL. [DOI] [PubMed] [Google Scholar]
- 41.Lowe G. Alcohol and state-dependent learning. Subst Alcohol Actions Misuse. 1983;4:273–282. [PubMed] [Google Scholar]
- 42.Petersen RC, Ghoneim MM. Diazepam and human memory: influence on acquisition, retrieval, and state-dependent learning. Prog Neuropsychopharmacol. 1980;4:81–89. doi: 10.1016/0364-7722(80)90064-8. [DOI] [PubMed] [Google Scholar]
- 43.Colpaert FC. A method for quantifying state-dependency with chlordiazepoxide in rats. Psychopharmacology (Berl) 1986;90:144–146. doi: 10.1007/BF00172887. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa Y, Iwasaki T. Involvement of benzodiazepine/GABA-A receptor complex in ethanol-induced state-dependent learning in rats. Brain Res. 1995;686:70–76. doi: 10.1016/0006-8993(95)00453-w. [DOI] [PubMed] [Google Scholar]
- 45.Stein L, Berger BD. Paradoxical fear-increasing effects of tranquilizers: evidence of repression of memory in the rat. Science. 1969;166:253–256. doi: 10.1126/science.166.3902.253. [DOI] [PubMed] [Google Scholar]
- 46.Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 48.Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, et al. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Ferando I, Mody I. Interneuronal GABAA receptors inside and outside of synapses. Curr Opin Neurobiol. 2014;26:57–63. doi: 10.1016/j.conb.2013.12.001. This work presents an overview of interneuronal GABAAR physiology and pharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor delta subunit. Epilepsia. 2002;43(Suppl 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- 51.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mansfield JH, Harfe BD, Nissen R, Obenauer J, Srineel J, Chaudhuri A, Farzan-Kashani R, Zuker M, Pasquinelli AE, Ruvkun G, et al. MicroRNA-responsive 'sensor' transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nat Genet. 2004;36:1079–1083. doi: 10.1038/ng1421. [DOI] [PubMed] [Google Scholar]
- 53.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banigan MG, Kao PF, Kozubek JA, Winslow AR, Medina J, Costa J, Schmitt A, Schneider A, Cabral H, Cagsal-Getkin O, et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS One. 2013;8:e48814. doi: 10.1371/journal.pone.0048814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol Psychiatry. 2011;69:188–193. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stumm RK, Zhou C, Schulz S, Hollt V. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J Comp Neurol. 2004;469:107–118. doi: 10.1002/cne.10997. [DOI] [PubMed] [Google Scholar]
- 57**.Somogyi P, Katona L, Klausberger T, Lasztoczi B, Viney TJ. Temporal redistribution of inhibition over neuronal subcellular domains underlies state-dependent rhythmic change of excitability in the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120518. doi: 10.1098/rstb.2012.0518. This paper discusses the cellular basis of state-dependent neuronal firing by focusing on specific populations of hippocampal interneurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 59.McIntyre DC, Stenstrom RJ, Taylor D, Stokes KA, Edson N. State-dependent learning following electrical stimulation of the hippocampus: intact and split-brain rats. Physiol Behav. 1985;34:133–139. doi: 10.1016/0031-9384(85)90091-5. [DOI] [PubMed] [Google Scholar]
- 60.Girden E. Cerebral mechanisivis in conditioning under curare. American Journal of Psychology. 1941;53:397–406. [Google Scholar]
- 61.Bliss DK, Sledjeski M, Leiman AL. State-dependent choice behavior in the Rhesus monkey. Neuropsychologia. 1971;9:51–59. doi: 10.1016/0028-3932(71)90061-3. [DOI] [PubMed] [Google Scholar]
- 62.Stokes KA, McIntyre DC. Lateralized state-dependent learning produced by hippocampal kindled convulsions: effect of split-brain. Physiol Behav. 1985;34:217–224. doi: 10.1016/0031-9384(85)90109-x. [DOI] [PubMed] [Google Scholar]
- 63.Corcoran KA, Donnan MD, Tronson NC, Guzman YF, Gao C, Jovasevic V, Guedea AL, Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci. 2011;31:11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64**.Ritter P, Born J, Brecht M, Dinse HR, Heinemann U, Pleger B, Schmitz D, Schreiber S, Villringer A, Kempter R. State-dependencies of learning across brain scales. Front Comput Neurosci. 2015;9:1. doi: 10.3389/fncom.2015.00001. The authors summarize evidence suggesting that oscillatory activity defines brain states from synaptic level to communication across brain areas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kletzkin M, Swan K. The effects of meprobamate and pentobarbital upon cortical and subcortical responses to auditory stimulation. J Pharmacol Exp Ther. 1959;125:35–39. [PubMed] [Google Scholar]
- 66.Sadowski B, Longo VG. Electroencephalographic and behavioural correlates of an instrumental reward conditioned response in rabbits. A physiological and pharmacological study. Electroencephalogr Clin Neurophysiol. 1962;14:465–476. doi: 10.1016/0013-4694(62)90052-4. [DOI] [PubMed] [Google Scholar]
- 67.A. L, D.K. B, I. P, M.R. R. Electrophysiological correlates of drug dissociation. Federation Proceedings. 1967:363. [Google Scholar]
- 68.Dejean C, Boraud T, Le Moine C. Opiate dependence induces network state shifts in the limbic system. Neurobiol Dis. 2013;59:220–229. doi: 10.1016/j.nbd.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 69.Newman EL, Gillet SN, Climer JR, Hasselmo ME. Cholinergic blockade reduces theta-gamma phase amplitude coupling and speed modulation of theta frequency consistent with behavioral effects on encoding. J Neurosci. 2013;33:19635–19646. doi: 10.1523/JNEUROSCI.2586-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vyazovskiy VV, Kopp C, Bosch G, Tobler I. The GABAA receptor agonist THIP alters the EEG in waking and sleep of mice. Neuropharmacology. 2005;48:617–626. doi: 10.1016/j.neuropharm.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 71.Sussillo D, Abbott LF. Generating coherent patterns of activity from chaotic neural networks. Neuron. 2009;63:544–557. doi: 10.1016/j.neuron.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72**.Yada Y, Kanzaki R, Takahashi H. State-Dependent Propagation of Neuronal Sub-Population in Spontaneous Synchronized Bursts. Front Syst Neurosci. 2016;10:28. doi: 10.3389/fnsys.2016.00028. This study shows state-dependent generation of various spatiotemporal firing patterns in cortical neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reus VI, Weingartner H, Post RM. Clinical implications of state-dependent learning. Am J Psychiatry. 1979;136:927–931. doi: 10.1176/ajp.136.7.927. [DOI] [PubMed] [Google Scholar]
- 74.Silberman EK, Putnam FW, Weingartner H, Braun BG, Post RM. Dissociative states in multiple personality disorder: a quantitative study. Psychiatry Res. 1985;15:253–260. doi: 10.1016/0165-1781(85)90062-9. [DOI] [PubMed] [Google Scholar]
- 75.Dorahy MJ, Corry M, Shannon M, Webb K, McDermott B, Ryan M, Dyer KF. Complex trauma and intimate relationships: the impact of shame, guilt and dissociation. J Affect Disord. 2013;147:72–79. doi: 10.1016/j.jad.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Renard SB, Pijnenborg M, Lysaker PH. Dissociation and social cognition in schizophrenia spectrum disorder. Schizophr Res. 2012;137:219–223. doi: 10.1016/j.schres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 77*.Maat A, van Montfort SJ, de Nijs J, Derks EM, Kahn RS, Linszen DH, van Os J, Wiersma D, Bruggeman R, Cahn W, et al. Emotion processing in schizophrenia is state and trait dependent. Schizophr Res. 2015;161:392–398. doi: 10.1016/j.schres.2014.11.027. State-dependency of emotion processing was found to be dependent on the disease state. [DOI] [PubMed] [Google Scholar]
- 78.Ross SM, Schwartz CW. State-dependent learning and its implications for treatment of drug-abusers. Psychiatr Q. 1974;48:368–373. doi: 10.1007/BF01562159. [DOI] [PubMed] [Google Scholar]